Abstract
Background
Delay discounting is a potential etiological factor in adolescents’ alcohol use, making it important to understand its antecedents. Family disorganization might contribute to delay discounting, but few studies have tested this relation. Moreover, because delay discounting is heritable, the effects of family disorganization on delay discounting might be moderated by adolescents’ genetic risk for delay discounting. Thus, the current study examined the role of family disorganization, in interaction with genetic risk, in predicting adolescents’ delay discounting and subsequent alcohol use.
Methods
Adolescents participated in four waves of data collection. Adolescents self-reported their family disorganization at T1, completed a delay discounting questionnaire at T3, and self-reported their alcohol use both at T2 (covariate) and T4 (outcome). Using results from an independent sample (Gray & MacKillop, 2014), we created a polygenic risk score consisting of dopaminergic genes to index genetic risk for delay discounting.
Results
Greater family disorganization predicted adolescents’ greater delay discounting, but only for adolescents with low levels of genetic risk for delay discounting. Adolescents with high and mean levels of genetic risk for delay discounting showed elevated delay discounting regardless of their family’s disorganization. Greater delay discounting prospectively predicted adolescents’ greater alcohol use. Finally, the effects of family disorganization on adolescents’ alcohol use were mediated through delay discounting, but only for adolescents with low levels of genetic risk.
Conclusions
Results suggest multiple pathways to delay discounting. Although there are genetically influenced pathways to delay discounting, family disorganization might represent an environmental pathway to delay discounting (and subsequent alcohol use) for a subset of adolescents at low genetic risk. These findings reinforce the utility of family interventions for reducing adolescents’ delay discounting and alcohol use, at least for a subgroup of adolescents. Because higher family organization did not buffer against delay discounting among adolescents with high genetic risk, future research should explore other early environmental influences that could protect these high-risk adolescents from developing these risky behaviors.
Keywords: Family Disorganization, Delay Discounting, Adolescence, Alcohol Use, Gene by Environment Interaction
Introduction
Sher’s (1991) empirically-supported “deviance-proneness” model posits that children’s heritable predispositions for behavioral undercontrol interact with the family environment to influence risk for later alcohol use and disorder (Chassin et al., 1993). This behavioral undercontrol phenotype is multi-faceted and has been operationalized in various ways. Among other components, research suggests that this phenotype includes increased delay discounting (also known as choice impulsivity; Hamilton et al., 2015). Delay discounting is defined as a preference for small immediate rewards over larger delayed rewards (Kirby and Marakavic, 1995) and has been associated with a variety of addictive behaviors (e.g., MacKillop et al., 2011).
Role of Delay Discounting in Adolescent Alcohol Use
Research demonstrates that high delay discounting concurrently predicts adolescents’ heavier alcohol use and prospectively predicts greater adolescent alcohol involvement (Fernie et al., 2013; Field et al., 2007). Adolescents with high delay discounting are likely at greater risk for addictive behaviors because they opt for the immediate rewards of alcohol consumption in spite of potential long-term consequences of alcohol use. Given that delay discounting is an important determinant of later alcohol use (e.g., Khurana et al., 2013; Kim-spoon et al., 2014), identifying its earlier predictors could have implications for preventive interventions aimed at reducing this risky behavior (e.g., Gray & MacKillop, 2015).
Role of Family Disorganization
Disorganized families have difficulty sticking to regular schedules and routines. They are also characterized by undependable and unpredictable caregivers who have trouble keeping promises (Bloom, 1985). Some researchers posit that such inconsistent family environments shape an unpredictability schema that fosters a propensity for immediate, guaranteed gratification over long-term, less-certain gains (Ross & Hill, 2002). Indeed, research has shown that parents’ inconsistency in providing promised rewards (Schneider et al., 2014) as well as interactions with experimenters who were perceived as unreliable (Kidd et al., 2013) predicted children’s higher delay discounting and lowered delay of gratification. Similarly, Patak and Reynolds (2007) found that adolescents who reported more uncertainty about obtaining delayed rewards showed greater delay discounting than did adolescents who reported more certainty about obtaining delayed rewards.
These findings suggest that family disorganization might influence delay discounting because disorganized families create uncertainty about receiving rewards. That is, adolescents from unpredictable, disorganized families may be less certain that delayed rewards will actually be received, which may foster a general tendency to prefer immediate rewards over larger but more delayed rewards. No previous studies have directly tested the relation between family disorganization and delay discounting. However, both early life adversity and family history of substance disorder, which are potential correlates of family disorganization, have been linked to faster discounting of delayed rewards (Acheson et al., 2011; Lovallo et al., 2013). To address this key gap in the literature, the current study tested whether family disorganization would predict adolescents’ delay discounting.
Gene-Environment Interaction
Research shows that adolescents’ delay discounting is heritable (Anokhin et al., 2011). Thus, in considering how family disorganization may influence delay discounting, it is also important to consider whether the effects of family disorganization on delay discounting might vary depending upon adolescents’ pre-existing levels of genetic risk for delay discounting.
The dopaminergic system warrants particular attention both in understanding the heritability of delay discounting and operationalizing genetic risk for delay discounting (Gray & MacKillop, 2014). Research from both animal and human literatures suggests that dopamine and the functioning of dopamine-modulated frontostriatal circuits are related to delay discounting (Dalley & Roiser, 2012). For instance, rats that displayed greater delay discounting had significantly greater transcription of genes coding the D1 and D5 dopamine receptors in the medial prefrontal cortex when compared to rats with lower delay discounting (Loos et al., 2009). In human imaging studies, variations in dopamine receptor availability have been linked to differences in delay discounting (Oberlin et al., 2015). Research also showed that differences in delay discounting performance were predicted by single nucleotide polymorphisms (SNPs) within dopaminergic genes such as DRD2, DRD4, and COMT (Eisenberg et al., 2007; Gray & MacKillop, 2014; Boettiger et al., 2007). Given the importance of dopaminergic genes in delay discounting, for the current study we created a dopaminergic polygenic score to index genetic risk for delay discounting using the results from an independent sample (Gray & MacKillop, 2014).
