Abstract
Purpose
To determine the level of epithelial membrane protein-2 (EMP2) expression in preretinal membranes from surgical patients with proliferative vitreoretinopathy (PVR) or epiretinal membranes (ERMs). EMP2, an integrin regulator, is expressed in the retinal pigment epithelium and understanding EMP2 expression in human retinal disease may help determine whether EMP2 is a potential therapeutic target.
Methods
Preretinal membranes were collected during surgical vitrectomies after obtaining consents. The membranes were fixed, processed, sectioned, and protein expression of EMP2 was evaluated by immunohistochemistry. The staining intensity (SI) and percentage of positive cells (PP) in membranes were compared by masked observers. Membranes were categorized by their cause and type including inflammatory and traumatic.
Results
All of the membranes stained positive for EMP2. Proliferative vitreoretinopathy–induced membranes (all causes) showed greater expression of EMP2 than ERMs with higher SI (1.81 vs. 1.38; P = 0.07) and PP (2.08 vs. 1.54; P = 0.09). However all the PVR subgroups had similar levels of EMP2 expression without statistically significant differences by Kruskal-Wallis test. Inflammatory PVR had higher expression of EMP2 than ERMs (SI of 2.58 vs. 1.38); however, this was not statistically significant. No correlation was found between duration of PVR membrane and EMP2 expression. EMP2 was detected by RT-PCR in all samples (n = 6) tested.
Conclusions
All studied ERMs and PVR membranes express EMP2. Levels of EMP2 trended higher in all PVR subgroups than in ERMs, especially in inflammatory and traumatic PVR. Future studies are needed to determine the role of EMP2 in the pathogenesis and treatment of various retinal conditions including PVR.
Keywords: proliferative vitreoretinopathy, epithelial membrane protein-2, EMP2, retinal pigment epithelium, fibrosis, retinal detachment, therapy, epiretinal membrane
Proliferative vitreoretinopathy (PVR) is the most frequent cause of recurrent retinal detachment following surgical repair, which has been reported to occur in up to 10% of cases.1,2 Ocular trauma increases the risk of developing PVR, with rates of up to 43% following ocular perforation.3
Many risk factors for PVR have been identified including vitreous hemorrhage, severe trauma, longstanding retinal detachments, extensive retinal detachments, the use of intraocular tamponade such as gas during surgery, extensive use of cryotherapy or photocoagulation, and surgical failures.1,2 The extent of ocular/retinal damage most likely causes increased inflammation and cytokine production, inducing an increase in PVR. Many cytokines such as IL-1, IL-6, IL-8, TNF-α, IFN-γ, and monocyte chemoattractant protein-1 (MCP-1) are increased in eyes with PVR; however, the level of cytokine production has not been found to directly correlate with PVR severity.4
The pathogenesis of PVR is complex, and the retinal pigment epithelium (RPE) cells have been implicated as one of the several cell types that play a key role in disease pathogenesis.5 Following trauma or retinal detachment, RPE cells are released into the vitreous, or these cells can be stimulated to migrate to the vitreous from their subretinal location. These RPE cells can then proliferate, de-differentiate, and undergo an epithelial to mesenchymal transformation (EMT), to help create the preretinal membranes of PVR.4 The RPE cells also likely cause membrane contraction and tractional forces that can result in recurrent retinal detachments and vision loss.2,6
Prevention of membrane growth and contraction is a principal goal in inhibiting the PVR response. In an in vitro experimental PVR model, we have demonstrated that the activation of focal adhesion kinase (FAK) through ligation of integrins (α1, α2, and α3) is a crucial control point for collagen gel contraction.7 The tetraspan (4-TM) superfamily is a class of proteins that controls the types of intracellular trafficking and signaling molecules assembled with integrins and other receptor complexes.8 Our laboratory has found that epithelial membrane protein-2 (EMP2), a member of this 4-TM family, regulates specific integrin distribution and signaling though FAK.9 Epithelial membrane protein is highly expressed in RPE10 and is a member of the growth arrest–specific gene 3/peripheral myelin protein 22 (GAS3/PMP22) 4-TM protein family.10–16 Therefore, as EMP2 is a regulator of integrins, and highly expressed in the RPE (a common cell found in intraocular fibrosis), it was chosen as a target in rabbit model of PVR. In these studies, an antibody against EMP2 is found to inhibit the development of PVR.17
In this study, we verified the expression of EMP2 in human preretinal membranes from eyes with PVR secondary to retinal detachment and self-limited preretinal membranes from epiretinal membrane (ERM), and we determine whether the level of expression of this protein may correlate with disease etiology or severity.
