Abstract
Objective To determine how between- and within-person variability in perceived sleep quality were associated with adolescent diabetes management. Methods A total of 236 older adolescents with type 1 diabetes reported daily for 2 weeks on sleep quality, self-regulatory failures, frequency of blood glucose (BG) checks, and BG values. Average, inconsistent, and daily deviations in sleep quality were examined. Results Hierarchical linear models indicated that poorer average and worse daily perceived sleep quality (compared with one’s average) was each associated with more self-regulatory failures. Sleep quality was not associated with frequency of BG checking. Poorer average sleep quality was related to greater risk of high BG. Furthermore, inconsistent and daily deviations in sleep quality interacted to predict higher BG, with more consistent sleepers benefitting more from a night of high-quality sleep. Conclusions Good, consistent sleep quality during late adolescence may benefit diabetes management by reducing self-regulatory failures and risk of high BG.
Keywords: adolescents, diabetes management, sleep quality, type 1 diabetes
Successful management of type 1 diabetes is a regulatory phenomenon that requires adherence to a complicated daily regimen (Hood, Peterson, Rohan, & Drotar, 2009). Adolescents must persist in multiple regimen activities (e.g., counting carbohydrates, checking blood glucose [BG], determining insulin dose) continually throughout the day, making it an especially difficult regulatory task. Given the daily nature of diabetes management in type 1 diabetes, adolescents may benefit from a restorative process, such as sleep. Poor sleep quality (the feeling of being tired on waking or that sleep is not restful or restorative) in late adolescence and emerging adulthood is associated with difficulties in emotion and stress regulation (Galambos, Howard, & Maggs, 2011), and performance on other complex regulatory tasks such as academic achievement (Short, Gradisar, Lack, & Wright, 2013). By extension, poor quality sleep may be associated with daily failures to regulate one’s cognitions, emotions, and behaviors surrounding diabetes that serve to support adherence and good glycemic control. The present study examined associations of daily perceived sleep quality with daily diabetes management among late adolescents with type 1 diabetes.
Recent research has suggested that sleep, as measured via polysomnography, actigraphy, and self-reports, is an important predictor of glycemic control (Borel et al., 2013; Perfect et al., 2012), and that youth with type 1 diabetes have poorer sleep compared with their peers without diabetes (Perfect et al., 2012). Specifically, youth with diabetes display shorter sleep duration, poorer sleep quality, and worse sleep architecture as demonstrated by both self-report and objective measures (Barone & Menna-Barreto, 2011; Jauch-Chara, Schmid, Hallschmid, Born, & Shultes, 2008; Perfect et al., 2012). Compared with those without diabetes, youth with diabetes spend more time in lighter stages of sleep (i.e., N2), which is associated with greater percentage of time spent in hyperglycemia and with poorer glycemic control (Perfect et al., 2012). Impaired sleep may undermine adolescents’ abilities to regulate their diabetes both physiologically and behaviorally.
Late adolescence is an especially important time to study the relation between diabetes management and sleep quality, as they both deteriorate during this time of development. First, both sleep quantity and quality often worsen in response to changes in circadian rhythms and an inability to sleep for sufficient length (Colrain & Baker, 2011). Eighty-seven percent of American adolescents get less than optimal average levels of sleep (<8 hr/night; Morselli, Leproult, Balbo, & Spiegel, 2010). Late adolescents are also at risk for inconsistent sleep (good sleep one night, poor the next), which predicts the occurrence of risk behaviors like conduct problems (Lin & Yi, 2015). Second, adolescence is a period when glycemic control and adherence to the diabetes regimen often deteriorate for youth with type 1 diabetes (King, Berg, Butner, Butler, & Wiebe, 2014; Luyckx & Seiffge-Krenke, 2009). Insulin sensitivity decreases (and thus glycemic variability increases) owing to hormonal fluctuations (Amiel, Sherwin, Simonson, Lauritano, & Tamborlane, 1986; Tfayli & Arslanian, 2007). Moreover, late adolescents and young adults are the age groups least likely to meet the American Diabetes Association (ADA)-recommended diabetes targets for glycemic control (Miller et al., 2015).
It is important to examine daily links between sleep quality and diabetes management given the daily regulatory challenge of managing diabetes and the demonstrated associations between sleep quality and diabetes management. The broader literature on sleep suggests that daily differences in sleep have implications for health during adolescence (e.g., body mass index; Moore et al., 2011). Variation in the quantity and quality of sleep is greatest during adolescence (Dahl and Lewin, 2002; Telzer, Goldenberg, Fuligni, Lieberman, & Galván, 2015), with “intra-individual differences in sleep duration often exceeding between-person differences” (p. 17, Telzer et al., 2015). Studies suggest that nightly deviations from one’s average sleep duration may be as detrimental as poor average levels of sleep for adolescents’ health and development (Fuligni & Hardway, 2006; Telzer et al., 2015). Less is known, however, about how variations in nightly sleep quality relate to health outcomes during adolescence. Fuligni and Hardway (2006) suggested that assessing daily variability in sleep quality would provide a clearer picture as to why fluctuations in sleep influence some adolescents more than others. Daily variations in self-reported sleep quality may be especially informative in understanding daily diabetes management given the potential disruptions in crucial cognitive (e.g., continual management of daily complicated tasks) and physiological (e.g., BG regulation) processes implicated by poor quality sleep.
