Abstract
Background
Surgery combined with chemotherapy is an important therapy for non-small cell lung cancer (NSCLC). However, chemotherapy drug resistance seriously hinders the curative effect. Studies show that DNA repair genes ERCC1 and BRCA1 are associated with NSCLC chemotherapy, but their expression and mechanism in NSCLC chemotherapy drug-resistant cells has not been elucidated.
Material/Methods
NSCLC cell line A549 and drug resistance cell line A549/DDP were cultured. Real-time PCR and Western blot analyses were used to detect ERCC1 and BRCA1 mRNA expression. A549/DDP cells were randomly divided into 3 groups: the control group; the siRNA-negative control group (scramble group); and the siRNA ERCC1 and BRCA1siRNA transfection group. Real-time PCR and Western blot analyses were used to determine ERCC1 and BRCA1 mRNA and protein expression. MTT was used to detect cell proliferation activity. Caspase 3 activity was tested by use of a kit. Western blot analysis was performed to detect PI3K, AKT, phosphorylated PI3K, and phosphorylated AKT protein expression.
Results
ERCC1 and BRCA1 were overexpressed in A549/DDP compared with A549 (P<0.05). ERCC1 and BRCA1siRNA transfection can significantly reduce ERCC1 and BRCA1 mRNA and protein expression (P<0.05). Downregulating ERCC1 and BRCA1 expression obviously inhibited cell proliferation and increased caspase 3 activity (P<0.05). Downregulating ERCC1 and BRCA1 significantly decreased PI3K and AKT phosphorylation levels (P<0.05).
Conclusions
ERCC1 and BRCA1 were overexpressed in NSCLC drug-resistant cells, and they regulated lung cancer occurrence and development through the phosphorylating PI3K/AKT signaling pathway.
MeSH Keywords: 1-Naphthylamine; Abnormalities, Drug-Induced; Genes, BRCA1
Background
Millions of people worldwide die of lung cancer every year. Lung cancer has the highest mortality rate among all malignant tumors. Its incidence and mortality in China are the highest in the world. In the past 20 years, lung cancer in China has become increasingly common in younger people, with a trend toward higher morbidity and mortality [1,2]. Lung cancer mainly includes small cell lung cancer (SCL) and non-small cell lung cancer (NSCLC), according to pathology, and the incidence of NSCLC accounts for more than 80% of all cases [3]. Lung cancer treatment has greatly advanced, including improved surgical technique and a variety of new chemotherapy drugs. Chemotherapy is an indispensable adjuvant therapy for lung cancer [4]. During the process of chemotherapy, however, lung cancer patients often develop secondary resistance after 10–14 months, which seriously limits the clinical effectiveness of chemotherapy. Overall, lung cancer patient survival and prognosis are still poor [5,6]. The mechanism of NSCLC drug resistance has not been fully defined. Research shows it may be related to the increasing ability of the body to inactivate chemotherapy drugs, compound formation, reducing drug accumulation, or DNA repair ability enhancement in the treatment process. DNA repair ability enhancement plays a key role in drug resistance [7,8].
Excision repair cross-complementary gene 1 (ERCC1) and breast cancer susceptibility gene 1 (BRCA1) are important members of the DNA repair-related gene system [9,10]. Studies showed that they can reflect the sensitivity to chemotherapy drugs, and their overexpression can affect the clinical curative effect of chemotherapy drugs in NSCLC [11,12]. However, ERCC1 and BRCA1 expression and related mechanism in NSCLC chemotherapy drug-resistant cell lines have not been fully elucidated. This study intended to provide an experimental basis for clinical lung cancer drug resistance through analyzing the expression and function of ERCC1 and BRCA1 in NSCLC drug-resistant cell lines.
Material and Methods
Main instruments and reagents
A549 and A549/DDP cell lines were purchased from the ATCC Cell Bank (USA). DMEM medium, fetal bovine serum (FBS), EDTA, and penicillin-streptomycin were bought from Hyclone (USA). Dimethyl sulfoxide and MTT powder were from Gibco. Tyresin-EDTA was from Sigma (USA). PVDF membrane was from Pall Life Sciences. Western blot-related chemical reagents were obtained from Beyotime. ECL reagent was from Amersham Biosciences. ERCC1, BRCA1, PI3K, AKT, phosphorylated PI3K, and phosphorylated AKT monoclonal antibodies, and HRP-tagged IgG secondary antibody were from Cell Signaling. Caspase 3 activity kits were purchased from Pall Life Sciences. RNA extraction kits and reverse transcription kits were from Axygen (USA). Other common reagents were purchased from Sangon. The Labsystem version 1.3.1 microplate reader was purchased from Bio-Rad (USA).