Studies examining gene-by-environment interactions have often demonstrated that the combination of adverse environments and high genetic risk places individuals at the greatest risk for a range of negative outcomes (i.e., a diathesis-stress model). For example, dopaminergic SNPs more strongly predicted higher delay discounting for those with lower socioeconomic status (Sweitzer et al., 2013) and for those reporting greater exposure to acute psychosocial stress compared to those who were not exposed to stress (White et al., 2009). Similar patterns have been shown for other facets of behavioral undercontrol. For example, poor parenting quality more strongly influenced children’s sensation seeking for those with the DRD4 7-repeat allele (Sheese et al., 2007), and higher paternal alcohol consumption more strongly predicted adulthood novelty seeking for those with the DRD4 two- or five-repeat allele (Lahti et al., 2010). Thus, it is possible that family disorganization will more strongly predict delay discounting for adolescents with a greater genetic vulnerability for delay discounting.
Family Disorganization, Delay Discounting, and Alcohol Use
Chaotic or disorganized family environments have also been linked to greater adolescent alcohol use (Stoolmiller et al., 2012), and this relation could be mediated by adolescents’ delay discounting. Research has yet to investigate mediation whereby adolescents’ family disorganization predicts delay discounting which in turn predicts greater alcohol use. However, as noted in the above two sections, past research has provided separate evidence for both of these possible links in the mediational chain. Moreover, similar mediation has been shown in research with closely related constructs. For example, Hill et al., (2008) found that a “future discounting” latent variable that included delay discounting as an indicator mediated the relation between environmental unpredictability and risk taking behavior among college students. Taken together, these findings suggest that family disorganization may indirectly influence adolescent alcohol consumption by fostering greater delay discounting.
Current Study
This study examined the impact of family disorganization on adolescents’ delay discounting and subsequent alcohol use in a longitudinal and genetically-informed analysis. To operationalize genetic risk for delay discounting, we created a dopaminergic polygenic risk score for delay discounting using results from an independent sample (Gray & MacKillop, 2014). We examined whether this polygenic risk score moderated effects of family disorganization on adolescents’ delay discounting. Based on the diathesis-stress framework, we hypothesized that family disorganization would predict adolescents’ delay discounting more strongly for adolescents with high levels of polygenic risk for delay discounting. We also tested whether delay discounting (directly) and family disorganization (indirectly through delay discounting) prospectively predicted adolescents’ later alcohol use. We expected that higher levels of delay discounting would predict greater levels of alcohol use. We also hypothesized an indirect effect of family disorganization on alcohol use (through delay discounting), but only for adolescents with high levels of polygenic risk (i.e., moderated mediation).
Materials and Methods
Participants
Participants were from the third generation (G3) of a larger study of familial alcoholism (e.g., Chassin et al., 1992). This study originally recruited 454 families including parents (G1s) and one biological child aged 11–15 (G2s). 54% of G1s had lifetime alcohol disorder. G2 adolescents and their G1 parents were interviewed annually from waves 1–3 and then every five years from waves 3–6. Children of G2s (G3s) were included at wave 5 and participated again at wave 6. Additional assessments of just the G3s were conducted about 18 months after wave 6, about four years after wave 6 (cognitive and personality assessment only), and about five years after wave 6 (substance use assessment only). The G3 wave 6 assessment and three post-wave-6 assessments were used in the current study and will be referred to as T1 (measure of family disorganization, Mage=12.09), T2 (measure of alcohol use covariate, Mage=13.45), T3 (measure of delay discounting, Mage=15.09), and T4 (measure of alcohol use outcome, Mage=16.18).
Participants were included if they were between the ages of 11–16 at T1, 12–18 at T3, and 13–19 at T4, if they provided genetic data, and if they were of non-Hispanic Caucasian race/ethnicity (given concerns about ethnicity-related heterogeneity in genetically-informed analyses; N = 120). These age inclusion criteria were chosen to allow us to test all hypotheses during the developmental period of adolescence while retaining as many participants as possible. Comparisons between included and excluded participants are shown in Table 1. There were no differences between included and excluded participants in delay discounting, gender, or parent substance use disorder diagnoses. However, compared to excluded participants, included participants were younger (by design), had higher IQ, greater levels of non-Hispanic Caucasian ancestry (by design, see measures section), greater levels of parental education, greater polygenic risk scores, greater family disorganization, and marginally less T4 alcohol use. Note that age and ancestry were selection criteria and, therefore, could be confounding variables that account for the differences between included and excluded participants. To test this possibility, we examined whether those four variables still differed across groups after controlling for age and ancestry. Results from MANCOVA revealed that included and excluded participants no longer differed with regards to their IQ (F=1.37, ns), parental education (F=0.26, ns), polygenic risk score (F=0.36, ns), family disorganization (F=0.23, ns), or T4 alcohol use (F=2.59, ns) after controlling for age and ancestry. Note that including only non-Hispanic Caucasian participants did not result in a restriction of range of study variables according to Levene’s Test for Equality of Variances.
Table 1.
Descriptive Statistics, t-tests, and χ2 Analyses Comparing Including vs. Excluded Participants
| Continuous Variables | N | Included M(SD) |
N | Excluded M(SD) |
t-test |
|---|---|---|---|---|---|
| T1 Age | 120 | 12.13(1.25) | 489 | 12.77(2.00) | 3.32** |
| Intelligence Quotient | 120 | 106.88(11.18) | 291 | 102.92(11.48) | −3.20** |
| Ancestry | 120 | 0.52(0.34) | 419 | −0.17(0.97) | −12.08** |
| Parents’ Education | 120 | 7.71(2.01) | 654 | 6.71(2.46) | −4.21** |
| Polygenic Risk for High Delay Discounting |
120 | 10.33(1.81) | 450 | 9.89(1.85) | −2.29* |
| T3 Delay Discounting | 120 | −4.85(1.44) | 422 | −4.84(1.43) | 0.04 |
| T1 Family Disorganization | 120 | 2.57(0.49) | 489 | 2.45(0.64) | −2.24* |
| Dichotomous Variables | % | % | χ2 | ||
| Gender | 120 | 45% Girls | 489 | 47.4% Girls | 0.23 |
| Parental SUD | 120 | 53.3% Parental SUD | 641 | 53.2 % Parental SUD | 0.001 |
| T2 Alcohol Use | 120 | 95% Never 1.7% 1–2 Times 0.8% 3–5 Times 1.7% More than 5 times, but less than once a month 0.8% 1–3 times a month 0% 1–2 times a week 0% 3–5 times a week 0% Every day |
458 | 88.9% Never 4.4% 1–2 Times 2.4% 3–5 Times 2.0% More than 5 times, but less than once a month 2.0% 1–3 times a month 0.4% 1–2 times a week 0% 3–5 times a week 0% Every day |
4.62 |
| T4 Alcohol Use | 111 | 87.4% Never 1.8% 1–2 Times 3.6% 3–5 Times 2.7% More than 5 times, but less than once a month 3.6% 1–3 times a month 0% 1–2 times a week 0.9% 3–5 times a week 0% Every day |
389 | 73.3% Never 6.7% 1–2 Times 5.4% 3–5 Times 2.8% More than 5 times, but less than once a month 7.2% 1–3 times a month 2.8% 1–2 times a week 1.8% 3–5 times a week 0% Every day |
11.81† |
Note.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.001.
