Abstract
Background
To evaluate the association between the isolated single umbilical artery (iSUA) and perinatal outcomes, including pregnancy outcomes and perinatal complications.
Material/Methods
We performed a meta-analysis of 15 eligible studies regarding the relationship between the iSUA and perinatal outcomes, including gestational age at delivery, nuchal cord, placental weight, small for gestational age (SGA), oligohydramnios, polyhydramnios, pregnancy-induced hypertension (PIH), gestational diabetes mellitus (GDM), preeclampsia, and perinatal mortality. The overall odds ratios (OR) or standardized mean difference (SMD) were calculated.
Results
The occurrence of nuchal cord was not found to be different between an iSUA and a three-vessel cord (TVC) fetus. For perinatal complications, the SGA, oligohydramnios, polyhydramnios, GDM, and perinatal mortality showed dramatic difference between women with an iSUA and women with a TVC fetus, which implied that the presence of iSUA significantly increased the risk of perinatal complications. For other perinatal complications, such as PIH and preeclampsia, no significant association was detected.
Conclusions
Our meta-analysis suggests that the presence of iSUA would increase the risk of perinatal complications such as SGA, oligohydramnios, polyhydramnios, GDM, and perinatal mortality. Therefore, pregnant women with an iSUA fetus have poorer perinatal outcomes and more attention should be given to the management of their pregnancy compared to women with a TVC fetus.
MeSH Keywords: Meta-Analysis, Perinatal Care, Pregnancy Outcome, Single Umbilical Artery
Background
Single umbilical artery (SUA) is characterized by the presence of one umbilical vein and the absence of one of the umbilical arteries instead of the normal umbilical cord containing one vein and two arteries (three-vessel cord), and is considered a malformation [1,2]. Among all deliveries, SUA is the most common anomaly of the umbilical artery and its incidence is approximately 0.5% to 1% [3]. It has been previously reported that the incidence of SUA was lowest in neonates and highest among aneuploidy fetuses with the rate ranging from 9% to 11% [4]. The precise explanation for the cause of SUA is not yet fully known and the most widely accepted hypotheses includes primary agenesis or later atrophy of one umbilical artery [5,6].
An association between the SUA and an increased risk of fetal defects and chromosomal abnormalities has been reported [7–9]. SUA is defined as an isolated SUA (iSUA), if no additional chromosomal or structural abnormalities occurs [2]. More than 90% of cases with SUA exhibit an isolated anomaly, based a report from the Spanish Society of Gynecology and Obstetrics [10].
A meta-analysis performed in 2013, evaluated the relationship of iSUA to fetal growth, aneuploidy, and perinatal mortality, and reported no significant association was detected between iSUA and fetal growth and perinatal mortality; with respect to aneuploidy, there was insufficient data [6]. However, a controversy exists concerning whether iSUA is associated with adverse perinatal outcomes, with other studies presenting different views [1,11,12]. For example, when compared to fetuses with a TVC, fetuses with an iSUA are considered at increased risk of adverse perinatal outcomes, such as perinatal mortality [1], growth restriction [13], preterm labor [11], and pregnancy complication [2].
Our meta-analysis, which focused on investigating the association between iSUA and pregnancy outcomes and perinatal complications was performed to resolve the previously observed inconsistencies and to provide a more reliable estimation of the association between the iSUA and multiple perinatal outcomes.
Material and Methods
Search strategy
We searched multiple databases, including PUBMED, MEDLINE and EMBASE for relevant literatures by using terms: “single umbilical artery” or “two umbilical vessels” or “SUA” and (“fetal” or “prenatal”) and (“three-vessel cord” or “normal umbilical cord” or “control” or “two umbilical arteries” or “three vessel disease”). All the studies published in English before July 31, 2015 were included. For the overlapped studies, the ones covering the most extensive information were selected.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) studies reporting iSUA and pregnancy outcomes; 2) participants in case group studies were singletons of at least 24 weeks’ gestation with iSUA identified by ultrasound scan [5]; 3) participants in control groups were single pregnancy with the normal three-vessel cord; 4) retrospective studies and prospective studies. The exclusion criteria were: 1) iSUA first diagnosed at birth; 2) studies were only on twin pregnancies; 3) the presence of any fetal malformation and/or marker of chromosomal abnormalities; and 4) absence of a SUA at delivery or by pathology report [14].
