Abstract
An extracellular β-glucosidase produced by Aspergillus terreus was identified, purified, characterized and was tested for the hydrolysis of soybean isoflavone. Matrix-assisted laser desorption/ionization with tandem time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF MS) revealed the protein to be a member of the glycosyl hydrolase family 3 with an apparent molecular mass of about 120 kDa. The purified β-glucosidase showed optimal activity at pH 5.0 and 65 °C and was very stable at 50 °C. Moreover, the enzyme exhibited good stability over pH 3.0–8.0 and possessed high tolerance towards pepsin and trypsin. The kinetic parameters K m (apparent Michaelis-Menten constant) and V max (maximal reaction velocity) for p-nitrophenyl-β-D-glucopyranoside (pNPG) were 1.73 mmol/L and 42.37 U/mg, respectively. The K m and V max for cellobiose were 4.11 mmol/L and 5.7 U/mg, respectively. The enzyme efficiently converted isoflavone glycosides to aglycones, with a hydrolysis rate of 95.8% for daidzin, 86.7% for genistin, and 72.1% for glycitin. Meanwhile, the productivities were 1.14 mmol/(L·h) for daidzein, 0.72 mmol/(L·h) for genistein, and 0.19 mmol/(L·h) for glycitein. This is the first report on the application of A. terreus β-glucosidase for converting isoflavone glycosides to their aglycones in soybean products.
Keywords: β-glucosidase, Aspergillus terreus, Characterization, Hydrolysis, Soybean isoflavones
1. Introduction
Isoflavones, a class of nonsteroidal estrogens, are diphenolic secondary metabolites of plants and are frequently abounded in soybeans (Glycine max). They have attracted considerable attention for their pharmacological effects, such as preventing certain cancers (breast, prostate, and colon) (Ravindranath et al., 2004; Ullah et al., 2016), decreasing the risk of cardiovascular diseases (Imai, 2015), improving bone health (Alekel et al., 2015), and preventing menopausal symptoms (Ko, 2013). Moreover, studies revealed that isoflavone aglycones, such as daidzein and genistein, are superior to isoflavone glycosides in various bioactivities. However, most of the isoflavones in natural food exist as isoflavone glycosides and are difficult to absorb in the intestines (Izumi et al., 2000). Thus, to obtain good bioavailability and pharmacological effects, isoflavone glycosides need to be hydrolyzed to aglycones.
β-Glucosidases (β-D-glucoside glucohydrolase, EC 3.2.1.21) mainly hydrolyze the β-1,4-glucosidic linkage in various disaccharides, oligosaccharides, alkyl-and aryl-β-D-glucosides (Kudou et al., 1991). They have attracted considerable interest due to their important roles in a variety of fundamental biological processes, such as the hydrolysis in glucosides of isoflavones (Matsuura and Obata, 1993), bio-ethanol production (Coughlan, 1985), pharmaceutical (Zhou et al., 2012) and flavor production (Gueguen et al., 1996). According to the amino acid sequences, β-glucosidases have been classified into glycoside hydrolase (GH) families GH1, GH3, GH5, GH9, GH30, and GH116 (http://www.cazy.org). The majority of fungal β-glucosidases belong to GH1 and GH3. The nutritional availability of foods and feeds can be improved for the release of vitamins, antioxidants, aglycones, and other beneficial compounds from their glycosides by adding β-glucosidases. Therefore, animal feeds are often treated with crude β-glucosidases (Cairns and Esen, 2010). Desired features which are important to the animal feed applications of β-glucosidases include high hydrolysis activity, good thermostability and pH stability, and high pepsin and trypsin tolerance.
The possibilities for different industrial applications of β-glucosidases justify the incessant search for new sources of microbial enzymes that can be produced with high structural stability in low economic value fermentation media (de Cassia Pereira et al., 2015). Fungal strains are known to be efficient producers of β-glucosidases (Souza et al., 2010). There have been several reports (Workman and Day, 1982; Rodionova et al., 1987; Nazir et al., 2008; Elshafei et al., 2014) about the purification of β-glucosidase from Aspergillus terreus; however, there have not been any investigations about their potential industrial applications. In this study, the newly isolated strain of fungus, A. terreus, can produce extracellular β-glucosidases with outstanding pepsin and trypsin tolerance, which indicates the potential applications in animal feeds. The absence of reports in scientific literature of this fact encouraged us to study the physicochemical characteristics and the industrial applicabilities of the A. terreus β-glucosidases (At-Bgls).
2. Materials and methods
2.1. Materials
p-Nitrophenyl-β-D-glucopyranoside (pNPG) and cellobiose were purchased from the Sigma-Aldrich Corp. (St. Louis, MO, USA). Pepsin (2500 U/mg) and trypsin (2500 U/mg) were purchased from the Shanghai Songon Company (Shanghai, China). Soybeans (Zhongdou-27) were provided by the Center of Soybean Research, Zhejiang Agricultural Academy (Hangzhou, China). Isoflavone glycosidic standards (daidzin, genistin, and glycitin) and isoflavone aglycone standards (daidzein, genistein, and glycitein) were purchased from the Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used were analytical-grade reagents unless otherwise stated.
2.2. Organism and culture condition
A. terreus (Trichocomaceae, Eurotiales, Ascomycota) was isolated from composting soil of the Jinhua region, located in Zhejiang Province, China, and was identified by its 18S DNA sequence. The strain was maintained in potato dextrose agar (PDA; 200 g potato, 20 g glucose, 20 g agar, 1000 ml distilled water) slant at 4 °C.
For the preparation of inoculum, an agar plug of 5 mm diameter cut out from the PDA containing mycelium was incubated into a 20-ml basal medium, which had been autoclaved at 121 °C for 20 min, containing 2 g/L (NH4)2SO4, 1 g/L KH2PO4, 0.4 g/L MgSO4·7H2O, and 0.5 g/L CaCl2. The culture was then incubated on a rotary shaker at 35 °C for 24 h.