N = 120. Polygenic risk, delay discounting, intelligence quotient, family disorganization, alcohol use, and ancestry are coded such that higher levels indicate greater levels of genetic risk for delay discounting, higher delay discounting, greater intelligence, greater family disorganization, greater alcohol use, and greater non-Hispanic Caucasian ancestry, respectively. The ancestry variable consists of factor scores saved from latent factors; latent factors are scaled such that 0 is the mean.
Recruitment
G1 alcoholic families were recruited using court records of DUI arrests, HMO wellness questionnaires, and community telephone screenings. Reverse directories and telephone screening were used to locate families without an alcoholic parent in the same neighborhoods as alcoholic families. G2 children of alcoholics (COAs) and non-COAs were matched in ethnicity, family structure, adolescent age, and socioeconomic status. See Chassin et al. 1992 for further details on recruitment.
Procedure
At T1 for the current analyses, G2 parents and G3 adolescents were interviewed at the family’s residence or at Arizona State University. At T2, adolescents were interviewed over the phone. At T3, adolescents participated in an online data collection. At T4, adolescents were interviewed about their substance use over the phone. G2 parents provided informed consent and G3 adolescents provided assent at all waves. Genetic data were collected with cheek brushing or saliva samples using Oragene collection kits. Genotyping was done through the Washington University Genome Sequencing Center. Details concerning genotyping and quality control are in Chassin et al. (2012).
Measures
Descriptive data for all measures are in Table 1.
Family disorganization
At T1, adolescents self-reported their family’s disorganization using seven items from the Family Process Scale (Bloom, 1985). Items assessed the extent to which families did not adhere to a regular schedule, could not count on promises made by family members, and had a hard time making plans because of unexpected events (1=strongly disagree, 5=strongly agree). Items were averaged to form a family disorganization composite (α=0.70). When considering the possible range of values on family disorganization, families in the study had low-moderate levels of family disorganization. Higher levels of the variable indicate greater levels of family disorganization.
Delay discounting
At T3, adolescents completed the Monetary Choice Questionnaire (MCQ; Kirby and Marakavic, 1995). Participants chose between an immediate reward and larger future reward (e.g., “Would you rather have $49 today or $60 in 89 days?”). Analyses used Kirby and Finch’s (2010) k (hyperbolic discount) parameter, where higher values reflect a greater tendency to choose immediate over larger future rewards. When considering the possible range of values for delay discounting, adolescents in the study generally reported moderate-high levels of delay discounting. Due to non-normality (skewness=4.79; kurtosis=26.94), this variable was log transformed. After the transformation, the variable was within the acceptable range of skewness (−0.55) and kurtosis (0.58).
Alcohol use
At T2 and T4, adolescents self-reported (0=never to 7=every day) how often they had consumed 3 or more drinks of any given beverage at one time within the past year. This alcohol measure was chosen because previous research shows that having three or more drinks in a row is an appropriate measure of binge drinking for adolescents (Donovan, 2009). Moreover, previous research suggested that delay discounting might be more closely related with substance misuse when compared with substance use alone (MacKillop et al., 2011). T2 alcohol use was used as a covariate in predicting T4 alcohol use to allow prospective prediction. T2 rather than T1 alcohol use was chosen as a covariate because it was the most proximal alcohol assessment to the T3 measure of delay discounting. Both alcohol use variables were substantially zero-inflated. At T2, 95% of adolescents had never consumed 3 or more drinks at once and at T4, 87.4% of adolescents had never consumed 3 or more drinks at once. See data analytic plan for a description of how this zero-inflation was handled. Also see Table 1 for the frequencies of each category of use for both alcohol use variables.
Polygenic risk score for delay discounting
We created a dopaminergic polygenic risk score based on results from an independent study by Gray and MacKillop (2014). Their study examined the associations between delay discounting as measured by the MCQ and a panel of 236 dopaminergic SNPs in a sample of weekly gamblers of European ancestry. Of the 11 SNPs that they found to be significantly associated with delay discounting, 8 SNPs were genotyped in our study. One of these SNPs was in high linkage disequilibrium (r2 > 0.80) with another SNP and was pruned. The remaining 7 SNPs (see Table 2) were coded additively (i.e., 0, 1, and 2; see Gray & MacKillop, 2014 for risk allele directions), and summed to form a dopaminergic polygenic risk score indexing genetic risk for delay discounting. Possible values of the continuous score ranged from 0–14, and therefore, adolescents in the current sample generally had moderate-high levels of polygenic risk (see Table 1). Higher polygenic risk scores indicate greater levels of genetic risk for delay discounting. Although not a main aspect of the current analyses, we also included standardized regression coefficients and p-values of the effect of each individual SNP on delay discounting in Table 2 for illustrative purposes.
Table 2.
Single Nucleotide Polymorphisms in the Dopaminergic Polygenic Risk Score for Delay Discounting and Regression Coefficients when Predicting Delay Discounting
| Single Nucleotide Polymorphism | Gene | β | p |
|---|---|---|---|
| rs3773678 | DRD3 | 0.07 | 0.44 |
| rs464049 | SLC6A3 | 0.05 | 0.57 |
| rs3756450 | SLC6A3 | 0.13 | 0.16 |
| rs10249982 | DDC | 0.20 | 0.03 |
| rs10499696 | DDC | 0.14 | 0.14 |
| rs2519154 | DBH | 0.21 | 0.02 |
| rs363338 | SLC18A2 | −0.07 | 0.44 |
Note. Standardized regression coefficients and p-values representing the effect of each SNP on delay discounting (i.e., 7 regressions with 1 SNP predictor each) in the full sample (N = 120) are also presented here for illustrative purposes, although not a main goal of the present study.