Materials and Methods
Ocular Tissues and Control Specimens
This study was approved by the institutional review board of the University of California, Davis, for using human PVR membranes. All procedures conformed to the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all participants before vitrectomy surgery. Thirty-five fresh tissue specimens were collected during pars plana vitrectomy from patients scheduled for surgery as part of standard of care after obtaining appropriate full informed consent. Inclusion criteria included any patients undergoing vitrectomy surgery for the removal of intraocular preretinal membranes with an adequate amount of membrane for analysis. Exclusion criteria included patients without enough membranes for evaluation. Proliferative vitreoretinopathy patients were clinically diagnosed with intraocular membranes secondary to retinal detachment, trauma, diabetes, or inflammation clinically diagnosed as PVR causing retinal traction. Spontaneous ERMs were from patients without previous or active retinal detachment or history of PVR. The surgical specimens were immediately fixed in 4% formalin and transported to the laboratory for processing and staining for grading EMP2 expression. For positive and negative controls, donor tissues were also fixed in 4% formalin within 24 to 48 hours postmortem.
Membranes were categorized by cause and type of preretinal membrane: PVR, idiopathic ERM, PVR-trauma, PVR-inflammatory, and PVR-PDR (proliferative diabetic retinopathy). Of note, all PVR patients had a history of rhegmatogenous retinal detachment, including the patients with PDR. Duration of the membrane was recorded from the time of diagnosis to date of surgical membrane retrieval.
Tissue Preparation for Immunohistochemistry
The membranes were fixed and processed for paraffin embedding by using routine immunohistochemical processing. The membranes were sectioned at 5-μm thickness.
Immunohistochemistry
Sections were then stained for protein expression including expression of EMP2 by immunohistochemical analysis. For immunostaining, specimens were deparafinnized in xylene and hydrated in ethanol. Sections were then permeabilized in 5% normal goat serum (Sigma-Aldrich Corp., St. Louis, MO, USA) in tris buffered saline with tween 20. Rabbit anti-human EMP2 primary antibody was applied with a 1:800 dilution in blocking buffer. Sections were washed before the addition of a goat anti-rabbit secondary antibody (Vectastain ABC; Vector Laboratories, Burlingame, CA, USA) with a dilution of 1:200 in TBS. The protein of interest was detected with Vector VIP (Vector) and a methyl green (Vector) counterstain was applied.
Rating of Immunohistochemistry
Four masked observers (LKG, SM, LM, SP) rated the slide for its staining intensity (SI) and percentage positive cells (PP) on the basis of a previously described protocol.18 Briefly, for SI, the rating scale ranged from 0 with no staining to 4 with a maximum expression, which was equal staining to the positive control (Fig. 1). The PP was also rated for the extent of staining on the specimen from 0 (minimal positive cells) to 4 (equaling the positive control) for maximum percentage.
Figure 1.
Immunohistochemistry showing EMP2 staining at ×40 (violet) in two examples of PVR (A, B) with nuclear counterstaining (blue). Bottom row shows positive and negative controls for comparison.