After incubation, the mycelium was stained with methylene blue, rinsed, and examined under a microscope to prove its purity, and was then broken into pieces using a homogenizer. An aliquot (1 ml) of the homogenate was incubated into 100 ml culture media, which had been autoclaved at 121 °C for 20 min, containing 30 g/L wheat bran, 4 g/L (NH4)2SO4, 1 g/L KH2PO4, 0.4 g/L MgSO4·7H2O, and 0.5 g/L CaCl2. Submerged fermentation was carried out in 250-ml flasks under continuous shaking (180 r/min) at 35 °C for 5 d with the flask sealed with sterile gauze and a cap of kraft paper in order to avoid excessive water evaporation and microorganism contamination. The culture suspension was squeezed through sterile absorbent gauze and centrifuged at 8000 r/min for 30 min at 4 °C. Thus, the cell-free culture supernatant was used for enzyme activity measurements and for the purification of β-glucosidase.
2.3. Enzyme purification and molecular mass determination
The cell-free culture supernatant was concentrated at 4 °C using Amicon Ultra-15 30K filter devices (Millipore, USA) and then dialyzed against a 20 mmol/L Tris-HCl buffer (pH 7.5). The dialyzed enzyme solution was loaded onto a HiTrap Q HP column (1.6 cm×2.5 cm, Amersham Pharmacia, Uppsala, Sweden) which was pre-equilibrated with a 20 mmol/L Tris-HCl buffer (pH 7.5). The column was first eluted with an equilibration buffer (two bed volumes) followed by a linear gradient of 1 mol/L NaCl at 2.0 ml/min. Then the fractions with high specific activity were pooled and concentrated using an Amicon Ultra-0.5 centrifugal Filter Unit with Ultracel-3 membrane (Millipore, USA).
The apparent molecular mass of the denatured protein was estimated by sodium dodecyl sulfate-polyarylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) with 5% (w/v) stacking polyacrylamide gel and 10% (w/v) separating gel which run at 120 V. The protein content in the fractions was determined using Lowry’s method.
2.4. Enzyme activity assay
The β-glucosidase activity was determined using pNPG as substrate. The reaction mixture contained 100 μl of appropriately diluted enzyme, 100 μl of pNPG (10 mmol/L), and 300 μl of McIlvaine buffer (100 mmol/L, pH 5.0). After incubation at 50 °C for 10 min, the reaction was stopped by adding 500 μl of 0.5 mol/L Na2CO3 and the absorbance was read at 405 nm for the amount of p-nitrophenol released. One unit of β-glucosidase activity was defined as the amount of enzyme that produced 1 μmol of p-nitrophenol per minute under the conditions of the assay. The experiments were performed in triplicate.
2.5. Effects of pH and temperature
The optimum pH of the purified β-glucosidase was determined at 50 °C for 10 min in the range from pH 3.0 to 8.0 using a 100 mmol/L McIlvaine buffer containing 10 mmol/L pNPG. For the pH stability study, the enzyme was pre-incubated in the above buffer systems for 60 min at 25 °C. The residual activities at different pH levels were determined under standard assay conditions after the incubation.
The optimal temperature of the enzyme was measured at the temperature range from 35 to 85 °C for 10 min in a McIlvaine buffer (100 mmol/L, pH 5.0) containing 10 mmol/L pNPG. For the thermal stability study, the purified enzyme was pre-incubated at different temperatures (50, 60, and 70 °C) in the absence of substrate. After incubation for different intervals (5–60 min), the residual activity was evaluated. All experiments were performed in triplicate.
2.6. Kinetic parameters
The apparent Michaelis-Menten constant (K m) and the maximal reaction velocity (V max) for the purified β-glucosidase were assessed from Lineweaver-Burk plots using pNPG or cellobiose as substrates. The enzyme was incubated in McIlvaine buffer (100 mmol/L, pH 5.0) with the substrate in concentrations ranging from 1.25 to 18.00 mmol/L of pNPG or 1.25–20.00 mmol/L of cellobiose at 65 °C for 10 min.
2.7. Tolerance to pepsin and trypsin
Glycine-HCl buffer (100 mmol/L, pH 3.0) containing 0.1% (1 g/L) pepsin and Tris-HCl buffer (100 mmol/L, pH 7.0) containing 0.1% (1 g/L) trypsin were prepared. To evaluate the tolerance to pepsin, the purified β-glucosidase (1 mg/ml) was incubated in glycine-HCl buffer solution for different intervals (30, 60, 120 min) with a β-glucosidase/pepsin mass ratio of 1:1 and 1:0.2, respectively. Similarly, the purified enzyme (1 mg/ml) was incubated in Tris-HCl buffer solution with a β-glucosidase/trypsin mass ratio of 1:0.1 and 1:0.02, respectively. After incubation, the residual activities were measured under standard assay conditions.
