Abstract
Background
The usefulness of Des-γ-carboxyprothrombin (DCP) has been indicated in areas where hepatitis C virus is prevalent. DCP has yet to be used in China. The aim of this study was to evaluate the usefulness of DCP in Chinese patients with hepatocellular carcinoma (HCC) predominantly caused by hepatitis B.
Material/Methods
329 subjects with HCC and 371 subjects without HCC that all underwent surgery were consecutively enrolled. Serum AFP and plasma DCP levels in all subjects and 153 healthy volunteers were measured and analyzed.
Results
Of 329 subjects with HCC, 258 (78.4%) were HBsAg positive. The median level of plasma DCP was 853.72 mAU/mL in subjects with HCC, 26.43 mAU/mL in subjects without HCC, and 29.91 m AU/mL in healthy volunteers. A cut-off DCP value of 87 mAU/mL yielded the optimal sensitivity of 74.80% and a specificity of 83.33% for differentiating subjects with HCC from subjects without HCC. The combination of AFP of 21.33 ng/mL and DCP of 87 mAU/mL had a sensitivity of 82.60% for tumors no larger than 2 cm, as well as a sensitivity of 90% for tumors larger than 5 cm.
Conclusions
The combination of DCP and AFP yielded great improvement in sensitivity in differentiating subjects with HCC from subjects without HCC. These two markers may be incorporated in the protocol for surveillance and diagnosis of HCC in the high-risk Chinese population.
MeSH Keywords: alpha-Fetoproteins; Carcinoma, Hepatocellular; Hepatitis B virus
Background
Hepatocellular carcinoma (HCC) is the fifth-most prevalent malignancy worldwide and the second leading cause of cancer-related deaths [1,2]. More than 700,000 newly diagnosed cases occur every year; unfortunately, about 75–80% of these cases occur in the Asian Pacific region, and especially in China [3,4].
HCC is one of the most difficult to treat malignances. Surgical resection and liver transplantation are thought to be its curative therapies. However, fewer than 30% of all patients are eligible candidates due to poor liver function or advanced disease at the time of diagnosis [5,6]. To achieve the most from available therapies, the most promising strategy is to diagnose and treat high-risk populations early on since HCC might be detected in an early stage and result in long-term survival [7,8].
The most prevalent surveillance protocol in China has been ultrasonography (US) and serum alpha-fetoprotein (AFP) [7]. Serum AFP has been widely used as a tumor biomarker since its advent in 1950s in China. An elevated serum AFP is known to be associated with HCC, but not always. According to reported data, 50–60% of all patients test positive for AFP. AFP may also be elevated in certain patients with chronic hepatitis or cirrhosis, [9,10], thus resulting in AFP’s poor sensitivity and specificity at diagnosing and postoperatively surveilling HCC. An ideal alternative or complement to AFP as a sensitive biomarker is crucial to detecting HCC.
Des-γ-carboxyprothrombin (DCP) is a well-established biomarker for HCC in Japan and South Korea [11–15]. Meta analysis showed that DCP shows more diagnostic accuracy than AFP, especially in diagnosing early stage HCC [16]. During the past decade, some studies have examined the clinical usefulness of DCP in Chinese patients with HCC, but they have not sufficiently demonstrated its usefulness due to limited samples and controls. DCP has yet to be used clinically in China until now [17–19]. Theoretically, the clinical significance of DCP might vary considerably for patients with HCC due to different etiologies in different geographic regions. Although hepatitis C is the underlying cause of HCC in Japan and Western countries, hepatitis B causes up to 85% of HCC in China [7]. In order to further investigate the clinical utility of DCP in detecting HCC in Chinese patients, the current study was conducted with a large cohort of patients with HCC and different controls.
Material and Methods
Study subjects
A total of 700 patients who were diagnosed with a hepatopancreatobiliary disease and who had undergone surgery were consecutively enrolled from the Institute of Hepatobiliary Surgery, Southwest Hospital, Third Military Medical University between September 2008 and September 2011. In addition, 153 healthy volunteers were also included in the study. All subjects and volunteers provided written informed consent. Ethical approval for this study was obtained from the local Institutional Review Board.
Subjects receiving surgery were divided into two groups: (i) subjects with HCC (n=329); and (ii) subjects without HCC, with either benign or malignant hepatopancreatobiliary disease (n=371). None of the subjects were receiving and/or had received vitamin K therapy.
HCC was clinically diagnosed based on international guidelines [19,20] and pathologic findings from resected specimens were confirmed for all 329 subjects. No subjects had received any previous therapy to treat HCC such as TACE, RFA, PEI, or resection and they underwent surgery for the first time at this Hospital. HBV infection was diagnosed based on a positive result for hepatitis B surface antigen (HBsAg), and HCV infection was confirmed based on a positive result for anti-HCV and HCV RNA.
To examine the usefulness of DCP in diagnosing HCC, a cohort with a hepatopancreatobiliary disease other than HCC based on enhanced imaging findings that were undergoing surgery at this hospital were used. All of these subjects had been diagnosed by clinical findings as well as pathologic findings. Detailed diagnostic information was also recorded (see Supplementary Tables 1, 2). All of the subjects with or without HCC were treated depending on their condition and stage of their disease.
