Abstract
Objective: Despite excellent survival prognosis, children treated for craniopharyngioma experience significant morbidity. We examined the role of hypothalamic involvement (HI) in excessive daytime sleepiness (EDS) and attention regulation in children enrolled on a Phase II trial of limited surgery and proton therapy. Methods: Participants completed a sleep evaluation (N = 62) and a continuous performance test (CPT) during functional magnetic resonance imaging (fMRI; n = 29) prior to proton therapy. Results: EDS was identified in 76% of the patients and was significantly related to increased HI extent (p = .04). There was no relationship between CPT performance during fMRI and HI or EDS. Visual examination of group composite fMRI images revealed greater spatial extent of activation in frontal cortical regions in patients with EDS, consistent with a compensatory activation hypothesis. Conclusion: Routine screening for sleep problems during therapy is indicated for children with craniopharyngioma, to optimize the timing of interventions and reduce long-term morbidity.
Keywords: cancer and oncology, neuropsychology, sleep
Craniopharyngiomas are intracranial tumors of low histological grade that arise during embryonic development (Garre & Cama, 2007) and account for 6% of all intracranial tumors diagnosed during childhood (NCI, 2015). Despite 5- and 10-year survival rates greater than 90% (NCI, 2015), survivors experience substantial long-term morbidity.
Treatment approaches for craniopharyngioma may include surgical resection with or without adjuvant radiation therapy. Maximal surgical resection is done with the goal of preventing tumor recurrence and minimizing the need for adjuvant therapy; however, this approach may result in substantial morbidity from damage to proximal neural structures, including the optic chiasm, hypothalamus, and mammillary bodies. Conservative surgical resection is attempted with the goal of relieving symptoms while preserving the function of surrounding neural structures; however, these patients frequently require adjuvant radiation therapy to treat residual tumor or subsequent progression. The consequences of central nervous system (CNS)-directed radiation therapy for treatment of CNS malignancies are well-known and include endocrinopathies, neurocognitive deficits, second malignancies, and damage to microvasculature that is associated with increased risk for later ischemic events. There is a need to reduce the deleterious impact of disease and treatment, so as to promote a high quality of life. Studies that clarify the onset and trajectory of adverse functional outcomes in survivors of childhood craniopharyngioma will inform treatment modifications, improve caregiver education to facilitate treatment decision-making, and inform interventions to ameliorate difficulties and improve quality of life.
Craniopharyngiomas are located in the sellar region of the brain and have both cystic and solid components. These tumors often involve or displace the optic chiasm, pituitary gland, and hypothalamus. Visual impairment, pituitary dysfunction, and endocrinopathies are frequently present at diagnosis (Muller, 2008). Notably, disruption of hypothalamic-pituitary function is present in 52–87% of cases (Muller, 2008). Symptoms including visual impairment and hydrocephalus related to tumor expansion may improve following surgical intervention (Caldarelli, Massimi, Tamburrini, Cappa, & Di Rocco, 2005). In contrast, symptoms of hypothalamic dysfunction, including obesity, behavior changes, fatigue, and sleep dysfunction, have been found to persist in 65–80% of patients following treatment (Poretti, Grotzer, Ribi, Schonle, & Boltshauser, 2004).
A retrospective study reported clinically significant rates of self-reported insomnia symptoms and poor sleep efficiency in 25% of adult survivors of childhood brain tumors (Zhou, Manley, Marcus, & Recklitis, 2016). Another study found that compared with a population-based group matched for age, sex, and zip code, adult survivors of childhood brain tumors were 2.7 times more likely to report sleep-onset problems (Nolan et al., 2013). Clinically significant rates of fatigue or excessive daytime sleepiness (EDS) have been identified in up to one-third of adult survivors of pediatric craniopharyngioma (Muller et al., 2006; Poretti et al., 2004). Endocrine deficits and obesity due to metabolic dysfunction may contribute to obstructive sleep apnea, which further impacts sleep quality (Muller, 2010). Results from a case series of three adolescent survivors of craniopharyngioma with self-reported daytime hypersomolence found significantly lower mean 24-hr plasma melatonin levels as compared with historical controls (Lipton et al., 2009). Another study found that lower melatonin levels correlated with increased self-reported fatigue in survivors (Muller, Handwerker, Wollny, Faldum, & Sorensen, 2002). Impairments in melatonin secretion are important to consider, as melatonin is a hormone primarily related to sleep onset as well as appropriate circadian rhythmicity (Gooley, 2011). Pre- and post-surgical hypothalamic tumor involvement (HI) is associated with adverse long-term outcomes in several domains (de Vile et al., 1996; Muller et al., 2011; Sterkenburg et al., 2015). Given the critical role of the hypothalamus in the regulation of biological and circadian rhythms, it is reasonable to hypothesize an association between HI and sleep outcomes in survivors of craniopharyngioma.
Importantly, healthy, adequate sleep is essential in memory consolidation, attentional processing, executive function, and emotion regulation (Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, 2012; Born & Wilhelm, 2012; Walker & van der Helm, 2009; Weissman, Roberts, Visscher, & Woldorff, 2006). Furthermore, adequate sleep has been demonstrated to be critical for both tissue renewal and recovery of neural processes. Sleep difficulties in children undergoing treatment for CNS tumors are particularly important to address, as sleep disruption has been found to be related to tumor growth in mouse models (Clanton et al., 2011; Hakim et al., 2014; Mandrell et al., 2012). With the vulnerability to deficits in these areas in pediatric CNS tumor patients, it is unknown at this time what additional role healthy sleep may play in neurocognitive performance.
The majority of studies examining neurocognitive outcomes in patients treated for childhood craniopharyngioma have documented evidence of disease and treatment-related deficits in specific domains, including executive function and memory (Carpentieri et al., 2001; Di Pinto, Conklin, Li, & Merchant, 2012; Waber et al., 2006), although studies examining global outcomes (e.g., intelligence) do not consistently find problems (Pierre-Kahn et al., 2005). Compared with age- and gender-matched controls, adult survivors of childhood craniopharyngioma had significantly worse performance on measures of attention, processing speed, and memory, and increased HI was found to be predictive of worse performance in the patient group (Fjalldal et al., 2013). In a study of 15 craniopharyngioma survivors, Ozyurt and colleagues found that patients with more extensive post-surgical hypothalamic lesions performed worse on measures of executive function and adaptive skills (Ozyurt, Thiel, et al., 2014). Studies using functional magnetic resonance imaging (fMRI) to examine the neural mechanisms underlying cognitive and behavioral deficits in survivors have found altered patterns of neural activation in frontal cortical regions that have been demonstrated to receive direct or indirect input from the hypothalamus (Lemaire et al., 2011). Ozyurt and colleagues showed that hypothalamic damage resulted in altered neural activation in the medial prefrontal cortex during an emotional face recognition task in a cohort of 10 survivors compared with 10 healthy controls matched for age (Ozyurt, Lorenzen, et al., 2014).