Parent substance use disorder (SUD)
At T1, adolescents’ G2 biological parents reported their lifetime alcohol/drug abuse or dependence by DSM-IV criteria using the Computerized Diagnostic Interview Schedule (C-DIS; Robins et al., 2000). Spousal reports with Family History Research Diagnostic Criteria (Endicott et al., 1975) were used for non-interviewed parents. We classified adolescents as having a parent with an SUD if one or both biological parents had a lifetime alcohol or drug disorder. This dichotomous variable was used as a covariate. As expected for a study that oversampled G1 parent alcoholism, there were relatively high rates of lifetime substance use disorders among the G2 parents (53.3%).
Other covariates
Adolescents self-reported their age and gender (0=Female, 1=Male). Adolescents’ IQ was measured using the Kaufman Brief Intelligence Test (Kaufman & Kaufman, 1990). In our sample, the average IQ score (106.88) is within the average range according to standardized norms. The highest level of education earned by either of the adolescents’ parents was utilized to index their socioeconomic status because previous research suggests that this variable may be the most stable and sensitive indicator of socioeconomic status for adolescents (Williams & Collins, 1995).
Adolescents’ ancestry was included as a covariate to control for population admixture. Thirty-seven SNPs in our dataset are ancestry informative markers that distinguish between non-Hispanic Caucasian and Mexican/Mexican/American ancestry, which are the two most represented ethnic groups in the current sample and geographical location of the study (Tian et al., 2008). Using these SNPs, we conducted a principal components analysis which showed that the first component explained 18.99% of the variance (eigenvalue = 7.03), the second explained 3.36% (eigenvalue = 1.24), and the third explained 3.11% (eigenvalue = 1.02). We used the 32 SNPs with loadings > 0.30 on the first principal component as indicators of a one-factor model using maximum likelihood estimation. The model fit the data well: χ2(464) = 824.99, p < 0.001, RMSEA = 0.03, CFI = 0.94, SRMR = 0.03. Factor scores were saved and used in all subsequent analyses. This ancestry variable was highly correlated with adolescents’ self-reported ethnicity in the larger sample (r = 0.86, p < 0.001), confirming its validity. Higher factor scores indicate higher levels of non-Hispanic Caucasian versus Mexican/Mexican-American ancestry.
Data Analytic Plan
We used Mplus Version 7.11 (Muthén and Muthén, 1998–2013) with full information maximum likelihood estimation to include participants with incomplete data on endogenous variables. Models used a robust sandwich estimator (TYPE=COMPLEX), to account for non-normality and to obtain standard errors and chi-square statistics adjusted for the clustering of siblings within families.
Due to zero-inflation of the T4 alcohol use outcome variable, we compared preliminary models with different distributional assumptions including zero-inflated poisson, zero-inflated negative binomial, negative binomial hurdle, continuous, and ordered-categorical variable modeling. Comparing relative fit indices (AIC, BIC, and -2 log likelihood) supported treating alcohol use as an ordered-categorical variable (using Monte Carlo integration). Therefore, absolute fit indices for the final model were not available.
We first estimated a path model to determine main effects on delay discounting. In this model, we estimated paths from polygenic risk and family disorganization to delay discounting. We also estimated paths from polygenic risk, family disorganization, delay discounting, and baseline alcohol use to T4 alcohol use. Finally, we estimated paths from all covariates to delay discounting and to T4 alcohol use.
Because gene-environment correlations can confound tests of gene-by-environment interaction, we examined the zero-order correlation between polygenic risk and family disorganization prior to testing the hypothesized interaction, similar to a previous study (see Results; Nikolas et al., 2010). To test the interaction between polygenic risk and family disorganization in predicting delay discounting, we estimated a path from the cross-product of polygenic risk and family disorganization to delay discounting. In addition, we estimated paths from all polygenic risk-by-covariate cross-products and all family-disorganization-by-covariate cross-products to delay discounting to control for potential confounders of this interaction (as recommended by Keller, 2014).
To test whether the gene-environment interaction was spuriously influenced by scaling, we re-tested the interaction following monotone transformations of the two interaction and outcome variables (Young-Wolff et al., 2011).
To probe moderation by polygenic risk, we calculated simple slopes at one SD below the mean, at the mean, and at one SD above the mean (referred to as low, average, and high levels of polygenic risk for brevity; Cohen et al., 2003).
Finally, we used the joint significance test to test the mediated effect of family disorganization on adolescent alcohol use through adolescent delay discounting. This choice was based on previous research suggesting that the joint significance test is the best approach for balancing Type 1 error and statistical power (MacKinnon et al., 2002).
Results
Zero-Order Correlations
Zero-order correlations are presented in Table 3. In support of the validity of the polygenic risk score, polygenic risk for higher delay discounting was significantly correlated with higher delay discounting. However, polygenic risk was not correlated with family disorganization, suggesting that this gene-environment correlation would be unlikely to bias our test of gene-environment interaction (Nikolas et al., 2010). Family disorganization was not correlated with alcohol use or delay discounting. However, as expected, T4 alcohol use was higher among older adolescents, those with higher earlier levels of drinking, and those with higher levels of delay discounting (p = 0.08). The magnitude of the correlations among predictors suggested no evidence of multicollinearity.
Table 3.
Zero-Order Correlations Among Study Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T4 Alcohol Useb | 1 | ||||||||||
| 2. T3 Delay Discounting | 0.24† | 1 | |||||||||
| 3. T1 Family Disorganization | −0.04 | 0.05 | 1 | ||||||||
| 4. Polygenic Risk Scorea | 0.04 | 0.19* | −0.09 | 1 | |||||||
| 5. T2 Alcohol Useb | 0.41** | −0.02 | 0.06 | 0.03 | 1 | ||||||
| 6. Gender | 0.06 | 0.06 | −0.16* | 0.06 | 0.01 | 1 | |||||
| 7. Ancestry | −0.37** | −0.01 | 0.09 | −0.06 | −0.13* | −0.02 | 1 | ||||
| 8. Age | 0.56** | −0.09 | −0.03 | −0.02 | 0.30** | −0.02 | −0.21** | 1 | |||
| 9. Parental SUD | 0.21 | −0.16ⱡ | 0.07 | −0.02 | 0.02 | −0.12* | −0.04 | 0.03 | 1 | ||
| 10. Intelligence Quotient | −0.12 | −0.05 | −0.06 | 0.01 | −0.03 | −0.15* | 0.09 | −0.27** | −0.08 | 1 | |
| 11. Parental Education | −0.20 | −0.13† | −0.08 | −0.10† | −0.12* | 0.04 | 0.20** | −0.07 | −0.16* | 0.14* | 1 |
Note.
p < 0.10.
p < 0.05.
p < 0.01.