2.8. Protein identification by a matrix-assisted laser desorption ionization tandem time using a flight mass spectrometry
The band of glucosidase was manually excised from SDS-PAGE stained with Coomassie Brilliant Blue (CBB), and digested in gel overnight with trypsin as described by Oda et al. (2006). The gel piece was washed with Millipore pure water three times, destained twice with 50% acetonitrile in 25 mmol/L ammonium bicarbonate (Ambic) until the CBB disappeared, then reduced with 10 mmol/L dithiothreitol (DTT) in 25 mmol/L Ambic, alkylated with 55 mmol/L iodoacetamide in 25 mmol/L Ambic, dried twice with 100% acetonitrile, and digested overnight at 37 °C with sequencing grade modified trypsin (Promega, Madison, WI, USA) in 25 mmol/L Ambic. The peptides were extracted twice with 0.1% trifluoroacetic acid in 50% acetonitrile. Samples were then spotted onto a freshly cleaned target plate and after air-drying, the crystallized spots were analyzed. Matrix-assisted laser desorption/ionization with tandem time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF MS) analysis was performed using an ABI Sciex 5800 MALDI-TOF/TOF MS Analyzer (AB SCIEX, Framingham, MA, USA). Thereafter, a database search was conducted using the MASCOT program online (http://www.matrixscience.com) against National Center for Biotechnology Information nonredundant (NCBInr) databases. The searching parameters were set as follows: tryptic digestion, one missed cleavage allowed, carbamidomethylation of cysteines as fixed modification, methionine oxidation as variable modification, all peptides monoisotopic, peptide mass tolerance of 100 ppm (10−6), and fragment mass tolerance of 0.3 Da. Only significant hits, as defined by the MASCOT probability analysis (P<0.05), were accepted.
2.9. Isoflavone extraction from soybean flour and enzymatic hydrolysis
Soybeans were ground to powder using a coffee grinder and then defatted using petroleum ether, with a Soxhlet extractor. The defatted soybean flour (1 g) was extracted with 5 ml 80% ethanol at 70 °C for 2 h. The slurry was then centrifuged at 12 000 r/min for 15 min, and the supernatant was filtered through a 0.45-μm filter and stored at 4 °C for further study.
To evaluate the potential application of At-Bgl in our study, the commonly used commercial β-glucosidase Novozyme 188 was used to hydrolyze the isoflavone glycosides. A 250-μl reaction mixture containing 50 μl soybean isoflavone extract was hydrolyzed with 0.025, 0.050, and 0.075 U of individual β-glucosidase, respectively, in McIlvaine buffer (pH 5.0) in a thermostatically controlled incubator at 50 °C for 10 min. The reaction was stopped by boiling for 5 min. A control reaction of extract samples without enzyme was set up in the same manner. The determination of isoflavone in hydrolyzed samples was determined by high performance liquid chromatography (HPLC) as previously described (Li et al., 2012). HPLC analyses were performed using a Waters HP1100 HPLC system (Milford, MA, USA) equipped with a Diamonsil C18 column (5 μm, 250 mm×4.6 mm; Dima Co., Ltd., Orlando, FL, USA), and six types of high purity soybean isoflavones, daidzin, glycitin, genistin, daidzein, glycitein, and genistein, were used as standards. The productivity (mmol/(L·h)) of isoflavone aglycones was defined as the increase in the concentration produced in 1 h (Yeom et al., 2012). The hydrolysis effect was calculated by the following equation (Song et al., 2011): hydrolysis rate=100%×(isoflavone glycosides in control samples−isoflavone glycosides in hydrolyzed samples)/isoflavone glycosides in the control samples.
2.10. Statistical analysis
Statistical analysis was performed using the one-way analysis of variance (ANOVA) procedure of the SPSS 19.0 statistical software package (IBM, Armonk, New York, USA). Treatment means were compared using Duncan’s multiple range tests. It was declared significant when P<0.05.
3. Results and discussion
3.1. Purification of A. terreus β-glucosidase
The A. terreus culture mycelium was proved purity (Fig. S1) and the procedures to purify β-glucosidase from the crude extract of A. terreus are presented in Table 1. β-Glucosidase was purified from the culture supernatant of A. terreus by ultrafiltration and anion exchange, and the enzyme was 11.45-fold purified to a specific activity of 25.73 U/mg with a yield of 7.62%. The results suggest that β-glucosidase may be a minor component in the culture supernatant, and the production may be related to the production medium (Nazir et al., 2008). After purification, the enzyme showed a single band in SDS-PAGE, with the apparent molecular mass of about 120 kDa (Fig. 1). Thus the enzyme was within the broad range of fungal β-glucosidase (35 to 250 kDa) (Song et al., 2011).
Table 1.
Purification of β-glucosidase from A. terreus
| Purification step | Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Purification (fold) | Recovery (%) |
| Crude extract | 115.14±1.82 | 258.83±4.12 | 2.25±0.07 | 1.00±0.02 | 100.00±0.82 |
| Ultra-filtraction | 58.06±1.11 | 194.84±2.74 | 3.36±0.53 | 1.49±0.03 | 75.28±0.75 |
| HiTrap Q HP | 0.77±0.02 | 19.72±0.88 | 25.73±0.62 | 11.45±0.53 | 7.62±0.18 |
Data represent the mean±SD (n=3)
Fig. 1.
