Abstract
Background
Gastrointestinal motility disorder is the main clinical manifestation in functional dyspepsia (FD) patients. Electroacupuncture is effective in improving gastrointestinal motility disorder in FD; however, the underlying mechanism remains unclear. It has been demonstrated that interstitial cells of Cajal (ICC) are pacemaker cells in the gastrointestinal tract, and the pacemaker potential is transmitted to nearby cells through gap junctions between ICC or ICC and the smooth muscle. Therefore, this study aimed to assess the effects of electroacupuncture on ICC ultrastructure and expression of the gap junction protein connexin 43 (Cx43) in FD rats.
Material/Methods
The animals were randomized into 3 groups: control, model, and electroacupuncture. Electroacupuncture was applied at Zusanli (ST36) in the electroacupuncture group daily for 10 days, while no electroacupuncture was applied to model group animals.
Results
Ultrastructure of ICC recovered normally in gastric antrum and small intestine specimens was improved, with Cx43 expression levels in these tissues significantly increased in the electroacupuncture group compared with the model group.
Conclusions
These findings indicated that electroacupuncture is effective in alleviating ICC damage and reduces Cx43 levels in FD rats, and suggest that ICC and Cx43 are involved in electroacupuncture treatment in rats with FD to improve gastrointestinal motility disorders.
MeSH Keywords: Dyspepsia, Electroacupuncture, Interstitial Cells of Cajal
Background
Functional dyspepsia (FD) is a clinical syndrome defined by symptoms thought to originate from the gastroduodenal region in the absence of organic, systemic, or metabolic disease likely to explain the manifestations [1]. FD prevalence ranges from 8% to 23% in Asia [2]. According to the Rome III consensus, the main FD symptoms include bothersome postprandial fullness, early satiety, epigastralgia, and epigastric burning. FD is a common morbidity and greatly impacts the quality of life, healthcare infrastructure usage, and socioeconomic costs [3]. The underlying pathophysiology of FD is incompletely understood and may be heterogeneous, possibly including mechanisms such as hypersensitivity to gastric distension [4], impaired meal accommodation [5], and delayed gastric emptying [6].
FD treatment most often requires the use of medications for controlling the symptoms. However, as a recurrent disorder with various etiopathogenesis mechanisms, treatments with established efficacy for patients with FD are still limited [7,8]. Acupuncture is an essential part of Traditional Chinese Medicine (TCM), which has been used for thousands of years. Electroacupuncture is a technique combining acupuncture with the electric current. A number of studies provided evidence for a beneficial effect of acupuncture on FD [9]. Acupuncture or electroacupuncture is able to alter gastrointestinal motility functions and improve gastrointestinal motility disorders [10]. ST36 belongs to the gastric meridian according to traditional acupuncture theories, which is believed to be one of the best acupuncture points specifically selected for gastrointestinal diseases [11].
Interstitial Cells of Cajal (ICC), which mediate input from the gastrointestinal motor nervous system to the smooth muscle, are pacemaker cells in the gastrointestinal tract [12]. ICC generate spontaneous electrical slow waves and regulate rhythmic peristalsis. Loss of ICC typically causes gastrointestinal dysmotility [13]. Increasing evidence suggests that ICC depletion and damage, network disruption and channel apathies may lead to aberrant slow wave initiation and conduction [14]. ICC have close contact with nerve varicosities and smooth muscle cells; they form gap junctions with each other, providing a route for the diffusion of low molecular weight materials as an important intercellular signal communication between these types of cells. Thus, gap junctions have a crucial role in mediating the synchronized contraction of smooth muscle cells. The gap junction protein connexin 43 (Cx43) is the most important protein in this process and present in ICC with convincing co-localization with c-kit [15]. Lack of Cx43 expression may be partly responsible for smooth muscle motility dysfunction [16]. Cx43 also has close relations with acupuncture, with high Cx43 density found at acupuncture points; in addition, gap junction intercellular communication may play an important role in the function of meridians [17]. Therefore, Cx43 may be an important mediator in the mechanisms of acupuncture and meridians [18].
In this study, a rat model of FD was established to investigate the effects of electroacupuncture on ICC ultrastructure and Cx43 expression.