Data extraction
According to the inclusion and exclusion criteria, the candidate studies were evaluated independently by two authors. In addition, we manually examined the reference lists for potentially relevant studies. The following information was extracted from the eligible studies: first author, year of publication, design type of study, study population, numbers of participants in case and control groups, age, gravidity and parity of maternal, body mass index (BMI) of neonatal, fetal gender, and perinatal mortality.
Statistical analysis
In our study, perinatal outcomes were estimated by pregnancy outcomes including gestational age at delivery, nuchal cord, and placental weight [5,15–17] and perinatal complications including small for gestational age (SGA), oligohydramnios, polyhydramnios, pregnancy-induced hypertension (PIH), gestational diabetes mellitus (GDM), preeclampsia, and perinatal mortality [1,2,5,11–15,18,19]. Gestational age at delivery was defined based on the description in a previous study [14]; and SGA was defined as a birth weight less than the 10th percentile [12,15]. The statistical analysis was conducted using STATA 12 software (STATA Corp LP, College Station, Texas, USA) and p value <0.05 was considered statically significant.
In our meta-analysis, we applied the Mantel-Haenszel (M-H) fixed-effects model for the calculation of I2 index, which was used to assess the heterogeneity among eligible studies. For dichotomous data, when I2 index was less than 50%, the Mantel-Haenszel (M-H) fixed-effects model was adopted to calculate the OR and 95% CI. Otherwise, the DerSimonian and Laird (D-L) random-effects model was chosen. With respect to the continuous data, when I2 index was less than 50%, the Inverse-Alteration (I-V) fixed-effects model was used to calculate the SMD and 95% CI. Otherwise, the DerSimonian and Laird (D-L) random-effects model was applied.
For the calculation of OR or SMD and 95% CI, the data from the control group (participants with a TVC fetus) served as reference). Forest plots were generated to summarize the results. An OR >1 or SMD >0 for any of the perinatal outcomes signified that, compared to TVC fetuses, there was an increased risk of a perinatal outcome of fetuses with an iSUA. Begg’s funnel plots and Egger’s tests were conducted to examine publication bias. The noticeable asymmetry in funnel plots provided the evidence for the publication bias and the significance level was set at 0.05 for Egger’s tests.
Results
Study characteristics
Overall, 56 papers were retrieved from the three databases after the first search, from which 14 studies that were irrelevant to human were excluded. Another 27 studies that did not meet the inclusion criteria were also excluded; leaving 15 studies [1,2,5,11–13,15,17,18,20–25] included in our meta-analysis. The selection process and reasons for exclusion are described in Figure 1. The eligible studies included two prospective studies and 13 retrospective studies. The main characteristics of all the 15 included studies are displayed in Table 1.
Figure 1.

Flow diagram of study selection and specific reasons for exclusion from the meta-analysis.
Table 1.
Summary of characteristics of studies included in the meta-analysis.
