Abstract
Background
Cardiac resynchronization therapy (CRT) with biventricular epicardial (BV‐CS) or endocardial left ventricular (LV) stimulation (BV‐EN) improves LV hemodynamics. The effect of CRT on right ventricular function is less clear, particularly for BV‐EN. Our objective was to compare the simultaneous acute hemodynamic response (AHR) of the right and left ventricles (RV and LV) with BV‐CS and BV‐EN in order to determine the optimal mode of CRT delivery.
Methods
Nine patients with previously implanted CRT devices successfully underwent a temporary pacing study. Pressure wires measured the simultaneous AHR in both ventricles during different pacing protocols. Conventional epicardial CRT was delivered in LV‐only (LV‐CS) and BV‐CS configurations and compared with BV‐EN pacing in multiple locations using a roving decapolar catheter.
Results
Best BV‐EN (optimal AHR of all LV endocardial pacing sites) produced a significantly greater RV AHR compared with LV‐CS and BV‐CS pacing (P < 0.05). RV AHR had a significantly increased standard deviation compared to LV AHR (P < 0.05) with a weak correlation between RV and LV AHR (Spearman rs = −0.06). Compromised biventricular optimization, whereby RV AHR was increased at the expense of a smaller decrease in LV AHR, was achieved in 56% of cases, all with BV‐EN pacing.
Conclusions
BV‐EN pacing produces significant increases in both LV and RV AHR, above that achievable with conventional epicardial pacing. RV AHR cannot be used as a surrogate for optimizing LV AHR; however, compromised biventricular optimization is possible. The beneficial effect of endocardial LV pacing on RV function may have important clinical benefits beyond conventional CRT.
Keywords: cardiac resynchronization therapy, ventricular contractility, endocardial pacing, biventricular acute hemodynamic response
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for symptomatic heart failure (HF) patients with significantly impaired left ventricular (LV) function and prolonged QRS duration; however, 30–40% of patients fail to derive clinical benefit.1 Attempts to improve CRT response have focused on improving both preprocedural2, 3, 4, 5 and intraprocedural6, 7, 8 predictors of response, as well as alternate forms of CRT delivery.9, 10, 11, 12
LV acute hemodynamic response (AHR) is a frequently used tool for CRT clinical research studies. Improvements in LV and/or right ventricular (RV) hemodynamics with CRT have been demonstrated13, 14, 15, 16; however, the latter are often neglected with respect to CRT optimization. Furthermore, the RV AHR has been shown to positively correlate with cardiac index, which supports the hypothesis that improvement of this acute metric may have an important and positive contribution on cardiac output.14 It is biologically plausible that biventricular (BV) optimization with simultaneous attention to the hemodynamic changes in both ventricles may be clinically beneficial. Biventricular endocardial pacing (BV‐EN) has been shown to improve LV AHR compared to epicardial pacing11, 12, 17, 18; however, the effect of BV‐EN on RV AHR is less clear. A recent canine study demonstrated that the RV myocardium plays a key role in work redistribution and improved LV pump function with CRT but RV AHR was not significantly improved by LV‐only or BV epicardial pacing,19 despite simultaneous increases in LV AHR. However, the effect of BV‐EN pacing on RV AHR was not addressed in that study.
The aim of the current study was to investigate the effect of epicardial and endocardial CRT on simultaneous RV and LV AHR. We hypothesized that: (1) BV‐EN has a greater capacity to improve RV AHR more than conventional epicardial pacing; and (2) RV AHR could also be optimized concurrently to LV AHR, resulting in improved overall cardiac function. To test these hypotheses, and to investigate if RV AHR could be used as a surrogate for LV AHR, we performed a comparison of BV AHR to various pacing protocols including epicardial pacing via the coronary sinus in LV‐only (LV‐CS) and biventricular modes (BV‐CS) as well as BV‐EN.
Methods
This study complies with the Declaration of Helsinki, the protocol was approved by the local ethics committee, and informed consent was obtained from each patient. Ten patients with conventional CRT criteria (New York Heart Association [NYHA] Class II–IV drug refractory HF, LV ejection fraction ≤35%, and QRS ≥ 120 ms) with previously implanted CRT systems, were recruited for an acute electrophysiology/pacing study. Patients were intentionally selected with a phenotype of suboptimal response to CRT as they have the most to gain by alternate strategies: Male patients with ischemic etiology and QRS duration 120–150 ms.20 Patients with a mechanical aortic valve or significant peripheral vascular disease were excluded.
Baseline assessment prior to original implant included clinical assessment (NYHA functional class), 12‐lead electrocardiogram, and 2D echocardiography (echo). Etiology of HF was confirmed on the basis of clinical history, coronary angiography, and cardiac magnetic resonance (CMR) imaging. An improvement of ventricular AHR by >10% defined an acute hemodynamic responder.6
Invasive Hemodynamic Study
Patients underwent an invasive study at least 3 months following implantation of the CRT device. Light sedation was used and a steerable 6F Livewire decapolar catheter (St. Jude Medical St. Paul, MN, USA) was passed via the femoral artery retrogradely to perform endocardial pacing from multiple sites within the LV cavity. Intravenous heparin (70 U/kg) was administered to achieve systemic anticoagulation (target‐activated clotting time 300–350 seconds). A 0.014‐inch diameter high‐fidelity Certus PressureWire (St. Jude Medical) was placed retrogradely into the LV cavity via the femoral or radial artery to assess real‐time mean LV dPdtmax using the accompanying PhysioMon software (RADI Medical Systems, part of St. Jude Medical). An RV pressure wire was inserted via cannulation of the femoral vein and catheterization of the right heart to measure simultaneous RV dPdtmax. Atrial pacing (or RV pacing for atrial fibrillation) at 5–10 beats/min above the intrinsic rate was used as the baseline and was a constant for each patient throughout different pacing modes. At least 20 seconds was respected after any change in pacing protocol, in order to achieve hemodynamic stabilization prior to recording pressure measurements for 20 seconds. The mean dPdtmax for each pacing protocol was determined by averaging the dPdtmax over the recorded period following removal of beats affected by ventricular extra systoles (Fig. S1). The AHR was defined to be the percentage change in the maximal increase in the rate of change of pressure (denoted dPdtmax) with the intervention protocol as compared with its associated baseline protocol. To minimize the known effect of baseline drift in dPdtmax throughout the case, the baseline was reassessed prior to every change in pacing modality.