Taken together, these findings document increased risk for sleep disruption and neurocognitive deficits in survivors of childhood craniopharyngioma. Studies of sleep outcomes are generally focused on long-term outcomes using retrospective design or case series. Studies using fMRI to examine neural mechanisms in craniopharyngioma are limited by small cohorts. Prospective studies are needed to clarify the onset and trajectory of morbidity. Further specification of the neural mechanisms underlying cognitive deficits may inform treatment decision-making and intervention.
The current study prospectively examines the impact of HI in sleep and neurocognitive performance in children undergoing treatment for craniopharyngioma in the context of a Phase II trial of limited surgical resection and proton therapy. We have previously demonstrated that more than half of these patients meet criteria for EDS on a multiple sleep latency test (MSLT) before treatment with proton therapy and that patients with two or more sleep-onset REM periods (SOREMP) on MSLT performed significantly worse on global and specific measures of intelligence when compared with patients with fewer than two sleep-onset REMs (Graef et al. 2015). This is an important consideration, as EDS (as indicated by shortened sleep-onset latency [SOL] as well as SOREMP) likely interferes with children’s ability to engage in preferred and social activities and may have a negative impact on academic performance, adjustment, and other aspects of daily functioning following completion of treatment for CNS tumors (Gapstur, Gross, & Ness, 2009; Mandrell et al., 2012; Rosen, Shor, & Geller, 2008; Verberne, Maurice-Stam, Grootenhuis, Van Santen, & Schouten-Van Meeteren, 2012).
We extend these findings by further clarifying the association between sleep dysfunction and neurocognition using fMRI and by examining the role of HI in these outcomes before adjuvant proton therapy. We hypothesized that greater extent of pre-surgical HI would be associated with increased sleep dysfunction, given prior research demonstrating that HI is a predictor of long-term sleep outcomes (de Vile et al., 1996; Fjalldal et al., 2013; Muller, 2010). Our second hypothesis was that patients with clinical symptoms of hypothalamic dysfunction (i.e., EDS) or with more extensive of HI would demonstrate decreased performance compared with patients without EDS and those with a less extensive HI, respectively, during an fMRI task measuring attention regulation. Finally, we hypothesized that patterns of neural activation would differ between groups based on extent of HI and/or the presence of EDS.
Method
Between March 2013 and July 2015, 80 individuals diagnosed with childhood craniopharyngioma were enrolled in a multi-institutional, Phase II trial of proton therapy for craniopharyngioma (ClinicalTrials.gov Identifier: NCT01419067). Patients between 0 and 21 years of age were eligible for enrollment. Craniopharyngioma was diagnosed by histology, cytology, or neuroimaging. This study was approved by the institutional review board. Informed consent and assent were obtained for all protocol-based procedures. Before receiving proton therapy, patients completed several baseline correlative studies, including neurocognitive testing, a sleep exam consisting of a clinical evaluation by a pediatric sleep specialist plus nocturnal polysomography (NPSG), and fMRI. All baseline correlative studies were conducted at a single center (St. Jude Children’s Research Hospital). Patients were excluded from neurocognitive testing if they spoke no English or spoke English as a second language or if they had a visual or sensorimotor impairment that precluded participation. Patients <6 years old were excluded from the baseline sleep exam. Patients <8 years old were excluded from fMRI exams. The analyses included in the current manuscript are restricted to those patients meeting inclusion criteria for the baseline sleep exam.
Table I details the demographic and clinical characteristics for the overall cohort (N = 62) and stratified by fMRI completion status (eligible and completed scan = +fMRI (n = 29); eligible and did not complete scan = −fMRI (n = 10). Of the 64 patients meeting criteria for this analysis, two did not complete the baseline sleep exam (one = illness, one = unknown). Sixty-five percent of the overall group was White and 18% were Black. The majority (82%) were not Hispanic or Latino. Of the 25 participants in the +fMRI cohort with available handedness data, 79% were right-handed. Of the 62 participants who completed a sleep study, 54 had surgery before the baseline cognitive assessment (median time from surgery to assessment = 2.55 months; range = 14 days to 3.7 years).
Table I.
Demographic and Clinical Characteristics for the Overall Cohort and the Cohort Completing fMRI
| MSLT (N = 62) | +fMRI (n = 29) | −fMRI (n = 10) | pa | |
|---|---|---|---|---|
| % | % | % | % | |
| Sex | 0.651 | |||
| Male | 51.6 | 51.7 | 60.0 | |
| Female | 48.4 | 48.3 | 40.0 | |
| Hypothalamic involvement | 0.242 | |||
| Grade 0 | 12.9 | 17.2 | 20.0 | |
| Grade 1 | 30.6 | 37.9 | 10.0 | |
| Grade 2 | 56.5 | 44.8 | 70.0 | |
| Surgical procedure | 0.196 | |||
| Catheter only – craniotomy | 4.8 | 10.3 | 10.0 | |
| Catheter only – burr hole | 12.9 | 41.4 | 20.0 | |
| Resection – craniotomy | 51.6 | 24.1 | 50.0 | |
| Resection – transphenoidal | 17.7 | 24.1 | 20.0 | |
| None | 12.9 | |||
| Laterality | 0.153 | |||
| Left | 12.9 | 4.5 | 30.0 | |
| Right | 51.6 | 37.9 | 50.0 | |
| Midline | 17.7 | 24.1 | 20.0 | |
| Bifrontal | 4.8 | 10.3 | ||
| CSF diversion | 0.951 | |||
| Yes | 29 | 69.0 | 70.0 | |
| No | 71 | 31.0 | 30.0 | |
| Diabetes insipidus | 0.777 | |||
| Yes | 54.8 | 55.2 | 50.0 | |
| No | 45.2 | 44.8 | 50.0 | |
| Visual acuity (right) | 0.314 | |||
| No deficits | 67.7 | 79.3 | 60.0 | |
| Reduced, no functional impairment | 16.1 | 13.8 | 40.0 | |
| Reduced, functional impairment | 11.3 | 3.4 | ||
| Blind | 4.8 | 3.4 | ||
| Visual acuity (left) | 0.484 | |||
| No deficits | 69.4 | 86.2 | 70.0 | |
| Reduced, no functional impairment | 9.7 | 6.9 | 20.0 | |
| Reduced, functional impairment | 14.5 | 3.4 | ||
| Blind | 6.5 | 3.4 | 10.0 | |
| Excessive daytime sleepiness | 0.045 | |||
| Yes | 75.8 | 69.0 | 100.0 | |
| No | 24.2 | 31.0 | . | |
| Epworth Sleepiness Scale – Parentb | 0.908 | |||
| Impaired (total score >10) | 40.4 | 37.9 | 60.0 | |
| Unimpaired (total score ≤9) | 59.6 | 62.1 | 40.0 | |
| M ± SD | M ± SD | M ± SD | ||
| Age at exam | 11.0 ± 4.0 | 13.1 ± 3.6 | 12.1 ± 3.1 | 0.449 |
| Full-Scale IQ | 99.5 ± 20.4 | 105.0 ± 14.7 | 93.3 ± 14.4 | 0.037 |
| Total number of surgeries | 1.4 ± 1.0 | 1.0 ± 0.8 | 1.6 ± 0.8 | 0.044 |
| MSLT mean sleep-onset latency | 8.0 ± 5.8 | 9.6 ± 5.9 | 5.4 ± 5.0 | 0.053 |
| MSLT total number of SOREMP | 1.2 ± 1.4 | 0.7 ± 1.1 | 2.0 ± 1.6 | 0.006 |
Note. MSLT = Multiple Sleep Latency Test; +fMRI = eligible and completed fMRI exam; −fMRI = eligible and did not complete fMRI exam; CSF = cerebrospinal fluid; SOREMP = sleep-onset REM periods. Hypothlamic involvement was assessed before surgical intervention. Clinical and treatment variables reference procedures completed before study baseline. Surgical variables reference the most extensive procedure completed before study baseline.