| Study | Study population | Type of study | Number* | Maternal age* | Gravidity* | Parity* | BMI* (kg/cm) | Fetal sex* (male%) | Perinatal mortality*% |
|---|---|---|---|---|---|---|---|---|---|
| Mladen Predanic (2005) | American | Retrospective case-control study | 84/ 84 | (31.2±5.2)/(33.4±4.7) | [2(1,3)]/ [2 (1, 2)] | [0(0,1)]/[0 (0,1)] | NR | NR | NR |
| Annette E. Bombrys (2008) | American | Retrospective case-control study | 255/ 289 | (26.9±6.2)/(24.9±5.9) | (2.7±1.7)/(2.9±2.4) | (1.2±1.4)/(1.3±1.4) | NR | NR | NR |
| Shu-Chi Mu (2008) | Taipei | Retrospective case-control study | 14/ 28 | (30.7±3.9)/(29.5 ± 4.2) | (1.9±1.0)/(2.0±1.1) | (1.3±0.5)/(1.5±0.6) | NR | NR | NR |
| Amanda L. Horton (2010) | American | Retrospective cohort study | 68/ 68 | (27.3±4.6)/(25.4±5.1) | NR | NR | (24.2±1.1)/(6.1±1.3) | 47/ 51.4 | NR |
| Lynn Murphy Kaulbeck (2010) | Canada | Retrospective cohort study | 725/ 196024 | NR | NR | NR | NR | NR | 3.35/ 0.39 |
| Meiling Hua (2010) | American | Retrospective cohort study | 392/ 63655 | (29.8±6.4)/(30.2±6.3) | (2.5±1.3)/(2.7±1.6) | (1.0±1.1)/(1.1±1.2) | NR | 46.0/ 50.8 | NR |
| Shilpa Chetty-John (2010) | American | Prospective study | 263/ 41415 | (25.02±5.6)/(24.08±6.0) | – | – | NR | 41.1/ 49.8 | NR |
| Shimon Burshtein (2011) | Russia | Retrospective cohort study | 243/ 194566 | (28.5±5.6)/(28.5±5.9) | – | – | NR | NR | 6.6/0.9 |
| Mohamed Ibrahim Khalil (2013) | Saudi Arabia | Retrospective cohort study | 159/ 35026 | (31.41±6.3)/(31.57±6.5) | (4.5±2.6)/(4.5±2.7) | (2.8±2.0)/(2.8±2.0) | (24.1±4.56)/(24.5±5.01) | NR | 3.14/0.99 |
| Eran Ashwal (2014) | Israel | Retrospective cohort study | 91/ 182 | (28.8±5.1)/(28.8±4.5) | [2(2–3)]/[2(1–3)] | [1(0–2)]/[1(0–2)] | NR | 38.5/ 47.8 | NR |
| Joel Baron (2014) | Israel | Prospective case-control study | 29/ 29 | (29.7±5.3)/(30.3±5.2) | NR | NR | NR | NR | NR |
| Lorena Mesquita Caldas(2014) | Brazil | Retrospective case-control study | 134/ 759 | (30.8±6.7)/(30.3±6.6) | NR | NR | NR | NR | NR |
| S. Doğan (2014) | Turkey | Retrospective cohort study | 77/ 95 | (30.3±5.1)/(29.6±5.9) | (1.9±1.0)/(2.1±1.2) | (0.7±1.0)/(0.76±0.8) | NR | NR | NR |
| Fırat Tülek (2015) | Turkey | Retrospective cohort study | 93/ 100 | (27.1±5.37)/(27.4±5.28) | (3.9±2.0)/(3.8±1.0) | (2.1±1.0)/(2.3±1.2) | (28.3±4.28)/(28.3±3.38) | NR | NR |
| Mariella Mailath-Pokorny (2015) | Austria | Retrospective cohort study | 136/ 500 | 28.6±6.5 | 2.7±1.6# | 1.1±1.1# | – | 48.1# | NR |
(Isolated SUA/normal umbilical artery)
total (all the fetal); BMI – body mass index; NR – no refer; ‘–’ – cloud not collect from article.