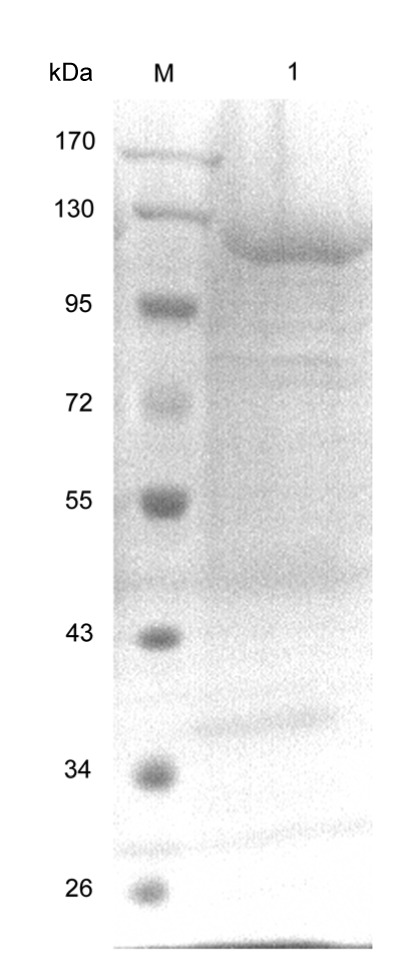
SDS-PAGE of A. terreus β-glucosidase
M, protein molecular weight marker (Thermo Fisher Scientific, Inc.); Lane 1, purified A. terreus β-glucosidase
3.2. Optimal pH and temperature, and stability
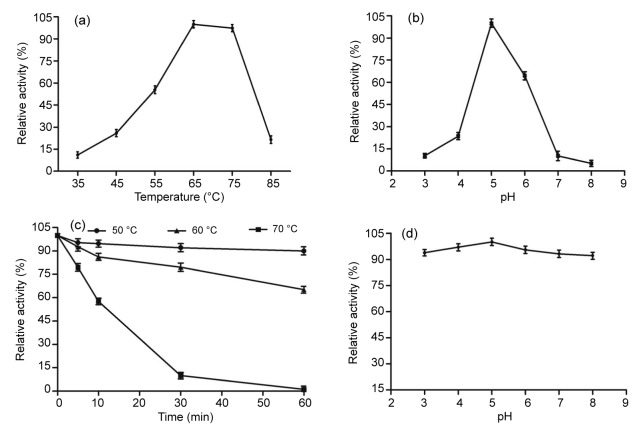
The pH optima of most fungal β-glucosidases have a range between pH 4.0 and 7.5 (Cairns and Esen, 2010). In this study, the purified β-glucosidase exhibited the highest activity at pH 5.0 and was sensitive to pH, while the activity decreased rapidly below pH 5.0 and above pH 7.0 (Fig. 2b). Based on the relative activity of 100% for the β-glucosidase at pH 5.0, only 23.6% and 64.5% of the maximum activity remained at pH 4.0 and 6.0, respectively. The optimal pH of the enzyme is similar to that of enzyme from A. terreus (Nazir et al., 2008), A. niger (Fujita et al., 2015), Penicillium piceum (Gao et al., 2013), and Rhizomucor miehei NRRL 5282 (Krisch et al., 2012). However, the enzyme was found to be very stable in the pH range from 3.0 to 8.0, remaining above 90% of the maximum activity, though it showed a significant decrease with pH above or below 5.0 (P<0.05; Fig. 2d). Before reaching the distal part of the intestinal tract and exerting their catalytic effects, enzymes must retain activity during transport through the stomach and the upper part of the intestinal tract (Peng et al., 2014). The mean pH (fed ad libitum) values of the porcine stomach and small intestine are 4.4 and 6.1–6.7, respectively (Merchant et al., 2011). Therefore, the At-Bgl should have high potential for use as catalysts in animal feeds.
Fig. 2.
Characterization of the purified A. terreus β-glucosidase
(a) Effect of temperature on A. terreus β-glucosidase activities. The activity was measured in McIlvaine buffer (100 mmol/L, pH 5.0) using pNPG as the substrate. (b) Effect of pH on A. terreus β-glucosidase activities. The enzyme activities at various pH values were measured at 50 °C for 10 min using pNPG as the substrate. (c) Thermostability of A. terreus β-glucosidase. The residual activity was measured using pNPG as the substrate after pre-incubation without substrate at 50, 60, and 70 °C for different periods of time. (d) pH stability of A. terreus β-glucosidase. The residual activities were measured at optimal conditions against pNPG after incubation at various pH values, 25 °C for 1 h. The error bars represent the standard deviation (SD), with n=3
The purified β-glucosidase had an optimal temperature of 65 °C (Fig. 2a), which was slightly higher than the previous reports (Li et al., 2012; 2013; Fujita et al., 2015; Kaur and Chadha, 2015; Zhao et al., 2015). In addition, the enzyme showed more than 97% relative activity at 75 °C, and showed no significant difference from the activity at 65 °C (P>0.05), while other fungal counterparts were generally inactivated at 70 °C (Li et al., 2013; Fujita et al., 2015; Kaur and Chadha, 2015; Zhao et al., 2015). Moreover, the At-Bgl showed good thermostability (Fig. 2c), maintaining 90.0% and 65.1% of the initial activity after incubation at 50 and 60 °C for 1 h, respectively, though it decreased significantly after incubation at 50, 60, or 70 °C for 5 min (P<0.05). These superior properties make this purified enzyme more advantageous for industrial processes, since most applications of β-glucosidase require high temperatures (50 °C or above) (Li et al., 2012).
3.3. Specific activity and kinetic parameters
Kinetic parameters of the purified At-Bgl for pNPG and cellobiose at pH 5.0 and 65 °C were estimated, respectively. The K m and V max values of the enzyme are shown in Table 2. The results indicate that the enzyme has greater affinity towards pNPG than cellobiose, and the hydrolysis of pNPG was about eight times faster than that determined on cellobiose. It is worth mentioning that the K m constant was slightly lower than those estimated for the At-Bgl in other reports, with a K m value of 2.5 mmol/L (Elshafei et al., 2014) and 14.2, 4.34, 11.1 mmol/L (Nazir et al., 2008), respectively. To our best knowledge, there are reports about another two At-Bgls with lower K m values of 0.78 mmol/L (Workman and Day, 1982) and 1.25 mmol/L (Rodionova et al., 1987), respectively. However, one of important drawbacks of those two enzymes is their low reaction temperatures, with optimal conditions of 50 °C (Workman and Day, 1982) and 60 °C (Rodionova et al., 1987), respectively. Therefore, those At-Bgls are still not enough for satisfying biotechnological applications, and the purified At-Bgl in our study displayed high potential for industrial applications.
Table 2.
