Abstract
Background:
Systemic candidiasis is a major complication in neutropenic cancer patients undergoing treatment. Most systemic fungal infections emerge from endogenous microflora so the aim of the present study was to identify Candida species isolated from the different regions of body in neutropenic patients in compare with the control group.
Methods:
A total of 309 neutropenic cancer patients and 584 patients without cancer (control group) entered in the study. Molecular identification of clinical isolates was performed by PCR-RFLP technique.
Results:
Twenty-two out of 309 patients had candidiasis (7.1%). Male to female ratio was 1/1 and age ranged from 23 to 66 years. Colorectal cancer and acute myeloid leukemia (AML) were the most common cancers. Candida albicans was the most prevalent Candida species among neutropenic patients (50%) and control group (57.9%). Mortality rate in cancer patients was 13.6% in comparison with control group (5.2%).
Conclusion:
Since candidiasis is an important cause of morbidity and mortality in neutropenic patients, precise identification of Candida species by molecular techniques can be useful for the appropriate selection of antifungal drugs particularly in high risk patients.
Key Words: Candidiasis, Neutropenic patients, Candida species
Systemic candidiasis is an important complication in neutropenic patients and those undergoing treatment for cancer (1). This infection has increased persistently over the past three decades and represents a significant cause of morbidity and mortality among high risk individuals (2). The predisposing factors for systemic candidiasis in neutropenic patients with hematological malignancies differ according to the level of immune suppression and role of the underlying neoplastic process (3, 4). Neutropenia may initiate due to radiation, bone marrow failure (aplastic anemia and myelodysplasia), chemotherapy, and replacement of hematopoietic cells by malignant cells in the bone marrow (3, 5). The digestive tract is the main entrance of Candida species in patients with acute neutropenia and leukemia and a region of endogenous microflora. Invasion of Candida to bloodstream may occur through disruption of the normal anatomical barriers. Candida infections may present as oropharyngeal candidiasis, esophagitis, candidemia, acute or chronic disseminated candidiasis among this population (4, 6, 7). The aim of the present study was to identify Candida species isolated from the different regions of body in neutropenic patients in compare with the control group. Due to the different susceptibilities of the conventional antifungal drugs such as fluconazole and itraconazole, timely and precise identification of Candida spp. would be noteworthy for successful treatment of the infection.
Methods
Isolates: From March 2014 to August 2015, a total of 309 neutropenic patients with suspected candidiasis from two university hospitals were included in the present study. In addition, we provided a control group without cancer comprised of 584 concurrent hospitalized patients in the ICU (274 patients), transplantation ward (169 patients), and general medicine ward (141 patients) who had no any cancer or cancer history. After sampling, all specimens were examined by direct microscopic examination (DM) with 10% potassium hydroxide (KOH), and culture on sabouraud glucose agar (Difco, Detroit, MI, USA), and CHROMagar Candida (Paris, France).
Molecular identification
DNA extraction: The genomic DNA of all isolates was extracted using FTA ® Elute MicroCards (Whatman Inc., Clifton, NJ, USA) (8), following the manufacturer's instructions. Briefly, a loopful of a single colony was suspended in 80-100 μl of distilled water and 5 μl of the suspension was transferred to a disc of FTA card (4 mm in diameter) and incubated at 25°C for at least 5 h. The dried papers were eluted in 400 μl sterile water for 10 seconds, then the paper was transferred to a new microtube containing 40 μl distilled water and incubated at 95 ° C for 15 min. The paper discs were removed and the water including DNA was used for PCR and stored at - 20 °C.
Polymerase chain reaction (PCR): Identification of Candida spp. was performed using the already delineated PCR-RFLP profiles (9-11). Briefly, the ITS1-5.8SrDNA-ITS2 region was amplified using PCR mixture including 5μl of 10 × reaction buffer, 0.4 mM dNTPs, 1.5 mM MgCl2, 2.5 U of Taq polymerase, 30 pmol of both ITS1 (5′ -TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′ -TCC TCC GCT TAT TGA TAT GC-3′) primers (10), and 2μl of extracted DNA in a final volume of 50μl. The PCR cycling conditions comprised: initial denaturation at 94 ° C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 45 s, and extension at 72 ° C for 1 min, with a final extension at 72 °C for 7 min.