To ensure that healthy volunteers did not have HCC, these subjects had to have no masses according to ultrasound or CT/MRI imaging and a total AFP below the reference level of 10 ng/mL. None of the subjects was taking vitamin K at the time of enrollment. A normal liver function test and a test for hepatitis infection were also performed.
Blood samples
A 10-mL sample of peripheral blood was obtained before surgery or at the time of enrollment and immediately centrifuged into serum and plasma and then stored in aliquots in a freezer at −80°C.
Assays of serum AFP and plasma DCP
Serum AFP was examined using a commercially available immunometric assay (ST AIA-PACK AFP, Tosoh, Tokyo, Japan) with enhanced chemiluminescence at the Southwest Hospital Clinical Diagnostic Center. Plasma DCP levels were detected using an electrochemiluminescence immunoassay (ED036, Eisai Co., Tokyo, Japan) in accordance with the manufacturer’s instructions and manual [21]. The optimal cut-off value for DCP to distinguish between normal healthy subjects and patients with HCC was previously determined to be 40 mAU/mL [14,22].
Statistical analysis
A P value <0.05 in a 2-tailed test was deemed to indicate statistical significance for all calculations. Data are presented as mean ± standard deviation and/or median (range) as appropriate. A Wilcoxon rank-sum test and chi-square test were used for analysis of continuous and categorical data, respectively. Receiver-operating characteristic (ROC) curves were created for AFP, DCP, and a combination of AFP and DCP to determine the optimal cut-off value for differentiating subjects with HCC, subjects without HCC, and healthy controls. The area under the ROC curves (AUROC) with the positive (+LR) and negative (−LR) likelihood ratio was examined for the aforementioned biomarkers. A bivariate normal distribution was assumed for the two markers. Box plots were created and analysis of variance was used to compare descriptive statistics for the transformed tumor markers. Youden’s index was calculated to serve as an index of sensitivity and specificity. All statistical calculations were performed using SPSS® version 22.0 for Windows® (SPSS, Chicago, Illinois, USA).
Results
General information
The 329 subjects with HCC had a mean age of 47.0±10.9 years and consisted of 293 males (89.1%) and 36 females (10.9%). Of the subjects, 258 (78.4%) tested positive for HBsAg and 3 (0.9%) tested positive for anti-HCV while 68 (20.7%) were negative for both (Table 1). Single tumors were found in 281 subjects (85.4%). The median size of the tumor was 6.0 cm with a range from 1.0 to 19.0 cm. A tumor was found in the left lobe in 79 subjects (24.0%), in the right lobe in 229 (69.6%), in both lobes in 15 (4.6%), and in the caudate lobe in 6 (1.8%). All of the 329 subjects underwent surgery which was specifically described in Supplementary Table 1.
Table 1.
Demographic data and levels of DCP and AFP in subjects with HCC and controls.
| Variate | Subjects with HCC (329 cases) | Subjects without HCC (371 cases) | Normal subjects (153 cases) |
|---|---|---|---|
| Age (years) (mean ±SD) | 47.0±10.9 | 52.3±11.1 | 32.70±5.24 |
| Gender (male/female) | 293/36 | 191/180 | 128/25 |
| HBs Ag | P1<0.001 | P2<0.001 | |
| Positive/negative | 258/71 | 90/280* | (0/153) |
| Anti-HCV | P1<0.001 | P2<0.001 | |
| Positive/negative | 3/326 | 6/365 | (0/153) |
| Plasma DCP value (mAU/mL) | P1<0.001 | P2<0.001 | |
| Median | 853.72 | 26.43 | 29.91 |
| Minimum | <10.00 | <10.00 | <10.00 |
| Maximum | >200000.00 | 179297.83 | 102.03 |
| Serum AFP value (ng/mL) | P1<0.001 | P2<0.001 | |
| Median | 375.86 | 3.00 | 7.00 |
| Minimum | 0.24 | 0.00 | 0.00 |
| Maximum | 1939000 | 2185.44 | 30.00 |
Missing=1; P1 – subjects with HCC vs. subjects without HCC; P2 – subjects with HCC vs. normal subjects.
This study enrolled 371 subjects without HCC who were diagnosed with a benign or malignant hepatopancreatobiliary disorder. These subjects served as disease controls and underwent surgery depending on their condition. Of these subjects, 90 tested positive for HBsAg and 6 tested positive for anti-HCV. One subject was excluded due to missing data, and the remaining subjects were all negative for HBsAg, anti-HBc and anti-HCV. The subject’s diagnosis was based on pathologic findings and roughly classified into one of four categories: a common liver disease (33 subjects, 8.9%), a rare liver disease (13 subjects, 3.5%), pancreatic disease (79 subjects, 21.3%), or biliary disease (246 subjects, 66.3%) (Supplementary Table 2).
All healthy volunteers were blank controls (mean age: 32.70±5.24 years; M/F ratio=128/25) with a normal biochemistry and were negative for HBsAg, anti-HBc, and anti-HCV.