RNA Isolation/RT-PCR
Quantitative real-time PCR was performed on the isolated RNA by using the Quantitect probe RT-PCR kit (QIAGEN, Valencia, CA, USA) on the DNA Engine Opticon Monitor 2 (MJ Research, Inc., South San Francisco, CA, USA). For EMP2 genomic RNA detection, the long terminal repeat (LTR) forward primer (5′-TCCTCTCCACCATTCTCT-3′), LTR reverse primer (5′-AAACCTCTCTCCCTGCTTCA-3′), and the fluorogenic probe (6-carboxyfluorescein-5′-TTCTTCATCTTCGTGCT-3′-tetramethyl carboxyrhodamine) were used for the RT-PCR. The quantity of EMP2 was calculated by interpolation from a standard curve generated by running in parallel serial dilutions of known quantities of full-length EMP2 cDNA incorporated into the EGFP-N3 vector. The levels of EMP2 mRNA were normalized against the housekeeping gene GAPDH by using the QIAGEN GAPDH Assay on Demand, which included both primers and probe. The GAPDH copy number was also calculated by interpolation from a standard curve generated from serial dilutions of a plasmid containing GAPDH cDNA (clone3869809; Open Biosystems, Huntsville, AL, USA).
Statistical Analysis
Kruskal-Wallis test was done to compare all groups. Masked observations were averaged and Wilcoxon rank sum test was performed on the data groups. Linear regression was performed on the scatterplots for age and duration versus PVR grading, and R values are reflected in the text and graph.
Results
Expression of EMP2 in Normal Human Retina
Normal retina shows clear EMP2 expression in the RPE layer of the retina in normal donor tissue (data not shown). This is consistent with previous reports.10 No significant expression was seen in the other retinal layers or the vitreous of normal eyes. The choroid also was shown to not express significant amounts of EMP2.
Expression of EMP2 in Human PVR Membranes
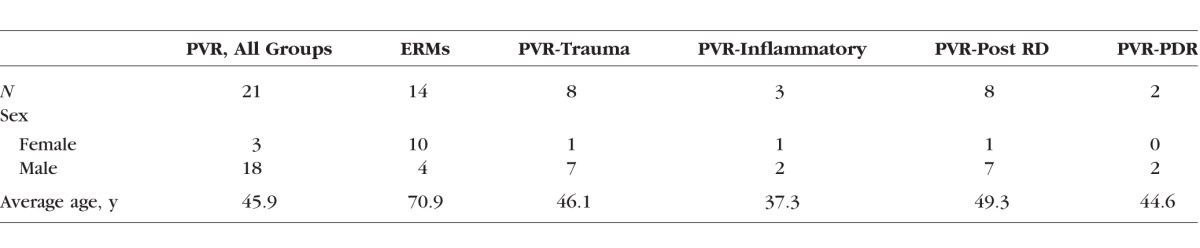
A total of 35 membranes were collected from patients undergoing vitrectomy surgery. Among them, 14 membrane samples were collected from patients undergoing vitrectomy surgery for ERM, while 21 membrane samples were obtained from patients undergoing surgery for PVR associated with retinal detachment, trauma, and inflammation (Table). Of note, this population had a higher representation of males than females (Table). Surgical membranes were collected including 14 ERMs, 12 idiopathic ERMs/macular puckers, and 2 ERMs associated with proliferative diabetic retinopathy.
Table.
Demographics of Patient Samples
EMP2 was highly expressed in preretinal membranes of patients with PVR (Fig. 1). EMP2 expression was noted to be variable in the many samples tested (Fig. 2). Therefore, the amount of EMP2 expression (staining) was evaluated. All of the membranes were graded by four masked observers for SI and PP as described in the Methods. All of the PVR membranes (21 of 21) were rated positive for SI and 81% (17 of 21) of PVR membranes were rated positive for EMP2 expression by PP rating. Proliferative vitreoretinopathy from all causes was rated with an average SI of 1.82 and PP of 2.08 (Figs. 3, 4).