Polygenic Risk Score Indexing Delay Discounting.
Frequency of drinking 3 or more drinks at once in the past year. Gender: 0=Female, 1=Male. All variables coded such that higher levels of the variable indicate higher levels of that construct.
Structural Equation Model
Main effects on delay discounting and alcohol use were tested prior to adding the gene-by-environment interaction in predicting delay discounting. Standardized main effects on delay discounting are presented below in parentheses. Table 4 (the final model) presents standardized path coefficients in predicting delay discounting, which includes the gene-by-environment, gene-by-covariate and environment-by-covariate interaction terms. Table 4 also presents the standardized main effect coefficients in predicting alcohol use (because there were no hypothesized interactions for alcohol use). See Figure 1 for a depiction of this final model. Interaction terms were probed by testing the simple slopes at 1 SD below the mean, at the mean, and 1 SD above the mean of the continuous polygenic risk score measure.
Table 4.
Standardized Path Coefficients from the Final Model
| Mediator | Outcome | |
|---|---|---|
| Delay Discounting (T3) | Alcohol Use (T4) | |
| Predictors | β(SE) | β(SE) |
| IQ | −0.13(0.08) | 0.11(0.12) |
| Ancestry | −0.01(0.08) | −0.22(0.08)* |
| Age | −0.13(0.10) | 0.49(0.09)** |
| Gender | 0.03(0.11) | 0.07(0.17) |
| Parents’ Substance Use Disorder (SUD) |
−0.18(0.09)† | 0.27(0.17) |
| Parents’ Education | −0.12(0.08) | −0.02(0.14) |
| Family Disorganization (T1) | 0.03(0.09) | −0.06(0.16) |
| Polygenic Risk Score (PRS) | 0.17(0.09)† | −0.06(0.20) |
| PRS-by-Family Disorganization | −0.17(0.09)* | -- |
| PRS-by-Parents’ SUD | −0.03(0.09) | -- |
| PRS-by-Age | 0.02(0.10) | -- |
| PRS-by-Gender | −0.02(0.11) | -- |
| PRS-by-Ancestry | 0.03(0.09) | -- |
| PRS-by-IQ | −0.06(0.07) | -- |
| PRS-by-Parents’ Education | −0.09(0.08) | |
| Family Disorganization-by-Parent SUD |
0.06(0.07) | -- |
| Family Disorganization-by-Age | 0.09(0.11) | -- |
| Family Disorganization-by-Gender | −0.05(0.09) | -- |
| Family Disorganization-by-Ancestry | 0.06(0.10) | -- |
| Family Disorganization-by-IQ | 0.03(0.07) | -- |
| Family Disorganization-by-Parents’ Education |
−0.06(0.09) | |
| Alcohol Use (T2) | -- | 0.24(0.07)** |
| Delay Discounting (T3) | -- | 0.37(0.17)* |
Note.
p < 0.10.
p < 0.05.
p < 0.001.
N = 120. Gender: 0=Female, 1=Male
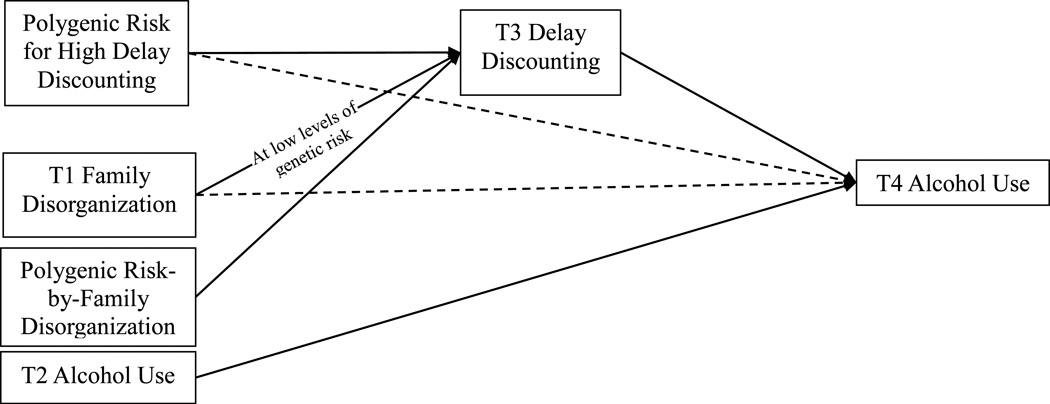
Figure 1.
Final Path Model. Text overlaid on arrow indicates from whom the path from family disorganization to delay discounting is significant (i.e., moderation). The same is not done for the path from polygenic risk to delay discounting because polygenic risk is considered the moderator. Only the alcohol use covariate is shown for ease of presentation. Solid lines indicate that the path is significant, dashed lines indicate that the path is non-significant. Alcohol use is defined as the frequency of drinking 3 or more drinks at once in the past year.
Prediction of delay discounting
The main effect model showed that higher levels of polygenic risk for delay discounting (β=0.18, p=0.04) predicted higher delay discounting (i.e., greater choice impulsivity). However, neither family disorganization (β=0.07, p=0.40) nor parent SUD (β=−0.18, p=0.06) significantly predicted delay discounting. No other covariates significantly predicted delay discounting.
After adding the interactions, results showed that polygenic risk interacted with family disorganization to predict delay discounting (even after controlling for covariates and testing for scaling related spuriousness; see Table 4).1 The pattern of the interaction was such that higher levels of family disorganization marginally significantly predicted higher levels of delay discounting at low levels of polygenic risk (β=0.21, p=0.09), but not at average (β=0.03, p=0.72) or high levels of polygenic risk (β=−0.15, p =0.28).