Results of DCP and AFP measurement
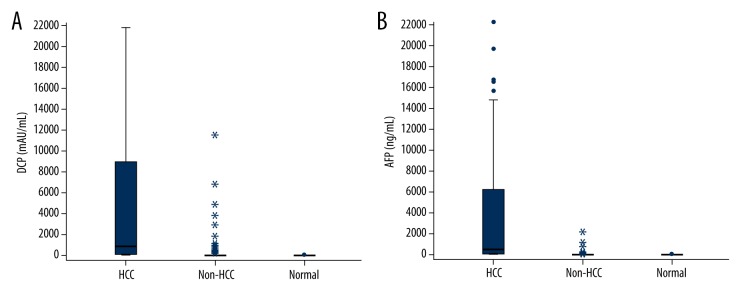
The median plasma concentration of DCP was 853.72 mAU/mL in subjects with HCC, 26.43 mAU/mL in subjects without HCC, and 29.91 mAU/mL in healthy volunteers. There was a marked difference in the level of DCP for subjects with HCC and the other two groups (P<0.001) (Table 1, Figure 1A). Median serum levels of AFP were 375.86 ng/mL in subjects with HCC, 3.00 ng/mL in subjects without HCC, and 7.00 ng/mL in healthy volunteers. A significantly higher level of AFP was seen in subjects with HCC than in the other two groups (Table 1, Figure 1B). The correlation between the levels of DCP and AFP in the 700 subjects and 153 healthy controls is shown in Figure 2. No association was seen in this cohort (r2=0.20).
Figure 1.
Levels of biomarkers in subjects with HCC and controls. (A), Box plots comparing levels of plasma DCP in subjects with HCC, subjects without HCC, and normal healthy subjects. Levels are presented as mAU/mL. (B), Box plots comparing levels of serum AFP in subjects with HCC, subjects without HCC, and normal healthy subjects. Levels are presented as ng/mL.
Figure 2.
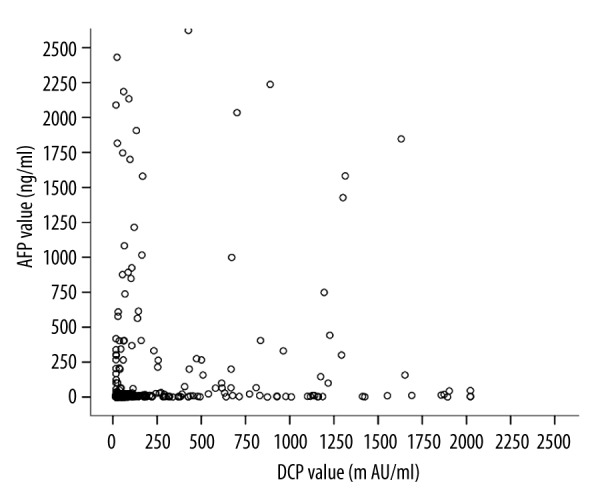
Correlation between serum AFP and plasma DCP values in 700 subjects and 153 normal controls. No correlation was found between the two markers (r2=0.20, P<0.0001).
In order to determine optimal cut-off values that could balance the false-positive and the false-negative rates with the best positive predictive value and that could best distinguish subjects with HCC from the other two groups, a ROC curve was created for DCP and for AFP. As shown in Table 2, when the cut-off value was set at 40 mAU/mL, the sensitivity was 83.00%, the specificity was 62.30%, and the Youden index (%) was 45.30% for subjects with HCC vs. subjects without HCC. In contrast, the sensitivity was 83.00%, the specificity was 66.00%, and the Youden index (%) was 49.00% for subjects with HCC vs. normal subjects. Interestingly, a cut-off DCP value of 87 mAU/mL yielded the optimal sensitivity of 74.80% and a specificity of 83.33% with a Youden index of 58.10% for differentiating subjects with HCC from subjects without HCC. The sensitivity was 74.80%, the specificity was 96.10%, and the Youden index was 70.90% for differentiating subjects with HCC from normal subjects. This indicated that a cut-off DCP level of 87 mAU/mL, and not 40 mAU/mL, should be used to diagnose HCC in the current subjects undergoing surgery (Table 2).
Table 2.
Sensitivity and specificity of DCP and AFP at different cut-off values.
| Cut-off value of DCP and AFP | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden index (%) |
|---|---|---|---|---|---|
| DCP (87 mAU/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 74.80 | 83.33 | 79.51 | 79.25 | 58.10 |
| Subjects with HCC vs. normal subjects | 74.80 | 96.10 | 97.45 | 65.63 | 70.90 |
| DCP (40 mAU/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 83.00 | 62.30 | 65.63 | 80.88 | 45.30 |
| Subjects with HCC vs. normal subjects | 83.00 | 66.00 | 83.01 | 66.01 | 49.00 |
| AFP (21.33 ng/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 70.60 | 93.80 | 92.31 | 75.27 | 64.40 |
| Subjects with HCC vs. normal subjects | 70.60 | 94.80 | 96.43 | 61.70 | 65.40 |
| AFP (400 ng/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 49.30 | 98.60 | 97.42 | 64.69 | 47.90 |
| Subjects with HCC vs. normal subjects | 49.30 | 100.00 | 100.00 | 49.68 | 49.30 |
| AFP (10 ng/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 77.00 | 80.40 | 80.48 | 76.97 | 57.40 |
| Subjects with HCC vs. normal subjects | 77.00 | 69.90 | 83.63 | 60.45 | 46.90 |
| AFP (21.33 ng/mL) + DCP (87 mAU/mL) | |||||
| Subjects with HCC vs. subjects without HCC | 88.90 | 80.50 | 79.07 | 89.73 | 69.40 |
| Subjects with HCC vs. normal subjects | 88.90 | 92.20 | 95.77 | 80.57 | 81.10 |
PPV – positive predictive value; NPV – negative predictive value.