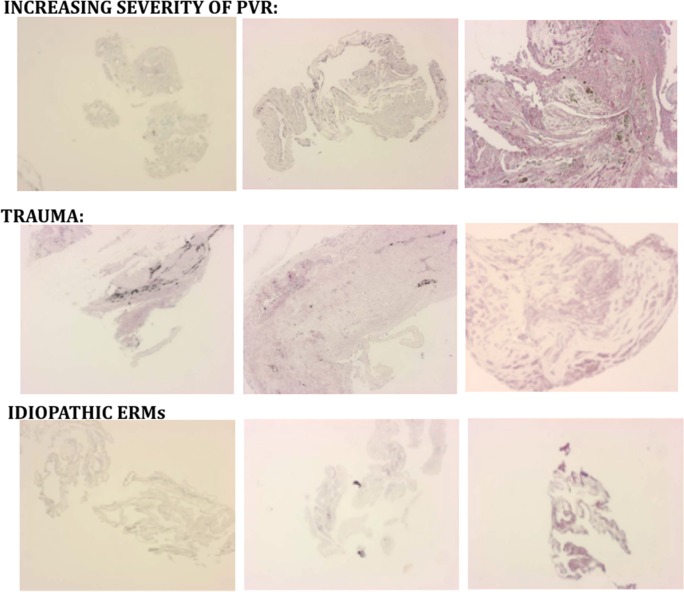
Figure 2.
Examples of EMP2 staining (violet) in PVR and ERM surgical specimens. Top row demonstrates increasing EMP2 staining with increased PVR membrane formation. Middle row shows increased EMP2 staining in traumatic PVR. Bottom row shows moderate EMP2 staining in spontaneous ERMs.
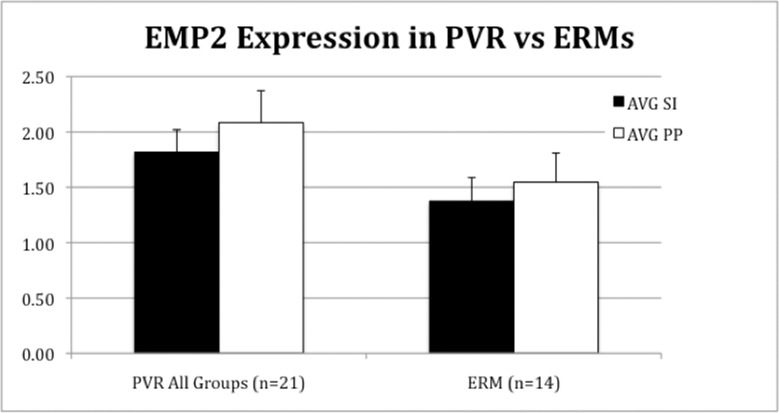
Figure 3.
Scoring of PVR and epiretinal membranes graded by four masked observers for EMP2 SI and PP as described in the Materials and Methods. EMP2 staining was found to be higher in PVR samples than in ERM patients.
Figure 4.
Scoring of PVR membranes for EMP2 SI and PP as described in the Materials and Methods. EMP2 was found to be higher in PVR associated with retinal detachments with inflammation (e.g., endophthalmitis, uveitis). The difference between groups was not found to be statistically significant by Kruskal-Wallis test.
Expression of EMP2 in Spontaneous ERMs
Spontaneous epiretinal membranes or macular puckers (ERMs) were also found to have high expression of EMP2. All of these membranes (14 of 14) were rated as positive for EMP2 by SI rating, and 93% (13 of 14) of ERMs were rated as positive by PP scale. Epiretinal membranes were rated with an average SI of 1.61 and an average PP of 1.74. Compared to all causes of PVR, ERMs had lower EMP2 SI (1.38 vs. 1.81; P = 0.07) and PP (1.54 vs. 2.08; P = 0.09) (Fig. 3). These P values were calculated by Wilcoxon rank sum test difference.
