Abstract
Background
There is increasing evidence that adenosine triphosphate (ATP), a well-known neurotransmitter and neuromodulator in the central nervous system, plays an important role as an extracellular chemical messenger in the cochlea.
Material/Methods
Using a whole-cell recording technique, we studied the effects of ATP on isolated Hensen’s cells, which are supporting cells in the cochlea, to determine if they are involved in the transduction of ions with hair cells.
Results
ATP (0.1–10 μM) reduced the potassium current (IK+) in the majority of the recorded Hensen’s cells (21 out of 25 cells). An inward current was also induced by high concentrations of ATP (100 μM to 10 mM), which was reversibly blocked by 100 μM suramin (a purinergic antagonist) and blocked by nifedipine (an L-type calcium channel blocker). After the cochleas were perfused with artificial perilymph solutions containing nifedipine and exposed to noise, the amplitude increase in the compound action potential (CAP) threshold and the reduction in cochlear microphonics was lower than when they were exposed to noise alone.
Conclusions
Our results suggest that ATP can block IK+ channels at a low concentration and induce an inward Ca2+ current at high concentrations, which is reversed by purinergic receptors. Nifedipine may have a partially protective effect on noise-induced hearing loss (NIHL).
MeSH Keywords: Adenosine Triphosphate; Hearing Loss, Noise-Induced; Kv Channel-Interacting Proteins; Nifedipine; Organizers, Embryonic
Background
The organ of Corti includes 2 types of cells: sensory cells (hair cells) and supporting cells [1]. The electromotive outer hair cells are believed to actively amplify the signal from the cochlea in response to low sound stimuli [2]. To maintain this highly sensitized function of hair cells, the surrounding supporting cells provide a suitable electrical and micromechanical environment for the hair cells to maintain homeostasis of the organ of Corti. Cell-to-cell connections through gap junctions between outer hair cells and supporting cells or between supporting cells and supporting cells were identified in different types of supporting cells (e.g., Hensen’s cells and Deiter’s cells), which were believed to play important roles in maintaining the normal physiological environment and functions of hair cells [3,4]. Gap junctions between the supporting cells and hair cells maintain the stable concentration difference of K+ between the endolymph and the perilymph by forming a K+ ion recycling pathway from the sensory hair cells to the endolymph [5]. ATP, existing in the endolymph and perilymph of the cochlea, is believed to be an important modulator of this ion transport mechanism [6].
Noise exposure elevates ATP levels in this cochlear compartment and changes the K+ conductance through P2X receptors by reducing the endocochlear potential (EP) [7]. In guinea pig cochlear fluids, extracellular ATP concentrations were elevated after a short 15-min noise exposure [8]. The observation that the efflux of K+ from the scala media is complemented by a P2Y receptor, a G protein-coupled ATP sensitive pathway, supports this hypothesis. This purinergic signaling mechanism is likely to provide the basis for a reactive homoeostatic regulatory mechanism that limits cochlear sensitivity under stressor conditions. Loss function of the ATP-gated P2X2 receptor (ligand-gated ion channel, purinergic receptor) which is expressed in sensory and supporting cells of the cochlea, may cause NIHL [9]. It is reasonable to speculate that ATP may influence the K+ currents of Hensen’s cells, although little is known about this suppressive effect.
Hearing loss due to noise exposure is a common sensorineural hearing impairment in industrialized countries [10]. White noise exposure at 120 dB SPL (sound pressure level) for 1 h induced a CAP (compound action potential) threshold shift and a CM (cochlear microphonic) potential amplitude shift [11]. Ionized calcium (Ca2+), a second messenger, plays a critical role in cellular function, including cell proliferation, differentiation, and apoptosis [12]. However, a sustained increase in intracellular Ca2+ levels is toxic to cells, leading to cell death [13]. Several factors may contribute to the elevated Ca2+ concentration of the cochlea, such as noise exposure and mechanical overstimulation, and acoustic trauma can increase the intracellular Ca2+, thus increasing the ototoxicity and causing narrowed chamber and increased permeability of the vessel, resulting in ischemia-hypoxia and energy metabolism disorders (e.g., ATP depletion) of the cochlea [14,15]. Therefore, it is reasonable to speculate that NIHL is related to the abnormal calcium homeostasis of the cochlea.