Material and Methods
Animals
A total of 36 Sprague-Dawley (SD) rats (18 males and 18 females, 8-weeks-old, 180–220 g) purchased from Laboratory Animal Center of Hunan University of Chinese Medicine were evaluated in this study. The animals were maintained under a 12-h light/12-h dark cycle at 20±2°C with a relative humidity of 50–70%. This study was approved by the Ethics Committee of Hunan University of Chinese Medicine and was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. After 3 days of adaptation, the rats were randomly divided into 3 groups, including the normal control, model (only modeling), and electroacupuncture (modeling followed by electroacupuncture) groups.
Establishment of the FD model
The functional dyspepsia rat model was established according to Hai-jun Guo et al. [19] A total of 6 rats were placed into 1 cage. Their tails were stimulated with sponge forceps at the last-third end to anger them and cause fighting, 30 minutes at a time, but without injury. The procedure was repeated 4 times (8:30, 10:30, 14:00, and 16:00) 1 day, for 7 continuous days.
Electroacupuncture treatment
After the model was established, rats in the electroacupuncture group received electroacupuncture treatment from the 8th day. Based on previous studies [20,21], Zusanli (ST36) was selected as the experimental acupoint, and was found according to Experimental Acupuncture [22]. The acupoint was punctured at 3–5 mm in depth. Electroacupuncture was provided by SDZ-Nerve and -Muscle Stimulator after fixing the rats (Suzhou Medical Sino-foreign Joint Venture Suzhou Hua Tuo Medical Instruments Co., Ltd., Suzhou, China). The filiform needles were sterile Hwato acupuncture needles for single use (25 mm and 0.30 mm in length and diameter, respectively) (Suzhou Medical Sino-foreign Joint Venture Suzhou Hua Tuo Medical Instruments Co., Ltd., Suzhou, China). Stimulation frequency was 3–4 Hz, with an intensity of 1–2 V in continuous wave (CW) mode to make the needle slightly vibrate, keeping the animal calm. The needles were retained for 30 min, once a day, and the treatment lasted for 10 days. The electroacupuncture practitioner had 10 years of acupuncture and TCM training, and 5 years of experience in academic and clinical acupuncture.
Treatment of control groups
There were 2 control groups: the normal control and model groups. All rats in the control groups were fixed once a day for 30 min without intervention, while the electroacupuncture group received electroacupuncture treatment.
Gastric and intestinal motility tests
Gastric remainder and small intestinal propulsion rate assessments were conducted as previously described to evaluate gastric and intestinal motility [23,24]. Before the experiments, rats were deprived of food for 24 h with free access to water. Then, the animals received by gavage 2 ml of black semisolid nutrient paste (containing 10 g of sodium carboxymethyl cellulose, 250 ml of distilled water, 16 g of milk powder, 8 g of sugar, 8 g of starch, and 2 g of activated carbon powder), and were killed 30 min later. The stomach and attached small intestine were then immediately exposed by laparotomy. After ligating the esophagogastric and gastroduodenal junctions, the whole stomach was carefully extracted and weighed (weight 1). Then, the stomach was opened, and the remaining black semisolid nutrient paste was washed by cooled normal saline. The stomach was weighed again (weight 2). The gastric remainder rate was calculated as: [(weight 1–weight 2)/weight 1]×100%. The small intestine was opened to evaluate the length containing the black semisolid nutrient paste and the small intestine’s propelling rate was calculated as: (length of small intestine containing the black semisolid nutrient paste/total length of small intestine) ×100%.