Comparative Quantification of EMP2 Expression in PVR Membranes
Proliferative vitreoretinopathy membranes were then compared by etiology of the PVR as follows: (1) PVR–all groups, from all patients with retinal detachment from any cause; (2) PVR-trauma, from patients with retinal detachment following penetrating ocular trauma; (3) PVR-inflammatory, from patients with retinal detachment following endophthalmitis or uveitis; (4) PVR–post RD, from patients with PVR from retinal detachment (RD) only; and (5) PVR-PDR, from patients with PDR and PVR. When comparing the membranes from these various causes of PVR membranes, inflammatory PVR was rated with the highest levels of EMP2 expression (Fig. 4). Traumatic PVR was also rated with high levels of EMP2 expression. Kruskal-Wallis test did not find any group to be significantly different from each other in terms of SI (P = 0.31) and PP (P = 0.39). However, inflammatory PVR had increased EMP2 expression as compared to idiopathic ERMs (SI: 2.58 vs. 1.38). Inflammatory PVR also had increased EMP2 expression as compared to PVR–post RD (SI: 2.58 vs. 1.52). Traumatic PVR versus PVR–post RD also had increased SI (1.78 vs. 1.52); however, these differences were not found to be statistically significant by Kruskal-Wallis test. Proliferative vitreoretinopathy associated with diabetic PDR was also found to have higher expression of EMP2 than ERMs and PVR–post RD; however, this group only had two samples, which limits statistical analysis.
Duration of PVR and EMP2 Expression and the Age of Patient
The EMP2 expression in these membranes was also evaluated by duration of the PVR membranes. Duration was calculated on membranes with a known start date (e.g., date of prior surgery, date of retinal detachment, date of trauma); 14 membranes of the total 24 PVR membranes had reliable dates of duration based on patient history. There was no significant trend for duration of PVR and EMP2 expression with an R2 coefficient of 0.008 (SI) and 0.013 (PP) (Fig. 5). Age of the patient was also compared to EMP2 expression and no significant association was found between the patient's age and EMP2 expression with an R2 coefficient of 0.021 (SI) and 0.0006 (PP).
Figure 5.
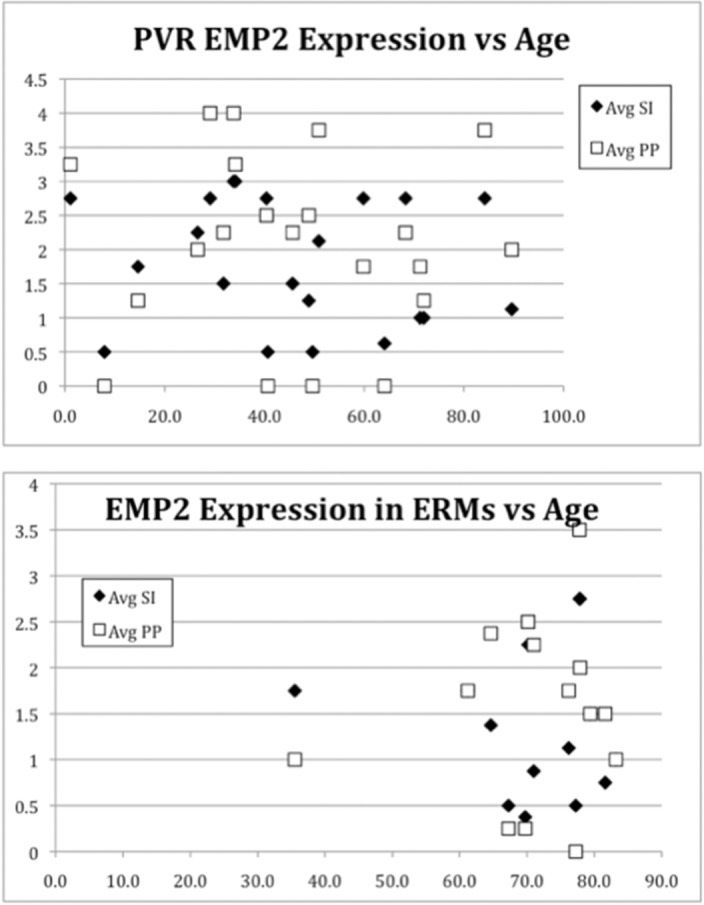
Increased EMP2 staining was not found to be associated with age or duration of PVR or ERM. Here EMP2 staining (SI and PP) is plotted versus the age of the patient with ERM or PVR. No significant trend for duration of PVR and EMP2 expression was found by regression analysis with an R2 coefficient of 0.008 (SI) and 0.013 (PP). Regression analysis of age and EMP2 expression also showed no significant correlation with an R2 coefficient of 0.021 (SI) and 0.0006 (PP).