aTwo-sided p-value from comparisons between + fMRI and −fMRI cohorts.
bN = 52.
Clinical Variables
Demographic and clinical data were systematically collected at baseline. HI was graded based on the earliest available pre-surgical MRI scan, according to the criteria proposed by Muller et al. (2011). Criteria were as follows: Grade 0: no HI; Grade 1: involvement of the anterior hypothalamus; and Grade 2: involvement of the anterior and posterior hypothalamic area (i.e., involving the mammillary bodies and the area beyond the mammillary bodies).
Sleep Evaluation
Patients were seen and evaluated by a pediatric sleep medicine specialist who reviewed a complete sleep history and performed a physical examination. Patients completed an NPSG exam performed on their usual sleep/wake schedule to assess sleep time, sleep stage distribution, and sleep efficiency, as well as to assess for sleep-related breathing disorders or periodic limb movements. The sleep evaluations were performed at a sleep center accredited by the American Academy of Sleep Medicine (AASM), conducted by experienced, registered sleep technologists, and interpreted by a board-certified sleep medicine physician. Exams were initiated within 1 hr of the patient’s usual bedtime. Data on brain activity, eye movement, muscle activity, and cardiac activity were collected using electroencephalogram (two- to four-channel), electrooculogram (two-channel), electromyogram (one-channel), and electrocardiogram, respectively. Data were collected with synchronized audio and video recording to document behavior and to detect snoring and other obstructive sounds. Respiratory data were collected using oro-nasal thermistors and/or nasal pressure transducers, and pulse oximetry. Some but not all patients were evaluated with end-tidal CO2 pressure. The length of the NPSG exams was commensurate with the patient's usual sleep duration.
The MSLT is a standardized test used to evaluate daytime sleepiness and measure sleep tendency in the absence of alerting factors. Studies were performed according to guidelines recommended by the AASM. Patients were given four or five nap opportunities at 2-hr intervals throughout the day in a quiet darkened room, with the exception of two patients with MSLT exams limited to only two naps. Patients were observed and instructed not to sleep at other times throughout the day. If sleep onset occurred during a nap opportunity, patients were allowed to sleep for 15 min before being awakened. If no sleep occurred after 20 min, the nap opportunity ended. The mean SOL was calculated as the arithmetic mean of all nap opportunities; the number of naps and the number of sleep-onset REM periods (SOREMP) were also recorded. EDS was defined as a mean SOL of ≤10 min and/or ≥2 SOREMP.
A modified version of the Epworth Sleepiness Scale (M-ESS) was administered to subjectively measure daytime sleepiness (Moore et al., 2009). Caregivers were asked to rank the propensity for the child or adolescent to fall asleep in various everyday situations (0 = no chance to 3 = high chance of dozing) for each of the eight items, with a maximum score of 24. Higher scores on the M-ESS indicate higher levels of EDS. Consistent with recommendations of Melendres, Lutz, Rubin, & Marcus (2004), a cut-off score of ≥10 reflected EDS (Melendres et al., 2004).
Neurocognitive Assessment
Participants completed a comprehensive neurocognitive assessment at study baseline. As part of this assessment, an age-standardized Full-Scale IQ (FSIQ) was derived for each patient using an age-appropriate Wechsler Intelligence Scale, with the majority completing the Wechsler Intelligence Scale for Children, 4th Edition, used for children aged 6 to 16 (Wechsler, 2003). These data are included in the current study to characterize global cognitive functioning for descriptive purposes.
MRI Exam
Participants completed an fMRI exam lasting 30–60 min and consisting of four functional tasks. All patients completed a video-based orientation and training program before the exam. MRI exams were performed with a 3T Siemens scanner using the standard quadrature head coil. Functional images of the whole brain were obtained with a T2*-weighted echoplanar imaging pulse sequence (field of view = 192 mm; matrix = 64 × 64; slice thickness = 5 mm; time to echo [TE] = 30 ms; 32 slices per volume). The acquisition time for one image volume (TR) was 2.06 s. High-resolution three-dimensional T1-weighted images were acquired for anatomic visualization (TR = 1.8 s, TE = 2.74 ms, flip angle = 15°, voxel size = 1 × 1 × 1 mm3).
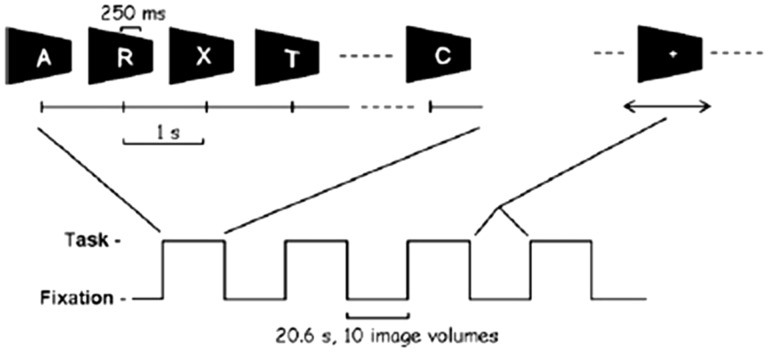
The details of the continuous performance test (CPT) task paradigm have been previously reported (Ogg et al., 2008). A 10.3-s baseline period of fixation was followed by four blocks with alternating periods of task and fixation. The duration of task and fixation periods was 20.6 s, and 10 imaging volumes were acquired per period. During the task, a series of 21 letters were presented in random order. Each letter was presented for 250 ms with a 1-s inter-stimulus interval. Please see Figure 1 for a depiction of the task. Participants were instructed to respond using a button press for every letter except the target letter X, which occurred at a fixed frequency of 10%. A small cross was shown at the center of the screen during the fixation blocks. Performance variables included reaction time (RT), number of omissions (failure to respond to a distractor), and number of commission errors (responding to an X). Performance accuracy (omissions only) and mean RT were calculated over all task trials. The experimental control software was programmed using Presentation software (v14.9 Neurobehavioral Systems, Albany, CA, USA) and synchronized by trigger pulse from the MRI scanner. Stimuli were projected onto a projection screen mounted at the rear of the magnet via an LCD projector and viewed by way of a mirror mounted to the head coil.