The sensed and paced atrioventricular (AV) and ventriculo‐ventricular (VV) settings of the device were optimized using echocardiography, 6 weeks after the original implantation; the mean of these values is shown in Table I. For the acute pacing study, a set AV delay of 100 ms was used for all patients. No AV or VV optimization was performed during this study due to the added time these require, and our intention to focus on endocardial pacing from multiple locations (VV pacing was simultaneous in all patients). Mean and standard deviation (SD) pressure metrics were also recorded for each heartbeat including the averaged peak systolic pressure (maximum pressure per beat), start diastolic pressure (minimum pressure per beat), and end diastolic pressure, which was defined to occur when the rate of increase of pressure traversed 100 mm Hg/s.
Table I.
Baseline Demographics, Device Settings, and CRT Response
| Demographics | |
|---|---|
| Pre‐CRT implantation | |
| Mean age (years) | 70 ± 7 |
| Male | 9 (100%) |
| Sinus rhythm | 6 (67%) |
| Ischemic heart disease | 7 (78%) |
| QRS duration (ms) | 152 ± 37 |
| LBBB morphology | 9 (100%) |
| NYHA class III | 7 (78%) |
| LV systolic function, EF by echo (%) | 30 ± 8 |
| RV systolic function: TAPSE (mm) | 13 ± 4 |
| RV basal diameter, diastole (mm) | 41 ± 8 |
| RV systolic function, EF by MRI (%) | 43 ± 10 |
| CRT settings | |
| Sensed AV delay (ms) | 120 ± 22 |
| Paced AV delay (ms) | 135 ± 15 |
| VV timing: LV ahead (ms) | 17 ± 13 |
| CRT response assessment at 6 months | |
| LV systolic function, EF by echo (%) | 32 ± 11 |
| RV systolic function: TAPSE (mm) | 14 ± 2 |
| RV basal diameter, diastole (mm) | 39 ± 10 |
| Echo responder | 3 (33%) |
| Clinical responder | 5 (56%) |
Averaged data are mean ± SD.
AV = atrio‐ventricular; CRT = cardiac resynchronization therapy; ECHO = echocardiography; EF = ejection fraction; LBBB = left bundle branch block; LV = left ventricular; MRI = magnetic resonance imaging; NYHA = New York Heart Association; RV = right ventricular; TAPSE = Tricuspid Annular Plane Systolic Excursion; VV = ventricular‐ventricular.
Pacing Protocols
Baseline dPdtmax was compared with paced dPdtmax for the following pacing protocols:
LV‐only epicardial CRT using the implanted LV lead (LV‐CS);
BV epicardial CRT using the RV lead and the implanted LV lead (BV‐CS);
BV endocardial pacing using the RV lead and the roving LV decapolar catheter (BV‐EN) in multiple positions at random.
BV AHR Optimization
BV AHR optimization involved a review of paired LV and RV AHR data in order to delineate the optimal protocols for both ventricles simultaneously. As conventional CRT optimization by AHR maximizes LV AHR only, BV AHR optimization (incorporating both LV and RV AHR metrics) will almost certainly involve some decrease in LV AHR. We therefore adhered to the following principle when selecting BV AHR optimization: the increase in RV AHR must be greater than the decrease in LV AHR, compared with the maximal LV AHR protocol (Fig. S2), as the effect of RV dPdtmax on RV function and subsequent clinical outcome is less clear than that of the LV.
To construct a BV optimization metric, which must be relative to the response achieved by the maximal LV AHR protocol, we denote the response achieved by conventional AHR and BV AHR optimization with subscripts LV and BV, respectively, and the comparative change in ventricular AHR from that of the maximal LV AHR protocol by ΔLV = (LV AHR)BV − (LV AHR)LV and ΔRV = (RV AHR)BV − (RV AHR)LV. Then it is proposed to use the ratio of the change in RV AHR (ΔRV) to the absolute change in LV AHR (|ΔLV|), giving the ratio ΔRV/|ΔLV|, as a quantitative measure of BV AHR performance relative to the conventional optimization method. This metric is applied to all protocols that did not maximize LV AHR and a protocol is deemed to be a viable alternative to the conventionally chosen protocol when the metric value is >1. The viable protocol that maximizes this BV AHR optimization metric is the chosen alternative BV optimization protocol. Conceptually, if one considers the set of points given by the relative response of a protocol compared to the maximal LV AHR protocol in the LV AHR, RV AHR Cartesian plane, then this process selects the protocol with the largest negative slope of the line joining the compromise protocol datum to the origin (Fig. S2).
Statistical Methods
Statistical quantities were computed using MATLAB (R2013b, The MathWorks, Cambridge, MA, USA). Values were presented as numbers and percentages for discrete variables and as mean ± SD for continuous variables. Bivariate correlations were quantified using both Pearson's parametric correlation coefficient (rp) and Spearman's nonparametric rank‐order correlation coefficient (rs). One‐way balanced repeated measures analysis of variance (ANOVA) was used to test for significant effects of the three pacing methods (LV‐CS/BV‐CS/best BV‐EN) on baseline hemodynamics, where “best BV‐EN” denotes maximal endocardially paced AHR for each patient averaged over all patients, as opposed to “average BV‐EN,” which denotes the mean endocardially paced AHR‐AHR for each patient averaged over all patients. Pairwise analysis for differences between epicardial pacing strategies (LV‐CS/BV‐CS) and endocardial pacing (best BV‐EN/average BV‐EN) was performed using a two‐tailed Student's t‐test. A P‐value <0.05 was considered statistically significant for all analyses.