Restriction fragment length polymorphism (RFLP): During the second step, PCR products were digested with the restriction enzyme HpaII (Fermentas, Vilnius, Lithuania).
Electrophoresis: Five microliters of each PCR amplicons and 10μl of RFLP products were separated by gel electrophoresis on 1.5 and 2% agarose gel (containing 0.5 μg/ml ethidium bromide), respectively.
Statistical Analysis: Data were analyzed using the SPSS software Version 17.0. Prevalence and types of Candida infection and their distribution were compared according to sex and age in patients and control group. Chi square and Independent sample t-test were used for analyses. A P-value of < 0.05 was considered significant.
Results
Twenty-two out of 309 patients had candidiasis (7.1%). Age range of patients was between 23 and 66 years (mean age, 44.5 years). Male to female ratio was 1/1. Colorectal cancer and acute myeloid leukemia (AML) were the most common cancers accounted for 50% of all cases. Cancer patients included 63.6% with organ and 36.4% with hematological malignancies. Clinical specimens were obtained from urine (59.1%), blood (18.2%), skin lesion (13.6%), soft tissue abscess (4.5%), and abdominal abscess (4.5%). The patients had been hospitalized in haematology ward (59.1%), and ICU (40.9%). Candida albicans was the most prevalent species (50%) followed by C. glabrata (36.3%), and C. tropicalis (13.6%) (fig1).
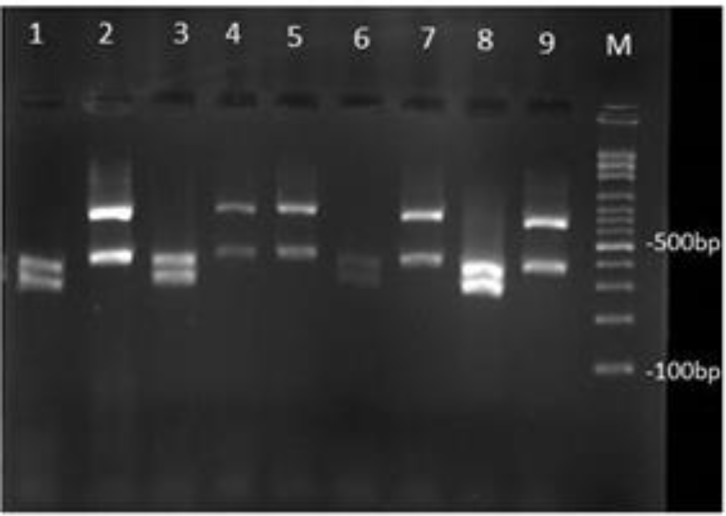
Figure 1.
Agarose gel electrophoresis of ITS-PCR products of various Candida species after digestion with HpaII. Lanes 1,3,6,8 are C. albicans, and Lanes 2, 4, 5, 7, 9 are C. glabrata, and Lane M: 100 bp DNA size marker
Table 1 summarizes the characteristics of all study patients. In the control group, 19 out of 584 patients (3.2%) were infected to different forms of candidiasis (Table 2). The mean age of patients in the control group was 35.4 years. In this group, Candida albicans was also the most common specie (57.9%) followed by C. parapsilosis (21%). There was no case with C. tropicalis infection among Candida strains isolated from the control group.
Table 1.