To further understand this interaction, regions of significance analyses were conducted to elucidate at what level(s) of polygenic risk the effect of family disorganization significantly predicted delay discounting. Tests were performed using the web utility developed by Preacher, Curran & Bauer (2006). Analyses revealed that higher levels of family disorganization significantly predicted higher levels of delay discounting only when genetic risk was less than or equal to 6.37, which is a little less than 2 SDs below the mean (β=0.38, p =0.05). Note that this value is within the actual range of scores of polygenic risk. In other words, the effect of family disorganization on delay discounting was significant at the lowest levels of genetic risk. See Figure 2 for a graphical display of the interaction.
Figure 2.
Interaction between family disorganization and polygenic risk for delay discounting in predicting delay discounting. Symbols represent predicted values on delay discounting given different observed values of genetic risk and family disorganization, and data points are only plotted for actual combinations of polygenic risk and family disorganization found in the data. Family disorganization is centered. Higher values represent greater family disorganization and greater delay discounting. High, average, and low levels of polygenic risk represent unstandardized simple slopes calculated at 1 standard deviation below the mean, at the mean, and 1 standard deviation above the mean. Unstandardized betas are shown because actual data points follow the scale of the raw data. The only significant simple slope is that at low levels (2 SDs below the mean) of polygenic risk.
Prediction of T4 alcohol use
Older age, higher levels of delay discounting, higher levels of Mexican/Mexican-American ancestry, and higher levels of T2 alcohol use significantly predicted higher levels of alcohol use at T4. No other covariates significantly predicted alcohol use. Polygenic risk, and family disorganization did not directly predict alcohol use at T4. See Table 4.
Mediated effects
Delay discounting mediated the relation between family disorganization and alcohol use, but only for adolescents whose polygenic risk for delay discounting was approximately (but less than) 2 SDs below the mean (e.g., moderated mediation; alow genetic risk=0.38, plow genetic risk=0.05; b=0.37, p=0.03). Because family disorganization did not predict delay discounting at any values higher than 2 SDs below the mean, this mediated effect was not significant at mean or high levels of polygenic risk for delay discounting.
Discussion
The present study examined the effects of family disorganization and genetic risk in predicting adolescents’ delay discounting and alcohol use in a high-risk sample using a genetically informed design. Results and implications of findings are discussed below.
Effects of Genetic Risk for Delay Discounting on Delay Discounting
The first finding of note was that the dopaminergic polygenic risk score, which was derived from an independent sample, positively predicted delay discounting. This supports the validity of the score and replicates Gray and MacKillop’s (2014) finding that these dopaminergic polymorphisms may play a role in delay discounting. This finding also replicates work from both animal and human studies which used a diverse range of methodologies to implicate dopaminergic signaling in impulsive choice (e.g., Eisenberg et al., 2007; Loos et al., 2009; Oberlin et al., 2015). Given the widespread lack of replication in the candidate gene literature (Ioannidis et al., 2001) this independent replication in a younger sample is noteworthy.
Gene-by-Environment Interaction
A gene-environment interaction was found, such that greater levels of family disorganization at T1 predicted greater levels of delay discounting at T3, but only for adolescents with low levels of polygenic risk for delay discounting (i.e., significant for adolescents whose polygenic risk was equal to or less than approximately 2 SDs below the mean). Importantly, this interaction was not an artifact of scaling and was unlikely to be biased by gene-environment correlation, given the lack of relation between polygenic risk and family disorganization. This increases confidence in the finding.
However, the nature of this interaction was not consistent with our prediction of a diathesis-stress interaction, where the negative influence of family disorganization would be exacerbated by high levels of genetic risk. Instead, the interaction was consistent with a “vulnerable-stable” model, where at-risk individuals are no longer responsive to environmental protections or risks and demonstrate maladaptive outcomes regardless of environmental influences (Luthar et al., 2000). Indeed, in our model, adolescents with high and mean levels of genetic risk evidenced high levels of choice impulsivity regardless of levels of familial disorganization.
In contrast, adolescents with low levels of genetic risk were influenced by familial disorganization in the expected manner. They showed greater delay discounting when their familial context was disorganized, but the least delay discounting when their familial context was organized. This pattern of findings suggests multiple pathways to adolescent delay discounting. For some adolescents, it might be genetic risk but not family environment that produces delay discounting. However, among adolescents at low genetic risk, family environments characterized by unpredictable and undependable reward schedules may teach them to value immediate (and more certain) reinforcements over larger delayed rewards. This effect of unpredictable reward contexts on delay discounting has been found in other studies (Schneider et al., 2014 Kidd, et al., 2013; Acheson et al., 2011).
Other studies have also reported interactions of this form. Both King and Chassin (2004) and Cleveland et al. (2010) found that adolescents with high levels of behavioral undercontrol had a greater probability of drug disorder or greater substance involvement regardless of the familial or neighborhood context, whereas adolescents with low levels of behavioral undercontrol were influenced by their familial and neighborhood contexts on these substance-use-related outcomes. Similarly, Wootton et al., (1997) found that parenting only protected against children’s conduct problems when children had low levels of callous-unemotional traits. Interestingly, two of these studies (King & Chassin, 2004; Wootton et al., 1997) as well as the current study were conducted with high-risk or clinical samples. Perhaps vulnerable-stable interaction patterns are more likely to be detected in such samples, where higher levels of risk are represented.
Effects of Delay Discounting on Alcohol Use
We also found that higher delay discounting prospectively predicted higher levels of adolescent drinking, over and above baseline drinking. This finding replicates previous studies that link greater delay discounting to a variety of adolescent alcohol outcomes (Fernie et al., 2013; Field et al., 2007). Adolescents who choose smaller but more immediate rewards may be attracted to the positive effects of alcohol (including stimulating effects, relaxation effects, and social benefits) regardless of any potential long-term consequences of underage drinking.
The current findings and the wider literature linking delay discounting with adolescent substance use reinforce the notion that choice impulsivity should be targeted in interventions for at-risk youth. Interestingly, a small but growing number of interventions focused on self-control and self-regulation training have demonstrated significant reductions in externalizing symptoms and problem behaviors (Riggs et al., 2006). The findings of the present study further corroborate the importance of choice impulsivity as an intervention target among adolescents.