Results
Patient Demographics (Table I)
Nine datasets were included in the analysis due to the recurrent dislodgement of the RV pressure wire during one procedure. The distribution of CRT devices was as follows: St. Jude Medical Quadra Assura (n = 3); Promote Quadra (n = 5); and Medtronic Consulta (n = 1). The mean age was 70 ± 7 years, mean QRS duration was 152 ± 37 ms, and all patients had left bundle branch block (LBBB). The majority of participants had cardiomyopathy secondary to ischemic heart disease (78%) and were in sinus rhythm (67%). Patients had impairment of both LV and RV systolic function prior to device implantation. Mean LV ejection fraction was 30 ± 8%, mean Tricuspid Annular Plane Systolic Excursion (TAPSE) was 13 ± 4 mm (normal > 16 mm21), mean RV basal diameter was 41 ± 8 (normal < 42 mm21), and mean RV systolic function measured with CMR was 43 ± 10% (normal range 40–68%). The mean time from CRT implantation to study participation was 15 ± 15 months. Patients were assessed for CRT response at 6 months following implantation. Response was determined using the Packer clinical composite score22 and with 2D echo, whereby an echo responder was defined as a patient with ≥15% reduction in end systolic volume. This cohort of patients had a high proportion of CRT nonresponse, six of nine (67%) as measured by echo and four (45%) as measured by clinical composite score. Endocardial pacing was performed in several positions from base to apex in each patient in a random fashion. The mean number of endocardially paced positions per patient was 8.4 ± 3.7 (Table S1).
Correlation of Interventricular Pressure and AHR Metrics
Biventricular and patient‐specific measurements of peak systolic pressure, start diastolic pressure, and end diastolic pressure are presented in Table S2 in the Supporting Information. RV and LV start and end diastolic pressure measurements displayed a positive correlation (start: rp = 0.77, rs = 0.84; end: rp = 0.79, rs = 0.82). Simple linear regression analysis of the start and end diastolic pressure relationships yielded coefficients of determination of r2 = 0.59 and r2 = 0.63, respectively (Fig. 1). Compared to the diastolic pressure measurements, peak systolic pressures had a reduced positive correlation (rp = 0.11, rs = 0.12 [Table S2]) and a reduced linear model coefficient of determination of r2 = 0.013 (Fig. 1A). Overall, LV and RV AHR were poorly correlated as can be qualitatively seen by the nonlinear distribution of data in Figure 2, and is quantitatively demonstrated by the correlation coefficient cohort averages of rp = 0.005 and rs = −0.055 (Table II). The lack of a linear relationship between LV and RV AHR was also displayed on an individual basis (Fig. S3) as demonstrated by the wide range of patient‐specific correlation values of Table II.
Figure 1.
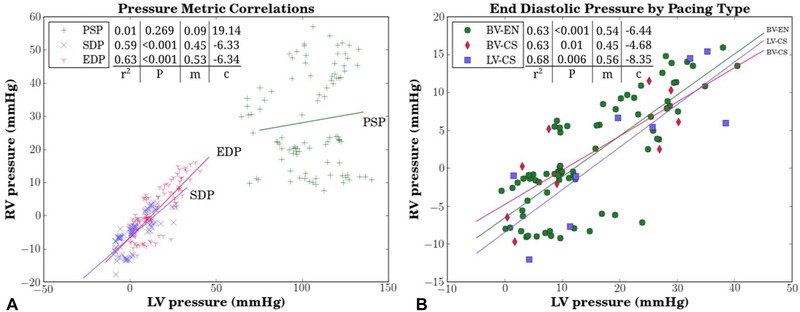
Scatterplot of paced LV and RV pressure data acquired from nine patients undergoing various pacing protocols (LV only‐coronary sinus = LV‐CS; biventricular‐coronary sinus = BV‐CS; biventricular endocardial pacing = BV‐EN). (A) Peak systolic (PSP), start diastolic (SDP), and end diastolic (EDP) pressures. Simple linear regression was applied to each pressure metric (m‐slope; c‐intercept). (B) Detailed analysis of EDP by pacing type. LV = left ventricular; RV = right ventricular.
Figure 2.
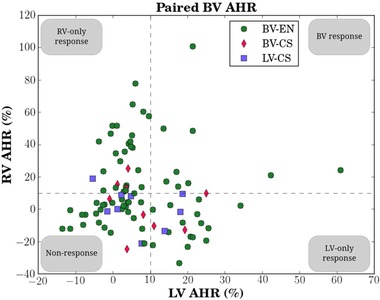
Scatterplot of all paired LV‐RV AHR data for nine patients, split by conventional coronary sinus pacing (a single protocol of LV‐CS/BV‐CS for each patient) and BV endocardial pacing (BV‐EN) protocols at multiple locations per patient. An AHR value >10% above baseline was used as the benchmark for a positive AHR, as indicated by the dashed lines.6 Using this cutoff, data points are thus divided into LV‐only response, RV‐only response, BV response, and nonresponse. AHR = acute hemodynamic response. Other abbreviations as in Figure 1.
Table II.
Correlation between RV and LV AHR
| LV AHR(%) | RV AHR(%) | Correlations | ||||
|---|---|---|---|---|---|---|
| ID | Maximum | Average | Maximum | Average | rP | rS |
| 1 | 3.9 | −1.0 ± 3.7 | 15.3 | 4.2 ± 7.2 | 0.56 | 0.54 |
| 2 | 61 | 19.5 ± 18.9 | 24.1 | 1.3 ± 14.4 | 0.79 | 0.72 |
| 3 | 34.2 | 10.3 ± 9.6 | 51.7 | 9.7 ± 23.6 | −0.04 | −0.14 |
| 4 | 14.3 | 5.1 ± 4.3 | 57.5 | 24.3 ± 24.9 | 0.08 | 0.05 |
| 5 | 13.8 | 6.2 ± 3.9 | 65.1 | 32.8 ± 24.0 | −0.19 | 0.19 |
| 6 | 25.6 | 17.6 ± 7.5 | 6 | −10.9 ± 10.9 | −0.41 | −0.25 |
| 7 | 7.6 | 0.3 ± 5.7 | 18.9 | 6.8 ± 9.1 | 0.40 | 0.37 |
| 8 | 21.4 | 0.2 ± 8.9 | 101 | 25.0 ± 35.5 | 0.77 | 0.76 |
| 9 | 25 | 12.4 ± 11.1 | 9.9 | 4.1 ± 5.7 | 0.91 | 1.00 |
| All | 61 | 7.6 ± 11.7 | 101 | 10.6 ± 24.4 | 0.00 | −0.06 |
Comparison of interventricular sensitivity of AHR across all pacing protocols (BV‐CS, LV‐CS, and BV‐EN). Individual and whole‐cohort maximum and averaged AHR (mean ± SD) were calculated for both ventricles.