Details of neutropenic patient with candidiasis
| No | Sex | Age | Hospital wards | Alive/ Deceased | Cancer of | Signs | Location body | WBC count (/µl) |
Neutrophil
(/µl) |
Neutrophil (%) | Candida spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 27 | Haematology | Alive | Breast | Breast lumps | Urine | 1650 | 800 | 48 | C. albicans |
| 2 | M | 39 | ICU | Alive | AML | Weakness | Blood | 2050 | 760 | 37 | C. glabrata |
| 3 | M | 57 | Haematology | Alive | Lung | Cough, Sputum | Urine | 2300 | 950 | 41 | C. albicans |
| 4 | M | 61 | Haematology | Alive | Colon | Gastrointestinal bleeding | Urine | 1400 | 670 | 47 | C. glabrata |
| 5 | F | 40 | ICU | Alive | Osteosarcoma | Pain in the lower femur | Urine | 2700 | 1150 | 42 | C. albicans |
| 6 | F | 34 | ICU | Deceased | AML | Weakness | Blood | 900 | 460 | 51 | C. albicans |
| 7 | F | 42 | ICU | Alive | Colon | Gastrointestinal bleeding | Blood | 3400 | 1200 | 35 | C. albicans |
| 8 | F | 61 | Haematology | Alive | Colon | Gastrointestinal bleeding | Urine | 1400 | 540 | 33 | C. albicans |
| 9 | F | 54 | Haematology | Alive | Breast | Breast lumps | Urine | 2150 | 1200 | 55 | C. albicans |
| 10 | M | 30 | Haematology | Alive | Colon | Gastrointestinal bleeding, Constipation | Urine | 1300 | 670 | 51 | C. glabrata |
| 11 | F | 43 | Haematology | Alive | Hodgkin's lymphoma | Lymphadenopathy | Skin lesion | 2400 | 1100 | 45 | C. tropicalis |
| 12 | M | 51 | ICU | Alive | Colon | Gastrointestinal bleeding | Soft tissue abscess | 1080 | 540 | 50 | C. glabrata |
| 13 | M | 47 | ICU | Deceased | Pancreas | Abdominal lumps | Abdominal abscess | 1800 | 920 | 51 | C. glabrata |
| 14 | M | 24 | Haematology | Alive | Multiple myeloma | Pain in the bones | Urine | 1300 | 450 | 34 | C. glabrata |
| 15 | F | 31 | Haematology | Alive | AML | Weakness | Skin lesion | 3100 | 1050 | 33 | C. tropicalis |
| 16 | F | 54 | Haematology | Alive | Stomach | Gastrointestinal bleeding, Abdominal pains | Urine | 1700 | 840 | 49 | C. albicans |
| 17 | M | 23 | ICU | Alive | Esophagus | Dysphagia | Urine | 2050 | 900 | 43 | C. tropicalis |
| 18 | M | 62 | Haematology | Alive | Colon | Gastrointestinal bleeding | Urine | 2700 | 1100 | 40 | C. albicans |
| 19 | F | 43 | ICU | Deceased | AML | Weakness | Blood | 1400 | 650 | 46 | C. glabrata |
| 20 | M | 40 | Haematology | Alive | AML | Asymptomatic | Urine | 2350 | 1200 | 51 | C. albicans |
| 21 | F | 66 | Haematology | Alive | Hodgkin's lymphoma | Lymphadenopathy, Abdominal pains | Skin lesion | 1900 | 740 | 38 | C. albicans |
| 22 | M | 50 | ICU | Alive | Lung | Hemoptysis | Urine | 2100 | 800 | 38 | C. glabrata |
Table 2.
Control group in the present study; patients with different forms of candidiasis without cancer
| No | Sex | Age | Hospital wards | Alive/ Deceased | Clinical site | Signs | WBC count (/µl) |
Neutrophil
(/µl) |
Neutrophil (%) | Candida spp. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 5 | ICU | Alive | Blood | Fever | 15300 | 10863 | 71 | C. albicans |
| 2 | F | 26 | ICU | Alive | Blood | Fever, Pain of joints | 13400 | 9246 | 69 | C. albicans |
| 3 | F | 18 | Transplantaion Ward | Alive | Urine | Painful urination | 6600 | 4554 | 69 | C. parapsilosis |
| 4 | M | 55 | Transplantaion Ward | Alive | Urine | Fever and chills | 8100 | 3969 | 49 | C. albicans |
| 5 | F | 63 | ICU | Alive | Blood | Fever and chills | 16900 | 9800 | 58 | C. albicans |
| 6 | F | 49 | ICU | Deceased | Blood | Fever | 19400 | 15520 | 80 | C. albicans |
| 7 | F | 38 | General ward | Alive | Vulvovagina | Vulvovaginal discharge | 10500 | 5670 | 54 | C. parapsilosis |
| 8 | F | 11 | Transplantaion Ward | Alive | Blood | Pain and tenderness | 9100 | 6825 | 75 | C. albicans |