Methods
A549 and A549/DDP cell culture and grouping
A549 and A549/DDP cell lines were preserved in liquid nitrogen and thawed in a 37°C water bath. After centrifugation at 1000 rpm for 3 min, the cells were cultured in fresh medium and maintained at 37°C and 5% CO2. A549 and A549/DDP cells were seeded into dishes at 1×106/cm2 and maintained in high-glucose DMEM containing 10% FBS, 100 U/ml penicillin, and 100 g/ml streptomycin. The 3rd to 8th generation cells in logarithmic phase were used for experiments. A549/DDP cells were randomly divided into 3 groups: the control group, the SiRNA-negative control group (scramble group), and the siRNA group.
ERCC1 and BRCA1 siRNA transfection
ERCC1 siRNA and BRCA1 siRNA were transfected to A549/DDP cells, respectively. ERCC1 siRNA primers: forward, 5′-ATATCTGCCAACGTGCTACGT-3′; reverse, 5′-CGTACTCGAATTCAGCTACGT-3′. ERCC1 siRNA negative control primers: 5′-ACTTCCGTAATGGACCTACGT-3′, 5′-ACCCTTCAATGGACACGGTTT-3′. BRCA1 siRNA primers: forward, 5′-CTGGATTTACGTCGACCTCAA-3′; reverse, 5′-CTTTGGCGCATAAGACCGT-3′. BRCA1 siRNA negative control primers: 5′-CTCTATGAACACCTAGCT-3′, 5′-ACCTTGAACTACCTAGCT-3′. After cell density reached 70–80%, ERCC1 siRNA and BRCA1 siRNA together with liposome were added to 200 μl serum-free medium, while negative control liposome was added to another medium for 15 min incubation at room temperature. Lipo2000 was then mixed with ERCC1 and BRCA1 siRNA for 30 min at room temperature. After changing cell medium to 1.6 ml serum-free medium, the mixture was added to each group and incubated in 37°C and 5% CO2 for 6 h. After changing the medium, the cells were then cultured for 48 h for the following experiment.
Real time PCR
Total RNA was extracted using Trizol and reverse transcribed to DNA according to the manufacturer’s instructions. Primers were designed by Primer 6.0 and synthesized by Invitrogen (Table 1). Real-time PCR was used to test target genes. The reaction was performed at 55°C for 1 min, followed by 35 cycles of 92°C for 30 s, 58°C for 45 s, and 72°C for 35 s. GAPDH was selected as the internal reference. The results were calculated by 2−ΔCt method.
Table 1.
Primer sequence.
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| GADPH | AGTACCAGTCTGTTGCTGG | TAATAGACCCGGATGTCTGGT |
| ERCC1 | ATGAACTTTCTCTGTCTTGG | TCACCG CCTCGGCTTGTCACA |
| BRCA1 | CTCCCACAGACTCTGTAAG | GCATTACCTGGGGCTGTAATT |
MTT assay
A549/DDP cells in logarithmic phase were seeded in 96-well plates at 3000/well and divided into control group, scramble group, and siRNA group, with 5 replicates each. After 48 h, 20 μl MTT solution at 5 g/L was added to each well for 4 h in the incubator. After removing the supernatant, 150 μl DMSO was added and the plate was vibrated for 10 min. The plate was read on a microplate reader at 570 nm to calculate the cell proliferation rate.
Caspase-3 activity detection
Caspase 3 activity was determined according to the manufacturer’s instructions. After enzyme digestion, the cells were centrifuged at 4°C and 600 g for 5 min. After being cracked on ice for 15 min, the cells were centrifuged at 4°C and 20 000 g for 5 min. The cells were detected at 405 nm to calculate caspase 3 activity after adding 2 mM Ac-DEVD-pNA. All experiments were repeated 3 times.