AHR = acute hemodynamic response; ID = anonymized patient number; rP = Pearson's correlation coefficient; rS = Spearman's rank correlation coefficient. SD = standard deviation. Other abbreviations as in previous tables.
Comparison of Epicardial versus Endocardial CRT on BV AHR (Table III and Figs. 3 and 4)
Table III.
Hemodynamic Data from HF and LBBB Patients (n = 9) during Baseline and CRT Pacing
| Variable | Baseline | LV‐CS | BV‐CS | BV‐EN | ANOVA (P‐Value) |
|---|---|---|---|---|---|
| LV start diastolic pressure (mm Hg) | 5.2 ± 11.9 | 5.5 ± 11.9 | 4.0 ± 10.4 | 5.3 ± 11.0 | 0.992 |
| LV end diastolic pressure (mm Hg) | 19.0 ± 15.4 | 20.1 ± 13.6 | 14.8 ± 12.7 | 19.8 ± 11.8 | 0.822 |
| LV peak systolic pressure (mm Hg) | 103.2 ± 19.8 | 103.6 ± 19.7 | 102.2 ± 18.5 | 107.3 ± 18.2 | 0.948 |
| LV dPdtmax (mm Hg/s) | 808.1 ± 247.2 | 856.2 ± 252.9 | 863.1 ± 243.4 | 989.5 ± 290.4 | 0.501 |
| LV AHR (%) | 0.0 ± 0.0 | 6.6 ± 8.6 | 8.4 ± 8.7 | 21.3 ± 17.6 | 0.036 |
| RV start diastolic pressure (mm Hg) | −5.1 ± 6.2 | −5.2 ± 6.8 | −4.4 ± 5.2 | −3.6 ± 5.5 | 0.933 |
| RV end diastolic pressure (mm Hg) | 1.7 ± 8.4 | 2.9 ± 9.3 | 2.0 ± 7.2 | 3.8 ± 7.9 | 0.946 |
| RV peak systolic pressure (mm Hg) | 29.4 ± 16.4 | 28.6 ± 15.5 | 30.1 ± 15.9 | 32.4 ± 15.6 | 0.961 |
| RV dPdtmax (mm Hg/s) | 341.4 ± 91.3 | 348.1 ± 107.5 | 348.2 ± 96.2 | 453.1 ± 173.3 | 0.176 |
| RV AHR (%) | 0.0 ± 0.0 | 0.9 ± 12.3 | 2.0 ± 15.9 | 35.0 ± 32.3 | 0.004 |
AHR = acute hemodynamic response; ANOVA = analysis of variance; BV‐CS = biventricular coronary sinus pacing; BV‐EN = biventricular endocardial pacing; HF = heart failure; LV‐CS = left ventricular coronary sinus pacing. Other abbreviations as in previous tables.
Figure 3.
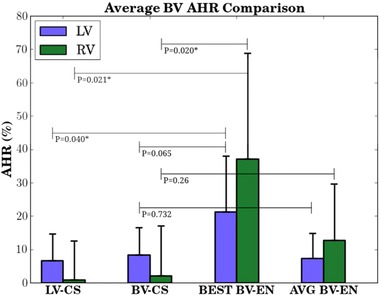
Bar chart (mean ± SD) comparing mean BV AHR resulting from LV coronary sinus (LV‐CS, n = 9), BV coronary sinus (LV‐CS, n = 9), and BV endocardial (BV‐EN, n = 76) pacing. Best BV‐EN significantly improved ventricular AHR over LV‐CS for both ventricles and over BV‐CS for the right ventricle. There was no statistical difference between LV‐CS and BV‐CS for either ventricle, nor was there a statistical difference between CS pacing and the average BV‐EN AHR for either ventricle. * denotes statistically significant. SD = standard deviation. Other abbreviations as in previous figures.
Figure 4.
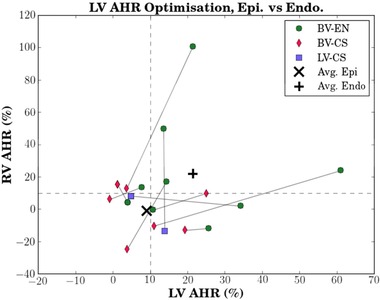
Patient‐specific paired biventricular AHR data using LV AHR data alone to find the best epicardial (LV‐CS or BV‐CS) pacing protocol and the best endocardial (BV‐EN) protocol per patient (plotted with its associated RV AHR). The resulting endocardial‐epicardial BV AHR pair for each patient is connected by the gray solid line. An AHR value >10% above baseline was used as the benchmark for a positive AHR, as indicated by the dashed lines.6 Mean of the best epicardial protocols (by LV AHR alone) show minimal/no change in RV‐AHR (∼0%) with approximately 10% improvement in LV AHR (X). This compared with a +21% improvement in LV AHR and similar improvement in the paired RV AHR with the mean of best endocardial protocols (+). Seven of nine patients improved LV AHR with BV‐EN compared to CS pacing. The axes are scaled differently. Abbreviations as in previous figures.
LV AHR was improved by 8.4 ± 8.7% from baseline by BV‐CS (P = 0.02) and 6.6 ± 8.6% by LV‐CS (P = 0.051) (Table III). Notably, RV AHR was not improved by either BV‐CS (2 ± 15.9%, P = 0.712) or LV‐CS (0.9 ± 12.3%, P = 0.832). The best BV‐EN, however, produced significantly improved AHR for both ventricles compared with conventional epicardial pacing (Fig. 3). The average LV and RV increase in AHR with BV‐EN was 21.4 ± 16.6% and 37.1 ± 31.8%, respectively (Fig. 3). In a pairwise comparison of pacing methods, best BV‐EN was a significant improvement in terms of RV AHR over BV‐CS (P = 0.021) and LV‐CS (P = 0.02). Best BV‐EN was also a significant improvement in terms of LV AHR over LV‐CS (P = 0.04) but not BV‐CS (P = 0.065).
