Abstract
Background
To identify the effects of microRNA (miR)-219-5p on morphine-induced apoptosis by targeting WEE1.
Material/Methods
Forty Balb/C mice (Toll-like receptor 9, TLR9 knockout) were randomly allocated to the experimental and control groups (20 in each group). The baseline miR-219-5p expression was detected using quantitative real-time PCR (qRT-PCR). After morphine was injected at 6 h on the 2nd and 6th days, experimental and control groups received miR-219-5p mimics or miRNA-negative control (NC), respectively, compound injection. Tissues and cells were later obtained from subjects in each group separately after mice were killed. TUNEL assay was used to investigate apoptosis in both groups. RAW264.7 cells were treated with miR-219-5p mimics and controls, respectively. After 24 h, 10 μM of morphine was added at 24 h. Cell apoptosis was assessed by flow cytometer. The WEE1 and Phospho-cdc2 (Tyr15) expressions were examined by Western blotting.
Results
MiR-219-5p expression in the experimental group was significantly lower than that in the control group (P<0.05). Mice injected with miR-219-5p mimic experienced an evident increase in apoptosis rate compared with the control group (P<0.05). The miR-219-5p NC group and the morphine group both presented an elevated apoptosis rate compared with the blank control group (both, P<0.05). The apoptosis rate in the miR-219-5p mimic group was 10.06%, remarkably lower than in the miR-219-5p NC group and blank control group (both P<0.05). WEE1 and Tyr15 protein expressions in the miR-219-5p NC group and morphine group were obviously stronger than those in the blank control group (all P<0.05). In the miR-219-5p mimic group, WEE1 and Tyr15 protein expressions were significantly lower compared with those in the miR-219-5p NC group and morphine group (all P<0.05).
Conclusions
Morphine significantly downregulated the expression of miRNA-219-5p, which targets WEE1 to suppress Tyr15 expressions and activate Cdc2, thus inhibiting the morphine-induced macrophage apoptosis.
MeSH Keywords: Apoptosis, Macrophage Activation, MicroRNAs, Morphine
Background
Opioids have been widely used for pain relief in humans for centuries; nevertheless, the long-term use of an opioid for the treatment of chronic non-cancer pain continues to be controversial due to benefits and adverse effects [1]. Opioids, including morphine, lead to cell apoptosis in various organ systems [1,2]. Endogenous morphine, in a parallel fashion to NO (nitric oxide)-coupled signaling systems, has been found to function as a regulator of metabolic homeostasis, energy metabolism, mitochondrial respiration, and energy production [3]. Morphine is a combined κ-, δ-, and μ-opioid receptor agonist, and it has very high affinity to the μ-opioid receptor. Its influence on opioid receptors in the central nervous system, including tolerance, analgesia and respiratory depression, has been well documented throughout past decades [4]. Morphine has been found to severely influence the immune system by modulating functions of variety of cells like phagocytes, T cells, and dendritic cells, and then lead to apoptosis in neuron, immune as well as cancer cells [5–7]. Dysregulation of apoptosis may contribute to various pathologic processes. For instance, excessive apoptosis damages the completeness of the endothelial barrier and is correlated with atherosclerosis, ischemia, autoimmune, neurodegeneration, cholestasis, inflammatory disease, and tumor-related metastasis [8,9]. Although opioid receptors are significantly involved in the processes of opioids-induced effects, the antagonists of opioid receptors can only partially block these effects [5]. Hence, the specific cellular and molecular mechanisms of opioids-induced apoptosis remain unclear.