The optimal cut-off values for AFP with different serum levels were also examined. Results showed that an AFP level of 21.33 ng/mL should be used to diagnose HCC. This yielded a sensitivity of 70.60%, a specificity of 93.80%, and a Youden index of 64.40% for differentiating subjects with HCC from subjects without HCC in contrast to a sensitivity of 70.60%, a specificity of 94.80%, and a Youden index of 65.40% for differentiating subjects with HCC from normal subjects (Table 2).
AUROC was used to evaluate the combination of the markers AFP and DCP at their optimal cut-off values to gauge any potential diagnostic advantage. The combination of AFP of 21.33 ng/mL and DCP of 87 mAU/mL had a higher sensitivity of 88.90% and a Youden index of 69.40% for subjects with HCC vs. subjects without HCC, as well as a higher sensitivity of 88.90% and a Youden index of 81.10% for subjects with HCC vs. normal subjects. There was a slight decrease in the specificity of this combination, but this was acceptable given the commensurate improvement of at least 14.1% in sensitivity compared to DCP or AFP alone (Table 2).
The ROC curves comparing DCP and AFP levels in subjects with HCC versus subjects without HCC indicated that the area under the ROC curve was 0.846 for DCP, 0.832 for AFP, and 0.890 for the combination of DCP and AFP (Figure 3).
Figure 3.
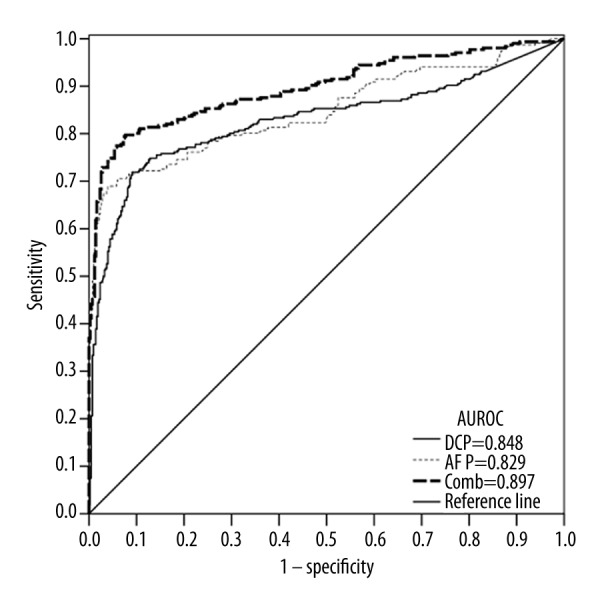
ROC curve comparing DCP and AFP levels in subjects with HCC versus subjects without HCC (including subjects with a benign or malignant hepatopancreatobiliary disease and normal healthy subjects). The curves show an optimal cut-off value for DCP of 87.00 mAU/mL and for AFP of 21.33 ng/mL. The area under the ROC curve was 0.848 for DCP, 0.829 for AFP, and 0.897 for the combination of DCP and AFP.
Sensitivity and specificity of DCP and AFP in detecting HCC in accordance with tumor size
The 329 subjects with HCC were categorized according to tumor size and the sensitivity and specificity of DCP and AFP were then estimated using ROC analysis. In accordance with clinical manifestations and the population studied, tumors were classified into six sizes ranging from ≤2.0 cm, >2.0 cm and ≤3.0 cm, >3.0 cm and ≤4.0 cm, >4.0 cm and ≤5.0 cm, >5.0 cm and ≤10.0 cm, and >10.0 cm.
The median value of plasma DCP for 329 subjects with HCC of differing sizes is shown in Table 3. Plasma DCP was 46.86 mAU/mL in subjects with a tumor ≤2.0 cm, 98.22 mAU/mL in subjects with a tumor >2.0 cm and ≤3.0 cm, 332.22 mAU/mL in subjects with a tumor >3.0 cm and ≤4.0 cm, 359.93 mAU/mL in subjects with a tumor >4.0 cm and ≤5.0 cm, 1766.53 mAU/mL in subjects with a tumor >5.0 cm and ≤10.0 cm, and 4162.12 mAU/mL in subjects with a tumor >10.0 cm. There was an obvious incremental increase in DCP values as the tumor size increased, but the same was not true for AFP (Table 3).
Table 3.
DCP and AFP levels (329 subjects with HCC) classified by tumor size, cirrhosis and HBsAg.