Transmission electron microscopy
Tissue samples from the small intestine 2 cm away from the pyloric antrum and 0.5 cm away from the pylorus obtained at the same site were fixed with 3% glutaraldehyde at room temperature. Then, the samples were washed overnight with 0.1 mol/L sodium cacodylate buffer [6% sucrose and 1.25 mmol/L CaCl2 (pH 7.4)] at 4°C and post-fixed with 1% osmium tetroxide in 0.05 mol/L sodium cacodylate buffer (pH 7.4) at 4°C for 2 h. The tissues were then stained with saturated uranyl acetate for 3.5 h at room temperature, dehydrated in graded alcohol and embedded in Eponate 12 resin (Ted Pella, Inc., USA). Next, tissue specimens were sectioned parallel and transversal to the long axis of the circular muscle layer. At suitable sites, 3-μm sections were cut and stained with 2% toluidine blue. After examination of toluidine blue-stained sections, ultrathin sections of selected areas were obtained with an ultra-microtome equipped with a diamond knife, mounted on 200-mesh grids, and stained with uranyl acetate and lead citrate. The grids were observed on an H-7500 electron microscope [25].
Immunohistochemistry for the expression of Cx43
Small intestine tissue samples were collected as described above, fixed with formaldehyde, paraffin embedded, cut into 3-μm thick sections, and collected on gelatinized slides for indirect immunohistochemistry. After deparaffinization and rehydration, the sections were treated with 3% H2O2 for 40 min, followed by 0.01 mol/L citric acid in saline buffer at 95–98°C for about 10 min. The sections were then placed in 7% non-fat milk for 30 min to block nonspecific background staining, and incubated overnight at 4°C with Cx43 antibody (1/1000, ADI, USA). After washing, the sections were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody. Diaminobenzidine (DAB) was employed to detect the immuno-complex, and hematoxylin was used for nuclear counterstaining. To ascertain staining specificity, the sections were incubated with nonspecific rabbit IgG, which was used as a negative control. After dehydration through an ascending alcohol gradient and clarification with xylene, the sections were mounted and examined on a Nikon Eclipse E600 microscope equipped with a CFI60 Infinity Optical System [26]. Images were analyzed with the Image Analysis software (Image Pro Plus 6.0), randomly chosen in all groups. The intensity of the immunohistochemical reaction was expressed as relative optical density (ROD) of diaminobenzidine brown reaction products, as described by Smolen [27,28].
Statistical analysis
Values given as are mean ±SD. Statistical analyses were performed by analysis of variance with the SPSS 20.0 software (SPSS, USA). P<0.05 was considered statistically significant.
Results
Effects of electroacupuncture on gastric and intestinal motility
Gastric remainder and small intestinal propulsion rates at treatment end are shown in Table 1. Compared with the normal group, gastric remainder rates in the model group were significantly increased (P<0.01). Compared with the model group, these values in the electroacupuncture group were significantly decreased (P<0.01). Compared with the normal control group, small intestinal propulsion rates in the model group were significantly decreased (P<0.05). Compared with the model group, these values were significantly increased in the electroacupuncture group (P<0.05).
Table 1.
Gastric remainder and small intestinal propulsion rates (mean ±SD).
| Group | N | Gastric remainder rate | Small intestinal propulsion rate |
|---|---|---|---|
| Normal group | 12 | 34.21±3.80 | 65.46±8.45 |
| Model group | 12 | 49.94±7.85## | 57.15±3.38# |
| Electroacupuncture group | 12 | 32.96±1.06** | 66.12±7.23* |
Compared with the normal group:
P<0.05,
P<0.01;
compared with the model group:
P<0.05,
P<0.01.