| 9 | M | 27 | ICU | Alive | Blood | Fever | 14000 | 9940 | 71 | C. albicans |
| 10 | M | 39 | ICU | Alive | Skin lesion | Inflammatory, Pruritus | 8200 | 5330 | 65 | C. parapsilosis |
| 11 | F | 41 | Transplantaion Ward | Alive | Urine | Fever and chills | 9450 | 6140 | 65 | C. albicans |
| 12 | M | 17 | ICU | Alive | Catheter | Fever | 21000 | 11130 | 53 | C. albicans |
| 13 | M | 14 | ICU | Alive | Blood | Fever | 11700 | 8892 | 76 | C. albicans |
| 14 | M | 55 | ICU | Alive | BAL | Cough, Chest pain | 11050 | 6630 | 60 | C. krusei |
| 15 | F | 69 | Transplantaion Ward | Alive | Blood | Fever | 14900 | 10280 | 69 | C. albicans |
| 16 | M | 27 | General ward | Alive | Urine | Fever | 8800 | 5016 | 57 | C. kefyr |
| 17 | F | 20 | General ward | Alive | Skin lesion | Pruritus | 7600 | 5320 | 70 | C. glabrata |
| 18 | F | 48 | General ward | Alive | Urine | Asymptomatic | 12650 | 7843 | 62 | C. parapsilosis |
| 19 | F | 51 | ICU | Alive | Perleche | Pruritus | 6550 | 4322 | 65 | C. glabrata |
Twelve patients (63.1%) were females and 7 control patients (36.8%) were males, age ranging from 5 to 69 years. Surprisingly, all Candida species that were isolated from blood stream were C. albicans. Mortality rate in cancer patients (13.6%) was significantly higher than the control group (5.2%).
Candida infection in cancer patients was greater than the control group [OR (CI 95%): 2.28 (1.21-4.28%), P=0.009] (table 3).
Table 3.
Statistical analysis of candidosis among neutropenic patients and control group
| Factors | Cancer(n=309) | Control(n=584) | P value |
|---|---|---|---|
| Age(year) | 44.50±12.91 | 35.42±18.83 | 0.076□ |
|
Sex
Male Female |
11(50.0%) 11(50.0%) |
7(36.8%) 12(63.2%) |
0.397□ |
|
Candidiasis
Yes No |
22(7.1%) 287(92.9%) |
19(3.3%) 565(96.7%) |
0.009□ |
Data Showed Mean±SD and n(%),: Used of Independent sample t test, : Used of Chi-Square
Discussion
Most fatal Candida infections result from endogenous host microbiota (9, 10). Colonization due to the non-Candida albicans spp. is increasing (2, 11, 12), and in recent years significant increase in frequency of blood stream isolated infection has been reported in particular Candida infection due to C. krusei, C. tropicalis and C. glabrata in high risk population, like patients with neutropenia is of serious concern. In the present study, we also isolated 2 out of 4 (50%) C. glabrata from cancer patients with candidemia.
However, no C. glabrata strain was isolated from the bloodstream infection in the control group. The intestinal tract is the main source for hematogenous Candida invasion (13-15). Mortality rate was 13.6% and 5.2% in neutropenic patients and control group, respectively. As expected, mortality rate in patients with candidemia was the highest in both groups. There has been a crucial shift in the causes of blood stream Candida infection from C. albicans toward non-albicans Candida species in neutropenic patients (4), but C. albicans was the most prevalent strain isolated from candidemia in the control group. Candidemia in neutropenic patients may be complicated by chronic disseminated candidiasis of eyes, spleen, liver, kidney, and abdomen (16). We also showed two patients (9.1%) with soft tissue abscess, and abdominal abscess as a result of chronic disseminated candidiasis.
Among the patients with candiduria, 7 patients (53.8%) had lower urinary tract symptoms (LUTS) (such as painful urination, increased frequency of urination, and incomplete voiding), 2 patients (15.4%) had upper urinary tract symptoms (UUTS) (including fever, chills, pain and tenderness, nausea, and vomiting), and 4 (30.7%) cases were asymptomatic, compared to the control group that 2 patients (40%) had UUTS, 2 patients (40%) with LUTS, and 1 patient (20%) was asymptomatic.