| Items | Median | Minimum | Maximum | |||
|---|---|---|---|---|---|---|
| DCP (mAU/mL) | AFP (ng/mL) | DCP (mAU/mL) | AFP (ng/mL) | DCP (mAU/mL) | AFP (ng/mL) | |
| Tumor size | ||||||
| ≤2.0 cm | 46.86 | 207.50 | <10.00 | 0.24 | 7369.15 | 14744.00 |
| >2.0 cm, ≤3.0 cm | 98.22 | 577.63 | <10.00 | 1.83 | 46825.61 | 30506.00 |
| >3.0 cm, ≤4.0 cm | 332.22 | 30.81 | <10.00 | 1.38 | >200000.00 | 230281.00 |
| >4.0 cm, ≤5.0 cm | 359.93 | 331.18 | <10.00 | 1.80 | 111170.15 | 1193000.00 |
| >5.0 cm, ≤10.0 cm | 1766.53 | 397.80 | <10.00 | 0.24 | >200000.00 | 794800.00 |
| >10.0 cm | 4162.12 | 1314.00 | <10.00 | 1.38 | >200000.00 | 1939000.00 |
| Liver cirrhosis | ||||||
| Present | 462.78 | 338.88 | <10.00 | 1.28 | 66576.16 | 497600.00 |
| Absent | 1168.34 | 163.10 | <10.00 | 0.24 | >200000.00 | 1939000.00 |
| HBsAg | ||||||
| Positive | 589.24 | 375.85 | <10.00 | 0.24 | >200000.00 | 1939000.00 |
| Negative | 1420.65 | 365.04 | <10.00 | 1.23 | >200000.00 | 622800.00 |
The sensitivity and specificity of DCP of 87 mAU/mL, AFP of 21.33 ng/mL, and a combination of both were examined in different tumor sizes. When the tumor was smaller than or equal to 3.0 cm, DCP had a relatively lower sensitivity than AFP. As the tumor size increased, the sensitivity of DCP also increased markedly and was much higher than AFP in subjects with HCC, as shown in Table 4. The sensitivity of the combination of the two tumor markers was higher than that of AFP or DCP alone for the entire range of tumor sizes. The sensitivity of the combined markers was 82.60% for tumors smaller than or equal to 2 cm in size. The combined markers had a sensitivity of more than 90% for tumors larger than 5 cm, and this was significantly higher than that of DCP or AFP alone (Table 4).
Table 4.
Sensitivity of plasma DCP and serum AFP in 329 subjects with HCC of differing sizes.
| Tumor size | DCP (87 mAU/mL) (%) | AFP (21.33 ng/mL) (%) | DCP (87 mAU/mL) + AFP (21.33 ng/mL) (%) |
|---|---|---|---|
| ≤2.0 cm (n=20) | 39.10 | 78.30 | 82.60 |
| >2.0 cm, ≤3.0 cm (n=37) | 54.80 | 74.20 | 83.90 |
| >3.0 cm, ≤4.0 cm (n=40) | 68.80 | 56.30 | 84.40 |
| >4.0 cm, ≤5.0 cm (n=39) | 68.60 | 62.90 | 88.60 |
| >5.0 cm, ≤10.0c m (n=142) | 85.30 | 71.30 | 91.20 |
| >10.0 cm (n=51) | 85.40 | 79.20 | 93.80 |
Discussion
This study involved a cross-sectional study of plasma DCP and serum AFP in subjects undergoing surgery for HCC predominantly caused by HBV. Results of this study indicated that DCP has a relatively higher sensitivity in detecting tumors larger than 3.0 cm. Results also indicated that DCP is a good candidate as a compliment to AFP in the diagnosis of HCC, regardless of tumor size. The combination of these two serum biomarkers could result in an optimal diagnostic accuracy for the Chinese population with hepatopancreatobiliary diseases.
DCP has been investigated widely since 1984 [23]. Its usefulness in diagnosing HCC at a reference level of 40 m AU/mL has been established by a number of retrospective and prospective studies [24]. However, most of these studies were completed in Japan or Western counties, where HCC is predominantly caused by HCV infection [24–26]. DCP levels may vary widely between forms of underlying liver disease, including ALD, NALD [27], and viral hepatitis [12]. Moreover, HCC occurring in China accounts for at least half of the new cases worldwide each year. Hence, there is an urgent demand for optimal serum biomarkers to help improve the early diagnosis of HCC [28]. Unfortunately, few studies have focused on the Chinese population, and these studies have also involved a small sample size and limited number of controls [17,18]. The current study included a consecutive cohort of 329 subjects with HCC who were undergoing curative surgery. This might be the largest sample size for a single-center study of HCC in China and the first report on all patients undergoing surgery. The subjects in the current study underwent different surgical procedures, including hepatectomy (317/329) and a liver transplant (12/329). The diagnosis of HCC was pathologically confirmed for each subject. Based on the inclusion criterion for surgery, this cohort should be classified as a cohort with early HCC, although the subjects did have a large tumor and they fell outside the Milan criteria for liver transplantation. ROC analysis indicated that DCP with a cut-off value of 40 m AU/mL had a sensitivity of 83.00% and a specificity of 62.30% in differentiating subjects with HCC from subjects without HCC and a sensitivity of 83.00% and sensitivity of 66.00% in differentiating subjects with HCC from normal healthy subjects. However, the current results indicated that DCP of 87 m AU/ml, and not the routine diagnostic level of 40 m AU/mL [14], should be used for Chinese patients. This higher level of DCP achieved a good balance between sensitivity and specificity with a higher Youden index than did the level of 40 m AU/mL.
