Abstract
Patient: Male, 25
Final Diagnosis: Ulcerative colitis and chronic fatigue syndrome
Symptoms: Colitis • profound fatigue • multi-joint pain • cognitive impairment • corneal keratitis
Medication: —
Clinical Procedure: VIP replacement therapy
Specialty: Family Medicine
Objective:
Unusual clinical course
Background:
Patients with multisymptom chronic conditions, such as refractory ulcerative colitis (RUC) and chronic fatigue syndrome (CFS), present diagnostic and management challenges for clinicians, as well as the opportunity to recognize and treat emerging disease entities. In the current case we report reversal of co-existing RUC and CFS symptoms arising from biotoxin exposures in a genetically susceptible individual.
Case Report:
A 25-year-old previously healthy male with new-onset refractory ulcerative colitis (RUC) and chronic fatigue syndrome (CFS) tested negative for autoimmune disease biomarkers. However, urine mycotoxin panel testing was positive for trichothecene group and air filter testing from the patient’s water-damaged rental house identified the toxic mold Stachybotrys chartarum. HLA-DR/DQ testing revealed a multisusceptible haplotype for development of chronic inflammation, and serum chronic inflammatory response syndrome (CIRS) biomarker testing was positive for highly elevated TGF-beta and a clinically undetectable level of vasoactive intestinal peptide (VIP). Following elimination of biotoxin exposures, VIP replacement therapy, dental extractions, and implementation of a mind body intervention-relaxation response (MBI-RR) program, the patient’s symptoms resolved. He is off medications, back to work, and resuming normal exercise.
Conclusions:
This constellation of RUC and CFS symptoms in an HLA-DR/DQ genetically susceptible individual with biotoxin exposures is consistent with the recently described CIRS disease pathophysiology. Chronic immune disturbance (turbatio immuno) can be identified with clinically available CIRS biomarkers and may represent a treatable underlying disease etiology in a subset of genetically susceptible patients with RUC, CFS, and other immune disorders.
MeSH Keywords: Colitis, Ulcerative; Fatigue Syndrome, Chronic; HLA-DR Antigens; Mycotoxins; Transforming Growth Factor beta1; Vasoactive Intestinal Peptide
Background
Over a million people in the United States alone are afflicted by a unique constellation of debilitating neural and cognitive symptoms known as chronic fatigue syndrome (CFS) [1]. Although this complex disorder affects an estimated 2.5% of the US population, CFS has historically not been well recognized by the medical community [2]. This is likely because the underlying disease pathophysiology is not understood, and the widely diverse associated symptoms have not been effectively defined as a recognizable disease entity. Hence, the disorder is not included in the majority of allopathic medical school textbooks or curricula [3]. Ulcerative colitis (UC) and the related Crohn disease (CD) are inflammatory bowel diseases (IBD) that affect over 1.4 million Americans [4]. In a subset of UC patients, standard-of-care anti-inflammatory therapies, including oral corticosteroids, do not induce remission. These patients are considered to have refractory ulcerative colitis (RUC) [5].
Individuals with RUC and/or CFS may seek treatment from complementary alternative medicine (CAM) providers, and reports of the success or failure of observation-based CAM treatments for these conditions are not routinely incorporated into the evidence-based medical literature. CFS thus remains a diagnosis of exclusion made only when other causes of the illness have been ruled out, and the patient meets subjective diagnostic and exclusionary criteria of Fukuda (1994) or other consensus clinical guidelines [6]. Similarly, refractory UC is diagnosed only after a patient has been subjected to the full range of standard-of-care pharmacotherapies (and accompanying adverse effects) with no evidence of remission [5].
It has recently been demonstrated that exposure of HLA-DR/ DQ genetically susceptible individuals to biotoxins (such as those from ciguatera fish, ticks, and “black molds”) can trigger CFS symptoms accompanied by distinctive transcriptomic signatures with quantifiable alterations in chronic inflammatory response syndrome (CIRS) biomarkers, including TGF-beta, C4a, and VIP [7,8]. The link between positive CIRS biomarkers, HLA-DR/DQ haplotype, and multi-system disorders in adult patients has been anecdotally observed but not yet reported in the medical literature. Here, we present the first case report of successfully resolved UC and CFS symptoms arising simultaneously in a previously healthy, genetically susceptible individual with biotoxin exposures.