The regulators of calcium homeostasis, including a calcium channel blocker and an indirect stimulator of the Ca2+ pump, are involved in the regulation of intracellular Ca2+. According to the effects of Ca2+ on cells and blood vessels, a typical calcium channel blocker, nifedipine, which can reduce Ca2+ influx and dilate blood vessels to relieve spasm and contraction induced by noise, was chosen to be studied in this experiment. The purposes of the present study were: 1) to research the influence of ATP on potassium current in isolated adult Hensen’s cells by use of the whole-cell recording technique; 2) to determine the effect of nifedipine (an L-type calcium channel blocker) on the cochlea of guinea pigs by examining EP; and 3) to identify whether it has a preventive effect on NIHL by reducing the concentration of intracellular Ca2+.
Material and Methods
Ethics statement
The entire experiment is done in the Academy of Military Medical Sciences (AMMS). Animal breeding and care and all experiments were performed in adherence to AMMS Experimental Animal Center guidelines and were approved by the Experimental Animal Ethics Committee of AMMS.
Isolation of Hensen’s cells from the cochlea
Pigmented guinea pigs (250–300 g) with a positive Preyer’s reflex were sacrificed using an overdose of sodium pentobarbital (50 mg/kg). The cochlea was then harvested and the bony shell of the cochlea was gently removed. The organ of Corti was dissected and incubated in Hanks’ balanced salt solution (HBSS). A cell suspension was obtained by digestion for 30 min with trypsin (0.25 mg/ml) in nominal Ca2+-free HBSS. The cell suspension was washed with HBSS and transferred into a perfusion chamber. Isolated Hensen’s cells were identified by their characteristic lipid inclusions and cultured on poly-L-lysine-coated coverslips (Figure 1) [16].
Figure 1.
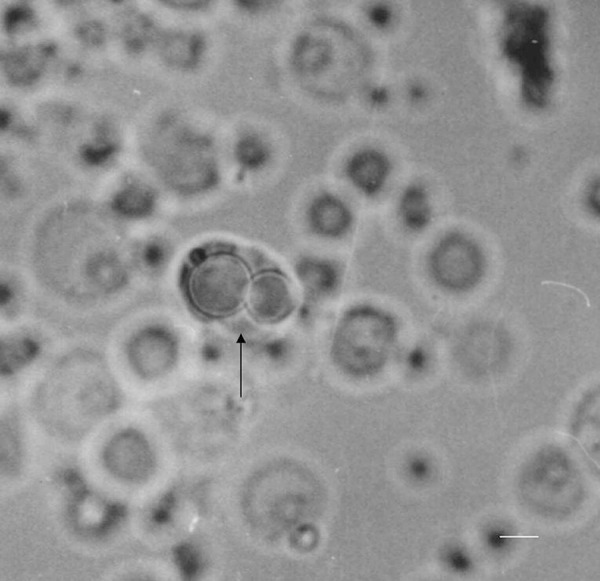
A typical Hensen’s cell isolated from the cochlea of an adult guinea pig (scale bar: 10 μm).
Preparation of solutions
For the patch clamp experiments, the extracellular solution contained the following (in mM): NaCl 125, KCl 2.5, CaCl2 2, MgCl2 1, NaHCO3 2.6, NaH2PO4 1.25, and glucose 10. The pipette filling solution for recording K+ currents contained the following (in mM): KCl 140, HEPES 10, MgCl2 1, and EGTA 10. All solutions were buffered to pH 7.3 with NaOH and the osmolarity was adjusted to 300 mOsm with glucose. ATP, TEA (tetraethylammonium), suramin, and nifedipine were purchased from Sigma. ATP and suramin were dissolved in the extracellular solution. Nifedipine was dissolved in dimethyl sulfoxide and then added to the extracellular solution.
Electrophysiological recordings
Pipettes were pulled using a 2-stage puller (Sachs-Flamming PC-84; Sutter Instruments, USA) to a resistance of 2–5 MΩ when filled with the pipette solution. Drugs were delivered to the cell using a micro-manifold consisting of 3 microtubules with a 100 μM diameter. The drugs were applied directly to single cells. The opening of the microtubule was placed approximately 1 mm from the recorded cell and the perfusion pressure of N2 (controlled using a BH-2 pressure injector made by Medical Systems Corp.) was adjusted to achieve rapid drug application while avoiding any mechanical disturbance to the recording. One of the microtubules was filled with the extracellular solution as a control and the others were filled with different concentrations of ATP as the test groups. Whole-cell recordings (Axopatch-1D; Axon Instruments Inc., USA) were performed at room temperature (21–25°C). Data acquisition and analysis were accomplished using pCLAMP 7.0 software (Axon instruments). The dose-response curve was fitted using Sigma Plot 2000 software (SPSS Inc., USA) with the logistic equation. Origin software 7.0 (OriginLab Inc., USA.) was used for the I–V graphic display and SAS 6.12 software (SAS Inc., USA) was used to conduct the 2-way analysis of variance (ANOVA). A P value less than 0.05 was considered statistically significant.