Poorer daily quality of sleep may put adolescents with type 1 diabetes at higher risk of experiencing self-regulatory failures related to diabetes (e.g., failing to check BG because of being distracted or not feeling like checking), poorer adherence behaviors (e.g., less frequent BG checks), and poorer daily BG. In support of the idea that sleep would be associated with more self-regulation failures, insufficient sleep quality has been associated with worsened daytime cognitive and emotional functioning in adolescents, which may contribute to poor adherence practices. That is, Benitez and Gunstad (2012) illustrated that adolescents who reported poorer overall sleep quality had poorer scores on tests of executive functions and attention, underlying capabilities that should minimize daily self-regulation failures (Short et al., 2013). Daily variations in sleep quality may compromise daily diabetes adherence, such as BG checking, which has been associated with better self-reported regulation skills (Berg et al., 2014). Moreover, poorer sleep quality also relates to increased food intake and unhealthy diets (Chaput, 2014). Finally, poor sleep quality may be directly associated with alterations in glucose homeostasis, and thus high BG values (Byberg et al., 2012; Perfect et al., 2012). Taken together, poor quality sleep may disrupt adolescents’ cognitive abilities to complete the daily complex regulatory tasks, adherence behaviors, and physiological homeostasis required to maintain glycemic control.
Further, although not studied within the context of diabetes, previous work has found that the combination of sufficient and consistent sleep related to improved self-control and less psychological strain, but not to either in isolation (Barber & Munz, 2010). Barber and Munz (2010) reported that inconsistent sleepers did not exhibit the benefits of good sleep. Thus, it may be the case that the regulatory benefits of daily sleep are not seen for self-regulation failures, BG checks, and BG regulation for those who report inconsistent sleep quality.
In the present study, we conducted a secondary analysis of a larger, ongoing data set to examine the associations between daily sleep quality and aspects of daily diabetes management, including daily self-regulatory failures, frequency of BG checking, and risk of high BG. We used a daily diary method to examine both between- and within-person differences in sleep quality related to these outcomes. We captured perceived sleep quality via three different facets: two that focused on individual differences in sleep quality (i.e., between-person average and inconsistent quality across a 14-day period), and one that represented within-person differences (daily deviations, or how an individual deviated daily from his or her own 14-day average quality). We first tested the main effects of each of these facets of perceived sleep quality on the diabetes management outcomes and then examined whether good daily sleep quality was most beneficial for those who had more consistent sleep quality across the 14 days. Based on the existing literature, we hypothesized that both between- and within-person differences in sleep quality would be associated with diabetes outcomes. At the between-person level, individuals who had higher average sleep quality and who had higher consistent sleep quality across the 2-week diary were expected to display better diabetes outcomes (i.e., fewer self-regulatory failures, more frequent BG checks, and lower risk of high BG levels). At the within-person level, we hypothesized that higher daily sleep quality would be associated with fewer self-regulatory failures, more BG checks, and lower risk of high BG on days following a night of higher quality sleep. As described above, we additionally tested an interaction of between-person (inconsistent) and within-person (daily deviations) effects of sleep quality to examine whether the daily benefits of good-quality sleep are greater for those who consistently report getting good-quality sleep.
Method
Participants
High school seniors with type 1 diabetes were recruited for a 2-year longitudinal study on diabetes management and self-regulation during late adolescence and emerging adulthood. Participants were recruited from outpatient pediatric endocrinology clinics in two southwestern U.S. cities by a research assistant in clinic, or by mail and phone. Of the qualifying 507 individuals approached, 301 (59%) initially agreed to participate. Of those who agreed, 247 teens (82%) completed baseline assessments. Reasons for not participating included being too busy in their senior year to participate (34%), lack of interest (33%), and 20% declined to give a reason.
Youth were eligible to participate if they had been diagnosed with type 1 diabetes for at least 1 year (M illness duration = 7.35 years, SD = 3.88), had English as their primary language, were in their final year of high school, lived with a caregiver (68.4% lived at home with both biological parents, 27.1% with one biological parent, and 4.5% lived with grandparents or adoptive parents), would be able to have regular contact with caregivers over the subsequent 2 years (consistent with objectives of the broader longitudinal study), and had no condition that would prohibit study completion (e.g., severe intellectual disability, blindness).