Effects of electroacupuncture on ICC ultrastructure
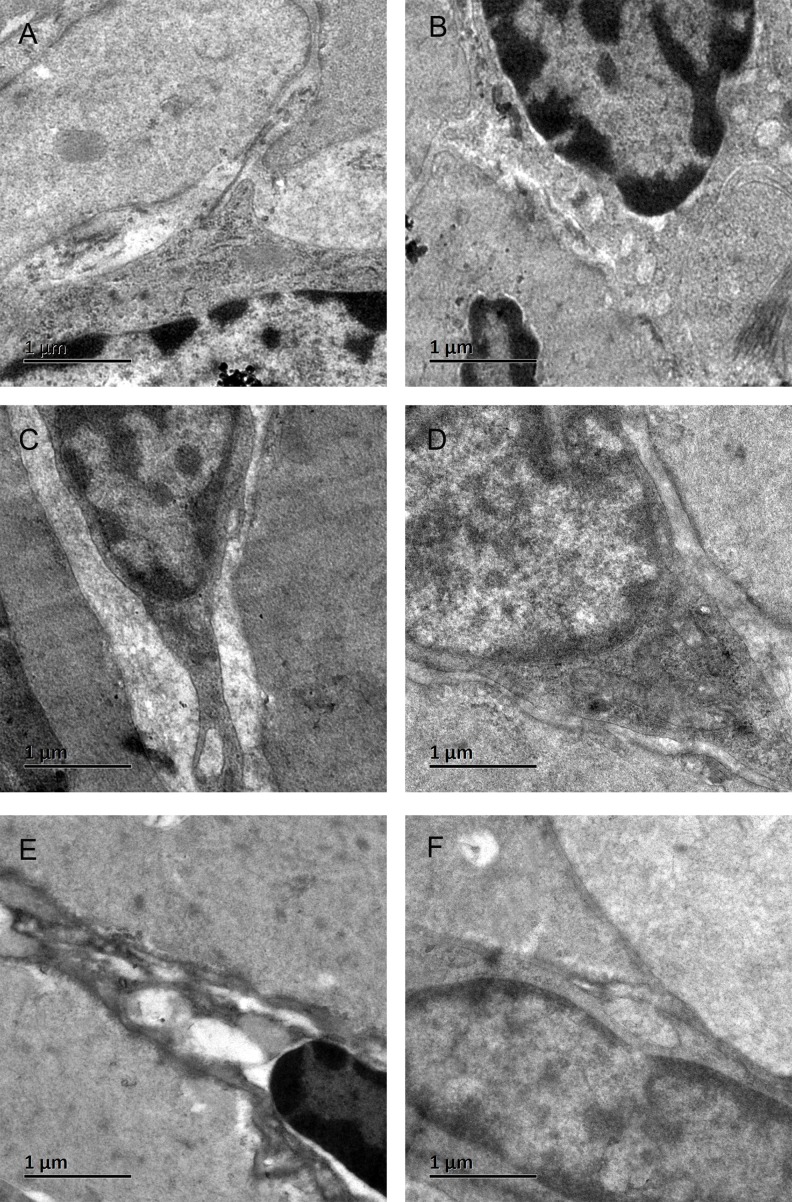
ICC in the normal group had triangular or fusiform shapes, with a higher electron-density cytoplasm compared with that of surrounding smooth muscle cells, and contained abundant cell organelles such as mitochondria, endoplasmic reticulum, and alveoli. Along the length of overlapping processes, ICC predominantly formed large gap junctions between adjacent cells, suggesting a functional relationship between these cell types (Figure 1A, 1D). However, an altered ICC ultrastructure (swollen or vacuolated mitochondria, lack of mitochondrial cristae, and dilated and vesiculated rER) was frequently observed in the model group. Damage of gap junctions between ICC and smooth muscle cells was also observed (Figure 1B, 1E). Interestingly, these ultrastructural abnormalities in the electroacupuncture group exhibited some improvement compared with the model group. However, the ultrastructural morphology had not returned to normal as observed in the normal group, and minor injury could be occasionally observed (Figure 1C, 1F).
Figure 1.
ICC ultrastructure. Compared with the normal group, ICC were seriously injured in the model group, while showing a nearly normal structure and minor injury in the electroacupuncture group. (A) ICC from small intestine in the normal group; (B) ICC from small intestine in the model group; (C) ICC from small intestine in the electroacupuncture group; (D) ICC from the antrum in the normal group; (E) ICC from the antrum in the model group; (F) ICC from the antrum in the electroacupuncture group.
Effects of electroacupuncture on Cx43 expression
Immunohistochemistry showed that compared with the normal group, Cx43 levels in the small intestine and antrum of the model group were significantly decreased (P<0.01). After electroacupuncture treatment, compared with the model group, Cx43 amounts in the small intestine and antrum were significantly increased (P<0.01) (Figures 2, 3 and Table 2).
Figure 2.