Evaluation of the association between iSUA and pregnancy outcomes
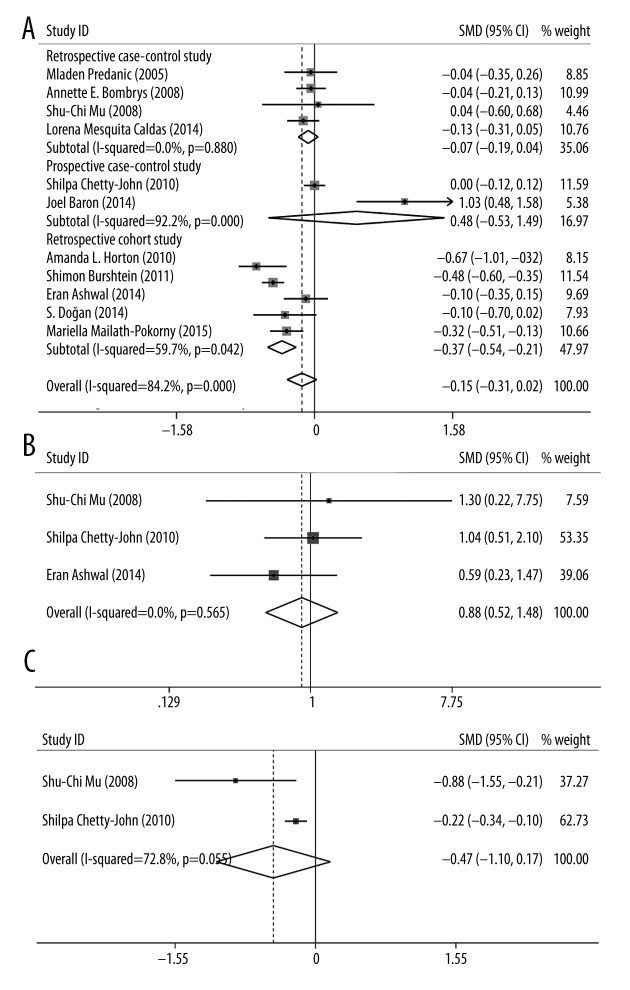
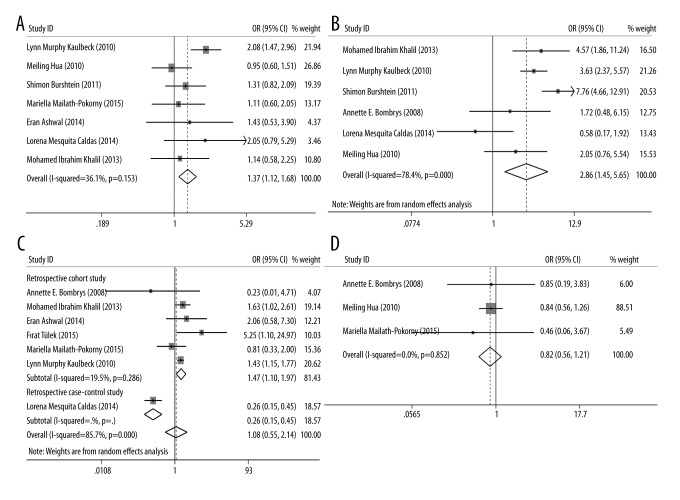
The association between iSUA and pregnancy outcomes, including gestational age, nuchal cord, and placental weight, were evaluated and the results are represented in Table 2. For gestational age, 11 eligible studies were included and the SMD was −0.145 (95% CI: −0.315–0.024, p=0.094, Figure 2A), suggesting that the gestational age in fetuses with iSUA was similar with that in TVC fetuses and no significant association was detected between iSUA and gestational age. For nuchal cord, three eligible studies were included and the OR was 0.880 (95% CI: 0.522–1.483, p=0.234, Figure 2B), indicating that there was no significant difference in the occurrence of nuchal cord between fetuses with iSUA and fetuses with TVC and no significant association was observed between iSUA and nuchal cord. For placental weight, two eligible studies were included and the SMD was −0.466 (95% CI: −1.097–0.166, p=0.148, Figure 2C), which signified that placental weight in fetuses with iSUA was similar to placental weight in TVC fetuses, and no significant association was found between iSUA and placental weight.
Table 2.
Meta-analysis for the outcome during pregnancy stage.