Western blot analysis
A549/DDP cells were cracked on ice for 15–30 min after adding cell lysis. After ultrasonication at 5 s ×4, the cells were centrifuged at 4°C and 10 000 g for 5 min and the supernatant was moved to a new EP tube. The protein was stored at −20°C. Total protein was separated by 10% SDS-PAGE electrophoresis and transferred to PVDF membrane by half-dry method. After blocking with 5% skim milk for 2 h, the membrane was incubated in various concentrations of ERCC1, BRCA1, PI3K, AKT, phosphorylated PI3K, and phosphorylated AKT antibody at 4°C overnight. After being incubated with secondary antibody at 1:2000, the membrane was exposure-imaged on X-ray film. Quantity One software was used to determine binding density. All experiments were repeated 4 times.
Statistical analysis
All statistical analyses were performed on SPSS 16.0 software. Measurement data are presented as mean ± standard deviation (χ2±SD) and compared using one-way ANOVA. P<0.05 was considered as statistically significant.
Results
ERCC1 and BRCA1 mRNA expression in A549 and A549/DDP
Real-time PCR results showed that ERCC1 and BRCA1 mRNA were overexpressed in A549/DDP, compared with A549 (P<0.05) (Figure 1).
Figure 1.
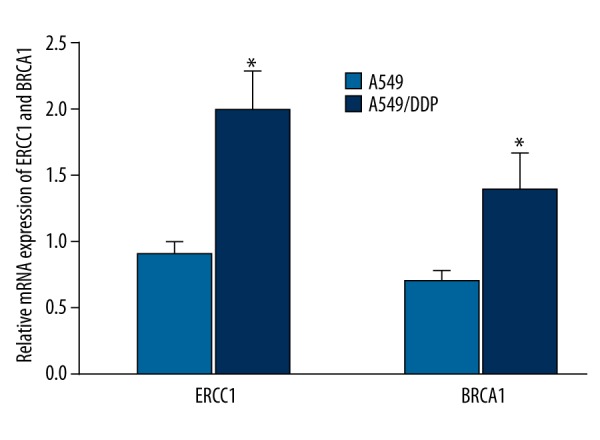
ERCC1 and BRCA1 mRNA expression in A549 and A549/DDP * P<0.05, compared with A549.
ERCC1 and BRCA1 protein expression in A549 and A549/DDP cells
Western blot analysis was used to compare ERCC1 and BRCA1 protein expression in A549 and drug-resistant cell line A549/DDP. We found that, similar to mRNA results, ERCC1 and BRCA1 protein expression was clearly elevated in A549/DDP compared with A549 (P<0.05) (Figures 2, 3), suggesting that ERCC1 and BRCA1 overexpression was closely related to NSCLC drug resistance.
Figure 2.
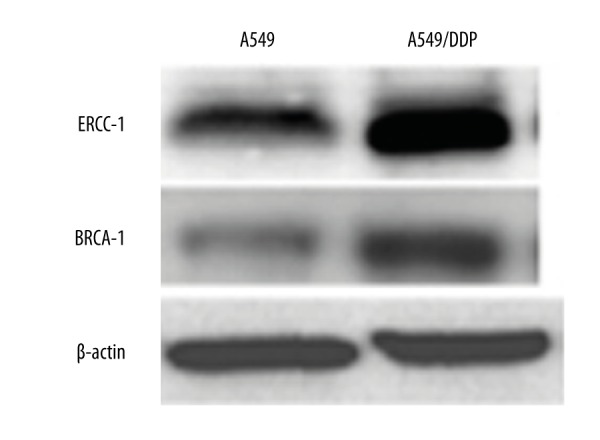
ERCC1 and BRCA1 protein expression in A549 and A549/DDP.
Figure 3.
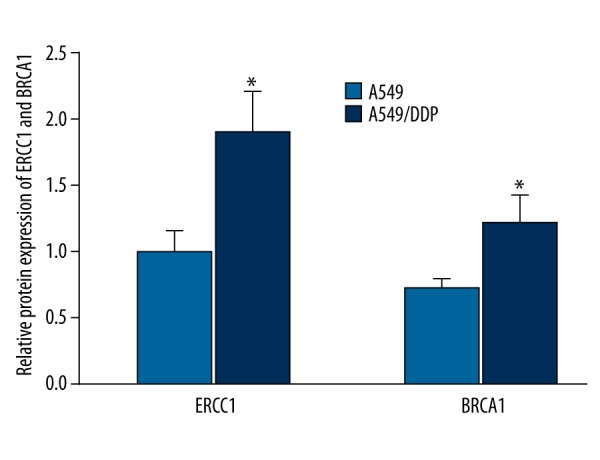
ERCC1 and BRCA1 protein expression analysis in A549 and A549/DDP cell. * P<0.05, compared with A549.
siRNA impact on ERCC1 and BRCA1 expression in A549/DDP
ERCC1 and BRCA1 siRNA were co-transfected to A549/DDP cells for 48 h. The results showed that ERCC1 and BRCA1 siRNA can significantly suppress ERCC1 and BRCA1 mRNA and protein expression in A549/DDP cells (P<0.05) (Figures 4, 5).