In recent years, a class of noncoding RNAs of 17–30 nucleotides, called microRNAs (miRNAs), belonging to the most abundant class of small RNAs in animals, were reported to act as key regulators of processes such as embryonic development, cell proliferation, cell growth, tissue differentiation, and apoptosis [10,11]. The miRNAs can decrease gene expressions through binding to the 3′ untranslated region (3′UTR) of target messenger RNAs (mRNAs) [12]. Several research groups have verified that miR-1d, miR-7, miR-148, miR-204, miR-210, miR-216, and miR-296 decrease apoptosis level, while miR-214, miR-486, miR-15, and miR-16 increase it [13–16]. There is accumulating evidence showing that miR-219 inhibits cell proliferation through observing that overexpression of miR-219 induced apoptosis in zebrafish [12,17]. MiR-219 regulates Sox6, PDGFRa, Hes5, ZFP238, and FoxJ3 levels during oligodendrocyte differentiation; furthermore, overexpression of miR-219-5p in glioma cell lines greatly suppresses proliferation, anchorage-independent growth, and glioma cell migration [18–20]. However, few reports have assessed the correlation between miR-219-5p and apoptosis. WEE1, a tyrosine kinase and a key regulator of cell cycle progression, is over-expressed in breast cancer and hepatocellular carcinoma, and is related to low disease-free survival rate in malignant melanoma patients [21,22]. The inhibition of WEE1, in breast cancer, leads to obvious decrease in cell proliferation and increase in apoptotic levels [23]. WEE1 may be a target gene of miR-219-5p, but the mechanism remains relatively unexplored [24]. Toll-like receptors (TLRs), a kind of pattern recognition receptor, has a distinct function in pathogen-associated molecular patterns (PAMPs) recognition and immune responses [25]. Furthermore, TLRs are key players in microglia activation and immune response in the central nervous system (CNS) [26]. Therefore, the aim of this study is to examine the effects of miR-219-5p on morphine-induced apoptosis by targeting WEE1 in TLR9 knockout Balb/C mice.
Material and Methods
Ethics statement
The animal experimental protocols followed the guidelines of Ningbo No. 2 Hospital and were approved by the Institutional Animal Ethics Committee. Efforts were made to minimize the suffering of animals.
Experimental animals
Balb/C male mice (TLR9 knockout) aged 6–8 weeks were provided by the Animal Center of Ningbo No. 2 Hospital. Mice were raised separately in the same animal lab with constant temperature (22°C) and humidity (relative humidity of 50%), and a regular 12-h light cycle. RAW264.7 cell lines were purchased from the Cell Bank of the Chinese Academy of Sciences.
Study design
Forty Balb/C mice (TLR9 knockout) were randomly assigned to 1 of the 2 groups: experimental group and control group (20 in each). Mice in the experimental group were treated with morphine dissolved in phosphate-buffered saline (PBS) buffer injected through the abdominal cavity twice a day for 8 days, with gradient dosages of 10 mg/kg, 20 mg/kg, 40 mg/kg, 80 mg/kg, 100 mg/kg, 120 mg/kg, 140 mg/kg, and 140 mg/kg each day. The control group received an equal volume of PBS and was treated in the same way as the experimental group. The experimental group received tail injection of miR-219-5p mimics with dosage of 7 mg/kg at 6 h after morphine injection on the 2nd and 6th days; the control group received an equal volume of synthetic miRNA-negative control compound injection.
Isolation and culture of mouse peritoneal macrophages
Three days before being killed, mice were treated with l ml of sterile thiol acetic acid through the abdominal cavity (injection into the intestine was avoided). Cervical dislocation was accomplished by placing a stout pair of scissors across the neck and rapidly pulling on the tail to break the neck of each mouse, and then the whole body was immersed in 70% ethyl alcohol for 3 to 5 s. Animals were placed at the autopsy table with all 4 limbs fixed with syringe needles, then tweezers were used to tear the skin of the belly to expose the parietal peritoneum, but not hurting it. Then, the abdominal peritoneum was washed using 70% ethyl alcohol; 10 ml of Eagle’s medium was injected through the abdominal cavity and, using our fingers, we kneaded the abdominal peritoneum on both sides. The abdominal peritoneum was raised with a needle head to make the body lean to one side; and the liquid was collected into the needle. The needle head was pulled out carefully; the liquid was transferred into a centrifuge tube and centrifuged at 250 g for 10 min at 4°C; supernatant was removed and 10 ml of Dulbecco Modified Eagle’s Medium (DMEM) was added. For cell culture purification, other leucocytes were removed from the broth several hours after vaccination. After washing twice, fresh DMEM was added and developed in the CO2 incubator at 37°C. After the isolation of peritoneal macrophages, each mouse was celiotomized to separate the liver and spleen. Tissues were preserved in liquid nitrogen at −80°C for further use.