The average SD of RV AHR across all patients was greater than that of the LV (±18% vs ±8%). The SD for the RV AHR under all three pacing methods was larger than the SD of the equivalent LV AHR (Fig. 3).
A patient‐specific comparison of the BV AHR performance of best epicardial and best endocardial pacing under conventional LV AHR optimization is presented in Figure 4. For epicardial pacing (LV‐CS/BV‐CS) and with respect to AHR (10% cutoff), there were three nonresponders, two RV‐only responders, four LV‐only responders, and zero BV responders. The average AHR for epicardial pacing was 9.1% and −1% for the LV and RV, respectively. For endocardial pacing (BV‐EN) and with respect to AHR, there was one nonresponder, one RV‐only responder, three LV‐only responders, and four BV responders. The average AHR for best BV‐EN pacing increased relative to epicardial pacing to 21.3% and 22.2% for the LV and RV, respectively. Overall, best BV‐EN as opposed to epicardial pacing increased the number of LV responders by 22% and the number of RV responders by 33%.
Biventricular Hemodynamic Optimization (Fig. 5)
Figure 5.
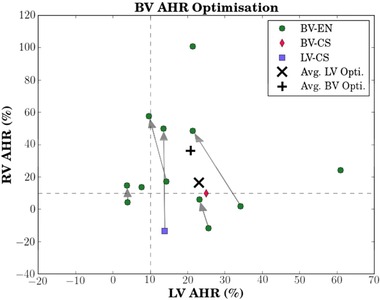
Alternative, patient‐specific biventricular AHR optimization. Here, the best patient protocol selected using LV AHR are compared to an alternative, BV AVR optimization protocol and connected by arrows where a viable alternative exists (five out of nine; Table A3). BV endocardial protocols were optimal in seven of nine (eight out of nine) cases under conventional (BV) optimization. The axes are scaled differently. Abbreviations as in previous figures.
BV‐EN produced 77% of the patient‐specific best LV AHR pacing protocols. Using the aforementioned BV AHR optimization metric, 56% patients had an alternative viable protocol (Table S3). Conventional LV AHR optimization resulted in one nonresponder, one RV‐only responder, four LV‐only responders, and three BV responders. In contrast, our alternative BV AHR optimization method resulted in zero nonresponders, three RV‐only responders, two LV‐only responders, and four BV responders. The averaged LV‐optimized AHR was 23% and 16% for the LV and RV, respectively, which can be compared with the averaged BV‐optimized AHR of 21% and 36% for the LV and RV, respectively. Thus, BV AHR optimization resulted in a greater than twofold increase in mean RV AHR at the relatively minor expense of an approximately 2% decrease in mean LV AHR compared to LV‐only AHR optimization.
Optimal LV Pacing Site Location (Fig. 6)
Figure 6.
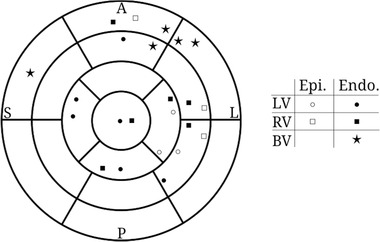
17‐Segment American Heart Association plot displaying the approximate location and pacing type (epicardial or endocardial) of the maximal achieved AHR for the LV (circle), RV (square), and BV (star; see Fig. 5). Note that four of nine patients did not have a viable compromise protocol for BV AHR optimization. Abbreviations as in previous figures.
The optimal site for the LV lead whilst delivering BV pacing was highly patient‐specific, scattered across most regions of the myocardium whether the optimal LV AHR alone or RV AHR alone was used. Where viable alternative BV optimization protocols existed, four of five (80%) had the LV lead position clustered in the basal‐mid‐ anterolateral position. LV lead positions were endocardial in 12 of 18 cases when looking at the optimal AHR (LV AHR alone, n = 9 and RV AHR alone, n = 9); the remaining six of 18 cases were in an epicardial position.
Discussion
To our knowledge, this study is the first to simultaneously analyze the effect of epicardial and endocardial pacing on LV and RV AHR in humans. The principal findings were:
Best BV‐EN produced a significant improvement in the AHR of both ventricles compared with conventional epicardial pacing;
Compromise optimization is possible and may be helpful in the improvement of overall biventricular function, i.e., by accepting a small reduction in LV AHR, a relatively greater improvement in RV AHR can often be achieved;
The measurement of RV AHR is more sensitive to pacing perturbations than LV AHR;
A weak correlation exists between the RV and LV AHR, thus the former cannot be used as a surrogate for the latter.
CRT is an efficacious therapy for selective patients with HF, yet this treatment is associated with a significant nonresponse rate. Reasons for poor response to CRT are well documented,23 including incomplete resynchronization leading to persistent dyssynchrony and suboptimal lead placement. While alternative epicardial pacing strategies are being considered,24 LV endocardial stimulation is increasingly being investigated as it has several advantages over conventional epicardial pacing: (1) there is no access constraint to myocardial sites subtended by the CS, which is important given the significant individual variation with regard to the optimal LV pacing site10, 18; (2) endocardial pacing produces more physiological activation of the LV and more favorable tissue repolarization.25, 26 Our findings are in keeping with previous clinical studies, which suggest that endocardial pacing produces a superior LV AHR compared with epicardial pacing, and that LV AHR is highly site‐specific.3, 18, 27 Indeed, patients with a suboptimal response to conventional CRT have been demonstrated to show both clinical and echocardiographic improvement from endocardially delivered LV stimulation for BV pacing.28
Importantly, our results suggest that the superior acute hemodynamic effects of endocardial pacing over conventional epicardial CRT may be extended to the RV as well (Fig. 2). As expected, the mean of the best achievable LV AHR increased from conventional epicardial BV pacing to endocardial BV pacing (Fig. 4). In addition, this increase was accompanied with a rise in the corresponding RV AHR inferring that some of the beneficial effects of biventricular pacing could be mediated through improvement in RV function.