The prevalence of candiduria is associated with antibiotic use (17), and varies in different hospital wards, being most prevalent in intensive care units (ICUs) (18) however, in the present study, only two patients with candiduria were hospitalized in ICU (in cancer patients) and also none of the patients in control group with candiduria hospitalized in ICU. Some studies showed that a low percentage (1-8%) of candiduric patients presents candidemia (19-21), however patients with candiduria in the present investigation did not shift toward bloodstream Candida infection except a patient undergoing kidney transplantation in the control group. In contrast to our findings, in many investigations C. parapsilosis complex was the main Candida species that is associated with candidiasis, containing candiduria (22-24), nevertheless, we did not isolate any C. parapsilosis complex from neutropenic patient whereas, 4 cases of C. parapsilosis (21%) isolates were identified in the control group. Afraseyabi et al. (25) isolated 19 Candida spp. from 60 cancer patients (31.6%). They reported gastrointestinal and breast cancer as the most frequent cancer whereas, colorectal cancer and acute myeloid leukemia (AML) were the most common cancers in the present study. Shokohi et al. (26) reported Candida albicans as the most common species among 80 neutropenic patients with candidosis (77.5%), followed by C.glabrata (15%), C. tropicalis (5%) and C. krusei (2.5%). Saltanatpouri et al. (27) reported C.albicans as the most prevalent Candida strain isolated from candidiasis in cancer patients. Brain tumor and esophageal cancer were the most frequent cancers in their investigation. Of the 68 blood samples collected from cancer patients, Kalantar et al. (28) showed that five (7.35%) were positive for Candida spp., 2 (40%) of which were identified as C. albicans and 3 (60%) were Candida non-albicans.
In conclusion, neutropenic population which has noticeable colonization with Candida spp particularly in different parts of the body and presence of C. glabrata, C. tropicalis or C. krusei should be considered as higher risk of mortality. Administration of fluconazole seems to be reasonable in preventing candidiasis due to C. albicans in neutopenic patients, but strategies to decrease Candida infections by nontriazole susceptible to Candida species like C. glabrata are unreliable. Due to the fact that candidiasis is connected with high morbidity and mortality rate among neutropenic patients, and emerging of antifungal resistance among Candida isolates, epidemiological data and susceptibility patterns of colonized Candida species may be useful for clinicians to select the best therapeutic choice for the management of infection among high-risk cases.
Acknowledgments
The authors express their appreciation to Al-Zahra and Seyed-Al-Shohada Hospitals’ personnel.
Funding: This study was not supported financially by any governmental or other public sources.
Conflict of Interest: None declared.
References
- 1.Uzun O, Ascioglu S, Anaissie EJ, Rex JH. Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis. 2001;32:1713–17. doi: 10.1086/320757. [DOI] [PubMed] [Google Scholar]
- 2.Safdar A, Chaturvedi V, Cross EW, et al. Prospective study of Candida species in patients at a comprehensive cancer center. Antimicrob Age Chemother. 2001;45:2129–33. doi: 10.1128/AAC.45.7.2129-2133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sipsas NV, Bodey GP, Kontoyiannis DP. Perspectives for the management of febrile neutropenic patients with cancer in the 21st century. Cancer. 2005;103:1103–13. doi: 10.1002/cncr.20890. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Gamaletsou MN. Treatment of fungal disease in the setting of neutropenia. Hematology Am Soc Hematol Educ Program. 2013;2013:423–7. doi: 10.1182/asheducation-2013.1.423. [DOI] [PubMed] [Google Scholar]
- 5.Bow EJ. Neutropenic fever syndromes in patients undergoing cytotoxic therapy for acute leukemia and myelodysplastic syndromes. Semin Hematol. 2009;46:259–68. doi: 10.1053/j.seminhematol.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Wiederhold N, Najvar L, Bocanegra R, Kirkpatrick W, Patterson T. Comparison of anidulafungin’s and fluconazole’s in vivo activity in neutropenic and non‐neutropenic models of invasive candidiasis. Clin Microbiol Infect. 2012;18:E20–3. doi: 10.1111/j.1469-0691.2011.03712.x. [DOI] [PubMed] [Google Scholar]