Artificial perfusion with nifedipine
After the guinea pigs were anesthetized, a small hole was drilled in the wall of the scala tympani and the scala vestibule in the basal turn of the cochlea. The prepared solution was perfused through the fenestra in the scala tympani and released through an outlet in the scala vestibule at a speed of 3 μl/min for 2 h. The right ear of the animals was exposed to white noise at 120 dB SPL for the noise exposure. Clicks at 10–90 dB SPL were used as acoustic stimuli. A recording electrode was placed on the round window niche. The reference electrode was placed in the neck muscle. Forty healthy hybrid guinea pigs were randomly divided into 4 groups. The perilymphatic spaces of the guinea pig cochleas were perfused with artificial perilymph solutions containing no or 0.5 μmol/L nifedipine with or without noise exposure for 2 h. CAP and CM were recorded from the round windows of the guinea pigs before and 120 min after perfusion.
Results
ATP depresses the outward K+ currents of Hensen’s cells
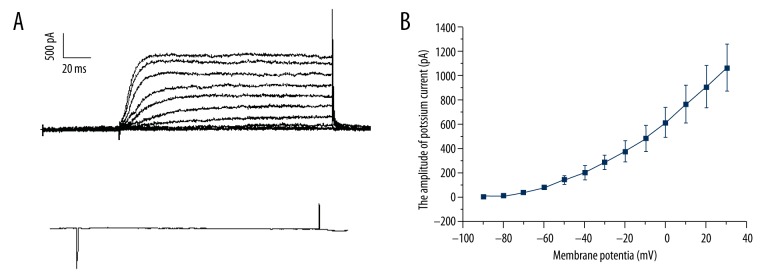
Voltage-dependent potassium currents (IK+) of Hensen’s cells were elicited using voltage steps from −90 to 60 mV (10 mV steps) (Figure 2A). A typical I/V curve of the IK+ recorded from Hensen’s cells is shown in Figure 2B. The outward currents could be totally blocked using Cs+ (140 mM) in the pipette and TEA (40 mM) in the bath solution, indicating that these currents are carried by K+ (Figure 2C).
Figure 2.
Outward K+ current recorded from a single isolated Hensen’s cell. (A) Typical raw data evoked by a voltage step from −90 to +60 mV (10 mV step). (B) I/V curve of IK+. (C) 40 mM TEA could block the IK+.
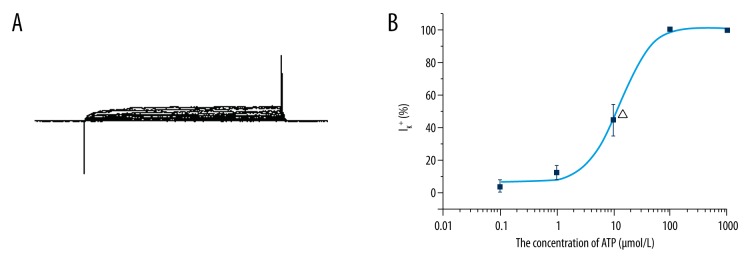
IK+ was significantly depressed by the direct application of ATP to the cell body and partially recovered at 2–3 min after ATP was washed out (Figure 3A). The reduction of IK+ was increased in an ATP-dependent manner from 0.1–10 μM. The mean [± standard deviation (SD)] suppressing rate of the different concentrations of ATP on the IK+ evoked using a 30 mV voltage was 3.51±3.8% (n=6) by 0.1 μM ATP, 12.58±4.62% (n=6) by 1 μM ATP, and 44.49±9.76% (n=9) by 10 μM ATP. The outward current was totally blocked by 100 μM (n=6) or 1 mM ATP (n=6). The concentration-response curve was fitted using the logistic equation (Figure 3B). The inhibition concentration (IC50) was 12.88±1.58 μM.
Figure 3.