Consistent with the patient population at participating clinics, 75.2% of the full sample (N = 247) identified as non-Hispanic White, 14.2% as Hispanic, 4.8% as African American, and the remainder identified as Asian/Pacific Islander, American Indian, or more than one race. Patients were 17.76 years old on average (SD = 0.39) and 60% were female. Parents had a range of educational backgrounds, with 12.9% of mothers and 18.2% of fathers having a high school education or less, 37.2% of mothers and 25.1% of fathers with some college or a vocational degree, and 34% of mothers and 46.3% of fathers with a bachelor’s degree or higher.
The present study analyzed baseline data from participants who responded to the daily diary. Of the 247 individuals enrolled in the full study, 236 completed the daily diary measures. Adolescents in this subsample were 17.77 years of age (SD = 0.39) on average and had been diagnosed with type 1 diabetes for an average of 7.34 (SD = 3.88) years. In this subsample, 62% of adolescents were female and 43% of patients reported using an insulin pump. Sixty-three percent of our analyzed sample was above the ADA age-specific recommendations (HbA1c < 7.5%) for glycemic control (M HbA1c = 8.27, SD = 1.62).
Procedure
The appropriate institutional review boards at each site approved the study, and all APA Codes of Conduct were followed. Parents provided informed consent for their child’s participation and adolescents completed assent or consent. Adolescents completed a 14-day online end-of-day diary measuring (among other variables) daily BG values, frequency of BG checking, and nightly sleep quality. On average, adolescents completed 11 days of the diary (SD = 3.59). Participants received the diary nightly via an e-mail link, and if it was not yet completed by 9:00 pm, they received a text message reminder. Adolescents were compensated $5 for each diary day completed. All baseline data were collected during the academic year (September–June) and data collection did not occur over holidays or school breaks.
Measures
Perceived Sleep Quality
At the end of each day, participants rated the perceived quality of their sleep the previous night (“How well did you sleep last night?”) on a scale of 1 (very badly) to 5 (very well). This measure was modeled after a similar item (“During the past month, how would you rate your sleep quality overall?”) from the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) that correlates highly (r = .83) with the global score. This item is frequently used in daily diary studies as an assessment of subjective sleep quality and is correlated with more objective measures of sleep (Krystal & Edinger, 2008). A single item was used given the necessity of short assessments in daily diary work to reduce subject burden (Iida, Shrout, Laurenceau, & Bolger, 2012), and because sleep was not the primary focus of the larger study.
Daily Self-Regulatory Failures
Adolescents completed eight items each day that were developed to capture failures in self-regulation involving cognitive, emotional, and behavioral processes surrounding BG checking. Prior research indicated that BG checking is a frequent problem in the daily lives of adolescents with type 1 diabetes (Berg et al., 2014; Hood et al., 2009). Teens reported on a 1 (strongly disagree) to 5 (strongly agree) scale on items such as “I kept putting off my BG testing,” “I had a lot going on and had a hard time figuring out the best time or place to do my BG tests,” and “Each time I was about to test my BG, I got distracted by something else” (see Author’s Citation for a complete list). A multilevel factor analysis revealed that the items fall on one factor both between and within persons (χ2 = 268.37, p < .001). A previous study demonstrated inter-item consistency reliability of the eight items was calculated via Hierarchical Linear Modeling random intercept models, with both time and item treated as nested levels, and was excellent (reliability of 0.98).
Daily Frequency of BG Checks
At the end of each day, adolescents entered that day’s BG values and time of each BG check into the online diary. Participants were instructed to enter these values into the diary directly from their glucometers. We used self-reported BG for many reasons: participating clinics did not routinely download glucometer data, the larger longitudinal study precluded physical downloads because teens were geographically mobile, and other technologies such as Bluetooth did not exist at the outset of the larger study.
Adolescents’ daily number of BG checks was captured as a count of the number of BG values reported for each day. The ADA generally recommends six to eight BG checks per day (ADA, 2014). Adolescents in our sample checked their BG an average of 3.65 times per day (SD = 1.58, Range = 0.36–10).
Daily Risk of High BG
The BG data were also used to compute the risk of having high BG, which was calculated using McCall and Kovatchev’s (2009) High BG Index. For each day, individuals’ BG values were used to calculate their daily risk of having high BG levels (i.e., above 180 mg/dl; scaled 0–100; M = 13.58, SD = 11.83, Range = 0–82.27). We focused on risk for high BG rather than average BG because the scaling of BG is not linear, or equivalent at each end of the scale (i.e., there is a greater range of high BG values [180–600+ mg/dl] than low [∼20–70 mg/dl]). We also calculated adolescents’ daily risk of low BG (values <70 mg/dl); the mean risk of low BG across the sample was quite low and the range restricted (Scaled 0–100; M = 1.43, Range = 0–11.99). The adolescents in our sample were at greater risk for high values, and thus we focused our analyses on risk of high BG. Across the daily diary, adolescents’ average BG reading was 188.17 (SD = 60.94).