The current study has some advantages over previous studies of DCP. This study enrolled a relatively comprehensive set of controls that included 371 subjects without HCC and 153 normal healthy volunteers. In previous studies reported, the controls were most often subjects with viral hepatitis and/or liver cirrhosis with an underlying viral infection or ALD or NALD. The accuracy with which DCP diagnosed HCC was repeatedly demonstrated under such conditions. However, the ability of DCP to differentiate HCC from other types of hepatobiliary disease has not been fully investigated, and this might substantially limit its use as a complement to AFP. Controls without HCC included subjects with a wide range of hepatopancreatobiliary diseases that included the common liver diseases of chronic severe hepatitis, focal nodular hyperplasia, hepatic cavernous hemangioma, and liver cirrhosis, as well as pancreatic cancer, hepatolithiasis, and bile duct cancer. Use of comprehensive controls with disease and normal controls indicated that a cut-off value of AFP 21.33 ng/mL and a cut-off value of DCP 87 mAU/mL had a sensitivity of 88.90% and specificity of 80.50% with a Youden index 69.40% in differentiating subjects with HCC from subjects without HCC. These two markers had a much higher sensitivity and specificity than DCP or AFP alone. The DCP level is known to not be associated with the serum level of AFP [29], and the current study corroborated that finding. The logical conclusion is that DCP is an optimal biomarker that complements AFP and that DCP greatly improves the sensitivity of detecting HCC predominantly caused by HBV. A previous study showed that the prognosis was poor when either AFP or DCP levels were high in Japanese patients with HBV [22]. This capability is ubiquitous, as demonstrated by different controls with underlying diseases and is not limited to viral hepatitis or liver cirrhosis.
There was a close correlation between plasma DCP levels and tumor size. However, the same was not true for AFP. Nakamura et al. reached the same conclusion [13]. The sensitivity of DCP of 87 mAU/mL and AFP of 21.33 ng/mL was further examined in 329 subjects with HCC of differing sizes. Results indicated that DCP had a lower sensitivity than AFP for a tumor ≤3.0 cm in size. As the tumor size increased, the diagnostic capability of plasma DCP increased markedly in comparison to that of serum AFP. This combination of two markers had a higher sensitivity than that of DCP or AFP alone for tumors of all sizes. Combining DCP and AFP yielded an overall sensitivity of 80–90%, and a sensitivity of 82.6% for tumors smaller than 2 cm in diameter. This finding has substantiated the use of DCP as a complement to AFP in diagnosing early HCC. The combination of these biomarkers is superior to the current protocol, and it offers promise in terms of surveillance.
There are some limitations to the current study. First, this study did involve a relatively large number of consecutive subjects with HCC, but all of those subjects underwent curative surgery. Hence, subjects with HCC in a more advanced stage were not included in this study. The intrinsic nature of this study design limits the generalizability of the results. Additional information that could have been collected includes the association between DCP values and more advanced HCC, major vessel invasion and metastasis, and the prognosis for subjects with HCC. A large-scale national program to survey DCP in Chinese patients with HCC should be conducted to complement this study. Second, the current controls without HCC included a relatively small proportion of subjects with liver cirrhosis (23/371) in comparison to previous studies. Most controls had a benign or malignant disease of the hepatopancreatobiliary system. Although this selection of controls will undoubtedly yield new useful information and expand knowledge, it restricts the range of evidence supporting the usefulness of DCP in differentiating HCC from HBV-related liver cirrhosis.
Conclusions
In conclusion, the current study involved a relatively large sample of subjects with HCC predominantly caused by HBV, and a comprehensive set of controls. This study indicated the diagnostic ability and usefulness of plasma DCP in Chinese patients undergoing surgery for HCC. In the current cohort, the optimal cut-off value for DCP was 87 mAU/mL. The combination of DCP with a cut-off value of 87 mAU/mL and AFP with a cut-off value of 21.33 ng/mL yielded great improvement in sensitivity in differentiating subjects with HCC from subjects without HCC. These two markers may be incorporated in the protocol for surveillance of HCC in high-risk subsets of the Chinese population.
Supplementary materials
Supplementary Table 1.
Tumor characteristics and surgical information of 329 cases of HCC.
| Catalogs | Items | Subdivisions | Results |
|---|---|---|---|
| Tumor features | Tumor number | Solitary | 281 (85.4%) |
| Multiple(≥2) | 48 (14.6%) | ||
| Tumor size | Mean | 6.87±3.58 | |
| Median | 6 (1,19) | ||
| Tumor site | Left lobe | 79 (24.0%) | |
| Right lobe | 229 (69.6%) | ||
| Bilateral lobe | 15 (4.6%) | ||
| Caudate lobe | 6 (1.8%)) | ||
| Treatment methods | Non-anatomic Hepatectomy | 114 (34.7%) | |
| Anatomic Hepatectomy | 203 (61.7%) | ||
| Segment I | 3 (0.9%) | ||
| Segment II | 1 (0.3%) | ||
| Segment V | 4 (1.2%) | ||
| Segment VI | 17 (5.2%) | ||
| Segment VII | 7 (2.1%) | ||
| Segment VIII | 4 (1.2%) | ||
| Segment II+III | 17 (5.2%) | ||
| Segment IV+VIII | 2 (0.6%) | ||
| Segment V+VI | 8 (2.4%) | ||
| Segment V+VIII | 6 (1.8%) | ||
| Segment VI+VII | 23 (7.0%) | ||
| Segment VII+VIII | 5 (1.5%) | ||
| Segment II+III+IV | 35 (10.6%) | ||
| segment IV+V+VIII | 6 (1.8%) | ||
| Segment V+VI+VII | 8 (2.4%) | ||
| Segment VI+VII+VIII | 3 (0.9%) | ||
| Segment I+II+III+IV | 5 (1.5%) | ||
| segment I+V+VI+VII | 1 (0.3%) | ||
| Segment V+VI+VII+VIII | 37 (11.2%) | ||
| Segment I+V+VI+VII+VIII | 1 (0.3%) | ||
| Segment II+III+IV+V+VIII | 5 (1.5%) | ||
| Segment IV+V+VI+VII+VIII | 5 (1.5%) | ||
| Transplantation | 12 (3.6%) | ||
| Orthotopic liver transplantation | 4 (1.2%) | ||
| Living-donor liver transplantation | 3 (0.9%) | ||
| Extracorporeal liver resection plus autotransplantation | 5 (1.5%) |
Supplementary Table 2.