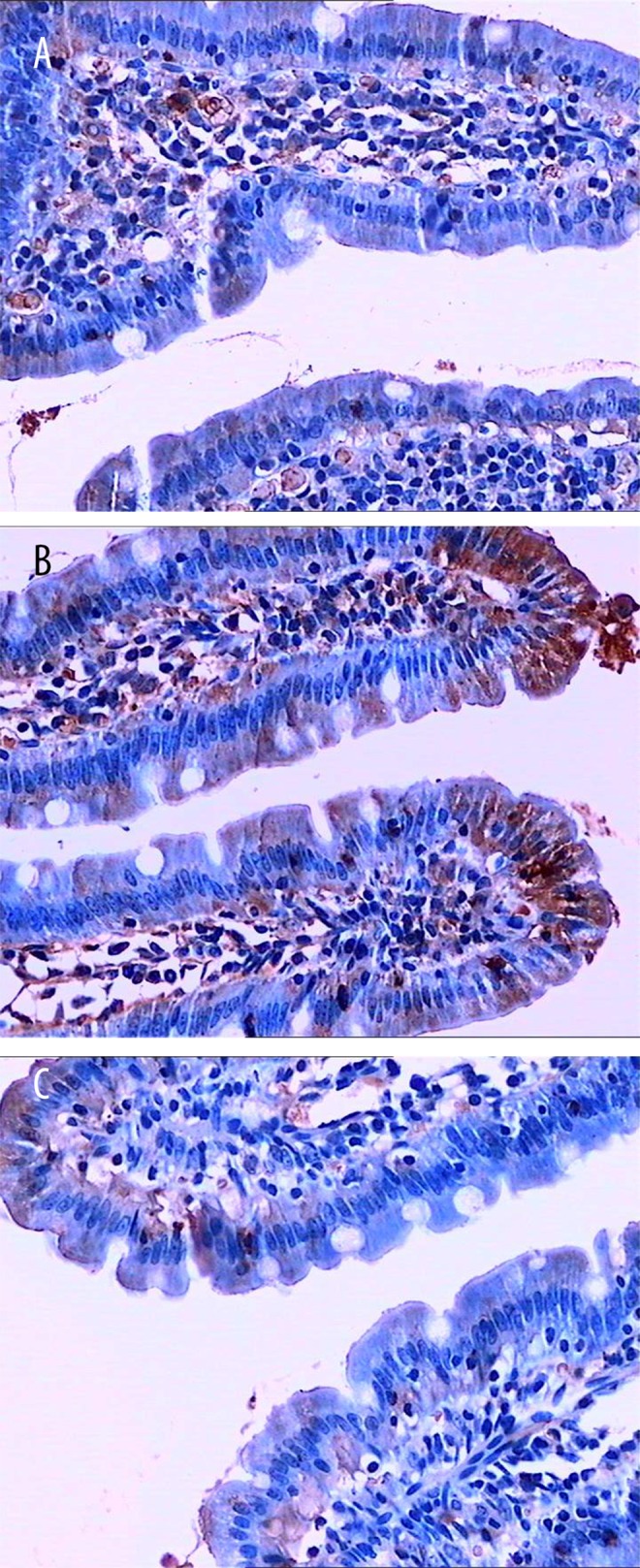
Cx43 expression in small intestine specimens (immunohistochemical detection). (A) Normal group; (B) Compared with the normal group, number and density of Cx43 positive cells were significantly decreased in the model group; (C) In the electroacupuncture group, the number of Cx43 positive were back to normal levels.
Figure 3.