| Analysis model | Analysis method | Number of studies | Total people (case/control) | Heterogeneity | OR or SMD | SMD | p-value | Publication bias | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | 95% CI | Begg | Egger | |||||||
| Gestational age | D-L random | 11 | 1361/238015 | 84.2 | 0.000 | −0.145* | −0.315 | 0.024 | 0.094 | 0.876 | 0.513 |
| Nuchal cord | M-H fixed | 3 | 368/41625 | 0.00 | 0.565 | 0.880 | 0.522 | 1.483 | 0.234 | 1.000 | 0.926 |
| Placental weight | D-L random | 2 | 277/41443 | 72.8 | 0.055 | −0.466* | −1.097 | 0.166 | 0.148 | 1.00 | – |
| SGA | D-L random | 11 | 1836/233155 | 69.5 | 0.000 | 2.514 | 1.806 | 3.505 | 0.000 | 0.640 | 0.886 |
| Oligohydramnios | M-H fixed | 3 | 427/194848 | 0.000 | 0.617 | 2.71 | 1.747 | 4.204 | 0.000 | 0.296 | 0.082 |
| Polyhydramnios | M-H fixed | 4 | 1218/425798 | 0.00 | 0.767 | 3.090 | 2.259 | 4.228 | 0.000 | 0.308 | 0.237 |
| PIH | D-L random | 7 | 1593/232880 | 85.7 | 0.000 | 1.097 | 0.921 | 1.308 | 0.229 | 0.548 | 0.678 |
| GDM | M-H fixed | 7 | 2135/490950 | 36.1 | 0.153 | 1.367 | 1.116 | 1.675 | 0.003 | 0.764 | 0.572 |
| Preeclampsia | M-H fixed | 3 | 783/64443 | 0.00 | 0.852 | 0.820 | 0.557 | 1.205 | 0.312 | 0.296 | 0.491 |
| Perinatal mortality | D-L random | 3 | 1127/425616 | 66.30 | 0.052 | 4.681 | 2.637 | 8.309 | 0.000 | 1.000 | 0.931 |
Figure 2.
Forest plot of study evaluating the association between iSUA and gestational age at delivery (A); nuchal cord (B); and placental weight (C).
Evaluation of the association between iSUA and perinatal complications
The association between iSUA and perinatal complications, including SGA, oligohydramnios, polyhydramnios, PIH, GDM, preeclampsia, and perinatal mortality, were also estimated and the results are shown in Table 2. For SGA, 11 eligible studies were included and the OR was 2.514 (95% CI: 1.806–3.505, p=0.000, Figure 3), indicating that the incidence of SGA in iSUA fetuses was significantly higher than that in TVC fetuses and iSUA was associated with the occurrence of SGA. For the oligohydramnios and polyhydramnios, three and four eligible studies were included respectively; the ORs were 2.71 and 3.090, respectively (for oligohydramnios: 95% CI: 1.747–4.204, p=0.000; for polyhydramnios: 95% CI: 2.259–4.228, p=0.000, Figure 4). These results suggest that the incidence of oligohydramnios or polyhydramnios in iSUA fetuses was significantly higher than in TVC fetuses, and iSUA increased the risk of oligohydramnios or polyhydramnios.
Figure 3.
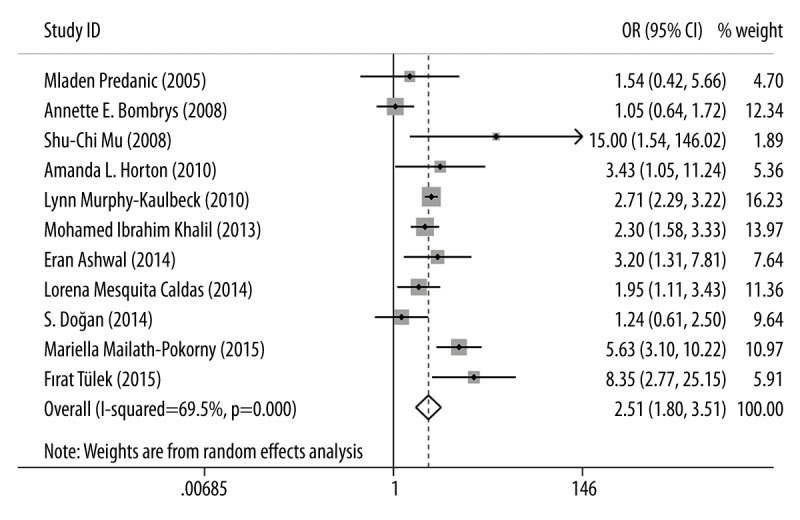
Forest plot of study estimating the association between iSUA and SGA.
Figure 4.

Forest plots of studies assessing the association between iSUA and oligohydramnios and polyhydramnios.