Detailed information for 371 cases of non-HCC hepatopancreatobiliary disease.
| Cataloge of diseases | Diagnosis | Cases | Gender (male/female) | Age (years, Mean ±SD) |
|---|---|---|---|---|
| Common liver diseases | Chronic severe hepatitis | 8 | 6/2 | 40.3±10.5 |
| Focal nodular hyperplasia, FNH | 4 | 3/1 | 47.7±18.4 | |
| Hepatic cavernous hemangioma | 6 | 1/5 | 40.4±7.4 | |
| Liver cirrhosis | 15 | 9/6 | 52.7±9.2 | |
| Rare liver diseases | 13 | 4/9 | 49.0±11.0 | |
| Hepatic hematoma | 2 | |||
| Hepatic lymphoepithelioma-like carcinoma | 1 | |||
| Intrahepatic biliary cystadenocarcinoma | 1 | |||
| Liver inflammatory myofibroblastic tumor | 1 | |||
| Liver metastasis from gastrointestinal stromal tumors, GIST | 1 | |||
| Primary biliary cirrhosis, PBC | 3 | |||
| Primary hepatic neuroendocrine carcinoma, PHNEC | 1 | |||
| Primary malignant fibrous histiocytoma, MFH | 1 | |||
| Wilson’s disease | 2 | |||
| Pancreatic diseases | Chronic pancreatitis and/or pancreatolithiasis | 8 | 8/0 | 42.8±10.3 |
| Pancreatic cancer | 71 | 50/21 | 55.5±11.1 | |
| Biliary diseases | Hepatolithiasis, HL | 121 | 39/82 | 48.7±12.2 |
| Carcinoma of gallblader | 6 | 3/3 | 60.4±12.6 | |
| Intrahepatic cholangiocarcinoma, ICC | 48 | 30/18 | 52.1±9.8 | |
| Hilar cholangiocarcinoma, HC | 43 | 25/18 | 58.6±8.3 | |
| Middle segment of the bile duct carcinoma | 5 | 3/2 | 73.3±9.9 | |
| Periampular carcinoma | 23 | 10/13 | 59.0±13.0 |
Abbreviations
- HCC
Hepatocellular carcinoma
- US
ultrasonography
- AFP
serum alpha-fetoprotein
- DCP
Des-γ-carboxyprothrombin
- TACE
transcatheter arterial chemoembolization
- RFA
radiofrequency ablation
- PEI
percutaneous ethanol injection
- HBsAg
hepatitis B surface antigen
- HCV
hepatitis B virus
- CT
computer tomography
- MRI
magnetic resonance imaging
- AUROC
area under the receiver operating characteristic curve
- ROC
receiver pperating characteristic curve
- ALD
alcoholic liver disease
- NALD
non-alcoholic liver disease
Footnotes
Competing interests
The authors declared that they have no competing interests.
Source of support: This study was supported by National S&T Major Project for Infectious Diseases of China (No. 2012ZX10002-017) and State Key Program of National Natural Science Foundation of China (No. 81230064)
References
- 1.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Song P, Xia J, et al. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov Ther. 2014;8:134–38. doi: 10.5582/ddt.2014.01025. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–22. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 7.Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603–11. doi: 10.1148/radiol.13130150. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Zhang CQ, Zhang YQ, et al. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–81. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 9.Bruce MG, Bruden D, McMahon BJ, et al. Clinical significance of elevated alpha-fetoprotein in Alaskan Native patients with chronic hepatitis C. J Viral Hepat. 2008;15:179–87. doi: 10.1111/j.1365-2893.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–29. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–48. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S, Nouso K, Sakaguchi K, et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–43. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamashiki N, Sugawara Y, Tamura S, et al. Diagnostic accuracy of alpha-fetoprotein and des-gamma-carboxy prothrombin in screening for hepatocellular carcinoma in liver transplant candidates. Hepatol Res. 2011;41:1199–207. doi: 10.1111/j.1872-034X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K, Imamura H, Matsuyama Y, et al. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795–804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus alpha-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res. 2014;44:E11–25. doi: 10.1111/hepr.12201. [DOI] [PubMed] [Google Scholar]
- 17.Cui R, Wang B, Ding H, et al. Usefulness of determining a protein induced by vitamin K absence in detection of hepatocellular carcinoma. Chin Med J (Engl) 2002;115:42–45. [PubMed] [Google Scholar]
- 18.Cui R, He J, Zhang F, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878–82. doi: 10.1038/sj.bjc.6601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song P, Feng X, Zhang K, et al. Perspectives on using des-gamma-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr. 2013;2:227–31. doi: 10.3978/j.issn.2304-3881.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 22.Meguro M, Mizuguchi T, Nishidate T, et al. Prognostic roles of preoperative alpha-fetoprotein and des-gamma-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:4933–45. doi: 10.3748/wjg.v21.i16.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebman HA, Furie BC, Tong MJ, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–31. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus alpha-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res. 2014;44(10):E11–25. doi: 10.1111/hepr.12201. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5–10. doi: 10.1177/1756283X10385964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beale G, Chattopadhyay D, Gray J, et al. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Song P, Gao J, et al. Perspectives on a combined test of multi serum biomarkers in China: Towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Drug Discov Ther. 2014;8:102–9. doi: 10.5582/ddt.2014.01026. [DOI] [PubMed] [Google Scholar]
- 29.Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Tumor characteristics and surgical information of 329 cases of HCC.