Figure 1.
Stimulus paradigm used for the CPT task. Reprinted with permission from Ogg et al. 2008.
Statistical Analysis
Statistical analysis of behavioral data was performed using SPSS 18 for Windows. Descriptive analyses of demographic and clinical variables were conducted to characterize the group and to compare participants with and without fMRI data to establish representativeness. All interval variable distributions were examined for normality to determine whether the use of parametric statistics was appropriate. Frequency comparisons (chi-square) were used to compare the proportion of participants with and without HI on demographic and clinical variables. Mean comparisons (e.g., two-sample t-test, one-way analysis of variance [ANOVA]) were used to compare groups on continuous variables. Results from tests examining the hypothesis that greater extent of pre-surgical HI would be associated with increased sleep dysfunction and that sleep dysfunction would predict decreased CPT performance were considered significant at p1sided ≤ 05.
fMRI data were preprocessed and statistically analyzed using the Statistical Parametric Mapping software (SPM 8; Wellcome Department of Imaging Neuroscience, London). Images were realigned to correct for interscan head motion (Ashburner, 2009), normalized to the Montreal Neurological Institute brain template (Brett, Johnsrude, & Owen, 2002) and smoothed with a 6-mm full width at half-maximum Gaussian kernel. The smoothed and normalized images were resliced to 2-mm isotropic resolution. Data from individual subjects were analyzed according to a fixed-effects general linear model, with task-related activity modeled as a box-car function convolved with the canonical hemodynamic response function (Friston et al., 1995). After parameter estimation, contrasts were generated reflecting the task > fixation and subsequently used as variables in second-level random-effect analyses to identify patterns of brain activation for the group as a whole. A two-sample independent t-test was used to examine patterns of activation by groups based on HI and groups based on EDS. Unless otherwise specified, activation was considered significant at p ≤ .05, family-wise error corrected for multiple comparisons and with a minimum cluster size of 5 voxels (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1994). The anatomical name and Brodmann area (BA) reported for each supratentorial cluster of activation were determined by visual comparison with the Talairach atlas (Talairach and Tournoux, 1988) and the MRI atlas of the cerebellum developed by Schmahmann et al. (1999).
We conducted an exploratory analysis on the fMRI data using effect size thresholds as opposed to conventional significance thresholds (i.e., p-values). Although we stress that this approach is used primarily to generate rather than test hypotheses, it is advantageous when used with small data sets to reduce the risk of Type II error. Specifically, we conducted two additional second-level random effects between-groups analyses using a liberal threshold (p ≤ .05 uncorrected, cluster threshold of 20 voxels). The contrast of interest was task > fixation. The first analyses compared the + EDS and –EDS groups and the second compared the +HI and –HI groups. Based on these analyses, we generated effect size plots for significant local maxima. We present only those clusters where the lower confidence interval bound is ≥0.4 standard deviations from the mean, a value that is consistent with a medium effect size.
Results
Participant Characteristics
Of the 62 participants who completed the sleep study, 23 did not meet eligibility criteria for participation in the fMRI exam (17 participants were under 8 years of age, two participants were not cleared for the 3T magnet, one participant did not speak English, and two participants had sensorimotor limitations). Data were successfully collected in 29 of the 39 eligible patients (74%). Reasons for missing fMRI data were as follows: scheduling difficulties = two, patient anxiety = three, and scan quality limited by motion = five). As detailed in Table I, participants in the + fMRI cohort had significantly fewer surgeries before study enrollment when compared with the –fMRI cohort (1.0 ± 0.8 vs. 1.6 ± 0.8, p = .044). Although the mean FSIQ in both groups was within age expectations, the mean FSIQ in the +fMRI cohort was significantly higher than that in the –fMRI cohort (105.0 ± 14.7 vs. 93.3 ± 14.4, p = .037). The+fMRI and –fMRI cohorts did not significantly differ on the extent of HI (p = 0.242). In contrast, there was a significantly greater frequency of +EDS participants in the –fMRI cohort compared with the +fMRI cohort (100% vs. 69%, respectively; p = .045).
Rates of EDS
Before adjuvant proton therapy, 76% of the overall group met MSLT-based criteria for EDS. The majority of patients (57%) had Grade 2 pre-surgical HI. Results of the chi-square analysis revealed a higher frequency of Grade 2 HI in participants with EDS compared with those without EDS (64% vs. 33%, p = 0.04; Table II). Results of one-way ANOVAs revealed a significant difference in MSLT mean SOL by HI grade (p < 0.0001). Post hoc comparisons revealed significant differences between Grade 0 (13.5 ± 4.8) and Grade 2 (6.1 ± 5.0; p = < .0001), and Grade 1 (9.2 ± 5.8) and Grade 2 (p = .05) only; as such, Grades 0 and 1 were combined for subsequent fMRI analysis, consistent with the approach of Ozyurt et al. (2014). There was no significant difference in the total number of SOREMPs by HI grade (p = .23).
Table II.
Frequency of Excessive Daytime Sleepiness by Hypothalamic Involvement
| +EDS n = 47 |
−EDS n = 15 | pa | |
|---|---|---|---|
| % | % | % | |
| Hypothalamic involvement | 0.04 | ||
| Grade 0 | 8.5 | 26.7 | |
| Grade 1 | 27.7 | 40.0 | |
| Grade 2 | 63.8 | 33.0 | |
Note. EDS = Excessive daytime sleepiness.
aOne-sided p-value from comparisons (chi-square) between +EDS and −EDS cohorts.