For GDM, seven eligible studies were included and the OR was 1.367 (95% CI: 1.116–1.675, p=0.003, Figure 5A), suggesting that the incidence of GDM in iSUA fetuses was significantly higher than in TVC fetuses, and iSUA increased the risk of GDM. For perinatal mortality, three eligible studies were included and the OR was 4.681 (95% CI: 2.637–8.309, p=0.000, Figure 5B), implying that the incidence of perinatal mortality in iSUA fetuses was significantly higher than in TVC fetuses, and iSUA increased the risk of perinatal mortality. For PIH and preeclampsia, seven and three eligible studies were included respectively, and the ORs were 1.082 and 0.820, respectively (for PIH: 95% CI: 0.547–2.141, p=0.229; for preeclampsia: 95% CI: 0.557–1.205, p=0.312, Figure 5C, 5D), revealing that there was no association between iSUA and PIH and preeclampsia.
Figure 5.
Forest plots of studies evaluating the association between iSUA and GDM (A); perinatal mortality (B); PIH (C); and preeclampsia (D).
Sensitivity analysis
We performed the sensitivity analysis to explore the effect of a single study on the overall meta-analysis by omitting individual trials sequentially. As shown in Supplementary Figure 1, no significant difference occurred after the omission of any study, which signified that our results were statistically reliable.
Publication bias
Begg’s plot and Egger’s test were applied for the evaluation of publication bias and the results are exhibited in Table 2. The p values of all analyses were higher than 0.05, indicating that no significant publication bias was observed in any individual investigation that was part of our meta-analysis.
Discussion
In this study we performed a meta-analysis that included 15 studies that considered the relationship between iSUA and perinatal outcomes, including gestational age at delivery, nuchal cord, placental weight, SGA, oligohydramnios, polyhydramnios, PIH, GDM, preeclampsia, and perinatal mortality. For the pregnancy outcomes, our results suggested that no significant difference was observed between women with an iSUA fetus and women with a TVC fetus, and there was no association between iSUA and pregnancy outcomes such as gestational age, nuchal cord, and placental weight. For perinatal complications, we compared the risk of SGA, oligohydramnios, polyhydramnios, GDM, and perinatal mortality between women with iSUA and TVC fetus, all the ORs were higher than 1 and p values were lower than 0.05, implying that the incidence of these complications was correlated with iSUA, and that iSUA may increase the risk of these complications. The comprehensive analysis in our study illustrated that compared with pregnant women with a TVC fetus, women with an iSUA fetus had poorer perinatal outcomes and were more likely to suffer from SGA, oligohydramnios, polyhydramnios, GDM, and perinatal mortality.
In terms of gestational age at delivery, a study by Shilpa et al. showed no significant difference between iSUA fetuses and TVC fetuses [5], results which were consistent with our study. As for the occurrence of nuchal cord, a retrospective cohort study published in 2014 suggested that there was no significant difference between iSUA fetuses and TVC fetuses [22], an observation also suggested by our study. However, for placental weight, the results of two previous studies [5,15] were opposite to that of our meta-analysis; the false positive in these two previous studies might be partially responsible for the inconsistency, and our meta-analysis might have eliminate the false positive by enlarging the sample size. With respect to perinatal complications, our results were in accordance with those of previous studies [1,11–13,18,24,25].
A previous meta-analysis [6] showed that fetuses with iSUA were more likely to suffer from SGA and perinatal mortality when compared with TVC fetuses, even if no statistical difference was detected for the two outcomes. Yet, the results of our meta-analysis suggest that the presence of iSUA significantly increased the risk of SGA and perinatal mortality, a finding inconsistent with the previous meta-analysis. As stated in the previous study, the limited sample size might be responsible for the non-significant result. The previous study included only seven eligible papers covering 928 pregnancies with iSUA, while our meta-analysis included 15 eligible papers covering 2763 pregnancy with iSUA. Additionally, in our meta-analysis, except for SGA and perinatal mortality, we also estimated the relationship between iSUA fetuses and other perinatal complication-related symptoms to increase the reliability of our conclusions.
Since large or extreme heterogeneity may lead to a misleading conclusion, the degree of heterogeneity is one of the main concerns in meta-analysis. In our study, large heterogeneity existed in the analysis of gestational age (I2=84.2%, p=0.000) and PIH (I2=85.7%, p=0.000). Heterogeneity may be caused by confounding factors among different studies. We performed subsequent subgroup analysis stratified by study type for gestational age and PIH. Our results (Figures 2A, 5C) showed that the two prospective case-control study [5,23] might be the source of heterogeneity for gestational age; and heterogeneity for PIH might have resulted from one retrospective case-control study [24]. Thus, we suggest that when pooling eligible studies for meta-analysis, the heterogeneity of different study types should be considered, and subgroup analysis based on study type should be performed if sufficient data is available.