ATP could block the IK+ evoked by voltage steps (−90 to +60 mV). (A) IK+ could be blocked by low concentrations of ATP. (B) the concentration-response curve of the ATP suppression effect on IK+ was fitted with the logistic equation. Note that the IC50 was 12.88±1.58 μM.
Suppression of IK+ by ATP is voltage-dependent
From the I/V curve (Figure 2A), it was observed that IK+ was activated at approximately −30 mV and the amplitude was saturated at approximately 30 mV. ATP inhibited the K+ current, but did not change the activation or saturation voltage of IK+. To determine whether the membrane potential had any effect on the inhibition of ATP (Figure 4), a 2-way ANOVA test was used to test the suppression rate of IK+ with different clamp voltages. We observed that the change in membrane potential had a significant interaction with the inhibition of ATP (F=46.95, P<0.05) compared to the average IK+ in the absence of ATP (Figure 4). Membrane potential significantly changed the amplitude of IK+ vs. the control group (n=6, F=50.03, P<0.05), and ATP significantly depressed the IK+ at increased concentrations (F=40.80, P<0.05). The change of membrane potential had a significant interaction with the inhibition of ATP (F=46.95, P<0.05).
Figure 4.
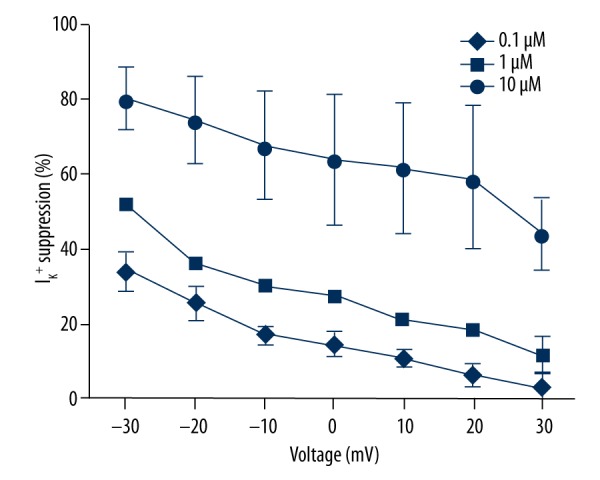
ATP-induced IK+ suppression (%)
A K+-dependent inward current is invoked by a high concentration of ATP and blocked by nifedipine
At concentrations of 100 μM, 1 mM, and 10 mM, ATP evoked an averaged inward current of 200 pA, 350 pA, and 470 pA (18 from 25 cells), respectively (Figure 5). No ATP-induced current could be recorded after IK+ was blocked using intracellular Cs+ (140 mM) or extracellular TEA (40 mM). This suggests that the inward current probably passes through the IK+ channels (n = 6) that are sensitive to ATP. The inward current could be reversely blocked using suramin (100 μM), a purinergic receptor antagonist, suggesting that the current was trigged by ATP through purinergic receptors. The inward current was also blocked by nifedipine (10 μM), a calcium channel blocker.
Figure 5.
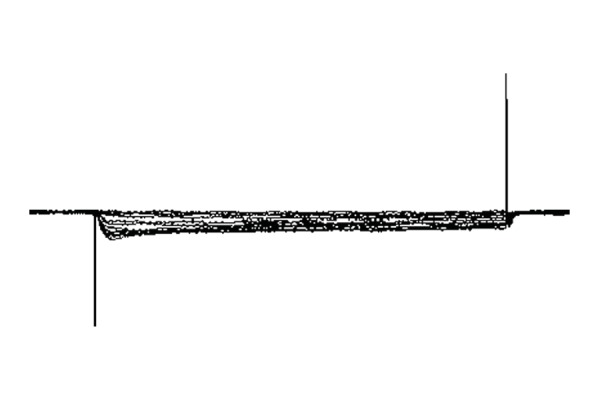
ATP-induced inward currents from isolated Hensen’s cells.
Nifedipine has a possible prophylactic effect against NIHL
The animals were randomly divided into 4 groups: 1) perfused with artificial perilymph solution (APS); 2) perfused with APS containing 0.5 μmol/L nifedipine (NIF); 3) perfused with APS and exposed to noise (APS+noise); and 4) perfused with APS containing 0.5 μmol/L nifedipine and exposed to noise (NIF+noise). There were no significant differences in the CAP thresholds or CM amplitudes before and after perfusion in the APS group (P>0.05). A rise in the CAP threshold and a reduction in the CM amplitude after perfusion were found in the other 3 groups (P<0.01). The increase in CAP thresholds after perfusion of these 3 groups was 28, 43.5, and 20 dB SPL, respectively (Figure 6). The reduction of CM amplitudes in the same 3 groups was 70.3%, 37.1%, and 21.1%, respectively (Figure 7). After perfusion with APS containing nifedipine and exposure to noise, the rise in the CAP threshold and the reduction in CM were lower than when exposed to noise alone (P<0.01).