Comparison with Previous Studies
The similar improvement in LV AHR with LV‐CS or BV‐CS configurations (Fig. 3) is in keeping with the findings of Lumens et al.19 Using computational modeling and experimental hemodynamic measurements in canines, they showed a nonsignificant difference in LV AHR improvement between LV‐CS and BV‐CS pacing, and a nonsignificant improvement in RV AHR from either epicardial pacing mode.19 The additional application of BV‐EN pacing in this study produced a substantial increase in maximal RV AHR, which may be linked to an enhanced ability to redistribute myocardial fiber work by altering the endocardial pacing location.19
The greater increase in RV AHR from BV‐EN as opposed to epicardial pacing is contrary to the findings of the acute LBBB canine model study of van Deursen et al.25 where only BV epicardial pacing led to a statistically significant increase in RV AHR. Several key factors may explain these seemingly opposing findings: (1) interspecies differences (canine vs human); (2) pericardial pressure differences—the canine experiment was performed in open‐chest dogs with the pericardium removed, whereas this study was conducted in vivo via cardiac catheterization; and (3) disease state—the canines were acute radiofrequency‐ablated LBBB models, whereas the study participants were chronic HF patients with long‐term implanted CRT devices (Table I).
We have shown for the first time that compromise BV optimization can be performed and may be useful in selected patients. Out of nine patients, five were found to have a viable BV‐optimized pacing response. While 77% (seven out of nine) of patients achieved maximal LV AHR from BV‐EN, this proportion increased to 88% (eight out of nine) when the alternative BV hemodynamics optimization was retrospectively applied (Fig. 5); thus, the ability to increase BV hemodynamic function appears to be feasible with endocardial pacing. BV optimization resulted in an average increase of RV AHR by 20% at the expense of only a 2% decrease in LV AHR, compared to conventionally optimized AHRs.
Clinical Relevance
AHR is an accurate reflection of acute pump function but whether changes in maximum dPdt translate into better clinical outcomes is yet to be proven in a large‐scale study. Our group is currently conducting a multicenter, international, randomized study (RADI CRT – NCT 01464502) to formally assess this. Whatever the outcome, an intra‐arterial measurement as a predictive tool nonetheless carries a risk of thromboembolic stroke. Thus it would be clinically advantageous to circumvent this risk by using a surrogate, such as the RV AHR. Our data, however, suggest that a weak correlation exists between LV AHR and RV AHR and does not support the use of RV AHR as a surrogate for LV function. This weak correlation may be due to an increased sensitivity of the RV AHR to pacing perturbations compared to the LV AHR (Fig. 3).
Endocardial pacing can increase not only the average LV AHR but also the corresponding RV AHR over epicardial pacing, and therefore incorporation of BV optimization to identify the optimal position for the LV lead may translate into improved long‐term clinical outcomes. However, if adopting a BV AHR approach, it is unknown what reduction in LV AHR, if any, is acceptable in order to improve RV AHR and maintain a positive clinical outcome. For example, ambiguity exists with patient no. 3 (Table S3 and Fig. 5) where an alternative viable protocol results in a reduction of LV‐AHR from 34% to 21% while increasing RV AHR from 2% to 49%. A larger clinical study to examine the long‐term clinical outcomes of BV optimization and the relationship between LV and RV AHR would be needed, and should include a thorough investigation of the clinical relevance of more complex BV optimization metrics than the simple metric adopted in this study.
Study Limitations
We intentionally selected ischemic patients for whom response to CRT was suboptimal; this clearly leads to selection bias, however, this patient group has the most to gain from alternate pacing strategies and incorporation of different AHR protocols (such as using the RV AHR in combination with the LV AHR rather than the latter alone).
Although we have studied a small number of patients, we have a large number of data points within this group, which we believe provides sufficient information for analysis and has resulted in statistically significant findings. Only limited substrate data were available and therefore we did not investigate the relationship between acute response and myocardial fibrosis, which is known to affect clinical outcomes from CRT.29 The AHRs reported in these patients are on average 15 months after CRT implantation. It is known that LV remodeling (whether positive or negative) often occurs between 3–6 months6; thus, AHR values at 15 months are unlikely to be the same as those at implantation and caution needs to be applied in the extrapolation of these findings to patient AHR at the time of implant. We also acknowledge that as a result of the anatomical restrictions of the coronary veins, we have more data points with endocardial pacing and therefore had a greater opportunity to achieve a better AHR with endocardial pacing.30 However, our results are similar to published data suggesting that endocardial pacing involves access to the fast‐conducting His‐Purkinje tissue and may be a preferential location for LV stimulation in delivering CRT. 25, 26, 31
An important assumption of the current manuscript is that a higher RV dPdtmax equates with an improvement in RV function. In support of this there are published data that the RV AHR positively correlates with the cardiac index supporting the hypothesis that improvement in this acute metric may have a positive and important contribution on cardiac output.14 It should be acknowledged, however, that this may not necessarily be the case in all HF patients where a decrease in LV function may lead to an increase in RV afterload and so higher RV pressure may not necessarily be a positive sign. Similarly the right side of the heart acts more as a volume and capacitance conduit than a high‐pressure pump, which exists on the left side, and therefore, the interpretation of the concept of RV or BV optimization may not be straightforward. Our findings of a change in the relation between RV and LV dPdtmax do, however, highlight the fact that changes in LV function are linked to RV function and vice versa.
Conclusions
BV‐EN pacing has greater capacity to improve RV as well as LV AHR compared with conventional epicardial pacing. A new metric of compromised biventricular optimization, where RV AHR is increased for a relatively smaller decrease in LV AHR, is feasible with BV‐EN pacing and may be of clinical benefit in patients with a poor CRT response to epicardial pacing. The weak correlation between LV and RV AHR across all tested pacing modes does not support the use of RV AHR as a surrogate measure of LV AHR.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1: Patient‐Specific Pacing Group Breakdown of the Total Dataset.