Analytic Plan
Multilevel modeling was used to address our questions regarding the associations between subjective sleep quality and three daily diabetes outcomes: daily self-regulatory failures, frequency of daily BG checks, and risk for high BG values. Given that we measured sleep quality and diabetes outcomes on a daily level, our data were structured such that days were nested within persons. All analyses were conducted using PROC MIXED in SAS v9.2.
To understand the between- versus within-person contributions of perceived sleep quality on diabetes outcomes, we computed three facets of sleep quality. First, we created a between-person variable, centered at the grand mean, that reflected a person’s average sleep quality across the daily diary. Higher values on this variable indicated that a person reported higher quality sleep on average across the 14-day diary than others in the sample. Second, we created another between-person variable, centered at the grand mean, which reflected inconsistent sleep quality. This variable was created by computing the standard deviation across all 14 days of each individual’s set of daily reports of perceived sleep quality. Higher values on this variable indicated that a person reported more inconsistent sleep quality across the 14-day diary than others in the sample. Lastly, we created a group-centered within-person daily sleep quality deviations variable, which captured the extent to which an individual reported better or worse sleep quality that day compared with his or her own average sleep quality. This variable was computed by subtracting each person’s individual mean from his or her daily reports of subjective sleep quality. Positive values on this variable indicated that a person perceived better sleep quality that day than his or her average. To determine the distribution of variance explained by between- versus within-person effects, separate multilevel models were run that solely included the outcome measures (intercept only models).
For each outcome variable (i.e., daily self-regulatory failures, daily BG checks, and daily risk for high BG values), we tested for main effects of the three facets of sleep and a between- × within-person interaction between daily sleep quality deviations and inconsistent sleep quality (while controlling for the main effect of average sleep quality). In each model, we controlled for insulin pump status (1 = uses insulin pump; 0 = no pump) and illness duration in years (grand mean centered) as both are known to be associated with diabetes management (see Author’s Citation). As an example of the structure of these analyses including the between- × within-person interaction, we have depicted the equation for risk of high BG values below; similar analyses were conducted for the other two diabetes outcomes (self-regulatory failures and BG checks):
 |
The daily risk for high BG was predicted from daily sleep quality deviations with β1ij representing the within-person effect of daily sleep quality on risk for going high (i.e., whether daily deviations in sleep quality were associated with daily variations in risk for going high). Between-person differences in average sleep quality and inconsistent sleep quality across the 14 days were represented by γ03 and γ04, respectively. Finally, the interaction of inconsistent and daily sleep deviations was represented by γ11.
Results
Preliminary Analyses
As can be seen in Table I, our sample of adolescents reported that on average they slept between “Okay” and “Pretty Well” across the 2-week daily diary. Greater average sleep quality was associated with less inconsistent (more consistent) sleep quality. Greater average sleep quality was also associated with fewer self-regulatory failures and lower risk of high BG. Inconsistent sleep quality also related to these outcomes, such that more inconsistent perceptions of sleep quality across the diary related to more self-regulatory failures and greater risk of high BG. Daily frequency of BG checks was not related to any of the three facets of sleep quality. Self-regulatory failures were associated with fewer BG checks and greater risk of high BG.
Table I.
Means, Standard Deviations, and Zero-Order Correlations
| Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| 1. Pump status | 43% | 1 | ||||||
| 2. Illness duration | 7.35 (3.88) | 0.07 | 1 | |||||
| 3. Average sleep quality (SQ) | 3.56 (0.78) | −0.05 | −0.12 | 1 | ||||
| 4. Inconsistent SQ | 0.75 (0.36) | −0.06 | 0.02 | −0.33** | 1 | |||
| 5. Self-regulatory failures | 2.09 (0.82) | 0.08 | 0.07 | −0.30** | 0.15* | 1 | ||
| 6. Risk of high BG | 13.58 (11.83) | −0.04 | 0.23** | −0.22** | 0.15* | 0.39** | 1 | |
| 7. Frequency of BG checks | 3.65 (1.58) | 0.11 | 0.05 | 0.09 | −0.07 | −0.39** | −0.27** | 1 |
Note. SQ = sleep quality. The “Daily deviations in sleep quality” variable was not included in Table I above because it is an individual-level variable, and if aggregated across the sample, the results would duplicate those for “Inconsistent SQ.”
*p < .01; **p < .001.
Daily Self-Regulatory Failures
To examine the variability in self-regulation failures accounted for by between- versus within-person variation, a fully unconditional model revealed that 71% of the variability in daily self-regulatory failures was between person and 28% was within person. Table II depicts the results for the conditional models. Linking sleep quality indices to self-regulation failures, there was a significant main effect of average sleep quality, indicating that those who reported higher quality sleep across the daily diary also reported fewer daily self-regulatory failures. Similarly, there was a main effect of daily sleep quality deviations, such that on days when adolescents reported better sleep quality the prior night, they had fewer self-regulatory failures. No main effect of inconsistent sleep quality was found, nor was an interaction of inconsistent sleep quality × daily sleep quality deviations.