Figure 6.
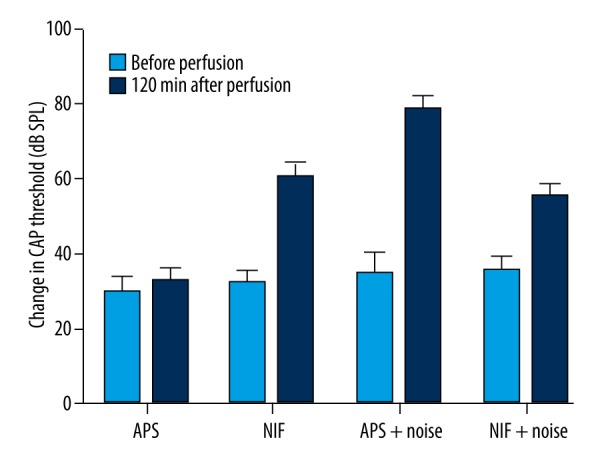
Change in CAP threshold (dB SPL, x ±SD, n=10). Rises in the CAP thresholds after perfusion were observed in NIF, APS+noise, and NIF+noise groups (P<0.01). After perfusion with APS containing nifedipine and exposure to noise (NIF+noise), the rise in the CAP threshold was lower than those observed following exposure to noise alone (P<0.01).
Figure 7.
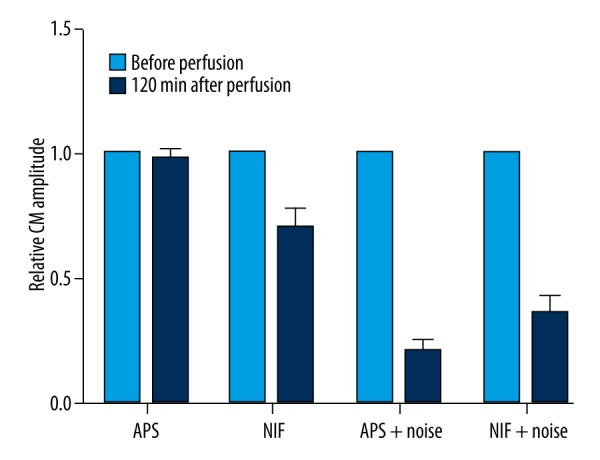
Relative CM amplitude (n=10). The reductions in the CM amplitudes after perfusion were observed in NIF, APS+noise, and NIF+noise groups (P<0.01). After perfusion with APS containing nifedipine and exposure to noise (NIF+noise), the reduction in the CM amplitudes was lower than those observed following exposure to noise alone (P<0.01).
Discussion
The present results show that low concentrations of ATP exerted a suppressive effect on the voltage-gated K+ currents (IK+) in isolated adult Hensen’s cells. This effect was voltage-dependent and suggests that ATP directly interacted with the K+ channel pore by binding to a site partway across the membrane electric field, although the site of action could not be determined in this experiment. The inhibition effect recovered quickly after ATP was removed, suggesting that the inhibition was reversible. This finding is consistent with the effect of ATP on the cells of the stria vascularis [17].
Previous studies have demonstrated that Hensen’s cells have a resting potential ranging from −60 to −100 mV [18,19]. A relatively high K+ concentration is required in the extracellular solution to maintain this resting potential. One possible source for the high K+ concentration in the endolymph could be the rich blood supply of the stria vascularis [20]. As a high concentration of extracellular K+ can depolarize Hensen’s cells [21], an outward K+ current during hyperpolarization could increase the concentration of K+ in the endolymph and maintain a high level of EP. Any factor that influences EP will result in hearing loss. Evidence suggests that ATP receptors are abundant in the organ of Corti of guinea pigs, including Hensen’s cells, outer hair cells, Deiter’s cells, pillar cells, and inner hair cells [22]. The introduction of extracellular ATP into the endolymphatic compartment of the guinea pig cochlea has a significant dose-dependent suppressive effect on EP and CM. In this experiment, ATP inhibited the outward flow of K+ from Hensen’s cells and this could decrease EP and CM [23], implying that ATP may modulate the outward K+ current of Hensen’s cells to maintain the high concentration of K+ in the endolymph and modulate hearing.