Figure 4.
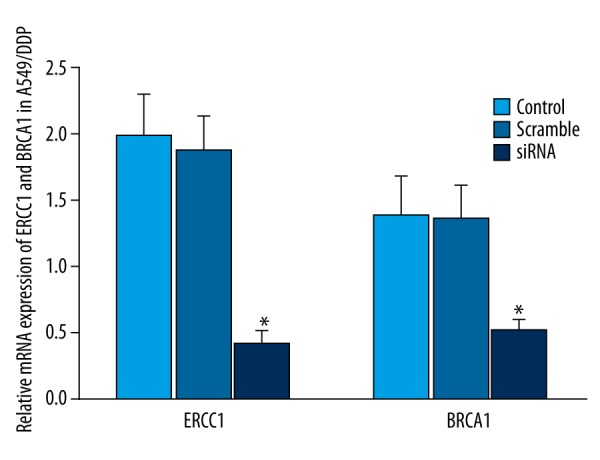
Effect of siRNA on ERCC1 and BRCA1 expression in A549/DDP. * P<0.05, compared with control.
Figure 5.
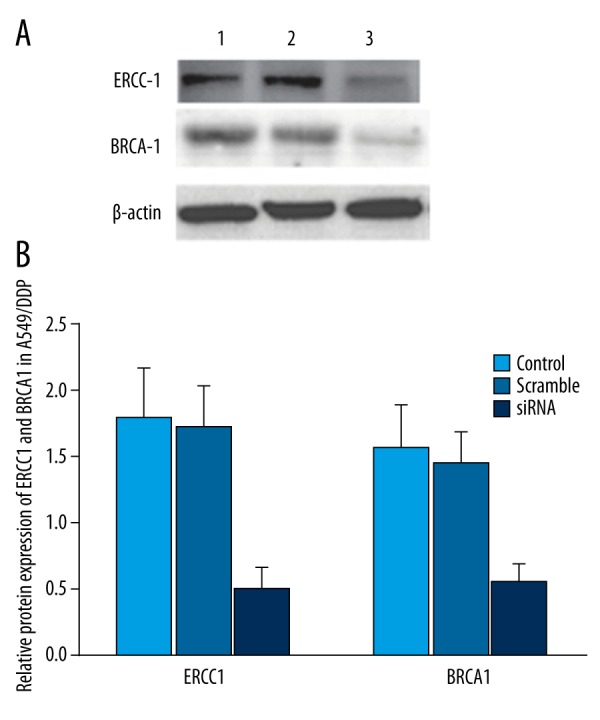
Effect of siRNA on ERCC1 and BRCA1 protein expression in A549/DDP. (A) ERCC1 and BRCA1 protein expression in A549/DDP. (B) ERCC1 and BRCA1 protein expression analysis in A549/DDP. 1 - control; 2 - scramble group; 3 - siRNA group. * P<0.05, compared with control.
Downregulating ERCC1 and BRCA1 expression impact on A549/DDP cell proliferation
MTT assay was used to determine the effect of downregulating ERCC1 and BRCA1 on A549/DDP cell proliferation. The results revealed that ERCC1 and BRCA1 siRNA co-transfection reduced ERCC1 and BRCA1 expression and obviously suppressed A549/DDP cell proliferation (P<0.05) (Figure 6), indicating that downregulating ERCC1 and BRCA1 in NSCLC facilitated suppressing drug-resistant cell proliferation.
Figure 6.