Case Report
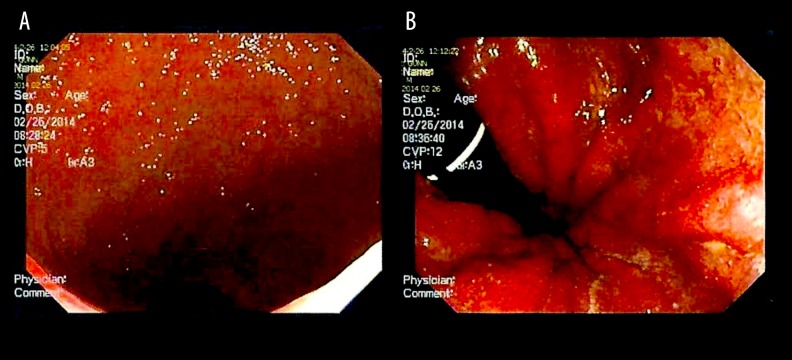
In July 2013, a 25-year-old man without co-existing diseases presented with recent onset of gastroenteritis and bloody diarrhea. The episode was preceded by persistent and increasing photophobia over approximately 2 years, but otherwise excellent health. Gastroenteritis symptoms resolved within a few weeks without treatment, but recurred in December 2013. The patient was treated with metronidazole without improvement, and an auto-immune disease workup [including IgA, IgG with subclasses, IgM, IgE, and anti-nuclear Ab (ANA)] was negative. Extended inflammatory bowel disease (IBD) panel results for the markers ACCA, ALCA, AMCA, gASCA, and pANCA were also negative and interpreted by the pathologist as “pattern not suggestive of inflammatory bowel disease.” However, a colonoscopy was performed in February 2014 (Figure 1A, 1B); pathology observations from the rectal biopsy were “chronic mucosal colitis with acute activity, consistent with previously clinically diagnosed chronic ulcerative colitis.” Mayo Colitis Scoring was moderate-to-severe, and the patient was started on mesalamine 4.8 g.
Figure 1.
(A) Colonoscopy image of the rectum showing granular edematous mucosa with hyperemia. (B) Colonoscopy image of the rectum showing granular edematous mucosa with hyperemia and frank blood. Mucosal changes are evident by the stippled pattern of light reflex.
Although colitis symptoms improved slightly, there was no remission after 6 months of treatment. During this same time period the patient’s overall health deteriorated as he developed multisystem symptoms meeting Fukuda 1994 criteria for CFS, including profound fatigue, post-exertional malaise, cognitive impairment, headaches, and multijoint pain. His sub-scale score results for physical and role functioning, as well as body pain, from the Medical Outcomes Study Short-Form General Health Survey (SF-36) were 2 SD below the mean and were consistent with CFS [9]. The UC and CFS symptoms were accompanied by severe photophobia complicated by corneal keratitis, as well as intractable abdominal pain resulting in an inability to work or engage in normal activities. Previously a 3-mile a day runner, he was house-bound and often bed-ridden. The addition of oral corticosteroids (budesonide) to the UC treatment regimen was accompanied by marked exacerbation of symptoms, including pancreatitis with serum lipase level peaking at 890 U/L (normal 30–210 U/L). The patient consumed no alcohol during his illness and had no previous history of pancreatitis, ruling out alcoholic pancreatitis. Serum IgG-4 levels were normal and steroids exacerbated rather than improved the patient’s symptoms, suggesting auto-immune pancreatitis (AIP) to be an unlikely etiology. There was no history of abdominal trauma and lipid levels were in the normal range. Due to the possibility of drug-induced pancreatitis, the decision was made to discontinue mesalamine and steroids, while evaluating alternative therapeutic options.
Seeking the root cause for his multisystem disorder (as well as alternatives to the conventional medicine RUC treatment algorithm, which includes immunomodulators, biologics such as infliximab, and colectomy) the patient consulted a functional medicine specialist. Extended autoimmune antibody profiling (Sjögren’s SS-A, SS-B, Smith (SM), RNP, SCL-70, JO-1, Centromere B, Rheumatoid factor, and DNA DS antibody) and urine mycotoxin testing (aflatoxins ochratoxins and macrocyclic trichothecenes) were performed. All autoantibody tests were negative, but urine mycotoxins were positive for trichothecene group. Environmental testing of the patient’s rental house revealed it to be a water-damaged building (WDB) contaminated with the toxic “black mold” Stachybotrys chartarum. After relocation to a new residence and a 4-times daily cholestyramine protocol, the patient showed some improvement, most notably with resolution of pancreatitis symptoms, and lipase levels returned to within the normal reference range (34 U/L). However, there was no remission from the colitis, so the patient was restarted on mesalamine 4.8 g.