fMRI-CPT
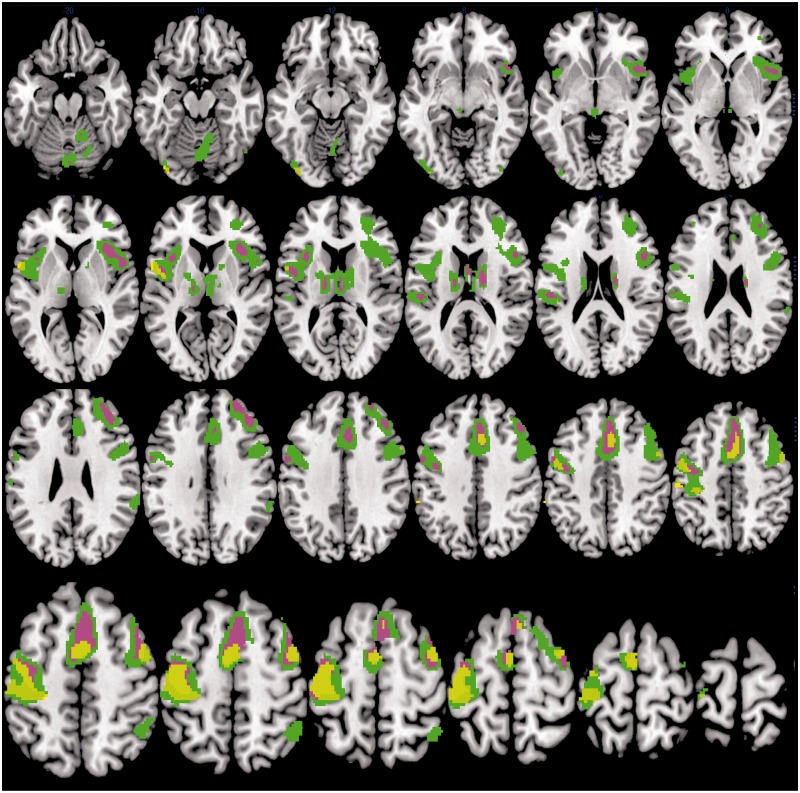
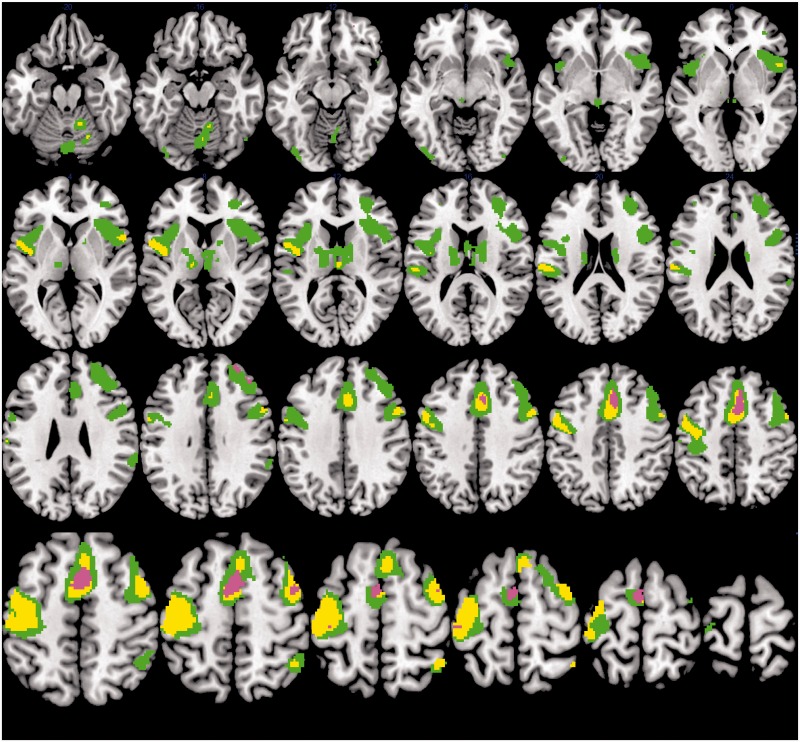
Accuracy on the fMRI-CPT was high for the overall group (% omission errors M = 6.55, SD = 6.42). There were no significant differences in performance accuracy (p = .23) or RT (p = .48) based on HI (low grade vs. high grade) or EDS (Table III). Sites of significant activation for the overall group during task performance are detailed in Table IV. Figure 2 depicts sites with significantly greater activation during task periods relative to fixation (CPT > fixation) for the overall group and separately for the +EDS and –EDS groups. Sites of significant task-related activation are shown separately for the overall group, the low–grade HI group, and the high-grade HI group in Figure 3. Between-group comparisons revealed no significant differences in neural activation between the low- and high-grade groups or the +EDS and –EDS groups; however, visual examination of the group composite images reveals a greater extent of spatial activation in the frontal cortical regions (superior frontal gyrus, dorsal frontal gyrus) for the +EDS group.
Table III.
FMRI-CPT Performance by Hypothalamic Involvement and Excessive Daytime Sleepiness
| Hypothalamic involvement |
Excessive daytime sleepiness |
|||||
|---|---|---|---|---|---|---|
| Low grade | High grade | −EDS | +EDS | |||
| M ± SD | M ± SD | pa | M ± SD | M ± SD | pa | |
| fMRI-CPT | ||||||
| Accuracy | 6.6 ± 5.8 | 6.5 ± 7.4 | 0.48 | 7.3 ± 7.0 | 6.2 ± 6.3 | 0.34 |
| Reaction time | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.23 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.44 |
Note. CPT = Continuous performance test.
aOne-sided p-value from mean comparisons between groups.
Table IV.
Regional Activation During fMRI-CPT
| BA | Talairach coordinates |
Volume |
Max T | ||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | mm3 | ||||
|
CPT > fixation |
|||||||
| R | Dorsal frontal gyrus | 6 | 4 | 18 | 44 | 56,152 | 9.74 |
| R | Superior/middle frontal gyrus | 6 | 42 | 0 | 58 | 9.21 | |
| R | Dorsal frontal gyrus | 6 | 2 | 6 | 50 | 8.86 | |
| L | Precentral gyrus | 4 | −46 | −26 | 64 | 29,168 | 8.53 |
| L | Precentral gyrus | 4 | −44 | −10 | 56 | 8.50 | |
| L | Precentral gyrus | 4 | −38 | −24 | 58 | 8.26 | |
| L | Superior temporal gyrus, lateral sulcus | 40 | −50 | −24 | 18 | 2,736 | 7.12 |
| L | Postcentral gyrus | 40/2 | −62 | −20 | 24 | 6.79 | |
| R | Cerebellum (VIIAt) | 2 | −78 | −22 | 6,360 | 6.88 | |
| R | Cerebellum (V) | 14 | −50 | −22 | 6.29 | ||
| R | Cerebellum (VI) | 30 | −52 | −28 | 6.23 | ||
| R | Thalamus (medial dorsal nucleus) | 2 | −16 | 12 | 8,544 | 6.47 | |
| R | Cerebral peduncle | 2 | −28 | 0 | 6.29 | ||
| L | Thalamus (medial dorsal nucleus) | −14 | −16 | 10 | 6.25 | ||
| L | Inferior occipital gyrus | 19 | −38 | −86 | −12 | 1,048 | 6.28 |
| R | Inferior parietal lobe | 40 | 44 | −52 | 56 | 1,352 | 6.00 |
| L | Cerebellum (VI) | −32 | −60 | −28 | 736 | 5.77 | |
| R | Supramarginal gyrus | 40 | 62 | −38 | 28 | 816 | 5.75 |
| R | Fusiform gyrus | 19 | 52 | −68 | −14 | 80 | 5.38 |
| R | Inferior/Middle occipital gyrus | 18 | 42 | −84 | −8 | 144 | 5.13 |
| R | Cuneus | 17 | 20 | −100 | 0 | 48 | 5.08 |
| L | Middle frontal gyrus | 46 | −36 | 46 | 22 | 64 | 5.04 |
|
Fixation > CPT |
|||||||
| R | Postcentral gyrus | 7 | 22 | −50 | 76 | 280 | 5.09 |
Note. R = Right, L = Left, BA = Brodmann area, Max T = maxiumum value of the T stastistic in the cluster.