Table II.
Multilevel Models of Sleep Quality (SQ) Facets Predicting Diabetes Outcomes
| Outcome | Parameter | b | SE | df | t | p |
|---|---|---|---|---|---|---|
| Daily self-regulatory failures | Intercept | 2.02 | 0.07 | 226 | 29.94 | <.0001*** |
| Pump status | 0.12 | 0.10 | 224 | 1.17 | .24 | |
| Illness duration | 0.007 | 0.01 | 226 | 0.55 | .58 | |
| Average SQ | −0.30 | 0.07 | 226 | −4.28 | <.0001*** | |
| Inconsistent SQ | 0.19 | 0.15 | 230 | 1.23 | .22 | |
| Daily SQ deviations | −0.04 | 0.02 | 2,285 | −2.12 | .03* | |
| Inconsistent × daily deviations | −0.06 | 0.04 | 2,285 | −1.36 | .17 | |
| Daily frequency of BG checks | Intercept | 3.50 | 0.14 | 223 | 25.23 | <.0001*** |
| Pump status | 0.42 | 0.21 | 220 | 1.99 | .05 | |
| Illness duration | 0.02 | 0.03 | 222 | 0.81 | .42 | |
| Average SQ | 0.23 | 0.15 | 225 | 1.57 | .12 | |
| Inconsistent SQ | −0.16 | 0.31 | 227 | −0.53 | .60 | |
| Daily SQ deviations | 0.003 | 0.04 | 2,209 | 0.06 | .95 | |
| Inconsistent × daily deviations | −0.11 | 0.10 | 2,206 | −1.06 | .29 | |
| Daily risk of high BG | Intercept | 14.22 | 0.96 | 209 | 14.76 | <.0001*** |
| Pump status | −1.45 | 1.47 | 205 | −0.98 | 0.33 | |
| Illness duration | 0.65 | 0.19 | 208 | 3.39 | 0.0008*** | |
| Average SQ | −2.56 | 1.02 | 213 | −2.51 | 0.01* | |
| Inconsistent SQ | 3.32 | 2.18 | 216 | 1.52 | 0.13 | |
| Daily SQ deviations | −0.46 | 0.34 | 2,131 | −1.35 | 0.18 | |
| Inconsistent × daily deviations | 2.28 | 0.86 | 2,125 | 2.64 | 0.0084** |
Note. Average SQ and Inconsistent SQ (standard deviation) were across the 14-day diary.
BG = blood glucose.
*p < .05; **p < .01; ***p < .001.
Frequency of BG Checking
A fully unconditional model revealed that 61% of the variability in frequency of BG checks was between person and 38% of the variability was within person. A model linking sleep quality indices to frequency of BG checks indicated that there were no significant main effects of any of the three facets of sleep quality nor was there an interaction in predicting frequency of BG checking (see Table II).
Daily Risk of High BG
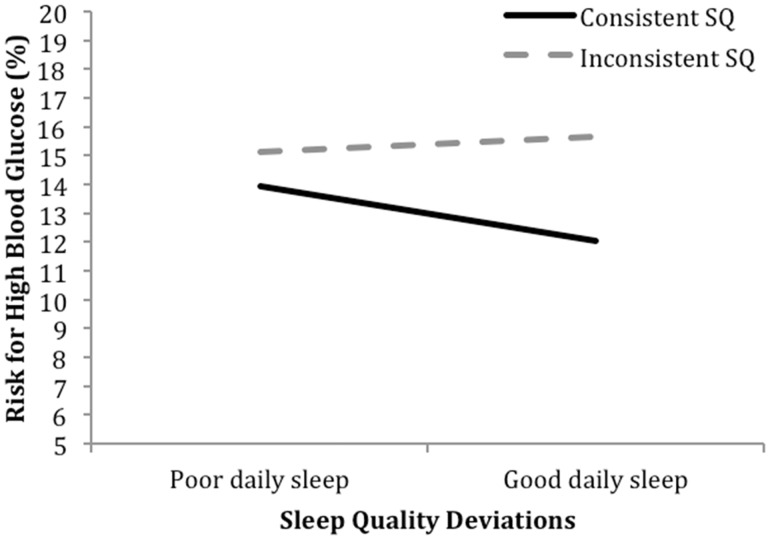
A fully unconditional model revealed that 54% of the variability in daily risk of high BG was between person and 46% was within person. A model linking sleep quality indicators to risk for high BG was conducted. Estimates for the conditional models are depicted in the middle section of Table II. First, longer duration of diabetes was significantly associated with higher daily risk of high BG values. Above this effect, there was a significant main effect of average sleep quality such that adolescents who had better sleep quality across the daily diary were at lower risk for having high daily BG. Neither daily sleep deviations nor inconsistent sleep quality was associated with high daily BG. However, including the between- × within-person interaction in the model revealed a significant inconsistent sleep quality × daily deviations in sleep quality interaction. As depicted in Figure 1, adolescents who reported more consistent sleep quality had lower risk for high BG values on days when they reported better sleep quality. In contrast, adolescents who reported less consistent sleep quality showed no association between daily sleep quality and high BG risk (simple slopes: pconsistent = 0.03, pinconsistent = 0.20).