MiR-219-5p expression detected using quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cultured peritoneal macrophages, as well as liver and spleen tissues, using Trizol Reagent (QIAGEN, Germany). RNA purity and concentration were measured by using an ultraviolet spectrophotometer and RNA integrity was confirmed using 1% agarose gel electrophoresis. Then, reverse transcriptional reaction was performed by applying 5 μl of RNA according to the manufacturer’s protocol. The miR-219-5p expressions after performing qRT-PCR in different groups were measured using the miScript II RT Kit (QIAGEN, Germany). Amplification of U6 was used as internal control. PCR primers used in our study were synthesized by Guangzhou Land Biological Technology Co., Ltd (Guangzhou, China). Designed PCR primers for miR-219-5p were: Forward primer: 5′-ACACTCCAGCTGGGTGATTGTCCAAACGCAAT-3′; Reverse primer: 5′-CTCAACTGGTGTCGTGGA3′; PCR primers for U6: Forward primer: 5′-CTCGCTTCGGCAGCACA-3′; Reverse primer: 5′-AACGCTTCACGAATTTGCGT-3′. The total PCR reaction system contains: 5×RT Buffer 4.0 μl, Enzyme mix 2.0 μl, Template total RNA 4.0 μl, SYBR Green master mix 10.0 μL, PCR Primer mix 2.0 μL, Diluter cDNA template 8.0 μL. The reaction conditions were: predenaturing (95°C, 10 min); denaturing (95°C, 10 s); elongation (60°C, 1 min); total 40 cycles. The amplification efficiency of the target gene was 97%, which was similar to the reference gene efficiency of 98%; thus, the ΔΔCT calculation method could be applied. The relative value of miR-219-5p was expressed using the formula:
Apoptosis detection by TUNEL
RAW264.7 cells were seeded into a 12-well plate (1×105/well) and developed in an incubator. The TUNEL kit (Roche, USA) was used to evaluate cell apoptosis. The cell culture medium was absorbed; cells were washed 3 times using PBS; cover glasses were fixed by using 4% paraformaldehyde for 60 min at room temperature. TUNEL was added on cover glasses and developed at room temperature for 10 min. The No. 1 and No. 2 TUNEL reaction liquids were mixed at the ratio of 1:9; the mixture was added in each cover glass and developed in a constant temperature of 37°C away from light for 1 h. The No. 3 TUNEL reaction liquid was added after the mixture was washed 3 times with PBS and reacted in a constant temperature of 37°C away from light for 30 min. The colored solution was mixed with stromal cells liquid at the ratio of 1:50 and added into a cover glass. Cells were colored and observed through a microscope and then the sheet was sealed and photographed.
Culture and transfection of RAW264.7 cells
RAW264.7 cell lines were incubated in DMEM containing 10% fetal bovine serum (FBS) at 37°C with 5% CO2, and the liquid was changed every 24–48 h. Cells in different growth periods were transferred on the top of 6-well plates. Four groups were assigned: (1) blank control group (no treatment); (2) morphine group (cell apoptosis induced by 10 μM of morphine, no transfection of miRNA); (3) miR-219-5p negative control (NC) group (cell apoptosis induced by 10 μM of morphine, and transfection of miR-219-5p NC); and (4) miR-219-5p mimic group (cell apoptosis induced by 10 μM morphine, and transfection of miR-219-5p mimics). When cells reached 80% confluence, the culture medium was refreshed. Lipofectamine TM2000 (Invitrogen, USA) and OPTI-MEM (Life Technologies, Gaithersburg, MD) were used to transfect cells with mimics and NC; after 8 h, the liquid was replaced; after 24 h, the cells were treated with morphine; and after 24 h and 48 h, cells were obtained for further apoptosis testing and Western blotting assay.
Dual luciferase reporter system
Twenty-four hours before transfection, RAW264.7 cell lines were incubated in 24-well plates with DMEM containing 10% FBS at the density of 1×105. The used culture medium was suctioned, and was washed by PBS 3 times. We added 300 μL OPTI-ME to transfect the culture medium, then placed it in the incubator with 5% CO2 at 37°C. Diluted Lipofectin2000 was placed at 20°C for 5 min; 20 mM miR-219-5p mimics and 1 μg WEE1 (3′UTR sequence of WEE1 cloned into a proper plasmid) were added into each plate (Invitrogen, Shanghai, China). Transfection of miR-NC was used as control. DMEM was then added up to 50 μL, and we kept it at 20°C for 5 min. The above reagents were mixed and kept at 20°C for 30 min. Then, 200 μL of mixture was transferred onto each plate, blended carefully, and incubated with 5% CO2 at 37°C for 6 h. After 48 h of transfection, the used culture medium was discarded, washed 3 times using PBS, then 200 mL PLB was added into each plate and swirled carefully at 20°C for 20 min, and the cell lysates were obtained. Afterwards, 30 μL of cell lysates were added onto the top of the fluorescent screen and the background value was read for 3 s. We added 50 μL of LARII reagents to each sample and mixed the sample, then the value was read for 3 s. After reading the value, 100 μL of Stop & Glo Reagent (Promega, USA) was added to each sample and mixed quickly; each mixture was transferred into the luminometer, and the results were obtained and recorded for 3 s.