Reverse Transcription–PCR
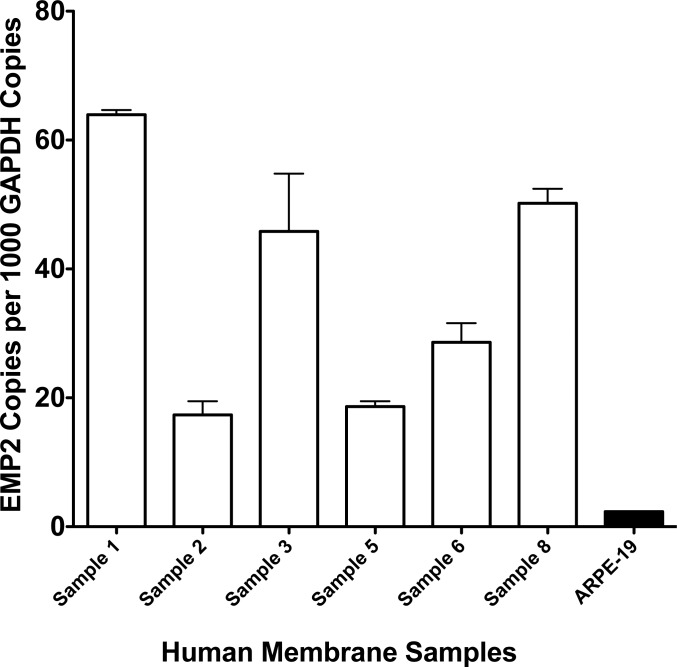
Proliferative vitreoretinopathy membrane samples from eight patients were divided, separating one for RNA samples and the other for fixation and histology. These samples, like the others reported, were found to be positive for EMP2 by immunohistochemical analysis. Quality RNA was obtained from six patient samples. All of these PVR samples were positive for EMP2 mRNA expression by RT-PCR testing (Fig. 6).
Figure 6.
Tissue samples from PVR surgery were used to isolate mRNA and test for EMP2 mRNA by RT-PCR. All samples tested were found to have significantly high copy number of mRNA for EMP2. The cell line ARPE-19, which is known to express EMP2, was used as a positive control.
Discussion
Integrins have been implicated in the pathogenesis of PVR formation, which has identified them as potential therapeutic targets in many studies.19–23 As integrins are known to mediate cell motility, it is not surprising that they may play a critical role in PVR. This study demonstrated that EMP2, a putative regulator of integrins, is significantly expressed in human PVR from all causes and with a noted trend of increased expression in inflammatory types of PVR.
In vitro studies have supported a role for EMP2 in membrane contraction.9 Animal models of PVR also have demonstrated the expression of EMP2 in preretinal PVR membranes.17 In addition, increased expression of EMP2 correlates with increased traction and PVR, and blocking anti-EMP2 antibodies decreases PVR formation.17,24
EMP2 was expressed significantly in most intraocular membranes tested, suggesting that RPE-derived cells are a significant or predominant cell type in PVR membranes and ERMs. Other cell types have been previously shown to also play a significant role in PVR membranes, including Müller cells, astrocytes, and microglial cells.25,26 As all membranes expressed EMP2, it appears that EMP2 may be expressed in more than just RPE-derived cells, and it is possible that other cell types that transition to a more fibroblast-like phenotype may then express EMP2. However, RPE-derived cells may play the main role in contractile forces and therefore may be a potential therapeutic target. Additional research is needed.