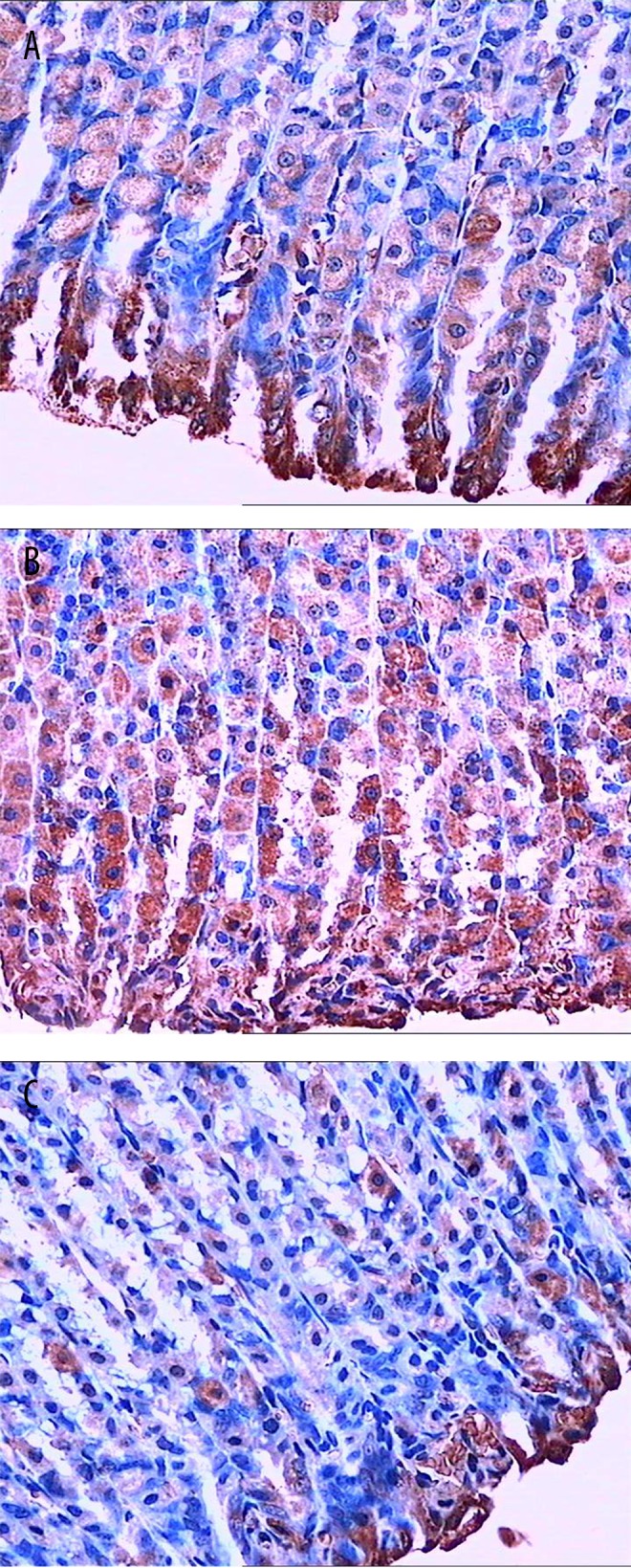
Cx43 expression in antrum samples (immunohistochemical detection). (A) Cx43 expression in the antrum of the normal group; (B) Compared with the normal group, number and density of Cx43 positive cells were significantly decreased in the model group; (C) In the electroacupuncture group, the number of Cx43 positive cells were back to normal levels.
Table 2.
Integrated optical density of Cx43 expression in the gastrointestinal tract (mean ±SD).
| Group | N | Small intestine samples | Antrum samples |
|---|---|---|---|
| Normal group | 12 | 4.13±1.46 | 2.93±1.02 |
| Model group | 12 | 2.00±0.75# | 1.26±0.70# |
| Electroacupuncture group | 12 | 5.39±2.93## | 3.38±2.51## |
Compared with the normal group:
P<0.05;
compared with the model group:
P<0.01.
Discussion
Acupuncture has been used to treat a variety of gastrointestinal problems in China for thousands of years. Electroacupuncture is more frequently used in clinical and research settings because of its reproducibility. Electroacupuncture is a cost-effective and minimally invasive procedure with a very low incidence of adverse effects [29]. The effect of electroacupuncture on gastrointestinal motility was found to be fairly consistent. Electroacupuncture is able to alter gastrointestinal motility functions and improve gastrointestinal motility disorders [10]. In this study, we demonstrated that electroacupuncture at ST36 decreased the gastric remainder rate and enhanced the small intestinal propulsion rate in FD rats. Therefore, this study focused on the mechanisms underlying electroacupuncture effects on gastrointestinal motility.