Kinetic parameters of the purified A. terreus β-glucosidase
| Substrate | V max (U/mg) | K m (mmol/L) |
| Cellobiose | 5.70±0.17 | 4.11±0.16 |
| pNPG | 42.37±0.74 | 1.73±0.04 |
Data represent the mean±SD (n=3)
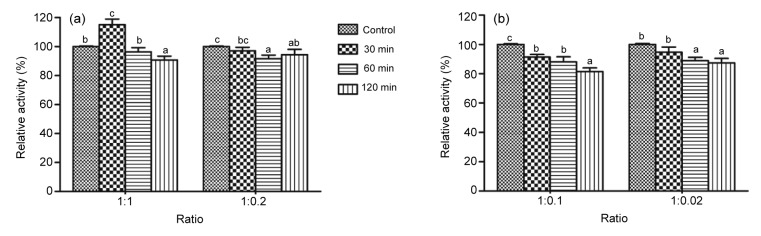
3.4. Tolerance to pepsin and trypsin
The tolerance to the gastrointestinal microenvironment is important for the application of feed additives (Peng et al., 2014). As shown in Fig. 3, the purified β-glucosidase maintained high activity after the treatment with pepsin or trypsin, indicating its high tolerance towards the animal digestive tract. The enzyme maintained more than 90% of the initial activity after the incubation with pepsin (in both 1:1 and 1:0.2 ratios) for 120 min. Moreover, the tolerance to trypsin is slightly lower than that to pepsin. The purified β-glucosidase retained 81.5% of the initial activity after incubating with trypsin (1:0.1) for 120 min and kept 87.4% of the initial activity when incubated with trypsin at a 1:0.02 ratio for 120 min.
Fig. 3.
Tolerance of A. terreus β-glucosidase to pepsin and trypsin
(a) The tolerance of A. terreus β-glucosidase to pepsin. The enzyme activity was measured at 65 °C, pH 5.0 (McIlvaine buffer, 100 mmol/L) against pNPG after being pre-incubated in glycine-HCl buffer (100 mmol/L, pH 3.0) containing 0.1% pepsin for different intervals (30, 60, and 120 min). (b) The tolerance of A. terreus β-glucosidase to trypsin. The enzyme activity was measured at 65 °C, pH 5.0 (McIlvaine buffer, 100 mmol/L) against pNPG after being pre-incubated in Tris-HCl buffer (100 mmol/L, pH 7.0) containing 0.1% trypsin for different intervals. The error bars represent the SD (n=3) and bars with different alphabets differ significantly (P<0.05)
3.5. Peptide sequence determination and protein identification
The purified samples of β-glucosidase were denatured and analyzed by SDS-PAGE and subjected to MALDI-TOF/TOF MS analysis. The results indicated that the inner peptide sequence of purified protein in the present showed 100% identity with a β-glucosidase from A. terreus NIH2624 (GenBank Accession No. X_P001212225), which belonged to the GH3. The matched fragments were HYILNE QEHFR, DEYGFAHFFPSEGAYER, VNEFVNVQR, and VEDASSYLYPEGLKR.
3.6. Hydrolysis of soybean isoflavone by A. terreus β-glucosidase
Enzymatic hydrolysis of β-glucosidase towards soybean isoflavone is an important application in the food and feed industry, which has been investigated by direct use of β-glucosidase from plants and microorganisms (Chuankhayan et al., 2007; Kaya et al., 2008; Yang et al., 2009; Song et al., 2011; Kim et al., 2012; Fang et al., 2014; Fujita et al., 2015). However, low solubility of isoflavones reduces the productivity of isoflavone aglycones in industrial production (Horii et al., 2009). To test the reactivity of At-Bgl toward isoflavone, the hydrolysis rate and the productivity were measured by HPLC using isoflavone flour extract as the substrate. The peaks of daidzin, genistin, glycitin, daidzein, genistein, and glycitein in soybean isoflavone flour extract were identified by comparison with commercial standards. The results showed that daidzin was the predominant isoflavones in soybean flour extract. However, after 10 min hydrolysis of At-Bgl, the peaks of daidzin, genistin, and glycitin were greatly decreased and the peaks of daidzein, genistein, and glycitein increased remarkably (Fig. 4b), which suggested that most of the glycosidic soybean isoflavones were converted to corresponding aglycones. As shown in Table 3, the At-Bgl exhibited higher hydrolytic activity than the commercial β-glucosidase cellobiase (Novozyme 188) toward daidzin, glycitin, and genistin (95.78% vs. 59.16%, 72.08% vs. 23.07%, and 86.74% vs. 54.15%, respectively). Thus, the At-Bgl offers a better advantage for the hydrolysis of isoflavone β-glycoside (daidzin, glycitin, and genistin) than Novozyme 188.
Fig. 4.
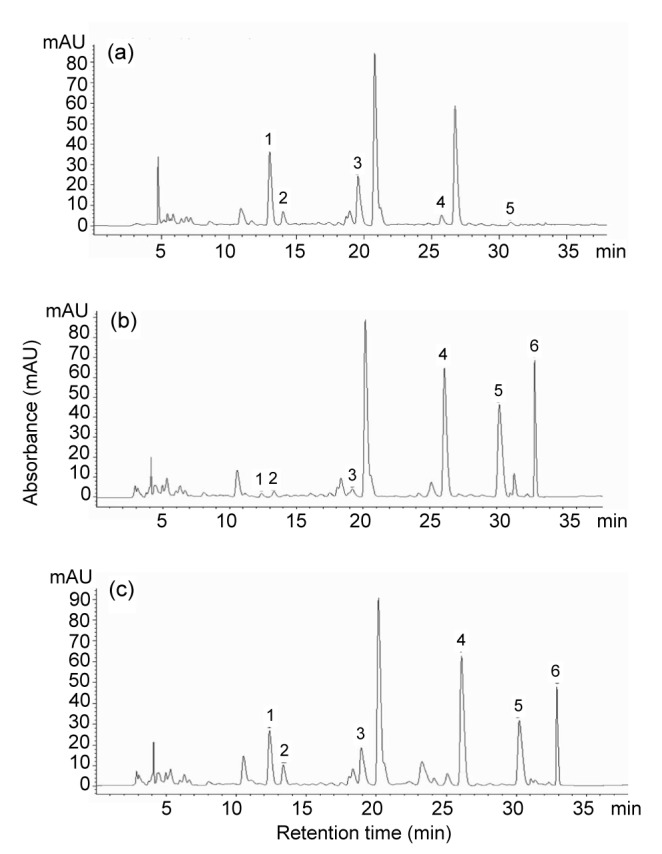
HPLC chromatograms of isoflavones
(a) Isoflavones in soybean flour extract; (b) Soybean flour extract hydrolysed with 0.075 U of A. terreus β-glucosidase at 50 °C for 10 min; (c) Soybean flour extract hydrolysed with 0.075 U of commercial β-glucosidase cellobiase Novozyme 188 at 50 °C for 10 min. 1, daidzin; 2, glycitin; 3, genistin; 4, daidzein; 5, glycitein; 6, genistein
Table 3.