Table S2: Individualized Interventricular Pressure Metrics Correlations.
Table S3: Conventional and Alternative CRT Optimizations of Biventricular Function.
Figure S1: Example processing of an individual pacing protocol using our bespoke software “pTool.” This software allows for the rapid removal of ectopic beat effects from the analyzed dataset. Ectopic beats were observed to have an influence on one beat prior‐ and up to two beats postectopic; thus, data from these beats were selected for removal from data analysis (shaded beat windows). Quantities of interest in the remaining beat windows were then analyzed to produce protocol‐averaged metrics (mean ± standard deviation), e.g., peak systolic pressure, start diastolic pressure, end diastolic pressure, and dPdtmax.
Figure S2: The datum corresponding to the maximal LV AHR protocol is located at the bottom right hand corner of each panel (i.e., (0,0)); the BV AHR of all other protocols are located relative to this data point. Viable compromise protocols are those that lie in the white triangular region, i.e., ΔRV −ΔLV, meaning that the enhanced RV response is greater than the diminished LV response. The vertical blue line indicates the 80% potential cutoff line as one possible strategy for selecting alternative protocols. Based on this BV optimized approach, three patients would have viable alternative protocols (patients 1, 5, and 6). AHR = acute hemodynamic response; BV = biventricular; LV = left ventricular; RV = right ventricular.
Figure S3: Scatterplot of all paired LV‐RV AHR data. The number is anonymized patient identification, and the green, red, and lilac colors correspond to BV‐EN, BV‐CS, and LV‐CS pacing, respectively. Acute ventricular response occurs for an AHR value >10% (indicated by the dashed lines).6 Note that patient 9 is the only patient not to improve via endocardial pacing as opposed to via the coronary sinus. This patient had the least number of endocardial protocols tested (n = 2) compared to all other patients (Table S1). AHR = acute hemodynamic response; BV = biventricular; CS = coronary sinus; EN = endocardial; LV = left ventricular; RV = right ventricular.
Disclosures: ERH and SAN receive funding from Boston Scientific. SAN is also supported by British Heart Foundation (PG/11/101/29212) and Wellcome Trust (WT 088641/Z/09/Z). CAR receives research funding and Honoraria from St. Jude Medical, Medtronic, and Boston Scientific. JMB receives funding through the NIHR Biomedical Research Centre at Guys' and St. Thomas' NHS Foundation Trust and the Rosetrees Trust. SC receives funding from St. Jude Medical.
References
- 1. Abraham WT, Fisher W. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 2. Derval N, Jais P. Optimizing hemodynamics in cardiac resynchronization therapy by left ventricular pacing site: You find only what you are looking for. J Am Coll Cardiol 2010; 56:782–783. [DOI] [PubMed] [Google Scholar]
- 3. Derval N, Bordachar P, Lim HS, Sacher F, Ploux S, Laborderie J, Steendijk P, et al. Impact of pacing site on QRS duration and its relationship to hemodynamic response in cardiac resynchronization therapy for congestive heart failure. J Cardiovasc Electrophysiol 2014; 25:1012–1020. [DOI] [PubMed] [Google Scholar]
- 4. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008; 117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 5. Sohal M, Amraoui S, Chen Z, Sammut E, Jackson T, Wright M, O'Neill MD, et al. Combined identification of septal flash and absence of myocardial scar by cardiac magnetic resonance imaging improves prediction of response to cardiac resynchronization therapy. J Interv Card Electrophysiol 2014; 40:179–190. [DOI] [PubMed] [Google Scholar]
- 6. Duckett SG, Ginks MR, Shetty AK, Bostock J, Gill JS, Hamid S, Kapetanakis S, et al. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol 2011; 58:1128–1136. [DOI] [PubMed] [Google Scholar]
- 7. Padeletti L, Pieragnoli P, Ricciardi G, Perrotta L, Grifoni G, Porciani MC, Lionetti V, et al. Acute hemodynamic effect of left ventricular endocardial pacing in cardiac resynchronization therapy: Assessment by pressure‐volume loops. Circ Arrhythm Electrophysiol 2012; 5:460–467. [DOI] [PubMed] [Google Scholar]
- 8. Lambiase PD, Rinaldi CA, Hauck J, Mobb M, Elliott D, Mohammad S, Gill JS, et al. Non‐contact left ventricular endocardial mapping in cardiac resynchronisation therapy. Heart 2004; 90:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jais P, Douard H, Shah DC, Barold SS, Barat JL, Clementy J. Endocardial biventricular pacing. Pacing Clin Electrophysiol 1998; 21:2128–2131. [DOI] [PubMed] [Google Scholar]
- 10. Bordachar P, Derval N, Ploux S, Garrigue S, Ritter P, Haissaguerre M, Jaïs P. Left ventricular endocardial stimulation for severe heart failure. J Am Coll Cardiol 2010; 56:747–753. [DOI] [PubMed] [Google Scholar]
- 11. Ginks MR, Shetty AK, Lambiase PD, Duckett SG, Bostock J, Peacock JL, Rhode KS, et al. Benefits of endocardial and multisite pacing are dependent on the type of left ventricular electric activation pattern and presence of ischemic heart disease: Insights from electroanatomic mapping. Circ Arrhythm Electrophysiol 2012; 5:889–897. [DOI] [PubMed] [Google Scholar]
- 12. Shetty AK, Sohal M, Chen Z, Ginks MR, Bostock J, Amraoui S, Ryu K, et al. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: An acute haemodynamic and electroanatomical study. Europace 2014; 16:873–879. [DOI] [PubMed] [Google Scholar]
- 13. Hegbom F, Hoff PI, Oie B, Følling M, Zeijlemaker V, Lindemans F, Ohm OJ. RV function in stable and unstable VT: Is there a need for hemodynamic monitoring in future defibrillators? Pacing Clin Electrophysiol 2001; 24:172–182. [DOI] [PubMed] [Google Scholar]
- 14. Dubin AM, Feinstein JA, Reddy VM, Hanley FL, Van Hare GF, Rosenthal DN. Electrical resynchronization: A novel therapy for the failing right ventricle. Circulation 2003; 107:2287–2289. [DOI] [PubMed] [Google Scholar]