Flow cytometer
The cells were developed up to 85%, and culture medium was removed and washed 2 times using PBS. Cells were treated by trypsin without EDTA for 30 s; serum was used to stop digestion, the cell suspension was beat and mixed, and collected in 2 ml micro-centrifuge tubes. Cells were washed 2 times using PBS (centrifugation in 2000 rpm for 5 min) and 1–5×105 of cells were collected. We added 500 μl of binding buffer suspension cells. Then, 5 μl of Annexin V-FITC was added and mixed; 5 μl Propidium Iodide was also added and mixed and the cells were incubated for an additional 15 min in the dark at room temperature. The mixture was then analyzed using flow cytometry.
Western blotting
The WEE1 and Phospho-cdc2 (Tyr15) expressions were examined by Western blotting. A nuclear protein and plasma protein extraction kit (Generay, Shanghai, China) was used to extract nuclear protein, which was separated using 11% polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto the top of the polyvinylidene fluoride (PVDF) membrane; PBS (PH 7.2) containing 6% dried skim milk was used to block at room temperate for 1–2 h; afterwards, the blocked PVDF membrane was placed in a freezer at 4°C overnight. Wee1 antibody and Tyr15 antibody (all at 1:500 dilution; Cell Signaling Technology) and Histone H3 antibody (at 1:1000 dilution; Cell Signaling Technology) were add as the primary antibody. After staying at room temperate for 1 h, it was washed with PBS, and human antibody against rabbit antibiotin-horseradish peroxidase (HRP) -IgG at 1:2500 dilution was added. At the same time, 40 μL of HRP antibodies at 1: 1000 dilution was added for reaction at room temperate for 1 h. Then, chemiluminescence reagent was added and immunoautoradiography was used to assess protein expressions.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Data are expressed as mean±standard deviation (SD). The Mann-Whitney U test was used to compare the relative values of miR-219-5p between the 2 groups. The comparison among other groups was estimated by t test. P<0.05 was considered as statistically significant.
Results
Morphine down-regulates miR-219-5p expression
As shown in Figure 1, we obtained mouse peritoneal macrophages treated with morphine. Our qRT-PCR results showed that the miR-219-5p expression in the experimental group was significantly lower than that in the control group (P<0.05). In vivo, miR-219-5p expressions in 2 important immune organs (the liver and spleen) were also estimated using qRT-PCR. The results demonstrated that miR-219-5p expression in the experimental group was significantly lower compared with that in the control group (all P<0.05).
Figure 1.
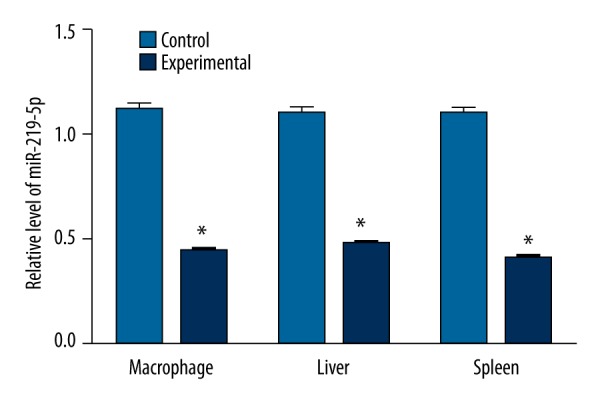
Expressions of microRNA-219-5p in macrophage, liver and spleen between control group and experimental group, * compared with control group, P<0.05.