Hydrolysis rates and productivities of A. terreus β-glucosidase and Novozyme 188 to soybean isoflavone
| Amount (U) | Enzyme | Hydrolysis rate (%) |
Productivity (mmol/(L·h)) |
||||
| Daidzin | Glycitin | Genistin | Daidzein | Glycitein | Genistein | ||
| 0.025 | At-Bgl | 32.76±0.28A | 27.58±0.10B | 28.78±0.13B | 0.41±0.02a | 0.08±0.01c | 0.24±0.02b |
| Nov 188 | 10.31±0.03C | 12.38±0.02A | 11.97±0.03B | 0.15±0.02a | 0.03±0.01c | 0.08±0.01b | |
| 0.050 | At-Bgl | 95.46±0.22A | 64.25±0.29C | 84.68±0.38B | 1.06±0.06a | 0.18±0.03c | 0.68±0.02b |
| Nov 188 | 34.04±0.04A | 20.58±0.28C | 28.52±0.29B | 0.57±0.02a | 0.07±0.01c | 0.19±0.02b | |
| 0.075 | At-Bgl | 95.78±0.20A | 72.08±0.08C | 86.74±0.04B | 1.14±0.01a | 0.19±0.01c | 0.72±0.03b |
| Nov 188 | 59.16±0.16A | 23.07±0.04C | 54.15±0.16B | 0.84±0.04b | 0.13±0.02a | 0.40±0.02c | |
The reactions were performed in McIlvaine buffer (100 mmol/L, pH 5.0) containing 50 μl soybean isoflavone flour extract and 0.025, 0.050, or 0.075 U β-glucosidase. All reactions were performed at 50 °C for 10 min. At-Bgl: β-glucosidase from Aspergillus terreus. Nov 188: commercial β-glucosidase cellobiase Novozyme 188. Data (mean±SD (n=3)) with different superscripts within a row differ significantly (P<0.05)
The results indicate that At-Bgl also showed better productivity than Novozyme 188 after incubation for 10 min with 0.075 U of At-Bgl. By comparison, the At-Bgl had the second highest productivity for daidzein (1.14 mmol/(L·h)) and genistein (0.72 mmol/(L·h)), much higher than that of β-glucosidase from A.oryzae (Horii et al., 2009), soybean okara (Chiou et al., 2010), and Pyrococcus furiosus (Yeom et al., 2012). In addition, the previously reported highest productivities for daidzein and genistein were 1.50 and 1.23 mmol/(L·h), respectively, produced by a β-glucosidase from Gongronella sp. (Fang et al., 2014). To the best of our knowledge, this is the first report of an At-Bgl hydrolyzing soybean isoflavones and its high hydrolysis rates of daidzin and genistin, and the second highest productivity for daidzein and genistein makes it a good candidate for hydrolysis of soybean isoflavones.
4 Conclusions
The purified β-glucosidase from A. terreus, a member of GH3, is a promising enzyme to be used as feed additives. The purified enzyme exhibited maximal activity at pH 5.0 and 65 °C. Moreover, the enzyme had multiple industrial desired properties, including thermostability, wide pH stability, and high tolerance to pepsin and trypsin. And its high efficiency to convert soybean isoflavone glycosides into aglycones was very attractive. Thus, At-Bgl might perform substantially to enhance nutritional value of soy products, such as soybean meal which is widely used in animal feeds. The study indicated that At-Bgl offers high potential for commercialization and is valuable in various industrial applications, especially in food and feeds.