Figure 1.
Daily sleep quality deviations × inconsistent sleep quality predicting daily risk of high blood glucose.
Note. The simple slope for inconsistent sleep quality is not significantly different from zero (p = .20).
Discussion
This study demonstrated that perceived quality of sleep over a 2-week period was related to several important aspects of daily diabetes management among older adolescents. This study was novel in its examination of daily associations of sleep quality with self-regulatory failures and risk for high BG. Higher self-reported sleep quality during the 14 days was associated with fewer self-regulatory failures and lower risk of high BG. Furthermore, even among those who generally reported good-quality sleep, reporting higher quality sleep than one’s average was associated with better diabetes management (i.e., fewer self-regulatory failures). Finally, inconsistent sleep quality and daily sleep quality interacted to predict risk of high BG, with more consistent sleepers benefitting the most from good-quality sleep by demonstrating lower risk for high BG.
Consistent with research on the role of sleep in supporting self-regulation (Barber & Munz, 2010), average sleep quality and daily deviations in sleep quality were associated with fewer daily self-regulation failures surrounding BG checking. BG checking is a key component of daily diabetes management because it informs other diabetes care behaviors (e.g., insulin dosing, food choices, exercise; Hood et al., 2009). These results extend the research linking poor sleep quality to a range of self-regulation difficulties in attention, memory, and emotion regulation (Benitez & Gunstad, 2012; Vriend et al., 2013) to the specific context of diabetes management. Reduced daily self-regulatory failures are important, as they are associated with better adherence to the diabetes regimen (Berg et al., 2014).
The lack of association between perceived sleep quality and a measure of adherence, the frequency of daily BG checks, was surprising. Several possible reasons exist for this finding. First, it is possible that BG checking itself may be a somewhat regimented behavior. It is not uncommon, and even suggested, to use meal times as a marker of when to test BG (ADA, 2014). Our data may suggest that adolescents use mealtimes as a guide for BG checks, as the average number of daily BG checks was 3.65. In addition, it is possible that there are other intervening factors at work that promote adherence behaviors in the face of poor quality sleep and the self-regulatory failures associated with poor sleep quality. For example, although adolescents may report failures to self-regulate such as not caring to check BG because of their mood, parents may provide the support needed to ensure that BG checking is accomplished. Future work should explore the co-occurrence of self-regulatory failures and the compensatory efforts of parents.
Good-quality daily sleep was associated with lower risk of high BG, consistent with work linking better sleep quality with glycemic control (Perfect et al., 2012). Further, consistent with Barber and Munz’s (2010) interaction between consistent sleep quality and daily sleep quality, we found that adolescents who reported more consistent sleep quality demonstrated the greatest benefit of better quality daily sleep in terms of risk of going high. In contrast, we did not find a significant interaction between daily sleep quality deviations and inconsistent sleep quality when examining self-regulatory failures or BG checks. Many differences in study design exist between Barber and Munz’s (2010) work and the present study that preclude direct comparisons (e.g., an examination of quantity vs. quality of sleep, different self-regulatory measures, and analyses that aggregated vs. examined daily effects). Future research is needed to more completely understand these different findings. In the present study, it appears that good-quality daily sleep is not sufficient to reduce the risk of high BG, but must occur together with consistent sleep quality. One implication of this finding is that targeting daily sleep quality (daily deviations) and inconsistent sleep quality may be important to improve BG outcomes for teens with type 1 diabetes.