Morphine-induced mouse peritoneal macrophages apoptosis was inhibited by miR-219-5p
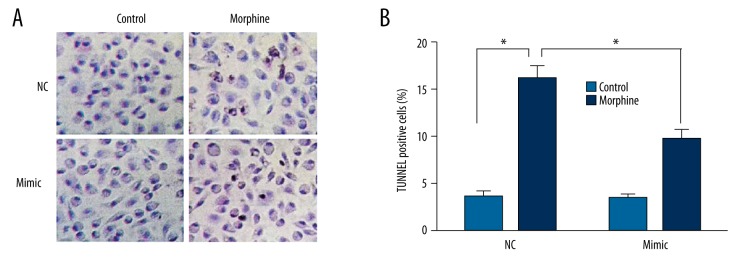
Cells were injected with miR-219-5p mimics after morphine rejection at 2 and 6 days through the caudal vein to explore the effects of morphine on miR-219-5p. Abdominal cavity macrophages were purified and obtained and cell apoptosis was examined. As shown in Figure 2, cell apoptosis was detected in the morphine group. The number and rate of cell apoptosis in mouse peritoneal macrophages, which were injected with miR-219-5p mimics, were significantly lower than those in the control group (P<0.05).
Figure 2.
MicroRNA-219-5p could inhibit the function of morphine-induced apoptosis; * P<0.05.
Target gene of miR-219-5p
The results of target gene of miR-219-5p from Target Scan software (http://www.targetscan.org) showed a 3′UTR sequence of WEE1 was matched with it (Figure 3A). The results of dual luciferase reporter system revealed that the luciferase activity was inhibited by adding miR-219-5p after cloning WEE1 3′UTR into luciferase reporter plasmids; while after the mutation of WEE1 3′UTR and miR-219-5p binding site, the luciferase activity could not be suppressed anymore (Figure 3B). The above results indicate that miR-219-5p is bound to the base of the WEE1 mRNA 3′ UTR region and that WEE1 may be the target gene of miR-219-5p.
Figure 3.
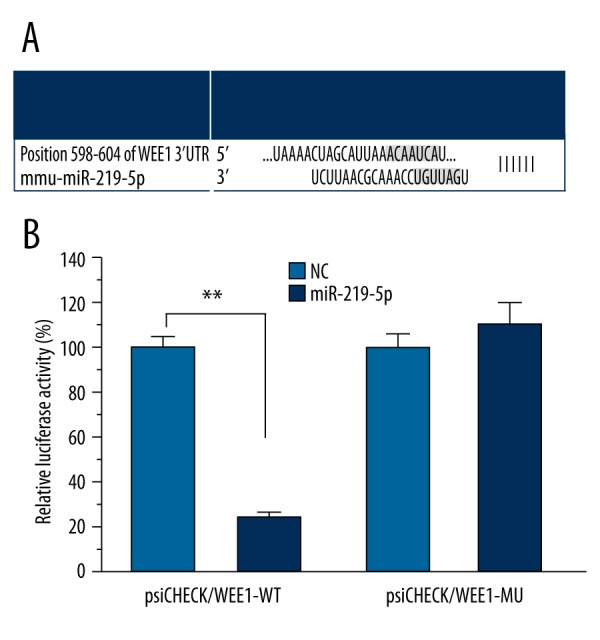
WEE1 may be a target gene of miR-219-5p (A: A 3′UTR sequence of WEE1 was matched with miR-219-5p detected using Target Scan software; B: Dual luciferase reporter system was applied for testing the gene activity. transfection of microRNA-219-5p and mutant WEE1 mRNA 3′ untranslated regions (3′UTR) expression vector group, the relative activity of luciferase was significantly decreased in transfection of microRNA-219-5p and wild type WEE1 mRNA 3′ UTR expression vector group (P<0.01), while no obvious change of the relative activity of luciferase was discovered in the transfection of microRNA-219-5p and mutant WEE1 mRNA 3′UTR expression vector group (P>0.05)).
MiR-219-5p inhibits morphine-induced macrophage apoptosis
As shown in Figure 4, the cell apoptosis analysis results showed that apoptosis rates of the miR-219-5p NC group and the morphine group were significantly higher than in the blank control group (all P<0.05). Cells apoptosis of the miR-219-5p mimic group, which had been transfected with miR-219-5p, was significantly lower than in the miR-219-5p NC group and the morphine group, and the difference was significant (both P<0.05).
Figure 4.