List of electronic supplementary materials
Fig. S1 Morphology of A. terrues culture mycelium
Footnotes
Project supported by the Innovation Team Program of Zhejiang Province (No. 2011R50025-12), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1500317) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Feng-ying YAN, Wei XIA, Xiao-xu ZHANG, Sha CHEN, Xin-zheng NIE, and Li-chun QIAN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Alekel DL, Genschel U, Koehler KJ, et al. Soy Isoflavones for Reducing Bone Loss Study: effects of a 3-year trial on hormones, adverse events, and endometrial thickness in postmenopausal women. Menopause. 2015;22(2):185–197. doi: 10.1097/GME.0000000000000280. (Available from: http://dx.doi.org/10.1097/GME.0000000000000280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cairns JRK, Esen A. β-Glucosidases. Cell Mol Life Sci. 2010;67(20):3389–3405. doi: 10.1007/s00018-010-0399-2. (Available from: http://dx.doi.org/10.1007/s00018-010-0399-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou TY, Lin YH, Su NW, et al. β-Glucosidase isolated from soybean okara shows specificity toward glucosyl isoflavones. J Agric Food Chem. 2010;58(15):8872–8878. doi: 10.1021/jf101848x. (Available from: http://dx.doi.org/10.1021/jf101848x) [DOI] [PubMed] [Google Scholar]
- 4.Chuankhayan P, Rimlumduan T, Svasti J, et al. Hydrolysis of soybean isoflavonoid glycosides by Dalbergia β-glucosidases. J Agric Food Chem. 2007;55(6):2407–2412. doi: 10.1021/jf062885p. (Available from: http://dx.doi.org/10.1021/jf062885p) [DOI] [PubMed] [Google Scholar]
- 5.Coughlan MP. The properties of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev. 1985;3(1):39–110. (Available from: http://dx.doi.org/10.1080/02648725.1985.10647809) [Google Scholar]
- 6.de Cassia Pereira J, Leite RSR, do Prado HFA, et al. Production and characterization of β-glucosidase obtained by the solid-state cultivation of the thermophilic fungus Thermomucor indicae-seudaticae N31. Appl Biochem Biotechnol. 2015;175(2):723–732. doi: 10.1007/s12010-014-1332-1. (Available from: http://dx.doi.org/10.1007/s12010-014-1332-1) [DOI] [PubMed] [Google Scholar]
- 7.Elshafei AM, Hassan MM, Morsi NM, et al. Purification and some kinetic properties of β-glucosidase from Aspergillus terreus NRRL 265. Afr J Biotechnol. 2014;10(84):19556–19569. [Google Scholar]
- 8.Fang W, Song R, Zhang X, et al. Characterization of a novel β-glucosidase from Gongronella sp. W5 and its application in the hydrolysis of soybean isoflavone glycosides. J Agric Food Chem. 2014;62(48):11688–11695. doi: 10.1021/jf502850z. (Available from: http://dx.doi.org/10.1021/jf502850z) [DOI] [PubMed] [Google Scholar]
- 9.Fujita A, Alencar SM, Park YK. Conversion of isoflavone glucosides to aglycones by partially purified β-glucosidases from microbial and vegetable sources. Appl Biochem Biotechnol. 2015;176(6):1659–1672. doi: 10.1007/s12010-015-1668-1. (Available from: http://dx.doi.org/10.1007/s12010-015-1668-1) [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Gao F, Zhang D, et al. Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresour Technol. 2013;147:658–661. doi: 10.1016/j.biortech.2013.08.089. (Available from: http://dx.doi.org/10.1016/j.biortech.2013.08.089) [DOI] [PubMed] [Google Scholar]
- 11.Gueguen Y, Chemardin P, Janbon G, et al. A very efficient β-glucosidase catalyst for the hydrolysis of flavor precursors of wines and fruit juices. J Agric Food Chem. 1996;44(8):2336–2340. (Available from: http://dx.doi.org/10.1021/jf950360j) [Google Scholar]
- 12.Horii K, Adachi T, Matsuda T, et al. Improvement of isoflavone aglycones production using β-glucosidase secretory produced in recombinant Aspergillus oryzae . J Mol Catal B Enzym. 2009;59(4):297–301. (Available from: http://dx.doi.org/10.1016/j.molcatb.2008.11.013) [Google Scholar]
- 13.Imai S. Soybean and processed soy foods ingredients, and their role in cardiometabolic risk prevention. Recent Pat Food Nutr Agric. 2015;7(2):75–82. doi: 10.2174/2212798407666150629123839. (Available from: http://dx.doi.org/10.2174/2212798407666150629123839) [DOI] [PubMed] [Google Scholar]
- 14.Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 15.Kaur A, Chadha BS. Penicillium janthinellum: a source of efficient and high levels of β-glucosidase. Appl Biochem Biotechnol. 2015;175(2):937–949. doi: 10.1007/s12010-014-1330-3. (Available from: http://dx.doi.org/10.1007/s12010-014-1330-3) [DOI] [PubMed] [Google Scholar]
- 16.Kaya M, Ito J, Kotaka A, et al. Isoflavone aglycones production from isoflavone glycosides by display of β-glucosidase from Aspergillus oryzae on yeast cell surface. Appl Microbiol Biotechnol. 2008;79(1):51–60. doi: 10.1007/s00253-008-1393-6. (Available from: http://dx.doi.org/10.1007/s00253-008-1393-6) [DOI] [PubMed] [Google Scholar]
- 17.Kim BN, Yeom SJ, Kim YS, et al. Characterization of a β-glucosidase from Sulfolobus solfataricus for isoflavone glycosides. Biotechnol Lett. 2012;34(1):125–129. doi: 10.1007/s10529-011-0739-9. (Available from: http://dx.doi.org/10.1007/s10529-011-0739-9) [DOI] [PubMed] [Google Scholar]
- 18.Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev. 2013;15(17):7001–7010. doi: 10.7314/apjcp.2014.15.17.7001. (Available from: http://dx.doi.org/10.7314/APJCP.2014.15.17.7001) [DOI] [PubMed] [Google Scholar]
- 19.Krisch J, Bencsik O, Papp T, et al. Characterization of a β-glucosidase with transgalactosylation capacity from the zygomycete Rhizomucor miehei . Bioresour Technol. 2012;114:555–560. doi: 10.1016/j.biortech.2012.02.117. (Available from: http://dx.doi.org/10.1016/j.biortech.2012.02.117) [DOI] [PubMed] [Google Scholar]