The present findings should be interpreted in light of several limitations. First, all primary measures were a form of self-report, and thus method variance may have influenced the results. Sleep quality was measured through self-reported recall of the prior night’s sleep, and future research is needed to understand what adolescents use to make these overall judgments, as they could be influenced by events that occurred during the day. Although participants reported on events that were separated in time, these reports were obtained concurrently and causal directions of association cannot be inferred. It is possible, for example, that inadequate diabetes management (i.e., out-of-range daily BG levels) could have disrupted sleep (Barone & Menna-Barreto, 2011; Jauch-Chara et al., 2008) and altered evaluations of sleep quality. Future research that supplements perceived sleep quality measures with objective metrics of sleep quality (e.g., sleep onset latency, sleep efficiency, frequency of night awakenings) that measures subjective sleep quality immediately on awakening, or that tests for reverse associations, would allow us to understand what it is that adolescents use when they report on sleep quality. Further, such research would assist in disentangling relations between sleep and diabetes management. Supplementing such research with wake and sleep times would allow us to also examine how BG just before sleep could affect sleep quantity and quality. Additionally, BG data (daily BG levels and number of checks) were based on adolescent daily reports of glucometer results, and therefore, it is possible that these data were inaccurate owing to transcription errors or social desirability. We used self-reported BG data as participating clinics did not routinely download glucometer data and logistical barriers in the larger longitudinal study precluded this practice as emerging adults were geographically scattered. Unfortunately, Bluetooth technology did not exist at the outset of the larger study. Future studies should examine how both daily sleep quality and quantity relate to objective markers of daily diabetes management (e.g., glucometer downloads or continuous glucose monitoring data). Finally, the sample was primarily Caucasian, reflecting demographic characteristics of those with type 1 diabetes, and all participants were seniors in high school. Results may not generalize to other age-groups or to racial minority patients.
These findings hold implications for youth with type 1 diabetes. If supported by future research, clinicians may wish to examine adolescents’ sleep practices and consider ways to promote consistently high-quality sleep. Maintaining or improving sleep quality may provide resources to prevent lapses in self-regulation and high BG excursions as late adolescents strive to manage diabetes on a daily basis. Given the importance of consistent sleep quality, it may be helpful to encourage adolescents and parents to consider sleep needs as they plan daily routines and obligations, and to develop consistent sleep habits (e.g., consistent sleep–wake timing). Furthermore, given the importance of daily fluctuations in sleep quality, it may be helpful to guide adolescents to evaluate day-to-day barriers to high-quality sleep and to problem-solve to minimize recurring barriers. Such factors may become increasingly important as late adolescents transition out of high school and into emerging adulthood, given that sleep patterns and diabetes management continue to change across this transition (Galambos et al., 2011).
Acknowledgments
The authors thank the physicians and staff at the Utah Diabetes Center, Mountain Vista Medicine, Children’s Medical Center Dallas, and the teens and parents who participated in this study.
Funding
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant number R01 DK092939, co-PIs Berg and Wiebe).
Conflicts of interest: None declared.
References
- American Diabetes Association. (2014). Standards of medical care in diabetes–2014. Diabetes Care, 37, S14–S80. doi:10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- Amiel S. A., Sherwin R. S., Simonson D. C., Lauritano A. A., Tamborlane W. V. (1986). Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. The New England Journal of Medicine, 315, 215–219. [DOI] [PubMed] [Google Scholar]
- Barber L. K., Munz D. C. (2010). Consistent-sufficient sleep predicts improvements in self–regulatory performance and psychological strain. Stress and Health, 27, 314–324. doi: 10.1002/smi.1292 [Google Scholar]
- Barone M. T., Menna-Barreto L. (2011). Diabetes and sleep: A complex cause-and-effect relationship. Diabetes Research and Clinical Practice, 91, 129–137. doi:10.1016/j.diabres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Benitez A., Gunstad J. (2012). Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. The Clinical Neuropsychologist , 26, 214–223. doi:10.1080/13854046.2012.658439 [DOI] [PubMed] [Google Scholar]
- Berg C. A., Wiebe D. J., Suchy Y., Hughes A. E., Anderson J. H., Godbey E. I., Butner J., Tucker C., Franchow E. I., Pihlaskari A. K., King P. S., Murray M. A., White P. C. (2014). Individual differences and day-to-day fluctuations in perceived self-regulation associated with daily adherence in late adolescents with type 1 diabetes. Journal of Pediatric Psychology , 39, 1038–1048. doi:10.1093/jpepsy/jsu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel A. L., Pépin J. L., Nasse L., Baguet J. P., Netter S., Benhamou P. Y. (2013). Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care, 36, 2902–2908. doi:10.2337/dcl 2-2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D. J., Reynolds C. F., Monk T. H., Berman S. R., Kupfer D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Byberg S., Hansen A. L., Christensen D. L., Vistisen D., Aadahl M., Linneberg A., Witte D. R. (2012). Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabetic Medicine, 29, e354–e360. doi:10.1111/j.1464-5491.2012.03711.x [DOI] [PubMed] [Google Scholar]