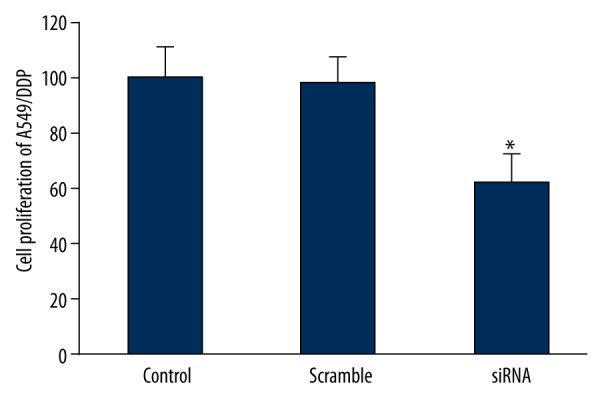
Effect of downregulating ERCC1 and BRCA1 expression on A549/DDP cell proliferation. * P<0.05, compared with control.
Effect of downregulating ERCC1 and BRCA1 expression on caspase 3 activity in A549/DDP
Downregulating ERCC1 and BRCA1 expression markedly increased caspase 3 activity in A549/DDP (P<0.05) (Figure 7), suggesting that downregulating ERCC1 and BRCA1 can mediate cell apoptosis and promote drug-resistant cell death.
Figure 7.
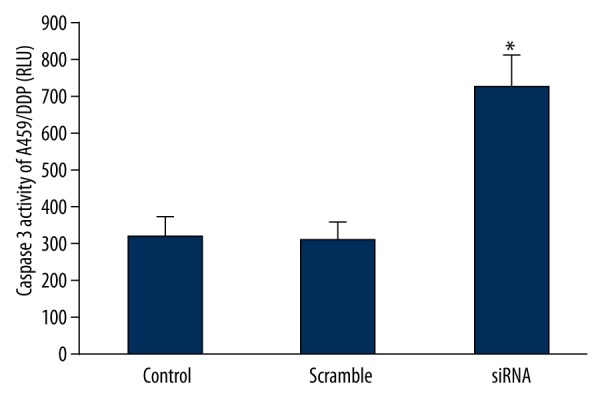
Effect of downregulating ERCC1 and BRCA1 expression on caspase 3 activity in A549/DDP. * P<0.05, compared with control.
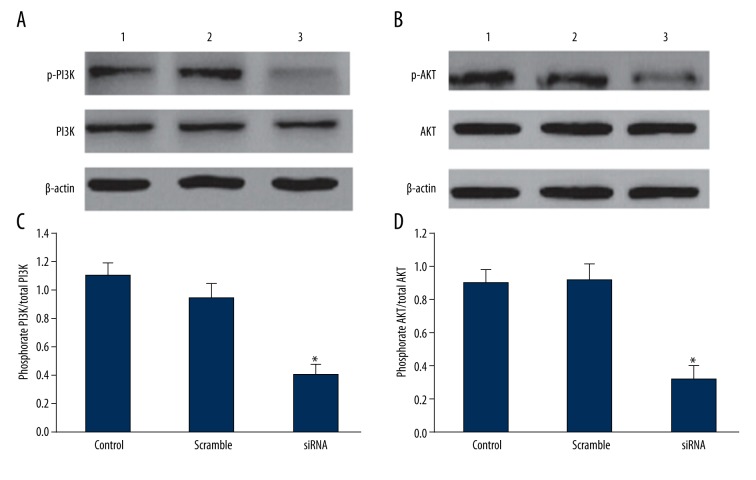
Effect of downregulating ERCC1 and BRCA1 expression on PI3K/AKT signaling pathway
Downregulating ERCC1 and BRCA1 significantly decreased PI3K and AKT phosphorylation levels (P<0.05), but PI3K/AKT total protein level showed no statistical changes (Figure 8), suggesting that ERCC1 and BRCA1 can regulate drug-resistant lung cancer cell proliferation through inhibiting PI3K/AKT phosphorylation level.
Figure 8.
Effect of downregulating ERCC1 and BRCA1 expression on PI3K/AKT signaling pathway. (A) Effect of downregulating ERCC1 and BRCA1 expression on PI3K expression. (B) Effect of downregulating ERCC1 and BRCA1 expression on AKT expression. (C) Effect of downregulating ERCC1 and BRCA1 expression on PI3K expression analysis. (D) Effect of downregulating ERCC1 and BRCA1 expression on AKT expression analysis. 1 - control; 2 - scramble group; 3 - siRNA group. * P<0.05, compared with control.