Indirect Effect of Family Disorganization on Alcohol Use
Moderated mediation analyses demonstrated that adolescents’ delay discounting mediated the relation between family disorganization and alcohol use, but only for adolescents with low levels of genetic risk for delay discounting. Previous studies have shown that chaotic family environments predicted adolescent alcohol consumption (Stoolmiller et al., 2012), but few have tested the mechanisms that mediate this relation. Our results suggest that family disorganization may increase the risk for later alcohol consumption by instilling a pattern of choice impulsivity favoring smaller immediate rewards over larger delayed rewards, but only for those at low genetic risk. In contrast, this mediated effect was not found for adolescents at high or mean levels of genetic risk for delay discounting. These adolescents had high choice impulsivity regardless of family disorganization, which subsequently increased their risky alcohol consumption.
Results suggest that improving organization in adolescents’ households might protect against future risky alcohol consumption by decreasing their choice impulsivity, but only for a subset of adolescents with low levels of genetic risk. Importantly, our study and previous work showed that children with high levels of behavioral undercontrol may not be protected by positive environmental influences (i.e., Cleveland et al., 2010; King & Chassin, 2004). Thus, an important topic of future research is to elucidate the factors that will effectively protect adolescents with high levels of genetic risk from manifesting delay discounting and subsequent alcohol consumption.
Lack of Parent SUD Prediction of Delay Discounting
Given the relation between genetic risk and delay discounting, it was somewhat surprising that parental SUD did not significantly predict adolescent delay discounting. Previous tests of such effects have been somewhat inconsistent, with more support from studies in adult samples (e.g., Acheson et al., 2011; Petry et al., 2002). One study that examined adolescents found only a marginally significant effect of family history on delay discounting (Herting et al., 2010). Similarly, studies that examined the effects of family history of SUD on adolescents’ neural responses to the monetary incentive delay task, a measure of reward processing, failed to find statistically significant effects (e.g., Bjork et al., 2008). Researchers speculated that the effect of parent SUDs on delay discounting and similar reward processing paradigms might not emerge until adulthood because these reward circuits are still in development during adolescence (Ernst et al. 2005). Alternatively, given that we found interactive influences between genetic risk and the environment in predicting delay discounting, the effect of parent SUD might only emerge for certain subgroups of adolescents as well.
Strengths, Limitations, and Summary
The current study had methodological strengths allowing for rigorous tests of hypotheses. The use of a high-risk sample likely strengthened our ability to detect effects because of greater variability in study constructs than are often found in normative samples. Moreover, rather than testing gene-environment interactions using an individual SNP, we used a polygenic risk score and we derived that score from an independent sample. We were also able to use longitudinal data and to consider an earlier measure of alcohol use in the model, so predictions of the alcohol use outcome were prospective. However, the study also had limitations. First, the small sample size might have reduced power, particularly for to detecting interactions. Second, the young ages of participants and somewhat low alcohol use rates are a limitation in testing predictors of alcohol use outcomes. However, note that alcohol misuse in adolescence is typically of low prevalence, yet still an extremely important phenomenon to study despite the low rates. Moreover, we specifically chose an alcohol misuse measure because research shows that it may have stronger links with delay discounting than alcohol use alone (e.g., MacKillop et al., 2011). Third, far more genetic polymorphisms than those used in this study likely influence delay discounting, and those should be included in future polygenic risk scores for delay discounting. Fourth, we limited our sample to non-Hispanic Caucasian participants given concerns about ethnicity-related heterogeneity in genetically-informed analyses, so findings may not generalize to individuals of other races and ethnicities. It would be informative for future research test these questions while utilizing more genetic polymorphisms selected based on functionality or genome-wide association studies, in different ethnic populations, using clinical samples, and/or using larger sample sizes.
In sum, the current study revealed that family disorganization is a risk factor for adolescents’ delay discounting and subsequent alcohol use. However, the extent of risk depends on levels of genetic risk for delay discounting. For adolescents with low levels of genetic risk, having a disorganized family life increases risk for delay discounting and for alcohol consumption. Conversely, adolescents at mean and high levels of genetic risk for delay discounting were more at-risk for delay discounting regardless of family disorganization, and this delay discounting also placed them at risk for alcohol consumption. Results reinforce the importance of interventions aimed at reducing delay discounting and suggest that interventions aimed at modifying family dynamics could also reduce delay discounting for adolescents at low genetic risk. More work is needed to uncover the environmental factors that may protect genetically-high-risk youth from developing risky patterns of delay discounting and heavy drinking.
Acknowledgments
This work was supported by Grants AA016213 and AA022097 to Laurie Chassin and AA023128 to Frances Wang from the National Institute of Alcohol Abuse and Alcoholism. Genotyping was supported by the Midwest Alcohol Research Center (P50 AA011998).
Footnotes
All the authors declare they have no conflict of interest.
To reduce concerns that the model may be underpowered because of the large number of predictors of delay discounting, we ran another model that omitted some of the paths that were not statistically significant. We ran the model without the non-significant gene-by-covariate and environment-by-covariate interaction terms in predicting delay discounting, reducing the number of predictors from 17 to 9. After removal, the results remained the same. Because including these interaction terms in the model constitutes a more conservative test of the gene-by-environment interaction, and because removing them did not make a difference, we retained all of the predictors for the final model.