- 7.Miceli MH, Díaz JA, Lee SA. Emerging opportunistic yeast infections. The Lancet Infect Dis. 2011;11:142–51. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 8.Borman AM, Linton CJ, Miles SJ, Campbell CK, Johnson EM. Ultra-rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology–a reusable DNA archiving system. Med Mycol. 2006;44:389–98. doi: 10.1080/13693780600564613. [DOI] [PubMed] [Google Scholar]
- 9.Safdar A, Armstrong D. Infectious morbidity in critically ill patients with cancer. Crit Car Clin. 2001;17:531–70. doi: 10.1016/s0749-0704(05)70198-6. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller MA. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis. 1996;22:S89–94. doi: 10.1093/clinids/22.supplement_2.s89. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi R, Badiee P, Badali H, et al. Use of restriction fragment length polymorphism to identify Candida species, related to onychomycosis. Adv Biomed Res. 2015;4:95. doi: 10.4103/2277-9175.156659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol. 2013;51:657–63. doi: 10.3109/13693786.2013.770603. [DOI] [PubMed] [Google Scholar]
- 13.Bernhardt H, Knoke M. Mycological aspects of gastrointestinal microflora. Scandinavian J Gastro Suppl. 1996;222:102–6. doi: 10.1080/00365521.1997.11720731. [DOI] [PubMed] [Google Scholar]
- 14.Safdar A, Armstrong D. Immune reconstitution Prospective evaluation of Candida species colonization in hospitalized cancer patients: impact on short-term survival in recipients of marrow transplantation and patients with hematological malignancies. Bone Marr Trans. 2002;30:931–5. doi: 10.1038/sj.bmt.1703732. [DOI] [PubMed] [Google Scholar]
- 15.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 16.Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 2010;51:295–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger M, Sweet S, Leibovici L, Pitlik S, Samra Z. Correlation between candiduria and departmental antibiotic use. J Hosp Infect. 2003;53:183–6. doi: 10.1053/jhin.2002.1354. [DOI] [PubMed] [Google Scholar]
- 18.Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:S72–5. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 19.Fiorante S, López-Medrano F, Lizasoain M, et al. Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kid Inter. 2010;78:774–81. doi: 10.1038/ki.2010.286. [DOI] [PubMed] [Google Scholar]
- 20.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–73. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–73. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linder N, Klinger G, Shalit I, et al. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother. 2003;52:663–7. doi: 10.1093/jac/dkg419. [DOI] [PubMed] [Google Scholar]
- 23.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–25. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh R, Parija S. Candida parapsilosis: an emerging fungal pathogen. The Indian J Med Res. 2012;136:671–3. [PMC free article] [PubMed] [Google Scholar]
- 25.Afraseyabi SH, Afkhamzadeh A, Sabori H, et al. Oral candidiasis amongst cancer patients at Quds hospitals in Sanandaj. Afr J Clin Exp Microbiol. 2011;12:129–32. [Google Scholar]
- 26.Shokohi T, Hashemi Soteh MB, Saltanat Pouri Z, Hedayati MT, Mayahi S. Identification of Candida species using PCR-RFLP in cancer patients in Iran. Indian J Med Microbiol. 2010;28:147–51. doi: 10.4103/0255-0857.62493. [DOI] [PubMed] [Google Scholar]
- 27.Saltanatpouri Z, Shokohi T, Hashemi Soteh MB, Hedayati MT. PCR-RFLP is a useful tool to distinguish between C Dubliniensis and C albicans in cancer patients in Iran. Inter J Hematol Oncol Stem Cell Res. 2010;4:14–18. [Google Scholar]
- 28.Kalantar E, Assadi M, Pormazaheri H, et al. Candida non albicans with a High Amphotericin B Resistance Pattern Causing Candidemia among Cancer Patients. Asian Pac J Cancer Prev. 2014;15:10933–35. doi: 10.7314/apjcp.2014.15.24.10933. [DOI] [PubMed] [Google Scholar]