Discussion
First-line chemotherapy drugs for NSCLC include platinum, such as cisplatin and carboplatin, while platinum resistance often leads to chemotherapy failure [13,14]. Platinum drugs can combine with nucleophilic DNA in tumor cells to form DNA-platinum adduct, thus inhibiting DNA replication, causing DNA damage, and ultimately inducing tumor cell death [15]. However, the DNA damage repair system can modify DNA damage to various extents, which affects the sensitivity of chemotherapy drugs. Therefore, the repair ability of DNA repair genes is closely related to the effectiveness of lung cancer chemotherapy. High repair ability induces chemotherapy resistance formation and causes chemotherapy failure [16].
In the clinical trials, ERCC1 and BRCA1 expression increased significantly in NSCLC patients with chemotherapy resistance. Thus, they can reflect the sensitivity of patients to chemotherapy drugs, as well as indicating prognosis [11]. However, the mechanism of ERCC1 and BRCA1 in NSCLC chemotherapy drug-resistant cells has not been fully defined. This research analyzed ERCC1 and BRCA1 mRNA and protein expression in NSCLC chemotherapy drug-resistant cells and drug-sensitive cells, and discovered that ERCC1 and BRCA1 are overexpressed in cell line A549/DDP, which is consistent with clinical findings [11,12]. Furthermore, we co-transfected ERCC1 and BRCA1siRNA to the drug-resistant cell line, thus confirming that downregulating ERCC1 and BRCA1 expression in NSCLC can regulate apoptosis, inhibit cell proliferation, and promote drug-resistant lung cancer cell death. The cellular DNA repair system includes base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), and double-strand break repair (DSBR). During DNA damage identification and removal, NER is the rate-determining step in the DNA repair pathway. ERCC1 and BRCA1 are important members of the NER pathway, which may reflect NER repair level through changing expression and activity reaction [17,18]. After removing the DNA-damaged part, ERCC1 and BRCA1 utilize ribonucleotide provided by nucleotide reductase-regulating subunits M1 to fill the gap, so as to complete DNA synthesis and repair. They are key materials in the DNA repair system [19]. Therefore, targeting BRCA1 and ERCC1 can significantly inhibit DNA synthesis and repair, leading to tumor cell death.
This study discovered that downregulating BRCA1 and ERCC1 suppressed PI3K/AKT phosphorylation levels to regulate chemotherapy-resistant lung cancer cell proliferation. The PI3K/Akt signaling pathway plays an important role in physiological and pathological process of tumor occurrence and development. In microvascular endothelial cells of NSCLC, endothelin-1 mediates the PI3K/AKT signaling pathway through tyrosine kinase C-SRC. It then activates C-Jun/AP-1 protein coupling and combines with the corresponding locus of COX-2 promoter, thus promoting COX-2 transcription and expression, and facilitating prostaglandin E2 (PGE2) biosynthesis and release, thereby accelerating angiogenesis [20]. PI3K/AKT can promote tumor angiogenesis, induce downstream signaling molecule structural changes, and the activate bypass signaling pathway and epithelial-mesenchymal transformation, which are related to drug resistance [21,22].
Conclusions
ERCC1 and BRCA1 are overexpressed in NSCLC drug-resistant cells, and regulate lung cancer occurrence and development through the PI3K/AKT pathway. Therefore, detecting ERCC1 and BRCA1 expression can not only help in NSCLC diagnosis and prognosis, but also provide a reference basis for individualized treatment in patients with advanced NSCLC.