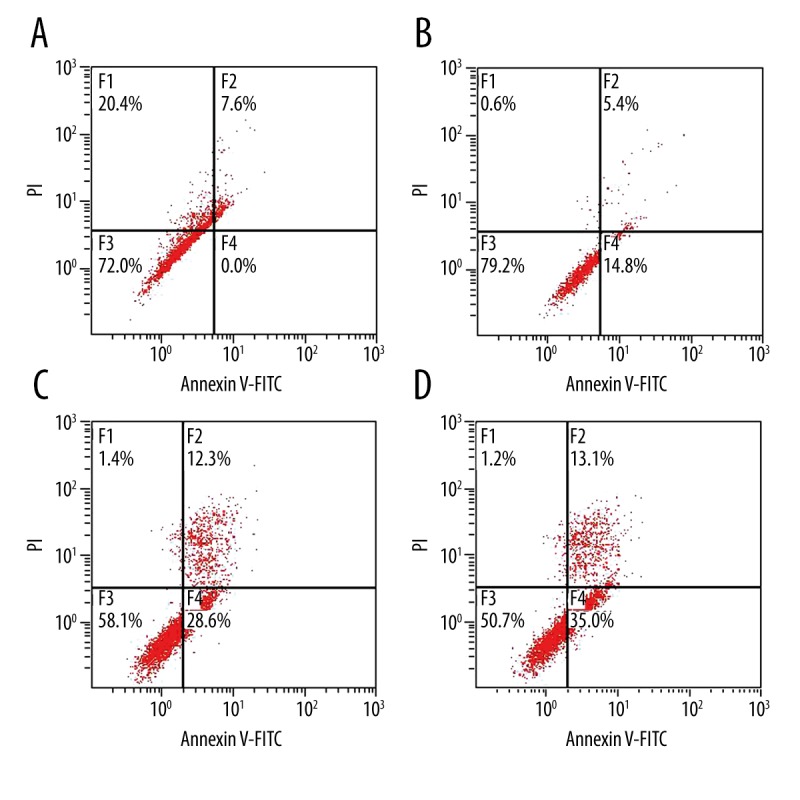
MicroRNA-219-5p could inhibit the function of morphine-induced apoptosis (A: Cell apoptosis in the blank control group; B: Cell apoptosis in the microRNA-219-5p mimic group; C: Cell apoptosis in the microRNA-219-5p negative control group; D: Cell apoptosis in the morphine group).
Expressions of WEE1 and Phospho-cdc2 (Tyr15)
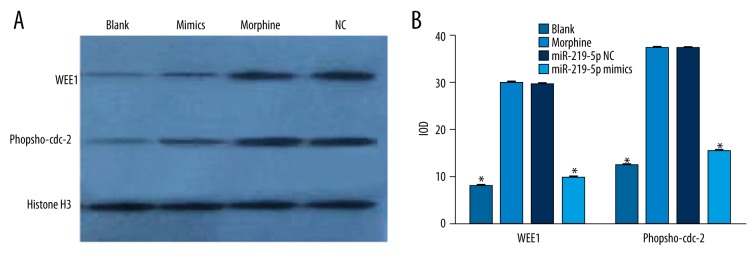
We examined the WEE1 and Tyr15 protein expressions in RAW264.7 cells after treatment with morphine. As shown in Figure 5, WEE1 protein expressions in the blank control group, miR-219-5p NC group, morphine group, and miR-219-5p mimic group were 7.65±0.15, 29.30±0.18, 29.52±0.20, and 9.51±0.29, respectively. Tyr15 protein expressions in the blank control group, miR-219-5p NC group, morphine group, and miR-219-5p mimic group were 12.16±0.13, 37.02±0.11, 37.10±0.12, and 15.15±0.20, respectively. The WEE1 and Tyr15 protein expressions in the miR-219-5p NC group and the morphine group were obviously higher than those in the blank control group (all P<0.05). In the miR-219-5p mimic group, WEE1 and Tyr15 protein expressions were significantly lower than in the miR-219-5p NC group and morphine group (all P<0.05). However, we observed no significant difference in WEE1 and Tyr15 protein expressions between the miR-219-5p NC group and morphine group (both P>0.05).
Figure 5.
WEE1 and Phospho-Cdc2 (Tyr15) expressions in RAW264.7 cells (A: Western Blots of WEE1 and Tyr15; B: Protein expressions of WEE1 and Tyr15); * compared with blank control group and miR-219-5p negative control group, P<0.05.