- 15. Thambo JB, Dos Santos P, De Guillebon M, Roubertie F, Labrousse L, Sacher F, Iriart X, et al. Biventricular stimulation improves right and left ventricular function after tetralogy of Fallot repair: Acute animal and clinical studies. Heart Rhythm 2010; 7:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quinn TA, Berberian G, Cabreriza SE, Maskin LJ, Weinberg AD, Holmes JW, Spotnitz HM. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol 2006; 291:H2380–H2387. [DOI] [PubMed] [Google Scholar]
- 17. Garrigue S, Bordachar P, Reuter S, Jaïs P, Haïssaguerre M, Clementy J. Comparison of permanent left ventricular and biventricular pacing in patients with heart failure and chronic atrial fibrillation: A prospective hemodynamic study. Card Electrophysiol Rev 2003; 7:315–324. [DOI] [PubMed] [Google Scholar]
- 18. Spragg DD, Dong J, Fetics BJ, Helm RH, Marine JE, Cheng A, Henrikson CA, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol 2010; 56:774–781. [DOI] [PubMed] [Google Scholar]
- 19. Lumens J, Ploux S, Strik M, Gorcsan J, Cochet H, Derval N, Strom M, et al. Comparative electromechanical and hemodynamic effects of left ventricular and biventricular pacing in dyssynchronous heart failure: Electrical resynchronization versus left‐right ventricular interaction. J Am Coll Cardiol 2013; 62:2395–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt O‐A, Cleland J, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013; 34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 21. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713. [DOI] [PubMed] [Google Scholar]
- 22. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001; 7:176–182. [DOI] [PubMed] [Google Scholar]
- 23. Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WHW. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol 2009; 53:765–773. [DOI] [PubMed] [Google Scholar]
- 24. Rinaldi CA, Burri H, Thibault B, Curnis A, Rao A, Gras D, Sperzel J, et al. A review of multisite pacing to achieve cardiac resynchronization therapy. Europace 2014:euu197. [DOI] [PubMed] [Google Scholar]
- 25. van Deursen CJM, van Geldorp IE, Rademakers LM, van Hunnik A, Kuiper M, Klersy C, Auricchio A, et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle‐branch hearts. Circ Arrhythm Electrophysiol 2009; 2:580–587. [DOI] [PubMed] [Google Scholar]
- 26. Hyde ER, Behar JM, Claridge S, Jackson T, Lee AWC, Remme EW, Sohal M, et al. Beneficial effect on cardiac resynchronization from left ventricular endocardial pacing is mediated by early access to high conduction velocity tissue: An electrophysiological simulation study. Circ Arrhythmia Electrophysiol 2015; 8:1164–1172. [DOI] [PubMed] [Google Scholar]
- 27. Shetty AK, Duckett SG, Ma YL, Kapetanakis S, Ginks M, Bostock J, Carr‐White G, et al. The acute hemodynamic response to LV pacing within individual branches of the coronary sinus using a quadripolar lead. Pacing Clin Electrophysiol 2012; 35:196–203. [DOI] [PubMed] [Google Scholar]
- 28. Morgan JM, Biffi M, Gellér L, Leclercq C, Ruffa F, Tung S, Defaye P, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): A prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J 2016:ehv723. [DOI] [PubMed] [Google Scholar]
- 29. Bleeker GB. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006; 113:969–976. [DOI] [PubMed] [Google Scholar]
- 30. Wichterle D, Vancura V. Statistical bias in seeking the left ventricular endocardial sweet spot for cardiac resynchronization therapy. J Am Coll Cardiol 2011; 57:1000. [DOI] [PubMed] [Google Scholar]
- 31. Strik M, Rademakers LM, van Deursen CJM, van Hunnik A, Kuiper M, Klersy C, Auricchio A, et al. Endocardial left ventricular pacing improves cardiac resynchronization therapy in chronic asynchronous infarction and heart failure models. Circ Arrhythm Electrophysiol 2012; 5:191–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1: Patient‐Specific Pacing Group Breakdown of the Total Dataset.
Table S2: Individualized Interventricular Pressure Metrics Correlations.
Table S3: Conventional and Alternative CRT Optimizations of Biventricular Function.
Figure S1: Example processing of an individual pacing protocol using our bespoke software “pTool.” This software allows for the rapid removal of ectopic beat effects from the analyzed dataset. Ectopic beats were observed to have an influence on one beat prior‐ and up to two beats postectopic; thus, data from these beats were selected for removal from data analysis (shaded beat windows). Quantities of interest in the remaining beat windows were then analyzed to produce protocol‐averaged metrics (mean ± standard deviation), e.g., peak systolic pressure, start diastolic pressure, end diastolic pressure, and dPdtmax.
Figure S2: The datum corresponding to the maximal LV AHR protocol is located at the bottom right hand corner of each panel (i.e., (0,0)); the BV AHR of all other protocols are located relative to this data point. Viable compromise protocols are those that lie in the white triangular region, i.e., ΔRV −ΔLV, meaning that the enhanced RV response is greater than the diminished LV response. The vertical blue line indicates the 80% potential cutoff line as one possible strategy for selecting alternative protocols. Based on this BV optimized approach, three patients would have viable alternative protocols (patients 1, 5, and 6). AHR = acute hemodynamic response; BV = biventricular; LV = left ventricular; RV = right ventricular.
Figure S3: Scatterplot of all paired LV‐RV AHR data. The number is anonymized patient identification, and the green, red, and lilac colors correspond to BV‐EN, BV‐CS, and LV‐CS pacing, respectively. Acute ventricular response occurs for an AHR value >10% (indicated by the dashed lines).6 Note that patient 9 is the only patient not to improve via endocardial pacing as opposed to via the coronary sinus. This patient had the least number of endocardial protocols tested (n = 2) compared to all other patients (Table S1). AHR = acute hemodynamic response; BV = biventricular; CS = coronary sinus; EN = endocardial; LV = left ventricular; RV = right ventricular.