Interstitial cells of Cajal are pacemaker cells, generating electrical slow waves that conduct to smooth muscle cells and provide a basis for the phasic contractions of muscle strips, as well as segmental and peristaltic contractions of gastrointestinal organs [30]. ICC play a role in motor regulation of the gastrointestinal tract by generating slow waves in smooth muscles, propagating the slow waves, translating the neural input, and detecting the stretching of the intestinal lumen [31,21]. ICC form a network in the gastrointestinal tract and are connected with smooth muscles via gap junctions [33]. Thus, disruption of ICC networks is associated with several gastrointestinal motility disorders [34]. Cx43, the most widely expressed gap junction protein, is associated with a number of physiological and pathological conditions [35]. Lack of Cx43 expression may be partly responsible for smooth muscle motility dysfunction [16]. As shown above, after electroacupuncture treatment, the altered ICC ultrastructure returned to a nearly normal state, and Cx43 protein levels were increased in FD Rats.
Conclusions
Overall, electroacupuncture is effective in alleviating the pathological change of ICC and reduces Cx43 levels in FD rats. These findings suggest that electroacupuncture could improve the ICC networks in the gastrointestinal to treat gastrointestinal motility disorders.
Footnotes
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Source of support: This study was supported by the National Basic Research Program of China (973 Program, No. 2015CB554502 and No. 2014CB543102), the National Natural Science Foundation of China (No. 81173326 and No. 81202770), the Colleges and Universities Innovation Platform Open Fund Project of Hunan Province (No. 12K087), the Innovation Foundation for Postgraduates of Hunan (No. CX2013B330), the Hunan University Science and Technology Innovation Team Foundation, and Key Discipline Fund of Acupuncture and Massage of Hunan Province
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Ghoshal UC, Singh R, Chang FY, et al. Epidemiology of uninvestigated and functional dyspepsia in Asia: Facts and fiction. J Neurogastroenterol Motil. 2011;17(3):235–44. doi: 10.5056/jnm.2011.17.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8(6):498–503. doi: 10.1016/j.cgh.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121(3):526–35. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 5.Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55(12):1685–91. doi: 10.1136/gut.2005.085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98(4):783–88. doi: 10.1111/j.1572-0241.2003.07389.x. [DOI] [PubMed] [Google Scholar]
- 7.Soo S, Forman D, Delaney BC, et al. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99(9):1817–22. doi: 10.1111/j.1572-0241.2004.30086.x. [DOI] [PubMed] [Google Scholar]
- 8.Xiao G, Xie X, Fan J, et al. Efficacy and safety of acotiamide for the treatment of functional dyspepsia: Systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:541950. doi: 10.1155/2014/541950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lima FA, Ferreira LE, Pace FH. Acupuncture effectiveness as a complementary therapy in functional dyspepsia patients. Arq Gastroenterol. 2013;50(3):202–7. doi: 10.1590/S0004-28032013000200036. [DOI] [PubMed] [Google Scholar]
- 10.Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. Auton Neurosci. 2010;157(1–2):31–37. doi: 10.1016/j.autneu.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H, Xu J, Li J, et al. Acupuncture for patients with functional dyspepsia: Study protocol of a randomised controlled trial. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-003377. pii: e003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296(1):G1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 13.Ueshima S, Nishida T, Koike M, et al. Nitric oxide-mediated injury of interstitial cells of Cajal and intestinal dysmotility under endotoxemia of mice. Biomed Res. 2014;35(4):251–62. doi: 10.2220/biomedres.35.251. [DOI] [PubMed] [Google Scholar]