- Chaput J. P. (2014). Sleep patterns, diet quality and energy balance. Physiology and Behavior , 134, 86–91. doi:10.1016/j.physbeh.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Colrain I. M., Baker F. C. (2011). Changes in sleep as a function of adolescent development. Neuropsychology Review, 21, 5–21. doi:10.1007/s11065-010-9155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. E., Lewin D. S. (2002). Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health, 31, 175–184. [DOI] [PubMed] [Google Scholar]
- Fuligni A. J., Hardway C. (2006). Daily variation in adolescents' sleep, activities, and psychological well-being. Journal of Research on Adolescence, 16, 353–378. [Google Scholar]
- Galambos N. L., Howard A. L., Maggs J. L. (2011). Rise and fall of sleep quantity and quality with student experiences across the first year of university. Journal of Research on Adolescence, 21, 342–349. doi:10.1111/j.1532-7795.2010.00679.x [Google Scholar]
- Hood K. K., Peterson C. M., Rohan J. M., Drotar D. (2009). Association between adherence and glycemic control in pediatric type 1 diabetes: A meta-analysis. Pediatrics, 124, e1171–e1179. doi:10.1542/peds.2009-0207 [DOI] [PubMed] [Google Scholar]
- Iida M., Shrout P. E., Laurenceau J., Bolger N. (2012). Using diary methods in psychological research. In Cooper H., Camic P. M., Long D. L., Panter A. T., Rindskopf D., Sher K. J. (Eds.), APA handbook of research methods in psychology (pp. 277–305). Washington, DC: American Psychological Association. [Google Scholar]
- Jauch-Chara K., Schmid S. M., Hallschmid M., Born J., Schultes B. (2008). Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care, 31, 1183–1188. doi:10.2337/dc07-1986. [DOI] [PubMed] [Google Scholar]
- King P. S., Berg C. A., Butner J., Butler J. M., Wiebe D. J. (2014). Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents' adherence. Health Psychology, 33, 424–432. doi:10.1037/a0032804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal A. D., Edinger J. D. (2008). Measuring sleep quality. Sleep Medicine, 9, S10–S17. [DOI] [PubMed] [Google Scholar]
- Lin W., Yi C. (2015). Unhealthy sleep practices, conduct problems, and daytime functioning during adolescence. Journal of Youth and Adolescence , 44, 431–446. doi:10.1007/s10964-014-0169-9 [DOI] [PubMed] [Google Scholar]
- Luyckx K., Seiffge-Krenke I. (2009). Continuity and change in glycemic control trajectories from adolescence to emerging adulthood relationships with family climate and self-concept in type 1 diabetes. Diabetes Care, 32, 797–801. doi:10.2337/dcOS-1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall A. L., Kovatchev B. P. (2009). The median is not the only message: A clinician's perspective on mathematical analysis of glycemic variability and modeling in diabetes mellitus. Journal of Diabetes Science and Technology, 3, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Foster N. C., Beck R. W., Bergenstal R. M., DuBose S. N., DiMeglio L. A., Maahs D. M., Tamborlane W. V.; for the T1D Exchange Clinic Network. (2015). Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D Exchange clinic registry. Diabetes Care , 38, 971–978. doi:10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- Moore M., Kirchner H. L., Drotar D., Johnson N., Rosen C., Redline S. (2011). Correlates of adolescent sleep time and variability in sleep time: The role of individual and health related characteristics. Sleep Medicine, 12, 239–245. doi:10.1016/j.sleep.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli L., Leproult R., Balbo M., Spiegel K. (2010). Role of sleep duration in the regulation of glucose metabolism and appetite. Best Practice and Research Clinical Endocrinology & Metabolism, 24, 687–702. doi: 10.1016/j.beem.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect M. M., Patel P. G., Scott R. E., Wheeler M. D., Patel C., Griffin K., Sorensen S. T., Goodwin J. L., Quan S. F. (2012). Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep, 35, 81–88. doi:10.5665/sleep.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher J. J., Ginter D. R., Sadowsky B. (1997). Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. Journal of Psychosomatic Research, 42, 583–596. [DOI] [PubMed] [Google Scholar]
- Short M. A., Gradisar M., Lack L. C., Wright H. R. (2013). The impact of sleep on adolescent depressed mood, alertness and academic performance. Journal of Adolescence, 36, 1025–1033. doi: 10.1016/j.adolescence.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Telzer E. H., Goldenberg D., Fuligni A. J., Lieberman M. D., Galvan A. (2015). Sleep variability in adolescence is associated with altered brain development. Developmental Cognitive Neuroscience, 14, 16–22. http://dx.doi.org/10.1016/j.dcn.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfayli H., Arslanian S. (2007). The challenge of adolescence: Hormonal changes and sensitivity to insulin. Diabetes Voice, 52, 28–30. doi:10.1093/jpepsy/jst033 [Google Scholar]
- Vriend J. L., Davidson F. D., Corkum P. V., Rusak B., Chambers C. T., McLaughlin E. N. (2013). Manipulating sleep duration alters emotional functioning and cognitive performance in children. Journal of Pediatric Psychology, 38, 1058–1069. [DOI] [PubMed] [Google Scholar]
- Wiebe D., Berg C., Korbel C., Palmer D. L., Beveridge R., Upchurch R., Lindsay R., Swinyard M. T., Donaldson D. L. (2005). Children s appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology, 30, 167–178. [DOI] [PubMed] [Google Scholar]