In December 2014, HLA-DR/DQ testing was performed, which revealed the [HLA-DRB1*04, HLA-DQB1*03, HLA-DRB4*01] haplotype associated with immune disturbances, including chronic inflammatory response syndrome (CIRS) [7]. In addition, serum biomarkers for CIRS were tested and showed highly elevated TGF-β and clinically undetectable VIP (Table 1). Intranasal VIP replacement therapy (50 mcg, 4 times a day) was initiated, and after 1 week of treatment the patient’s longstanding keratitis/photophobia began to resolve with visual acuity improved from 20/200 to 20/30. Follow-up CIRS biomarker testing in March 2015 revealed VIP level restored to the normal range at 29 pg/mL. However, the colitis symptoms persisted and TGF-beta, although decreased by 50%, remained elevated at 6980 pg/mL (Table 1). In addition, the patient’s C4a level was slightly increased from the normal range to 3807 n/mL indicating the presence of a persistent source of inflammation.
Table 1.
Laboratory test results for the patient’s CIRS biomarkers.
| Biomarker | Results 12/2014 | Results 03/2015 | Results 12/2015 | Reference Range | Marker significance | Insurance |
|---|---|---|---|---|---|---|
| HLA-DR/DQ | HLA-DRB1*04 HLA-DQB1*03 HLA-DRB4*01 |
N/A | N/A | N/A | Inherited tightly linked genes used as a DNA-based screening method for HLA haplotype sensitivity. The 4-3-53 haplotype is associated with increased relative risk to develop CIRS | Yes |
| TGF-β | 14,140 pg/mL | 6980 pg/ml | 3000 pg/ml | (344–2382 pg/mL) | An inflammatory marker produced by multiple immune cell types and elevated in chronic immune inflammatory conditions | Yes |
| C4a | 2599 pg/mL | 3807 ng/mL | 1063 ng/mL | (0–2830 ng/mL) | Immunologic marker elevated in patients with chronic immune inflammatory conditions | Yes |
| VIP | Undetectable | 29 pg/mL | 33 pg/mL | (20–42 pg/mL) | Anti-inflammatory neuropeptide critical for regulation of innate-immune response. Can be decreased or undetectable in chronic immune inflammatory conditions | Yes |
All testing was performed at LabCorp or Quest Diagnostics CAP/CLIA certified laboratories and billed to patient insurance.
As the patient had a history of periodontal disease, a biological dentist was consulted to screen for occult infections. These were identified in 2 left upper molars accompanied by extensive root resorption. Following tooth extractions, colitis symptoms began resolving within 6 weeks, and over the next 3 months his mesalamine dosage was tapered down to zero as normal bowel function returned. With relief from the colitis symptoms as well as joint pain and muscle soreness, the patient was able to begin a regular yoga-based, mind-body intervention-relaxation response (MBI-RR) program. Off all medications, and currently taking only multivitamin mineral supplementation, he has been in colitis remission for over 6 months. His most recent S-36 evaluation revealed subscale scores in the range of healthy controls (HC), indicating continued resolution of CFS symptoms. After the patient had returned to work and resumed daily exercise, CIRS biomarkers were tested in December 2015; the results showed normalized VIP and C4a levels with TGF-beta just outside the normal range (Table 1).
Discussion
Inflammagen triggering of an aberrant immune response has long been hypothesized as a possible disease mechanism for multisystem chronic diseases, including RUC and CFS. For example, Epstein-Barr virus subset CFS patients have been shown to improve remarkably after a course of valacyclovir antiviral therapy [10]. Hooper et al. demonstrated greater than 90% positivity rates for mycotoxins (aflatoxins, ochratoxins, and trichothecenes) in urine samples from CFS patients not found in normal controls [11,12]. More recently, a distinct plasma immune signature of dysregulated pro- and anti-inflammatory cytokine activation has been defined early in the course of disease in patients with acute onset but not those with longstanding CFS [13]. Although plasma cytokines and proteomic immune signatures are intriguing potential biomarkers for immune system disturbance, this testing is not readily available from most clinical laboratories and is often not covered by patient insurance. In addition, these tests do not elucidate the underlying pathophysiology or explain why some individuals develop symptoms after biotoxin and other inflammagen exposures while others (often from the same family) do not.