Discussion
Apoptosis, a programmed process of cell death, thereby removing damaged cells without provoking inflammation, is considered a vital component of tissue development and homeostasis [27,28]. It features chromatin condensation, DNA fragmentation, plasma membrane blebbing, and cell shrinkage due to decrease in cytoplasm and organelles. Ultimately, membrane-bound apoptotic bodies, which contain cytosol and processed organelles, are generated and removed by macrophages [29]. Inappropriate apoptosis is an influential factor in various pathologic processes, including excessive apoptosis that damages the completeness of the endothelial barrier; it is also correlated with atherosclerosis, infection, autoimmune and inflammatory diseases, and tumor-related metastasis [8,9]. Research focusing on the elucidation and analysis of the cell cycle machinery and signaling pathways discovered morphine-mediated apoptosis in neurons of immune cells and cancer cells [5–7]. Our study focused on the unknown specific cellular and molecular mechanisms for opioid-induced apoptosis. Our study demonstrates that morphine can down-regulate miR-219-5p expression and miR-219-5p can inhibit morphine-induced macrophage apoptosis, suggesting that miR-219-5p might be a potential target for morphine-induced macrophage apoptosis. The miR-219-5p is encoded by 2 loci: one mapped on chromosome 6 and another locus on chromosome 9 [20]. Adlakha et al. showed that the miR-219-5p expression decreases with increasing degree of brain malignancy [30]. There is also evidence showing that up-regulation of miR-219-5p inhibits cell growth and migration in glioblastoma cells via EGFR targeting [31]. Huang N et al. reported that miR-219-5p functions as a tumor suppressor through negative regulation of glypican-3 (GPC3) [12]. However, there are few reports exploring the correlation between miR-219-5p and apoptosis, and the mechanism is unclear.
Our results also revealed that miR-219-5p can inhibit morphine-induced mouse peritoneal macrophages, and found that WEE1 can serve as a target gene of miR-219-5p. Taken together, our results suggest that cell apoptosis can be induced by targeting WEE1. Moreover, in vitro study also identified that after the transfection with miR-219-5p mimic, WEE1 and Tyr15 protein expression were significantly decreased compared with both the miR-219-5p NC group and morphine group, showing that WEE1 and Tyr15 are involved in the morphine-induced apoptosis. Wee1 is a nuclear protein that reversibly phosphorylates Cdc2 at Tyr15, and then inactivates Cdc2/cyclin B complex [32,32]. In several reports, it was found that the increase in Cdc2 kinase activity during apoptosis was related to Cdc2 dephosphorylation; Cdc2 phosphorylation induced by Wee1 overexpression, which prevents apoptosis, has been discovered in several systems [23,34]. Notably, the activity of a cell cycle regulatory kinase, Cdc2, can trigger mitosis, which oscillates during the cell cycle, peaking during mitosis and dropping during interphase; this accurate regulation is ensured by the coordinated action of negative and positive regulators of Cdc2 catalytic activity as well as localization [35]. During interphase, starting from Cdc2 synthesis, the newly generated Cdc2 complexes remain inactive through phosphorylation on Cdc2 at Thr14 and Tyr15; these phosphorylations are induced by the Myt1 and Wee1 kinases [36]. At the G2/M transition, Cdc25, the dual-specificity phosphatase, is activated; however, Wee1 is inactivated [37]. Cdc25 dephosphorylates Thr14 and Tyr15, thus activating the Cdc2 complex and mitosis [35]. Over-expression of Cdc2 can promote oncogenesis, while down-regulation of Cdc2 expression can suppress cell proliferation, induce G2/M arrest, and trigger apoptosis [38]. Thus, we hypothesized that miRNA-219-5p negatively targets WEE1 through suppressing Tyr15 expression and activating Cdc2, thus leading to inhibition of morphine-induced macrophage apoptosis.
Conclusions
This is the first study assessing the functions of miRNA-219-5p on morphine-induced macrophage apoptosis. The results of the present study revealed that miRNA-219-5p could negatively target WEE1 to suppress Tyr15 expression and activate Cdc2, thus inhibiting morphine-induced macrophage apoptosis. Our findings indicate a potential target for the improvement of morphine-induced immunosuppression.
Acknowledgement
We would like to show our sincere gratitude to our instructors for their valuable advices. We also appreciate the reviewers who give us precious comments on this article.
Footnotes
Conflicts of interest
The authors declared that there were no conflicts of interest concerning the publication of this paper.