There were limitations to our meta-analysis. First, for the analysis of placental weight, only two eligible studies were retrieved; an analysis of a large sample size should be conducted to get a more reliable estimation. Second, as we discussed earlier, for the analysis of gestational age and PIH, large heterogeneity occurred, hence these results should be interpreted with caution.
Conclusions
To the best of our knowledge, our study is the most comprehensive meta-analysis evaluating the association of iSUA and perinatal outcomes. Our results suggest there is no significant association between iSUA and pregnancy outcomes, including gestational age at delivery, nuchal cord, and placental weight. In terms of perinatal complications, the incidence of PIH and preeclampsia has no correlation with the presence of iSUA, while the presence of iSUA could increase the risk of SGA, oligohydramnios, polyhydramnios, GDM, and perinatal mortality. Our results suggest that compared to pregnant women with a TVC fetus, women with an iSUA fetus have a poorer perinatal outcomes, and more attention should be paid to the management of pregnant women with an iSUA fetus.
Supplementary materials
Result of sensitivity analysis.
Footnotes
Declaration of conflicting interest
There is no special conflict of interest in our study.
Source of support: Departmental sources
References
- 1.Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010;116:843–50. doi: 10.1097/AOG.0b013e3181f0bc08. [DOI] [PubMed] [Google Scholar]
- 2.Tulek F, Kahraman A, Taskin S, et al. Determination of risk factors and perinatal outcomes of singleton pregnancies complicated by isolated single umbilical artery in Turkish population. J Turk Ger Gynecol Assoc. 2015;16:21–24. doi: 10.5152/jtgga.2015.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parilla BV, Tamura RK, MacGregor SN, et al. The clinical significance of a single umbilical artery as an isolated finding on prenatal ultrasound. Obstet Gynecol. 1995;85:570–72. doi: 10.1016/0029-7844(94)00451-I. [DOI] [PubMed] [Google Scholar]
- 4.Persutte WH, Hobbins J. Single umbilical artery: A clinical enigma in modern prenatal diagnosis. Ultrasound Obstet Gynecol. 1995;6:216–29. doi: 10.1046/j.1469-0705.1995.06030216.x. [DOI] [PubMed] [Google Scholar]
- 5.Chetty-John S, Zhang J, Chen Z, et al. Long-term physical and neurologic development in newborn infants with isolated single umbilical artery. Am J Obstet Gynecol. 2010;203:368.e1–7. doi: 10.1016/j.ajog.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskamp BJ, Fleurke-Rozema H, Oude-Rengerink K, et al. Relationship of isolated single umbilical artery to fetal growth, aneuploidy and perinatal mortality: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:622–28. doi: 10.1002/uog.12541. [DOI] [PubMed] [Google Scholar]
- 7.Dagklis T, Defigueiredo D, Staboulidou I, et al. Isolated single umbilical artery and fetal karyotype. Ultrasound Obstet Gynecol. 2010;36:291–95. doi: 10.1002/uog.7717. [DOI] [PubMed] [Google Scholar]
- 8.Saller DN, Jr, Keene CL, Sun CC, Schwartz S. The association of single umbilical artery with cytogenetically abnormal pregnancies. Am J Obstet Gynecol. 1990;163:922–25. doi: 10.1016/0002-9378(90)91097-v. [DOI] [PubMed] [Google Scholar]
- 9.Chow JS, Benson CB, Doubilet PM. Frequency and nature of structural anomalies in fetuses with single umbilical arteries. J Ultrasound Med. 1998;17:765–68. doi: 10.7863/jum.1998.17.12.765. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand E, Selbing A, Blomberg M. Comparison of first and second trimester ultrasound screening for fetal anomalies in the southeast region of Sweden. Acta Obstet Gynecol Scand. 2010;89(11):1412–19. doi: 10.3109/00016349.2010.517307. [DOI] [PubMed] [Google Scholar]