Results of a random-effects analysis, p ≤ .05, FWE-corrected, and cluster size ≥ 5 voxels. Volume of activation reported for regions that are also significant at p ≤ .05, cluster-corrected.
Figure 2.
Brain activity during the CPT task as compared with fixation for the overall group (N = 29), the +EDS group (n = 20), and the –EDS group (n = 9). Sites with significantly greater activation during task relative to fixation (CPT > fixation) are shown in green for the overall group, violet for the +EDS group, and yellow for the –EDS group. Random-effects analysis, p ≤ .05, voxel-corrected FWE, and cluster size of ≥5 voxels. Results are shown on an average structural image created from a template. Images are shown in neurological convention (left on left).
Figure 3.
Brain activity during the CPT task as compared with fixation depicted for the overall group (N = 29), the low-grade HI group (n = 16), and high-grade HI group (n = 13). Sites with significantly greater activation during task relative to fixation (CPT > fixation) are shown in green for the overall group, yellow for the low-grade HI group, and violet for the high-grade HI group. Random-effects analysis, p ≤ .05, voxel-corrected FWE, and cluster size of ≥5 voxels. Results are shown on an average image created from a template. Images are shown in neurological convention (left on left).
Exploratory analyses based on effect size thresholds revealed differences based on subgroup (Supplementary Table I and Figures 1 and 2). Compared with the +EDS group, greater activation was found in the –EDS in the left inferior occipital temporal gyri; right visual processing and association cortex (lingual gyrus, cuneus, and precuneus); and left precentral and postcentral gyri. Differences in activation between the low- and high-grade groups were seen in the left superior parietal lobe (low > high) and bilateral superior frontal gyrus (high > low).
Discussion
This study examined the role of HI on sleep and neurocognitive sequalae in a cohort of patients completing treatment for childhood craniopharyngioma, with the broader goal of gaining insight into predictors and underlying mechanisms of morbidity in these vulnerable survivors. To our knowledge, this is the first study that uses fMRI to examine underlying mechanisms related to disease and treatment sequalae in a cohort of patients who are naïve to radiation therapy.
Findings support our a priori hypothesis regarding the association of HI and daytime sleepiness in survivors. Patients with a greater extent of HI are more likely to meet objective, standardized criteria for clinically significant EDS. This finding underscores the predictive role of the extent of pre-surgical HI on outcomes related to quality of life in survivors. Results may also have implications for treatment decision-making, given that the adverse consequences of hypothalamic damage are present before adjuvant treatment.
We found no evidence of significant differences in fMRI-CPT performance based on greater extent of HI or EDS. Mean error rates for the fMRI-CPT ranged from 6% to 7% when examined by subgroups based on extent of HI or EDS. This finding is somewhat contrary to our expectations, given the findings of relative deficits in performance on sustained attention tasks in children with poor sleep and in patients with greater extent of HI. However, it is important to note that the 5-min duration of our task is much shorter than standardized continuous performance measures used to assess sustained attention in clinical settings. Further, given the significantly higher rate of EDS in participants in the –fMRI cohort compared with the +fMRI cohort limit, these findings may underestimate the difference in sustained attention performance between these groups.
Patterns of neural activation in the overall group were comparable with previous findings in healthy control studies using the fMRI-CPT task (Ogg et al., 2008). Results of between-group comparisons did not reach significance in analyses corrected for multiple comparisons; however, group differences are evident on visual inspection of contrasts displayed at the same statistical threshold, in areas previously found to be associated with performance on CPT tasks in healthy children and adults (Ogg et al., 2008; Wang et al., 2013). We stress that this is a preliminary analysis of data from an ongoing study and that differences in activation patterns between groups did not reach strict statistical thresholds corrected for multiple comparisons. Nonetheless, our findings are interesting, particularly in the context of similar performance between groups. Specifically, the finding of increased spatial extent of activation within the frontal lobe regions for the +EDS cohort is consistent with expectations based on a compensatory activation hypothesis (Shaywitz & Shaywitz, 2008). The low-grade HI group appeared to have a larger spatial extent of task-related activation in temporal and parietal regions when visually compared with the high-grade HI group, suggesting that the low-grade group has relied on different cortical regions to achieve a similar level of performance.
Exploratory between-groups comparisons were conducted in an effort to generate data to inform hypotheses to be tested in future studies. Results revealed preliminary evidence of differences between subgroups of at least a moderate effect size (90% confidence interval lower limit: d ≥ 0.3). We found a greater spatial extent of activation in the −EDS group when compared with the +EDS group in posterior brain regions, including the occipital and temporal cortex. Although preliminary, these results suggest compensatory activation because patients without EDS appear to be relying less on frontal brain regions for a sustained attention task, thus performance is less effortful and more automatic. Our findings differences in the pattern of activation in the low- and high-grade HI groups provide further support for the hypothesis that the groups may engage different cortical regions to achieve similar performance. Again, these findings are preliminary; however, they may provide direction for future analyses based on a priori regions of interest.
Limitations
Our findings should be considered in light of study limitations. Patients younger than 6 years old were excluded from MSLT, which limits generalizability. Similarly, findings from the fMRI exams may not reflect neural activation patterns for patients younger than 8 years of age. Group differences between the +fMRI and −fMRI cohort on demographic and clinical characteristics further limit the generalizability of our findings. Specifically, the higher rate of +EDS, higher mean number of surgeries, and lower mean FSIQ in the −fMRI cohort suggest that those participants who were eligible but did not complete the fMRI portion of the study were more impaired than those in the +fMRI cohort. The absence of data from a healthy control cohort limits our ability to interpret findings of equivalent performance in patient groups. Our sample size is consistent with recommendations for fMRI studies of healthy adults (Desmond & Glover, 2002); however, our ability to detect statistically significant differences between groups may be decreased due to developmentally based physiological differences (Gaillard, Grandin, & Xu, 2001). Finally, the study was limited by a single night PSG, which may be impacted by the first night effect and thus not provide the full picture of a child’s sleep that occurs in the habitual sleep environment (Verhulst, Schrauwen, De Backer, & Desager, 2006).