This study clearly established EMP2 expression in human PVR membranes and ERMs, by both immunohistochemistry and RT-PCR. We found a trend toward increased EMP2 expression when comparing all causes of PVR to ERMs; however, this did not prove to be statistically significant. We also found a trend of increased expression of EMP2 in inflammatory PVR, suggesting that inflammation upregulates EMP2 expression; however, the inflammatory PVR group was small with only three samples; two of these samples were RDs caused by endophthalmitis and one RD caused by uveitis (Vogt-Koyanagi-Harada syndrome). The two samples of PVR associated with PDR also had higher levels of EMP2 expression, which may represent increased inflammation associated with PDR especially with an RD. Traumatic PVR also showed increased levels of expression, which was not statistically significant. These data confirmed EMP2 expression in all intraocular membranes and suggetst a trend of expression with severe inflammation.
In this study, EMP2 expression was not affected by the duration of the PVR or the age of the patient. The obvious limitations of this current study were the small sample size and study design that included preretinal membranes from diverse etiology. A larger study with more defined inclusion and exclusion criteria from multiple centers with greater numbers of samples could further help confirm if EMP2 protein has increased expression in the RPE and cells that have undergone EMT. In addition, we do not know if EMP2 expression is unique to intraocular healing responses. EMP2 expression has not been studied in other healing and scarring responses. While we do not expect to find EMP2 expression in other tissue-healing responses, additional research is needed to answer these questions. Nonetheless, this study provided evidence that EMP2 is expressed in all intraocular membranes and may be a potential therapeutic target to prevent or treat vision loss caused by these membranes, as it proved to be in animal models of PVR.17 Increased understanding of PVR pathology and key molecular events will hopefully lead to new therapeutic interventions.
Acknowledgments
Supported by unrestricted departmental funds from Research to Prevent Blindness, Foundation Fighting Blindness (DGT), EY019909 (LKG, DGT).
Disclosure: D.G. Telander, P; A.K. Yu, None; K.I. Forward, None; S.A. Morales, P; L.S. Morse, None; S.S. Park, None; L.K. Gordon, P
References
- 1. Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998; 43: 3–18. [DOI] [PubMed] [Google Scholar]
- 2. Pastor JC,, de la Rua ER,, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002; 21: 127–144. [DOI] [PubMed] [Google Scholar]
- 3. Cardillo JA,, Stout JT,, LaBree L,, et al. Post-traumatic proliferative vitreoretinopathy: the epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology. 1997; 104: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 4. Asaria RH,, Charteris DG. Proliferative vitreoretinopathy: developments in pathogenesis and treatment. Compr Ophthalmol Update. 2006; 7: 179–185. [PubMed] [Google Scholar]
- 5. Oberstein SY,, Byun J,, Herrera D,, Chapin EA,, Fisher SK,, Lewis GP. Cell proliferation in human epiretinal membranes: characterization of cell types and correlation with disease condition and duration. Mol Vis. 2011. ; 17: 1794–1805. [PMC free article] [PubMed] [Google Scholar]
- 6. Kroll P,, Rodrigues EB,, Hoerle S. Pathogenesis and classification of proliferative diabetic vitreoretinopathy. Ophthalmologica. 2007; 221: 78–94. [DOI] [PubMed] [Google Scholar]
- 7. Morales SA,, Mareninov S,, Prasad P,, Wadehra M,, Braun J,, Gordon LK. Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway. Exp Eye Res. 2007; 85: 790–798. [DOI] [PubMed] [Google Scholar]
- 8. Tahara YR,, Sakamoto TR,, Oshima YR,, et al. The antidepressant hypericin inhibits progression of experimental proliferative vitreoretinopathy. Curr Eye Res. 1999; 19: 323–329. [DOI] [PubMed] [Google Scholar]