- 11.Khalil MI, Sagr ER, Elrifaei RM, et al. Outcomes of an isolated single umbilical artery in singleton pregnancy: a large study from the Middle East and Gulf region. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):277–80. doi: 10.1016/j.ejogrb.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Mailath-Pokorny M, Worda K, Schmid M, et al. Isolated single umbilical artery: Evaluating the risk of adverse pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2015;184:80–83. doi: 10.1016/j.ejogrb.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Burshtein S, Levy A, Holcberg G, et al. Is single umbilical artery an independent risk factor for perinatal mortality? Arch Gynecol Obstet. 2011;283:191–94. doi: 10.1007/s00404-009-1326-3. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Payo C, Gaitero A, Tamarit I, et al. Perinatal results following the prenatal ultrasound diagnosis of single umbilical artery. Acta Obstet Gynecol Scand. 2005;84(11):1068–74. doi: 10.1111/j.0001-6349.2005.00884.x. [DOI] [PubMed] [Google Scholar]
- 15.Mu SC, Lin CH, Chen YL, et al. The perinatal outcomes of asymptomatic isolated single umbilical artery in full-term neonates. Pediatr Neonatol. 2008;49:230–33. doi: 10.1016/S1875-9572(09)60016-4. [DOI] [PubMed] [Google Scholar]
- 16.Gornall AS, Kurinczuk JJ, Konje JC. Antenatal detection of a single umbilical artery: Does it matter? Prenat Diagn. 2003;23(2):117–23. doi: 10.1002/pd.540. [DOI] [PubMed] [Google Scholar]
- 17.Bombrys AE, Neiger R, Hawkins S, et al. Pregnancy outcome in isolated single umbilical artery. Am J Perinatol. 2008;25:239–42. doi: 10.1055/s-2008-1061504. [DOI] [PubMed] [Google Scholar]
- 18.Hua M, Odibo AO, Macones GA, et al. Single umbilical artery and its associated findings. Obstetr Gynecol. 2010;115:930–34. doi: 10.1097/AOG.0b013e3181da50ed. [DOI] [PubMed] [Google Scholar]
- 19.Prucka S, Clemens M, Craven C, McPherson E. Single umbilical artery: what does it mean for the fetus? A case-control analysis of pathologically ascertained cases. Genet Med. 2004;6:54–57. doi: 10.1097/01.gim.0000105743.91723.b0. [DOI] [PubMed] [Google Scholar]
- 20.Predanic M, Perni SC, Friedman A, et al. Fetal growth assessment and neonatal birth weight in fetuses with an isolated single umbilical artery. Obstet Gynecol. 2005;105:1093–97. doi: 10.1097/01.AOG.0000158108.51397.f5. [DOI] [PubMed] [Google Scholar]
- 21.Horton AL, Barroilhet L, Wolfe HM. Perinatal outcomes in isolated single umbilical artery. Am J Perinatol. 2010;27:321–24. doi: 10.1055/s-0029-1241732. [DOI] [PubMed] [Google Scholar]
- 22.Ashwal E, Melamed N, Hiersch L, et al. The impact of isolated single umbilical artery on labor and delivery outcome. Prenat Diagn. 2014;34:581–85. doi: 10.1002/pd.4352. [DOI] [PubMed] [Google Scholar]
- 23.Baron J, Weintraub AY, Sciaky Y, et al. Umbilical artery blood flows among pregnancies with single umbilical artery: A prospective case-control study. J Matern Fetal Neonatal Med. 2015;28(15):1803–5. doi: 10.3109/14767058.2014.968845. [DOI] [PubMed] [Google Scholar]
- 24.Caldas LM, Liao A, Carvalho MH, et al. Should fetal growth be a matter of concern in isolated single umbilical artery? Rev Assoc Med Bras. 2014;60:125–30. doi: 10.1590/1806-9282.60.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Dogan S, Özyüncü Ö, Atak Z, Turgal M. Perinatal outcome in cases of isolated single umbilical artery and its effects on neonatal cord blood gas indices. J Obstet Gynaecol. 2014;34:576–79. doi: 10.3109/01443615.2014.919578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Result of sensitivity analysis.