ATP is known to be the basis of Ca2+ current in numerous tissues, including the cochlea [24]. In this study, a high concentration of ATP evoked an inward current that could be blocked by nifedipine (i,e,, the blockage of L-type calcium channels), suggesting that a high likelihood that the inward current was carried by Ca2+; moreover, the inward current could be blocked by suramin, a purinergic receptor antagonist. This suggests that the inward current is reversed by ATP though purinergic receptors that could include a ligand-gated channel (P2X) or a G-protein-related channel (P2Y). As the inward current was carried by Ca2+, it is likely to be a G-protein-related channel.
After the cochlea was perfused with an APS, which contained nifedipine, and exposed to noise, the amplitude rise in the CAP threshold and reduction in CM were lower than with noise alone, showing that nifedipine may have protective effects on NIHL, which is consistent with previous experimental results [15]. Previous studies have demonstrated that endolymphatic Ca2+ plays an considerable role in hearing [25]. Noise exposure induces increased Ca2+ concentration along with linear auditory brainstem response (ABR) threshold shifts [18]. When Ca2+ channel blocker and noise exposure were introduced simultaneously, it was subtractive instead of simply additive, with a certain level of “counteraction” [15]. ATP increases the intracellular Ca2+ concentration ([Ca2+]i) in Hensen’s cells, and the influx of extracellular Ca2+ is essentially induced by increases of [Ca2+]i [26]. Calcium imbalance, in turn, damages the mitochondrial function and the increase of [Ca2+]i causes local vasoconstriction, resulting in hypoperfusion of the inner ear [27]. In most cells, the reduction of ATP is caused by mitochondrial damage and dysemia [28]. A study showed that the depletion of ATP, induced by traumatic noise exposure, could evoke hair cell death and was associated with Ca2+ influx [14]. In this study, we found that nifedipine could also block the inward Ca2+ current induced by ATP. Many studies have shown that the accrescence of intracellular calcium was associated with hearing loss and the calcium channel blockers could improve hearing loss caused by factors such as age, noise, and chemotherapy [29–31]. These findings indirectly demonstrate that nifedipine may have a possible prophylactic effect against NIHL.
Conclusions
In this study, we demonstrated that: 1) ATP inhibited the voltage-gated K+ channel. The inward current evoked by ATP at high concentrations was IK+-dependent and was triggered by ATP through a purinergic receptor. 2) Nifedipine may have a prophylactic effect against NIHL.
Footnotes
Source of support: This research was supported by the National Natural Science Foundation of China (No. 30070812); the Chinese Beijing Municipal Science and Technology Commission (No. Z131100004013019); and the Clinical Research Support Fund of Chinese People’s Liberation Army General Hospital (No. 2012FC-TSYS-4017)
References
- 1.Nam JH, Fettiplace R. Force transmission in the organ of Corti micromachine. Biophys J. 2010;98(12):2813–21. doi: 10.1016/j.bpj.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu N, Zhao HB. Modulation of outer hair cell electromotility by cochlear supporting cells and gap junctions. PLoS One. 2009;4(11):e7923. doi: 10.1371/journal.pone.0007923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blödow A, Ngezahayo A, Ernst A, Kolb HA. Calmodulin antagonists suppress gap junction coupling in isolated Hensen cells of the guinea pig cochlea. Pflugers Arch. 2003;446(1):36–41. doi: 10.1007/s00424-002-1004-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Liang C, Chen J, et al. Active cochlear amplification is dependent on supporting cell gap junctions. Nat Commun. 2013;4:1786. doi: 10.1038/ncomms2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Zhao HB. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010;6(2):221–29. doi: 10.1007/s11302-010-9184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Zhao HB. ATP activates P2X receptors to mediate gap junctional coupling in the cochlea. Biochem Biophys Res Commun. 2012;426(4):528–32. doi: 10.1016/j.bbrc.2012.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton-Jones RT, Vlajkovic SM, Thorne PR, et al. Properties of ATP-gated ion channels assembled from P2X2 subunits in mouse cochlear Reissner’s membrane epithelial cells. Purinergic Signal. 2015;11(4):551–60. doi: 10.1007/s11302-015-9473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121(1):10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 9.Yan D, Zhu Y, Walsh T, et al. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc Natl Acad Sci USA. 2013;110(6):2228–33. doi: 10.1073/pnas.1222285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16(2):235–45. doi: 10.1517/14728214.2011.552427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29(2):70–75. [PMC free article] [PubMed] [Google Scholar]