- 9. Morales SA,, Mareninov S,, Wadehra M,, et al. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009; 50: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wadehra M,, Sulur GG,, Braun J,, Gordon LK,, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003; 74: 106–112. [DOI] [PubMed] [Google Scholar]
- 11. Forbes A,, Wadehra M,, Mareninov S,, et al. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem. 2007; 282: 26542–26551. [DOI] [PubMed] [Google Scholar]
- 12. Wadehra M,, Goodglick L,, Braun J. The tetraspan protein EMP2 modulates the surface expression of caveolins and glycosylphosphatidyl inositol-linked proteins. Mol Biol Cell. 2004; 15: 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wadehra M,, Iyer R,, Goodglick L,, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002; 277: 41094–41100. [DOI] [PubMed] [Google Scholar]
- 14. Wadehra M,, Mainigi M,, Morales SA,, et al. Steroid hormone regulation of EMP2 expression and localization in the endometrium. Reprod Biol Endocrinol. 2008. ; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wadehra M,, Su H,, Gordon LK,, Goodglick L,, Braun J. The tetraspan protein EMP2 increases surface expression of class I major histocompatibility complex proteins and susceptibility to CTL-mediated cell death. Clin Immunol. 2003; 107: 129–136. [DOI] [PubMed] [Google Scholar]
- 16. Wang CX,, Wadehra M,, Fisk BC,, Goodglick L,, Braun J. Epithelial membrane protein 2 a 4-transmembrane protein that suppresses B-cell lymphoma tumorigenicity. Blood. 2001; 97: 3890–3895. [DOI] [PubMed] [Google Scholar]
- 17. Telander DG,, Morales SA,, Mareninov S,, Forward K,, Gordon LK. Epithelial membrane protein-2 (EMP2) and experimental proliferative vitreoretinopathy (PVR). Curr Eye Res. 2011; 36: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Remmele W,, Schicketanz KH. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer: computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol Res Pract. 1993; 189: 862–866. [DOI] [PubMed] [Google Scholar]
- 19. Robbins SG,, Brem RB,, Wilson DJ,, et al. Immunolocalization of integrins in proliferative retinal membranes. Invest Ophthalmol Vis Sci. 1994; 35: 3475–3485. [PubMed] [Google Scholar]
- 20. Zahn G,, Volk K,, Lewis GP,, et al. Assessment of the integrin alpha5beta1 antagonist JSM6427 in proliferative vitreoretinopathy using in vitro assays and a rabbit model of retinal detachment. Invest Ophthalmol Vis Sci. 2010; 51: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann S,, He S,, Jin M,, et al. A selective cyclic integrin antagonist blocks the integrin receptors alphavbeta3 and alphavbeta5 and inhibits retinal pigment epithelium cell attachment, migration and invasion. BMC Ophthalmol. 2005. ; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo L,, Yu W,, Li X,, et al. Targeting of integrin-linked kinase with a small interfering RNA suppresses progression of experimental proliferative vitreoretinopathy. Exp Eye Res. 2008; 87: 551–560. [DOI] [PubMed] [Google Scholar]
- 23. Proulx S,, Guerin SL,, Salesse C. Effect of quiescence on integrin alpha5beta1 expression in human retinal pigment epithelium. Mol Vis. 2003. ; 9: 473–481. [PubMed] [Google Scholar]
- 24. Morales SA,, Telander DG,, Mareninov S,, et al. Anti-EMP2 diabody blocks epithelial membrane protein 2 (EMP2) and FAK mediated collagen gel contraction in ARPE-19 cells. Exp Eye Res. 2012. ; 102: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garweg JG,, Tappeiner C,, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013; 58: 321–329. [DOI] [PubMed] [Google Scholar]
- 26. Tosi GM,, Marigliani D,, Romeo N,, Toti P. Disease pathways in proliferative vitreoretinopathy: an ongoing challenge. J Cell Physiol. 2014; 229: 1577–1583. [DOI] [PubMed] [Google Scholar]