- 20.Kudou S, Fleury Y, Welti D, et al. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill) Agric Biol Chem. 1991;55(9):2227–2233. (Available from: http://dx.doi.org/10.1080/00021369.1991.10870966) [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. (Available from: http://dx.doi.org/10.1038/227680a0) [DOI] [PubMed] [Google Scholar]
- 22.Li G, Jiang Y, Fan XJ, et al. Molecular cloning and characterization of a novel β-glucosidase with high hydrolyzing ability for soybean isoflavone glycosides and glucose-tolerance from soil metagenomic library. Bioresour Technol. 2012;123:15–22. doi: 10.1016/j.biortech.2012.07.083. (Available from: http://dx.doi.org/10.1016/j.biortech.2012.07.083) [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhao J, Shi P, et al. Molecular cloning and expression of a novel β-glucosidase gene from Phialophora sp. G5. Appl Biochem Biotechnol. 2013;169(3):941–949. doi: 10.1007/s12010-012-0048-3. (Available from: http://dx.doi.org/10.1007/s12010-012-0048-3) [DOI] [PubMed] [Google Scholar]
- 24.Matsuura M, Obata A. β-Glucosidases from soybeans hydrolyze daidzin and genistin. J Food Sci. 1993;58(1):144–147. (Available from: http://dx.doi.org/10.1111/j.1365-2621.1993.tb03231.x) [Google Scholar]
- 25.Merchant HA, McConnell EL, Liu F, et al. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur J Pharm Sci. 2011;42(1-2):3–10. doi: 10.1016/j.ejps.2010.09.019. (Available from: http://dx.doi.org/10.1016/j.ejps.2010.09.019) [DOI] [PubMed] [Google Scholar]
- 26.Nazir A, Soni R, Saini H, et al. Regulation of expression of multiple β-glucosidases of Aspergillus terreus and their purification and characterization. Bioresources. 2008;4(1):155–171. [Google Scholar]
- 27.Oda K, Kakizono D, Yamada O, et al. Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl Environ Microbiol. 2006;72(5):3448–3457. doi: 10.1128/AEM.72.5.3448-3457.2006. (Available from: http://dx.doi.org/10.1128/AEM.72.5.3448-3457.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Z, Wang A, Feng Q, et al. High-level expression, purification and characterisation of porcine β-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl Microbiol Biotechnol. 2014;98(12):5487–5497. doi: 10.1007/s00253-014-5560-7. (Available from: http://dx.doi.org/10.1007/s00253-014-5560-7) [DOI] [PubMed] [Google Scholar]
- 29.Ravindranath MH, Muthugounder S, Presser N, et al. Anticancer therapeutic potential of soy isoflavone, genistein. In: Cooper EL, Yamaguchi N, editors. Complementary and Alternative Approaches to Biomedicine. US: Springer; 2004. pp. 121–165. (Available from: http://dx.doi.org/10.1007/978-1-4757-4820-8_11) [DOI] [PubMed] [Google Scholar]
- 30.Rodionova NA, Tavobilov IM, Martinovich LI, et al. β-Glucosidases from cellulolytic fungi Aspergillus terreus, Geotrichum candidum, and Trichoderma longibrachiatum as typical glycosidases. Biotechnol Appl Biochem. 1987;9(3):239–250. doi: 10.1111/j.1470-8744.1987.tb00475.x. (Available from: http://dx.doi.org/10.1111/j.1470-8744.1987.tb00475.x) [DOI] [PubMed] [Google Scholar]
- 31.Song X, Xue Y, Wang Q, et al. Comparison of three thermostable β-glucosidases for application in the hydrolysis of soybean isoflavone glycosides. J Agric Food Chem. 2011;59(5):1954–1961. doi: 10.1021/jf1046915. (Available from: http://dx.doi.org/10.1021/jf1046915) [DOI] [PubMed] [Google Scholar]
- 32.Souza FHM, Nascimento CV, Rosa JC, et al. Purification and biochemical characterization of a mycelial glucose-and xylose-stimulated β-glucosidase from the thermophilic fungus Humicola insolens . Process Biochem. 2010;45(2):272–278. (Available from: http://dx.doi.org/10.1016/j.procbio.2009.09.018) [Google Scholar]
- 33.Ullah MF, Bhat SH, Husain E, et al. Pharmacological intervention through dietary nutraceuticals in gastrointestinal neoplasia. Crit Rev Food Sci Nutr. 2016;56(9):1501–1518. doi: 10.1080/10408398.2013.772091. (Available from: http://dx.doi.org/10.1080/10408398.2013.772091) [DOI] [PubMed] [Google Scholar]
- 34.Workman WE, Day DF. Purification and properties of β-glucosidase from Aspergillus terreus . Appl Microbiol Biotechnol. 1982;44(6):1289–1295. doi: 10.1128/aem.44.6.1289-1295.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Wang L, Yan Q, et al. Hydrolysis of soybean isoflavone glycosides by a thermostable β-glucosidase from Paecilomyces thermophila . Food Chem. 2009;115(4):1247–1252. (Available from: http://dx.doi.org/10.1016/j.foodchem.2009.01.038) [Google Scholar]
- 36.Yeom SJ, Kim BN, Kim YS, et al. Hydrolysis of isoflavone glycosides by a thermostable β-glucosidase from Pyrococcus furiosus . J Agric Food Chem. 2012;60(6):1535–1541. doi: 10.1021/jf204432g. (Available from: http://dx.doi.org/10.1021/jf204432g) [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Guo C, Tian C, et al. Heterologous expression and characterization of a GH3 β-glucosidase from thermophilic fungi Myceliophthora thermophila in Pichia pastoris . Appl Biochem Biotechnol. 2015;177(2):511–527. doi: 10.1007/s12010-015-1759-z. (Available from: http://dx.doi.org/10.1007/s12010-015-1759-z) [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Qian L, Ma H, Yu X, et al. Enhancement of amygdalin activated with β-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohydr Polym. 2012;90(1):516–523. doi: 10.1016/j.carbpol.2012.05.073. (Available from: http://dx.doi.org/10.1016/j.carbpol.2012.05.073) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Morphology of A. terrues culture mycelium