References
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bloom BL. A factor analysis of self-report measures of family functioning. Fam Process. 1985;24:225–239. doi: 10.1111/j.1545-5300.1985.00225.x. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Barrera M, Bech K, Kossak-Fuller J. Recruiting a community sample of adolescent children of alcoholics: a comparison of three subject sources. J Stud Alcohol Drugs. 1992;53:316–319. doi: 10.15288/jsa.1992.53.316. [DOI] [PubMed] [Google Scholar]
- Chassin L, Lee MR, Cho YI, Wang FL, Agrawal A, Sher KJ, Lynskey MT. Testing multiple levels of influence in the intergenerational transmission of alcohol disorders from a developmental perspective: the example of alcohol use promoting peers and μ-opioid receptor M1 variation. Dev Psychopathol. 2012;24:953–967. doi: 10.1017/S0954579412000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Pillow DR, Curran PJ, Molina BSG, Barrera M. Relation of parental alcoholism to early adolescent substance use: a test of three mediating mechanisms. J Abnorm Psychol. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. [DOI] [PubMed] [Google Scholar]
- Cleveland MJ, Collins LM, Lanza ST, Greenberg MT, Feinberg ME. Does individual risk moderate the effect of contextual-level protective factors? A latent class analysis of substance use. J Prev Interv Community. 2010;38:213–228. doi: 10.1080/10852352.2010.486299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/ Correlation Analysis for the Behavioral Sciences. 3rd. Mahwah, New Jersey: 2003. [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Donovan JE. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics. 2009;123:e975–e981. doi: 10.1542/peds.2008-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:1–14. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Anderson N, Spitzer RL. Family History Diagnostic Criteria. New York, NY: 1975. [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108:1916–1923. doi: 10.1111/add.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Genetic basis of delay discounting in frequent gamblers: examination of a priori candidates and exploration of a panel of dopamine-related loci. Brain and behavior. 2014;4:812–821. doi: 10.1002/brb3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Impulsive delayed reward discounting as a genetically-influenced target for drug abuse prevention: a critical evaluation. Front Psychol. 2015;6:1104. doi: 10.3389/fpsyg.2015.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schütz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG. Choice impulsivity: definitions, measurement issues, and clinical implications. Personal Disord. 2015;6:182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EM, Jenkins J, Farmer L. Family unpredictability, future discounting, and risk taking. J Socio Econ. 2008;37:1381–1396. [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test Manual. Minnesota: American Guidance Service; 1990. [Google Scholar]
- Keller MC. Gene× environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction. 2013;108:506–515. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd C, Palmeri H, Aslin RN. Rational snacking: young children’s decision-making on the marshmallow task is moderated by beliefs about environmental reliability. Cognition. 2013;126:109–114. doi: 10.1016/j.cognition.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, McCullough ME, Bickel WK, Farley JP, Longo GS. Longitudinal associations among religiousness, delay discounting, and substance use initiation in early adolescence. J Res Adolescence. 2015;25:36–43. doi: 10.1111/jora.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Chassin L. Mediating and moderated effects of adolescent behavioral undercontrol and parenting in the prediction of drug use disorders in emerging adulthood. Psychol Addict Behav. 2004;18:239–249. doi: 10.1037/0893-164X.18.3.239. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Finch JC. The hierarchical structure of self-reported impulsivity. Pers Individ Dif. 2010;48:704–713. doi: 10.1016/j.paid.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN. Modeling myopic decisions: Evidence for hyperbolic delay-discounting within subjects and amounts. Organ Behav Hum Decis Process. 1995;64:22–30. [Google Scholar]
- Lahti J, Räikkönen K, Ekelund J, Peltonen L, Raitakari OT, Keltikangas-Järvinen L. Novelty seeking: interaction between parental alcohol use and dopamine D4 receptor gene exon III polymorphism over 17 years. Psychiatr Genet. 2005;15:133–139. doi: 10.1097/00041444-200506000-00010. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, Spijker S, van Gaalen MM. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex. 2009;20:1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2013;37:616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mplus [computer program]. Version 7.11. Los Angeles, CA: Muthén & Muthén;; 2013. [Google Scholar]
- Nikolas M, Friderici K, Waldman I, Jernigan K, Nigg JT. Research gene× environment interactions for ADHD: synergistic effect of 5HTTLPR genotype and youth appraisals of inter-parental conflict. Behav Brain Funct. 2010;6:1–15. doi: 10.1186/1744-9081-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Albrecht DS, Herring CM, Walters JW, Hile KL, Kareken DA, Yoder KK. Monetary discounting and ventral striatal dopamine receptor availability in nontreatment-seeking alcoholics and social drinkers. Psychopharmacology. 2015;232:2207–2216. doi: 10.1007/s00213-014-3850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patak M, Reynolds B. Question-based assessments of delay discounting: do respondents spontaneously incorporate uncertainty into their valuations for delayed rewards? Addict Behav. 2007;32:351–357. doi: 10.1016/j.addbeh.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol Drugs. 2002;63:83–90. [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Riggs NR, Greenberg MT, Kusché CA, Pentz MA. The mediational role of neurocognition in the behavioral outcomes of a social-emotional prevention program in elementary school students: effects of the PATHS curriculum. Prev Sci. 2006;7:91–102. doi: 10.1007/s11121-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) Missouri: Washington University School of Medicine; 2000. [Google Scholar]
- Ross LT, Hill EM. Childhood unpredictability, schemas for unpredictability, and risk taking. Soc Behav Personal. 2002;30:453–473. [Google Scholar]
- Schneider S, Peters J, Peth JM, Büchel C. Parental inconsistency, impulsive choice and neural value representations in healthy adolescents. Transl Psychiatry. 2014;4:e382. doi: 10.1038/tp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Dev. Psychopathol. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Children of alcoholics: A critical appraisal of theory and research. Chicago: University of Chicago Press; 1991. [Google Scholar]
- Stoolmiller M, Wills TA, McClure AC, Tanski SE, Worth KA, Gerrard M, Sargent JD. Comparing media and family predictors of alcohol use: a cohort study of US adolescents. BMJ Open. 2012;2:e000543. doi: 10.1136/bmjopen-2011-000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Halder I, Flory JD, Craig AE, Gianaros PJ, Ferrell RE, Manuck SB. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: evidence for differential susceptibility. Soc Cogn Affect Neurosci. 2013;8:499–508. doi: 10.1093/scan/nss020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Gen. 2008;17:R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, Lawford BR, Morris CP, Young RM. Interaction between DRD2 C957T polymorphism and an acute psychosocial stressor on reward-related behavioral impulsivity. Behav Genet. 2009;39:285–295. doi: 10.1007/s10519-008-9255-7. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu Rev Sociol. 1995;21:349–386. [Google Scholar]
- Wootton JM, Frick PJ, Shelton KK, Silverthorn P. Ineffective parenting and childhood conduct problems: the moderating role of callous-unemotional traits. J Consult Clin Psychol. 1997;65:301–308. doi: 10.1037/0022-006x.65.2.292.b. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, Prescott CA. The influence of gene–environment interactions on alcohol consumption and alcohol use disorders: a comprehensive review. Clin Psychol Rev. 2011;31:800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]