In the present case, DNA-based biomarker (HLA-DR/DQ haplotype) testing was used as a screen for genetic susceptibility to developing chronic inflammation (Figure 2). Based on the patient’s positive HLA results, reflex testing for CIRS bio-markers associated with immune system disturbance was performed (Table 1). Results for TGF-β, C4a, and VIP were consistent with a multisystem and multisymptom disease representing the “final common pathway” in chronic immune activation reported by Shoemaker et al. [7,8]. We found that the CIRS biomarkers provided guidance as to the next steps that should be taken to eliminate residual toxin exposures, including vacating the WDB and initiating the cholestyramine protocol. CIRS biomarker results also served as objective measures for tracking treatment progress. For example, based on his clinical symptoms, and supported by the VIP biomarker showing undetectable levels in his serum, the patient was started on intranasal VIP 50 mcg 4 times daily as a targeted therapy. The response was excellent, particularly in the cornea, where keratitis and photophobia began resolving in a few days, with visual acuity improved from 20/200 to 20/30. These striking results are not surprising given that clinical applications for reestablishment of immune homeostasis using neuropeptides such as VIP are expanding and have been recently reported in diverse non-infectious inflammatory conditions such as sarcoidosis, pulmonary hypertension, and CIRS following exposure to water-damaged buildings [14–17]. In addition, VIP is known to be an anti-inflammatory neuropeptide secreted by corneal nerves as a stimulator of wound healing [18,19].
Figure 2.
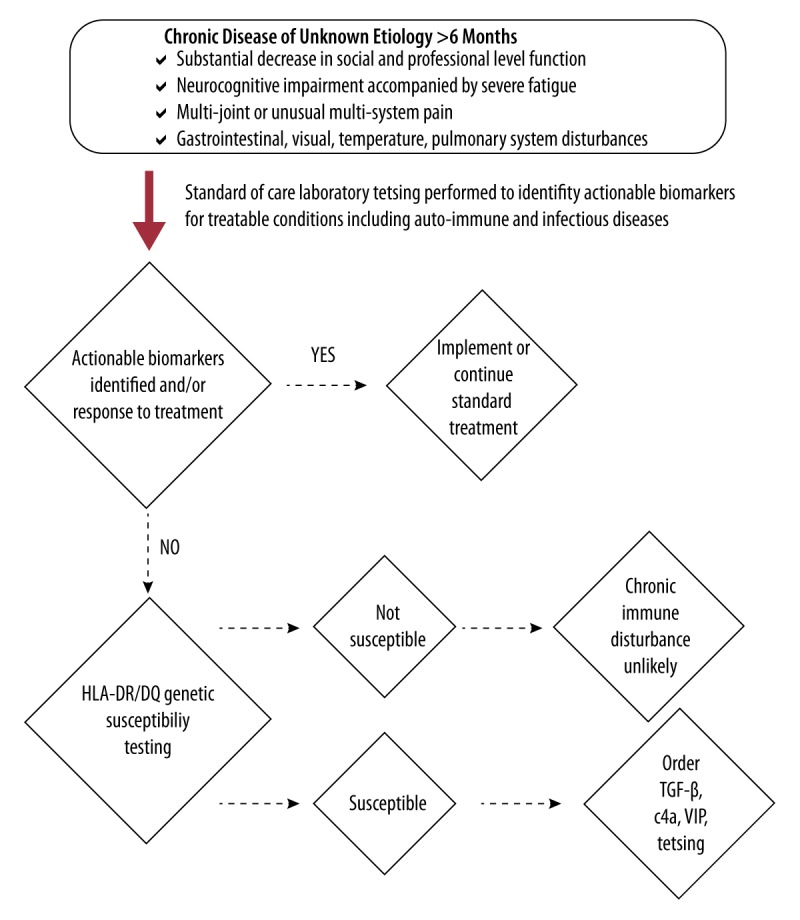
HLA-DR/DQ haplotype as a screening method for genetic susceptibility to develop chronic immune disturbance.
To address lingering colitis symptoms, a biological dentist was consulted. Oral-systemic health linkages increasingly point to chronic periodontal inflammation as a potential trigger for autoimmune spectrum diseases, including rheumatoid arthritis and coronary artery disease [20]. Successful tooth extractions were followed by improvement in the colitis and markedly increased energy levels allowing initiation of a yoga-based mind-body intervention-relaxation response (MBI-RR) program. Recent studies by Kuo and colleagues have shown significant clinical improvement in inflammatory bowel disease with non-pharmacological therapies such as meditation and yoga through regular elicitation of the relaxation response. Positive clinical effects are mirrored at the molecular level by differential gene expression changes after a 9-week MBI-RR program [21]. Now off medications and back to work, the patient continues to benefit from an ongoing wellness program including multivitamin mineral supplementation coordinated by his family practice and functional medicine clinics [22].