- 14.O’Grady G, Wang TH, Du P, et al. Recent progress in gastric arrhythmia: Pathophysiology, clinical significance and future horizons. Clin Exp Pharmacol Physiol. 2014;41(10):854–62. doi: 10.1111/1440-1681.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of cajal in bladder – a cell often misidentified as myocyte or myofibroblast. PLoS One. 2012;7(11):e48897. doi: 10.1371/journal.pone.0048897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth L, Maddur S, Puri P. Immunolocalization of the gap junction protein Connexin43 in the interstitial cells of Cajal in the normal and Hirschsprung’s disease bowel. J Pediatr Surg. 2000;35(6):823–28. doi: 10.1053/jpsu.2000.6851. [DOI] [PubMed] [Google Scholar]
- 17.Zheng CH, Huang GY, Zhang MM, et al. [Experimental study on expression of connexin 43 in meridians of rats]. Zhongguo Zhen Jiu. 2005;25(9):629–32. [in Chinese] [PubMed] [Google Scholar]
- 18.Zhao F, Cui S, Huang L. P6 Electroacupuncture improved QTc interval prolongation by upregulation of connexin43 in droperidol treated rats. Evid Based Complement Alternat Med. 2014;2014:926423. doi: 10.1155/2014/926423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling J, Wei L, Zhang Y, et al. [Effect of method of soothing liver and regulating qi on expression of gastrin and somatostatin in hypothalamus, castric antrum of functional dyspepsia rats]. Zhongguo Zhong Yao Za Zhi. 2010;35(22):3069–73. [in Chinese] [PubMed] [Google Scholar]
- 20.Chang XR, Lan L, Yan J, et al. Efficacy of acupuncture at acupoints of Foot-Yangming Meridian in the treatment of patients with functional dyspepsia: an analysis of 30 cases. Shijie Huaren Xiaohua Zazhi. 2010;18(8):839–44. [Google Scholar]
- 21.Chang XR, Yan J, Lin YP, et al. Influence of puncturing tsusanli point on the gastrointestinal hormone of the plasma of the patients with functional dyspepsia. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2001;9(5):283–84. [Google Scholar]
- 22.Yu SG, Guo Y. Experimental acupuncture. Shanghai Science and Technology Press; Shanghai, China: 2009. [Google Scholar]
- 23.Chen CY, Lee WJ, Chong K, et al. Impact of intracerebroventricular obestatin on plasma acyl ghrelin, des-acyl ghrelin and nesfatin-1 levels, and on gastric emptying in rats. Mol Med Rep. 2012;6(1):191–96. doi: 10.3892/mmr.2012.901. [DOI] [PubMed] [Google Scholar]
- 24.Zizzo MG, Mulè F, Amato A, et al. Guanosine negatively modulates the gastric motor function in mouse. Purinergic Signal. 2013;9(4):655–61. doi: 10.1007/s11302-013-9378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Du L, Xiao YT, et al. Disruption of interstitial cells of Cajal networks after massive small bowel resection. World J Gastroenterol. 2013;19(22):3415–22. doi: 10.3748/wjg.v19.i22.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang GY, Zheng CH, Yu WC, et al. Involvement of connexin 43 in acupuncture analgesia. Chin Med J (Engl) 2009;122(1):54–60. [PubMed] [Google Scholar]
- 27.Zhang ZB, Zhou DR, Song B. Gap junctional protein connexin 43 in rat detrusor muscle with unstable bladder. Chin Med J (Engl) 2008;121(17):1698–701. [PubMed] [Google Scholar]
- 28.Kopera I, Durlej M, Hejmej A, et al. Effects of pre- and postnatal exposure to flutamide on connexin 43 expression in testes and ovaries of prepubertal pigs. Eur J Histochem. 2010;54(2):e15. doi: 10.4081/ejh.2010.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Chen Y, Liu S, et al. Electroacupuncture regulates apoptosis/proliferation of intramuscular interstitial cells of cajal and restores colonic motility in diabetic constipation rats. Evid Based Complement Alternat Med. 2013;2013:584179. doi: 10.1155/2013/584179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders KM, Ward SM, Koh SD. Interstitial cells: Regulators of smooth muscle function. Physiol Rev. 2014;94(3):859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao HY, Kim DH, Ki JS, et al. Effects of lubiprostone on pacemaker activity of interstitial cells of cajal from the mouse colon. Korean J Physiol Pharmacol. 2014;18(4):341–46. doi: 10.4196/kjpp.2014.18.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BJ, Kwon YK, Kim E, et al. Effects of histamine on cultured interstitial cells of cajal in murine small intestine. Korean J Physiol Pharmacol. 2013;17(2):149–56. doi: 10.4196/kjpp.2013.17.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576(Pt 3):653–58. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain D, Moussa K, Tandon M, et al. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98(3):618–24. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 35.Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011;41(1):103–16. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]