| Catalogs | Items | Subdivisions | Results |
|---|---|---|---|
| Tumor features | Tumor number | Solitary | 281 (85.4%) |
| Multiple(≥2) | 48 (14.6%) | ||
| Tumor size | Mean | 6.87±3.58 | |
| Median | 6 (1,19) | ||
| Tumor site | Left lobe | 79 (24.0%) | |
| Right lobe | 229 (69.6%) | ||
| Bilateral lobe | 15 (4.6%) | ||
| Caudate lobe | 6 (1.8%)) | ||
| Treatment methods | Non-anatomic Hepatectomy | 114 (34.7%) | |
| Anatomic Hepatectomy | 203 (61.7%) | ||
| Segment I | 3 (0.9%) | ||
| Segment II | 1 (0.3%) | ||
| Segment V | 4 (1.2%) | ||
| Segment VI | 17 (5.2%) | ||
| Segment VII | 7 (2.1%) | ||
| Segment VIII | 4 (1.2%) | ||
| Segment II+III | 17 (5.2%) | ||
| Segment IV+VIII | 2 (0.6%) | ||
| Segment V+VI | 8 (2.4%) | ||
| Segment V+VIII | 6 (1.8%) | ||
| Segment VI+VII | 23 (7.0%) | ||
| Segment VII+VIII | 5 (1.5%) | ||
| Segment II+III+IV | 35 (10.6%) | ||
| segment IV+V+VIII | 6 (1.8%) | ||
| Segment V+VI+VII | 8 (2.4%) | ||
| Segment VI+VII+VIII | 3 (0.9%) | ||
| Segment I+II+III+IV | 5 (1.5%) | ||
| segment I+V+VI+VII | 1 (0.3%) | ||
| Segment V+VI+VII+VIII | 37 (11.2%) | ||
| Segment I+V+VI+VII+VIII | 1 (0.3%) | ||
| Segment II+III+IV+V+VIII | 5 (1.5%) | ||
| Segment IV+V+VI+VII+VIII | 5 (1.5%) | ||
| Transplantation | 12 (3.6%) | ||
| Orthotopic liver transplantation | 4 (1.2%) | ||
| Living-donor liver transplantation | 3 (0.9%) | ||
| Extracorporeal liver resection plus autotransplantation | 5 (1.5%) |
Supplementary Table 2.
Detailed information for 371 cases of non-HCC hepatopancreatobiliary disease.
| Cataloge of diseases | Diagnosis | Cases | Gender (male/female) | Age (years, Mean ±SD) |
|---|---|---|---|---|
| Common liver diseases | Chronic severe hepatitis | 8 | 6/2 | 40.3±10.5 |
| Focal nodular hyperplasia, FNH | 4 | 3/1 | 47.7±18.4 | |
| Hepatic cavernous hemangioma | 6 | 1/5 | 40.4±7.4 | |
| Liver cirrhosis | 15 | 9/6 | 52.7±9.2 | |
| Rare liver diseases | 13 | 4/9 | 49.0±11.0 | |
| Hepatic hematoma | 2 | |||
| Hepatic lymphoepithelioma-like carcinoma | 1 | |||
| Intrahepatic biliary cystadenocarcinoma | 1 | |||
| Liver inflammatory myofibroblastic tumor | 1 | |||
| Liver metastasis from gastrointestinal stromal tumors, GIST | 1 | |||
| Primary biliary cirrhosis, PBC | 3 | |||
| Primary hepatic neuroendocrine carcinoma, PHNEC | 1 | |||
| Primary malignant fibrous histiocytoma, MFH | 1 | |||
| Wilson’s disease | 2 | |||
| Pancreatic diseases | Chronic pancreatitis and/or pancreatolithiasis | 8 | 8/0 | 42.8±10.3 |
| Pancreatic cancer | 71 | 50/21 | 55.5±11.1 | |
| Biliary diseases | Hepatolithiasis, HL | 121 | 39/82 | 48.7±12.2 |
| Carcinoma of gallblader | 6 | 3/3 | 60.4±12.6 | |
| Intrahepatic cholangiocarcinoma, ICC | 48 | 30/18 | 52.1±9.8 | |
| Hilar cholangiocarcinoma, HC | 43 | 25/18 | 58.6±8.3 | |
| Middle segment of the bile duct carcinoma | 5 | 3/2 | 73.3±9.9 | |
| Periampular carcinoma | 23 | 10/13 | 59.0±13.0 |