Conclusions
This patient’s unusual clinical presentation of CIRS biomarker-positive RUC and CFS required a multidisciplinary medical team (family practice, gastroenterology, ophthalmology, biological dentistry, functional medicine, and pathology) to achieve successful treatment of multi-system symptoms arising from immune disturbance. Consistent communication between physicians allowed coordination of supportive care while continuing the search for, and elimination of, root causes of his disease. Further multi-disciplinary studies and case reports are clearly needed to firmly establish a link between disturbance of immune homeostasis and multi-system diseases such as RUC and CFS. Looking retrospectively at this patient’s illness, CIRS bio-marker testing at the first clinical presentation of immune disturbance, followed by immediate identification and elimination of biotoxin exposures, could have considerably shortened his illness. We therefore hope that in the future, genetically susceptible patients with reversible conditions arising from biotoxin-mediated immune disturbance (turbatio immuno) will be identified early in their disease course by CIRS biomarker testing so that clinicians can immediately implement effective treatment.
Acknowledgments
The authors would like to acknowledge the skill and expertise of the multidisciplinary team in the management of this case: Amy Myers MD (Functional Medicine), Kristen Held MD (Ophthalmology), Keith Berndtson MD (Family Medicine), James Jackson MD (Gastroenterology), and Candice Owens DDS (Biological Dentistry). In addition, we would like to thank Margaret Rockwood MD (Family Medicine) for her insights and critical reading of the manuscript.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References:
- 1.Jason A, Richmond J, Rademaker A, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129–37. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 2.Reeves W, Jones J, Maloney E, et al. Prevalence of CFS in metropoloitan and rural Georgia. Population Health Metrics. 2007;5:5. doi: 10.1186/1478-7954-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson TM, Peterson TW, Emerson S, et al. Coverage of CFS within US medical schools. Universal Journal of Public Health. 2013;1:177–79. [Google Scholar]
- 4.Loftus E. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.MacDermott R, Green J. Refractory ulcerative colitis treatment. Gastroenterology Hepatol. 2007;3:64–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda K, Straus S, Hickie I, et al. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Inter Med. 1994;12:954–59. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Ryan J, Quinzhong W, Shoemaker R. Transcriptomic signatures in whole blood of patients who acquire a chronic inflammatory response syndrome following an exposure to the marine toxin ciguatoxin. BMC Medical Genomics. 2015;8:15. doi: 10.1186/s12920-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoemaker R, House D, Ryan J. Defining the neurotoxin derived illness chronic ciguatera using markers of chronic systemic inflammatory disturbances: A case/control study. Neurotoxicol Teratol. 2010;32:633–39. doi: 10.1016/j.ntt.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald D, Pearlman T, Jovine U, et al. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med. 1996;101:364–70. doi: 10.1016/S0002-9343(96)00234-3. [DOI] [PubMed] [Google Scholar]
- 10.Lerner A, Beqaj S, Deeter R, Fitzgerald J. Valacyclovir treatment in Epstein-Barr virus subset chronic fatigue syndrome: Thirty-six months followup. In Vivo. 2007;21:707–14. [PubMed] [Google Scholar]
- 11.Brewer J, Thrasher J, Straus D, et al. Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins. 2013;5:605–17. doi: 10.3390/toxins5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer J, Thrasher J, Hooper D. Chronic illness associated with mold and mycotoxins; Is naso-sinus fungal biofilm the culprit? Toxins. 2014;6:66–80. doi: 10.3390/toxins6010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornig M, Montoya J, Klimas N, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1:e1400121. doi: 10.1126/sciadv.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado M, Ganea D. Anti-inflammatory neuropeptides: A new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–51. doi: 10.1016/j.bbi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasse A, Zissel G, Lutzen N, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–48. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 16.Petkov V, Mosgoeller W, Ziesche R, et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2001;111:1339–46. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoemaker R, House D, Ryan J. Vasoactive intestinal polypeptide (VIP) corrects chronic inflammatory response syndrome (CIRS) acquired following exposure to water-damaged buildings. Health. 2013;5:396–401. [Google Scholar]
- 18.Muller L, Marfurt C, Kruse F, Tervo T. Corneal nerves: structure, contents, and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Jian X, McClellan S, Barrett R, et al. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52(9):6154–61. doi: 10.1167/iovs.10-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebersole J, Dawson D, Morford L, et al. Periodontoal disease immunology: ‘Double indemnity’ in protecting the host. Periodontal. 2005;62:163–202. doi: 10.1111/prd.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo B, Bhasin M, Jacquart J, et al. Genomic and clinical effects associated with a relaxation response mind-body intervention in patients with irritable bowel syndrome and inflammatory bowel disease. PLoS One. 2015;10(4):e0123861. doi: 10.1371/journal.pone.0123861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maric D, Brkic S, Tomic S, et al. Multivitamin mineral supplementation in patients with chronic fatigue syndrome. Med Sci Monit. 2014;20:47–53. doi: 10.12659/MSM.889333. [DOI] [PMC free article] [PubMed] [Google Scholar]