- 12.Apati A, Berecz T, Sarkadi B. Calcium signaling in human pluripotent stem cells. Cell Calcium. 2016;59(2–3):117–23. doi: 10.1016/j.ceca.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Antony AN, Paillard M, Moffat C, et al. MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun. 2016;7:10955. doi: 10.1038/ncomms10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen FQ, Zheng HW, Hill K, Sha SH. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32(36):12421–30. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Niu YG, Li WX, et al. Interaction of a calcium channel blocker with noise in cochlear function in guinea pig. Acta Otolaryngol. 2012;132(11):1140–44. doi: 10.3109/00016489.2012.690534. [DOI] [PubMed] [Google Scholar]
- 16.Todt I, Ngezahayo A, Ernst A, Kolb HA. Inhibition of gap junctional coupling in cochlear supporting cells by gentamicin. Pflugers Arch. 1999;438(6):865–67. doi: 10.1007/s004249900109. [DOI] [PubMed] [Google Scholar]
- 17.Peng Y, Chen J, He S, et al. Release of ATP from marginal cells in the cochlea of neonatal rats can be induced by changes in extracellular and intracellular ion concentrations. PLoS One. 2012;7(10):e47124. doi: 10.1371/journal.pone.0047124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oesterle EC, Dallos P. Intracellular recordings from supporting cells in the guinea pig cochlea: DC potentials. J Neurophysiol. 1990;64(2):617–36. doi: 10.1152/jn.1990.64.2.617. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Sacchi J. Isolated supporting cells from the organ of Corti: Some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res. 1991;52(1):89–98. doi: 10.1016/0378-5955(91)90190-k. [DOI] [PubMed] [Google Scholar]
- 20.Wada J, Paloheimo S, Thalmann I, et al. Maintenance of cochlear function with artificial oxygen carriers. Laryngoscope. 1979;89(9 Pt 1):1457–73. doi: 10.1002/lary.5540890911. [DOI] [PubMed] [Google Scholar]
- 21.Sugasawa M, Erostegui C, Blanchet C, Dulon D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol. 1996;491(Pt 3):707–18. doi: 10.1113/jphysiol.1996.sp021251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32(3):128–41. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Munoz DJ, Thorne PR, Housley GD. P2X receptor-mediated changes in cochlear potentials arising from exogenous adenosine 5′-triphosphate in endolymph. Hear Res. 1999;138(1–2):56–64. doi: 10.1016/s0378-5955(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 24.Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14(6):526–29. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol. 2010;518(18):3785–802. doi: 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horváth T, Polony G, Fekete Á, et al. ATP-evoked intracellular Ca(2+) signaling of different supporting cells in the hearing mouse hemicochlea. Neurochem Res. 2016;41(1–2):364–75. doi: 10.1007/s11064-015-1818-4. [DOI] [PubMed] [Google Scholar]
- 27.Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol. 2003;8(4):207–21. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- 28.Nagashima R, Yamaguchi T, Kuramoto N, Ogita K, et al. Acoustic overstimulation activates 5′-AMP-activated protein kinase through a temporary decrease in ATP level in the cochlear spiral ligament prior to permanent hearing loss in mice. Neurochem Int. 2011;59(6):812–20. doi: 10.1016/j.neuint.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Yu YF, Wu WY, Xiao GS, et al. Effect of T-type calcium channel blockers on spiral ganglion neurons of aged C57BL/6J mice. Int J Clin Exp Med. 2015;8(9):15466–73. [PMC free article] [PubMed] [Google Scholar]
- 30.Bao J, Hungerford M, Luxmore R, et al. Prophylactic and therapeutic functions of drug combinations against noise-induced hearing loss. Hear Res. 2013;304:33–40. doi: 10.1016/j.heares.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waissbluth S, Daniel SJ. Cisplatin-induced ototoxicity: Transporters playing a role in cisplatin toxicity. Hear Res. 2013;299:37–45. doi: 10.1016/j.heares.2013.02.002. [DOI] [PubMed] [Google Scholar]