Source of support: This project was supported by NingboHealth Technology Project for Excellent Young and Middle-aged Talent, No. 2011-145; Ningbo Leader and Top Notch Person Training Project, No. 2012-131; and Zhejiang Medicine and Health Project for Excellent Young Talent, No. 2013-245, and Shanghai Three-Year Project of Traditional Chinese Medicine(ZYSNXD-CCYJXYY)
References
- 1.Li Y, Sun X, Zhang Y, et al. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem Biophys Res Commun. 2009;378:857–61. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 3.Kream RM, Stefano GB. Interactive effects of endogenous morphine, nitric oxide, and ethanol on mitochondrial processes. Arch Med Sci. 2010;6:658–62. doi: 10.5114/aoms.2010.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsiao PN, Chang MC, Cheng WF, et al. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Li H, Zhang Y, et al. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem Biophys Res Commun. 2010;391:426–30. doi: 10.1016/j.bbrc.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Li H, Li Y, et al. Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett. 2011;489:43–47. doi: 10.1016/j.neulet.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol. 2010;177:984–97. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discov Med. 2010;9:145–52. [PubMed] [Google Scholar]
- 9.Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Vasilescu C, Rossi S, Shimizu M, et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang N, Lin J, Ruan J, et al. MiR-219-5p inhibits hepatocellular carcinoma cell proliferation by targeting glypican-3. FEBS Lett. 2012;586:884–91. doi: 10.1016/j.febslet.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Zhang P, Chen Z, et al. MicroRNA-7 downregulates XIAP expression to suppress cell growth and promote apoptosis in cervical cancer cells. FEBS Lett. 2013;587:2247–53. doi: 10.1016/j.febslet.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Dai Y, Hitchcock C, et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc Natl Acad Sci USA. 2013;110:15043–48. doi: 10.1073/pnas.1307107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–46. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Narducci MG, Arcelli D, Picchio MC, et al. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sezary syndrome. Cell Death Dis. 2011;2:e151. doi: 10.1038/cddis.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang MC, Lv Y, Qi YT, et al. [Knockdown and overexpression of miR-219 lead to embryonic defects in zebrafish development]. Fen Zi Xi Bao Sheng Wu Xue Bao. 2008;41:341–48. [in Chinese] [PubMed] [Google Scholar]
- 18.Zhao X, He X, Han X, et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–26. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugas JC, Cuellar TL, Scholze A, et al. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao SA, Arimappamagan A, Pandey P, et al. miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One. 2013;8:e63164. doi: 10.1371/journal.pone.0063164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iorns E, Lord CJ, Grigoriadis A, et al. Integrated functional, gene expression and genomic analysis for the identification of cancer targets. PLoS One. 2009;4:e5120. doi: 10.1371/journal.pone.0005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnussen GI, Holm R, Emilsen E, et al. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS One. 2012;7:e38254. doi: 10.1371/journal.pone.0038254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creevey L, Ryan J, Harvey H, et al. MicroRNA-497 increases apoptosis in MYCN amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Mol Cancer. 2013;12:23. doi: 10.1186/1476-4598-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Yan W, Wang Y, et al. Effect of miR-219-5p on malignant phenotype of glioblastoma cells. Tumor. 2012;32:395–401. [Google Scholar]
- 25.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 26.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 27.Gregory CD, Pound JD. Microenvironmental influences of apoptosis in vivo and in vitro. Apoptosis. 2010;15:1029–49. doi: 10.1007/s10495-010-0485-9. [DOI] [PubMed] [Google Scholar]
- 28.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–41. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–48. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 30.Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol Cancer. 2014;13:33. doi: 10.1186/1476-4598-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene SB, Herschkowitz JI, Rosen JM. Small players with big roles: microRNAs as targets to inhibit breast cancer progression. Curr Drug Targets. 2010;11:1059–73. doi: 10.2174/138945010792006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Ye H, Wan L, et al. Millepachine, a novel chalcone, induces G2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo. Carcinogenesis. 2013;34:1636–43. doi: 10.1093/carcin/bgt087. [DOI] [PubMed] [Google Scholar]
- 35.Perry JA, Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leijen S, Beijnen JH, Schellens JH. Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Curr Clin Pharmacol. 2010;5:186–91. doi: 10.2174/157488410791498824. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya A, Hirai G, Koyama Y, et al. Dual-specificity phosphatase CDC25A/B inhibitor identified from a focused library with nonelectrophilic core structure. ACS Med Chem Lett. 2012;3:294–98. doi: 10.1021/ml2002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Huang Q, Dong J, et al. Overexpression of CDC2/CyclinB1 in gliomas, and CDC2 depletion inhibits proliferation of human glioma cells in vitro and in vivo. BMC Cancer. 2008;8:29. doi: 10.1186/1471-2407-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]