Implications
Before adjuvant proton therapy, the vast majority of patients in our study met criteria for clinically significant daytime sleepiness. These results have implications for the timing and focus of interventions to remediate difficulties in survivors. Routine clinical follow-up during the early survivorship period should screen for sleep problems, with a particular focus on EDS. Remediation of sleep dysfunction should be a priority, to maximize adherence to interventions (e.g., to promote physical activity and offset potential weight gain related to hypothalamic injury). Further, sleep restriction in healthy adolescents has been linked to functional outcomes, including decreased attention, learning difficulties, mood dysregulation, and increased consumption of foods with a high glycemic index (Baum et al., 2014; Beebe, Rose, & Amin, 2010; Beebe et al., 2013). This project makes a significant contribution to the literature on sleep in pediatric conditions, given the rarity of craniopharyngioma and challenges related to fMRI in children with supratentorial tumors. We successfully collected fMRI data on 71% of patients who completed baseline sleep exams and met MRI eligibility criteria, suggesting that functional neuroimaging studies are feasible in children 8 years of age or older with complex medical conditions.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/.
Funding
This work was supported by the National Cancer Institute (St. Jude Cancer Center Support, CORE, grant number P30 CA21765), the American Lebanese Syrian Associated Charities (ALSAC), and the National Cancer Institute (Pediatric Oncology Education Program, grant number R25CA23944).
Conflicts of interest: None declared.
Supplementary Material
References
- Ashburner J. (2009). Computational anatomy with the SPM software. Magnetic Resonance Imaging, 27, 1163–1174. doi: 10.1016/j.mri.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Astill R. G., Van der Heijden K. B., Van Ijzendoorn M. H., Van Someren E. J. (2012). Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychological Bulletin , 138, 1109–1138. doi: 10.1037/a0028204 [DOI] [PubMed] [Google Scholar]
- Baum K. T., Desai A., Field J., Miller L. E., Rausch J., Beebe D. W. (2014). Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry , 55, 180–190. doi: 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D. W., Rose D., Amin R. (2010). Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. Journal of Adolescent Health , 47, 523–525. doi: 10.1016/j.jadohealth.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D. W., Simon S., Summer S., Hemmer S., Strotman D., Dolan L. M. (2013). Dietary intake following experimentally restricted sleep in adolescents. Sleep , 36, 827–834. doi: 10.5665/sleep.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., Wilhelm I. (2012). System consolidation of memory during sleep. Psychological Research , 76, 192–203. doi: 10.1007/s00426-011-0335-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Johnsrude I. S., Owen A. M. (2002). The problem of functional localization in the human brain. Nature Reviews: Neuroscience , 3, 243–249. doi: 10.1038/nrn756 [DOI] [PubMed] [Google Scholar]
- Caldarelli M., Massimi L., Tamburrini G., Cappa M., Di Rocco C. (2005). Long-term results of the surgical treatment of craniopharyngioma: The experience at the Policlinico Gemelli, Catholic University, Rome. Child's Nervous System , 21, 747–757. doi: 10.1007/s00381-005-1186-5 [DOI] [PubMed] [Google Scholar]
- Carpentieri S. C., Waber D. P., Scott R. M., Goumnerova L. C., Kieran M. W., Cohen L. E., Kim F., Billett A. L., Tarbell N. J., Pomeroy S. L. (2001). Memory deficits among children with craniopharyngiomas. Neurosurgery , 49, 1053–1057; discussion 1057–1058. [DOI] [PubMed] [Google Scholar]
- Clanton N. R., Klosky J. L., Li C., Jain N., Srivastava D. K., Mulrooney D., Zeltzer L., Stovall M., Robison L. L., Krull K. R. (2011). Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer , 117, 2559–2568. doi: 10.1002/cncr.25797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vile C. J., Grant D. B., Hayward R. D., Kendall B. E., Neville B. G. R., Stanhope R. (1996). Obesity in childhood craniopharyngioma: Relation to post-operative hypothalamic damage shown by magnetic resonance imaging. Journal of Clinical Endocrinology and Metabolism , 81, 2734–2737. [DOI] [PubMed] [Google Scholar]
- Desmond J. E., Glover G. H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. Journal of Neuroscience Methods , 118, 115–128. [DOI] [PubMed] [Google Scholar]
- Di Pinto M., Conklin H. M., Li C., Merchant T. E. (2012). Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. International Journal of Radiation Oncology, Biology, Physics, 84, e363–e369. doi: 10.1016/j.ijrobp.2012.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjalldal S., Holmer H., Rylander L., Elfving M., Ekman B., Osterberg K., Erfurth E. M. (2013). Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. The Journal of Clinical Endocrinology and Metabolism , 98, 3253–3262. doi: 10.1210/jc.2013-2000 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Poline J. B., Grasby P. J., Williams S. C., Frackowiak R. S., Turner R. (1995). Analysis of fMRI time-series revisited. Neuroimage , 2, 45–53. doi: 10.1006/nimg.1995.1007 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Worsley K. J., Frackowiak R. S., Mazziotta J. C., Evans A. C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping , 1, 210–220. doi: 10.1002/hbm.460010306 [DOI] [PubMed] [Google Scholar]
- Gaillard W. D., Grandin C. B., Xu B. (2001). Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage , 13, 239–249. doi: 10.1006/nimg.2000.0681 [DOI] [PubMed] [Google Scholar]
- Gapstur R., Gross C. R., Ness K. (2009). Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors: A review. Oncology Nursing Forum , 36, 723–731. doi: 10.1188/09.ONF.723-731 [DOI] [PubMed] [Google Scholar]
- Garre M. L., Cama A. (2007). Craniopharyngioma: Modern concepts in pathogenesis and treatment. Current Opinion in Pediatrics , 19, 471–479. doi: 10.1097/MOP.0b013e3282495a22 [DOI] [PubMed] [Google Scholar]
- Gooley J. J., Saper C. B. (2011). Anatomy of the mammalian circadian system. In Kryger M. H., Roth T., Kryger M. H., Dement W. C. (Eds.), Principle and practice of sleep medicine (5th ed.). St. Louis, MO: Elsevier. [Google Scholar]
- Graef DM, Conklin H, Sykes A, Ashford J, Wise M, Mandrell BN, Merchant T, Indelicato D, Crabtree V. Excessive daytime sleepiness and neurocognitive performance in pediatric patients with craniopharyngioma. SLEEP Conference. Seattle, WA. June, 2015 [Google Scholar]
- Hakim F., Wang Y., Zhang S. X., Zheng J., Yolcu E. S., Carreras A., Khalyfa A., Shirwan H., Almendros I., Gozal D. (2014). Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Research , 74, 1329–1337. doi: 10.1158/0008-5472.CAN-13-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire J. J., Frew A. J., McArthur D., Gorgulho A. A., Alger J. R., Salomon N., Chen C., Behnke E. J., De Salles A. A. (2011). White matter connectivity of human hypothalamus. Brain Research , 1371, 43–64. doi: 10.1016/j.brainres.2010.11.072 [DOI] [PubMed] [Google Scholar]