Footnotes
Source of support: Departmental sources
References
- 1.Zaarour M, Weerasinghe C, Nazha B, et al. Epidermal growth factor receptor tyrosine kinase inhibitors in elderly patients with non-small cell lung cancer. Expert Rev Anticancer Ther. 2015;15:1–10. doi: 10.1586/14737140.2015.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Genestreti G, Grossi F, Genova C, et al. Third- and further-line therapy in advanced non-small-cell lung cancer patients: An overview. Future Oncol. 2014;10:2081–96. doi: 10.2217/fon.14.96. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Lan H, Su Y, Li J, Wan J. Clinicopathological significance and potential drug target of RUNX3 in non-small cell lung cancer: a meta-analysis. Drug Des Devel Ther. 2015;9:2855–65. doi: 10.2147/DDDT.S76358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasari R, De Carvalho A, Medellin DC, et al. Wittig derivatization of sesquiterpenoid polygodial leads to cytostatic agents with activity against drug resistant cancer cells and capable of pyrrolylation of primary amines. Eur J Med Chem. 2015;103:226–37. doi: 10.1016/j.ejmech.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maione P, Sacco PC, Sgambato A, et al. Overcoming resistance to targeted therapies in NSCLC: Current approaches and clinical application. Ther Adv Med Oncol. 2015;7:263–73. doi: 10.1177/1758834015595048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel J, Richters A, Getlik M, et al. Targeting drug resistance in EGFR with covalent inhibitors: A structure-based design approach. J Med Chem. 2015;58:6844–63. doi: 10.1021/acs.jmedchem.5b01082. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Qin G, Luo M, et al. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis. 2015;6:e1829. doi: 10.1038/cddis.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanimoto A, Yamada T, Nanjo S, et al. Receptor ligand-triggered resistance to alectinib and its circumvention by Hsp90 inhibition in EML4-ALK lung cancer cells. Oncotarget. 2014;5:4920–28. doi: 10.18632/oncotarget.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilen N, Tekin SB, Topdagi O. ERCC1 expression in non-small cell lung and esophageal cancer. Eurasian J Med. 2014;46:84–88. doi: 10.5152/eajm.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Qing Y, Guan W, et al. Predictive value of APE1, BRCA1, ERCC1 and TUBB3 expression in patients with advanced non-small cell lung cancer (NSCLC) receiving first-line platinum-paclitaxel chemotherapy. Cancer Chemother Pharmacol. 2014;74:777–86. doi: 10.1007/s00280-014-2562-1. [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, Yan X, Zhang G, et al. The predictive value of ERCC1 and p53 for the effect of panobinostat and cisplatin combination treatment in NSCLC. Oncotarget. 2015;6:18997–9005. doi: 10.18632/oncotarget.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Pan H, Liu D, et al. Excision repair cross complementation group 1 is a chemotherapy-tolerating gene in cisplatin-based treatment for non-small cell lung cancer. Int J Oncol. 2015;46:809–17. doi: 10.3892/ijo.2014.2784. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Chen P, Tang M, et al. Tumstatin 185-191 increases the sensitivity of non-small cell lung carcinoma cells to cisplatin by blocking proliferation, promoting apoptosis and inhibiting Akt activation. Am J Transl Res. 2015;7:1332–44. [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda S, Horinouchi H, Fujiwara Y, et al. Cytotoxic chemotherapy may overcome the development of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy. Lung Cancer. 2015;89:287–93. doi: 10.1016/j.lungcan.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Zou F, Xie G, Ma JA, et al. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: A case report. Oncol Lett. 2015;9:2239–43. doi: 10.3892/ol.2015.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyler A, Johansson A, Karlsson T, et al. Targeting glucosylceramide synthase induction of cell surface globotriaosylceramide (Gb3) in acquired cisplatin-resistance of lung cancer and malignant pleural mesothelioma cells. Exp Cell Res. 2015;336:23–32. doi: 10.1016/j.yexcr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Blein S, Barjhoux L, et al. GENESIS Investigators. Targeted sequencing of the mitochondrial genome of women at high risk of breast cancer without detectable mutations in BRCA1/2. PLoS One. 2015;10:e0136192. doi: 10.1371/journal.pone.0136192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Sun L, Li C, et al. Quantitative detection of methylation of FHIT and BRCA1 promoters in the serum of ductal breast cancer patients. Biomed Mater Eng. 2015;26(Suppl 1):S2217–22. doi: 10.3233/BME-151527. [DOI] [PubMed] [Google Scholar]
- 19.Dietel M, Johrens K, Laffert MV, et al. A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: A review focussing on clinical relevance. Cancer Gene Ther. 2015;22:417–30. doi: 10.1038/cgt.2015.39. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Zhu H, Xu X, et al. Inactivated Sendai virus induces apoptosis and autophagy via the PI3K/Akt/mTOR/p70S6K pathway in human non-small cell lung cancer cells. Biochem Biophys Res Commun. 2015;465:64–70. doi: 10.1016/j.bbrc.2015.07.130. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Yu S, Li H, et al. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett. 2015;589:2248–56. doi: 10.1016/j.febslet.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Zhao R, Chen M, Jiang Z, et al. Platycodin-D induced autophagy in non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathways. J Cancer. 2015;6:623–31. doi: 10.7150/jca.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]