- Lipton J., Megerian J. T., Kothare S. V., Cho Y. J., Shanahan T., Chart H., Ferber R., Adler-Golden L., Cohen L. E., Czeisler C. A., Pomeroy S. L. (2009). Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology , 73, 323–325. doi: 10.1212/WNL.0b013e3181af78a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell B. N., Wise M., Schoumacher R. A., Pritchard M., West N., Ness K. K., Crabtree V. M., Merchant T. E., Morris B. (2012). Excessive daytime sleepiness and sleep-disordered breathing disturbances in survivors of childhood central nervous system tumors. Pediatric Blood and Cancer , 58, 746–751. doi: 10.1002/pbc.23311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendres M. C., Lutz J. M., Rubin E. D., Marcus C. L. (2004). Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics, 114, 768–775. doi: 10.1542/peds.2004-0730 [DOI] [PubMed] [Google Scholar]
- Moore M., Kirchner H. L., Drotar D., Johnson N., Rosen C., Ancoli-Israel S., Redline S. (2009). Relationships among sleepiness, sleep time, and psychological functioning in adolescents. Journal of Pediatric Psychology , 34, 1175–1183. doi: 10.1093/jpepsy/jsp039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. L. (2008). Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Hormone Research, 69, 193–202. doi: 10.1159/000113019 [DOI] [PubMed] [Google Scholar]
- Muller H. L. (2010). Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: Review of the literature and perspectives. International Journal of Endocrinology , 2010, 519607. doi: 10.1155/2010/519607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. L., Gebhardt U., Teske C., Faldum A., Zwiener I., Warmuth-Metz M., Pietsch T., Pohl F., Sörensen N., Calaminus G.; Study Committee of KRANIOPHARYNGEOM 2000. (2011). Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: Results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. European Journal of Endocrinology , 165, 17–24. doi: 10.1530/EJE-11-0158 [DOI] [PubMed] [Google Scholar]
- Muller H. L., Handwerker G., Gebhardt U., Faldum A., Emser A., Kolb R., Sorensen N. (2006). Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes and Control , 17, 583–589. doi: 10.1007/s10552-005-9012-7 [DOI] [PubMed] [Google Scholar]
- Muller H. L., Handwerker G., Wollny B., Faldum A., Sorensen N. (2002). Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. The Journal of Clinical Endocrinology and Metabolism , 87, 3993–3996. doi: 10.1210/jcem.87.8.8751 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2015). Retrieved from http://www.cancer.net/cancer-types/craniopharyngioma-childhood/statistics [Google Scholar]
- Nolan V. G., Gapstur R., Gross C. R., Desain L. A., Neglia J. P., Gajjar A., Klosky J. L., Merchant T. E., Stovall M., Ness K. K. (2013). Sleep disturbances in adult survivors of childhood brain tumors. Quality of Life Research , 22, 781–789. doi: 10.1007/s11136-012-0208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg R. J., Zou P., Allen D. N., Hutchins S. B., Dutkiewicz R. M., Mulhern R. K. (2008). Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging , 26, 504–512. doi: 10.1016/j.mri.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Ozyurt J., Lorenzen A., Gebhardt U., Warmuth-Metz M., Muller H. L., Thiel C. M. (2014). Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiology of Learning and Memory , 111, 71–80. doi: 10.1016/j.nlm.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Ozyurt J., Thiel C. M., Lorenzen A., Gebhardt U., Calaminus G., Warmuth-Metz M., Muller H. L. (2014). Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. The Journal of Pediatrics, 164, 876–881.e874. doi: 10.1016/j.jpeds.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Pierre-Kahn A., Recassens C., Pinto G., Thalassinos C., Chokron S., Soubervielle J. C., Brauner R., Zerah M., Sainte Rose C. (2005). Social and psycho-intellectual outcome following radical removal of craniopharyngiomas in childhood: A prospective series. Child's Nervous System , 21, 817–824. doi: 10.1007/s00381-005-1205-6 [DOI] [PubMed] [Google Scholar]
- Poretti A., Grotzer M. A., Ribi K., Schonle E., Boltshauser E. (2004). Outcome of craniopharyngioma in children: Long-term complications and quality of life. Developmental Medicine and Child Neurology , 46, 220–229. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Shor A. C., Geller T. J. (2008). Sleep in children with cancer. Current Opinion in Pediatrics , 20, 676–681. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Doyon J., McDonald D., Holmes C., Lavoie K., Hurwitz A. S., Kabani N., Toga A., Evans A., Petrides M. (1999). Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage , 10(3 Pt 1), 233–260. doi: 10.1006/nimg.1999.0459 [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., Shaywitz B. A. (2008). Paying attention to reading: The neurobiology of reading and dyslexia. Development and Psychopathology , 20, 1329–1349. doi: 10.1017/S0954579408000631 [DOI] [PubMed] [Google Scholar]
- Sterkenburg A. S., Hoffmann A., Gebhardt U., Warmuth-Metz M., Daubenbuchel A. M., Muller H. L. (2015). Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: Newly reported long-term outcomes. Neuro-oncology, 17, 1029–1038. doi: 10.1093/neuonc/nov044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-planar sereotaxic atlas of the human brain. New York, NY: Thieme. [Google Scholar]
- Verberne L. M., Maurice-Stam H., Grootenhuis M. A., Van Santen H. M., Schouten-Van Meeteren A. Y. (2012). Sleep disorders in children after treatment for a CNS tumour. Journal of Sleep Research , 21, 461–469. doi: 10.1111/j.1365-2869.2011.00971.x [DOI] [PubMed] [Google Scholar]
- Verhulst S. L., Schrauwen N., De Backer W. A., Desager K. N. (2006). First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Archives of Disease in Childhood , 91, 233–237. doi: 10.1136/adc.2005.085365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber D. P., Pomeroy S. L., Chiverton A. M., Kieran M. W., Scott R. M., Goumnerova L. C., Rivkin M. J. (2006). Everyday cognitive function after craniopharyngioma in childhood. Pediatric Neurology , 34, 13–19. doi: 10.1016/j.pediatrneurol.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Walker M. P., van der Helm E. (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin , 135, 731–748. doi: 10.1037/a0016570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang Y., Xing W., Chen J., Liu C., Luo X. (2013). Altered neural circuits related to sustained attention and executive control in children with ADHD: An event-related fMRI study. Clinical Neurophysiology , 124, 2181–2190. doi: 10.1016/j.clinph.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2003). WISC-IV Wechsler Intelligence Scale for Children: Technical and Interpretative: Manual. San Antonio, TX: Pearson. [Google Scholar]
- Weissman D. H., Roberts K. C., Visscher K. M., Woldorff M. G. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience , 9, 971–978. doi: 10.1038/nn1727 [DOI] [PubMed] [Google Scholar]
- Zhou E. S., Manley P. E., Marcus K. J., Recklitis C. J. (2016). Medical and psychosocial correlates of insomnia symptoms in adult survivors of pediatric brain tumors. Journal of Pediatric Psychology, 41, 623–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.