Abstract
Background
The aim of this study was to determine the correlation between glucocorticoids (GCs) and insulin resistance (IR) in healthy individuals by conducting a systematic meta-analysis.
Material/Methods
A systematic literature review was conducted using 9 electronic databases. Only case-control studies investigating fasting plasma glucose (FPG) and IR were enrolled based on strictly established selection criteria. Statistical analyses were performed by Stata software, version 12.0 (Stata Corporation, College Station, Texas, USA).
Results
Among 496 initially retrieved articles, only 6 papers published in English were eventually included in this meta-analysis. A total of 201 healthy individuals (105 in GC group and 96 in control group) were included in the 6 studies. In 4 of these 6 studies, dexamethasone was used, and in the other 2 studies prednisolone was given. This meta-analysis revealed that the FPG, fasting insulin (FINS), and homeostasis model assessment of insulin resistance (HOMA-IR) levels in the GC group were all significantly higher than that in the control group (FPG: SMD=2.65, 95%CI=1.42~3.88, P<0.001; FINS: SMD=2.48, 95%CI=1.01~3.95, P=0.001; HOMA-IR: SMD=38.30, 95%CI=24.38~52.22, P<0.001).
Conclusions
In conclusion, our present study revealed that therapies using GCs might result in elevated FPG, FINS, and HOMA-IR, and thereby contribute to IR in healthy individuals.
MeSH Keywords: Anti-Inflammatory Agents, Glucocorticoids, Insulin Resistance, Meta-Analysis
Background
Glucocorticoids (GCs) are steroid hormones synthesized by the adrenal cortex and stimulated to release after the hypothalamic-pituitary-adrenal axis is activated, and also play a predominant role in the maintenance of resting- and stress-related homeostasis [1,2]. Except for their well-established influences on glucose metabolism, GCs are involved in nearly every cellular, molecular, and physiologic network of the body, and modulate broad aspects of physiologic functions, including cell survival, proliferation, reproduction, growth, cognition, and behavior, together with function of the central nervous system, immune system, and cardiovascular functions [3,4]. GCs exert their functions through binding to their intracellular receptor, known as the glucocorticoid receptor (GR), which is a ligand-inducible transcription factor that belongs to the nuclear receptor superfamily [5]. GCs are the most frequently prescribed class of anti-inflammatory drugs in treatment of chronic inflammatory diseases, such as rheumatoid arthritis, asthma, and inflammatory bowel disease, but the use of GCs as therapeutics is often restricted because of adverse effects [6–8]. The common adverse effects include hypertension, glucose intolerance, central adiposity, dyslipidemia, diabetes, insulin resistance (IR), and skeletal muscle wasting [9,10].
IR is correlated to multiple metabolic diseases, such as obesity, diabetes mellitus, and hypertension [11,12]. IR is defined as a clinical state characterized by a decrease in sensitivity and responsiveness to insulin, and therefore a given concentration of insulin elicits a suboptimal biological effect [13]. IR is characterized by the diminished capacity for insulin to stimulate intracellular signaling in adipose tissue, the liver, and skeletal muscle [14]. Impaired insulin signaling in these tissues leads to depressed glucose uptake, insufficient suppression of hepatic glucose output, compensatory hyperinsulinemia, and dyslipidemia, which together constitute metabolic syndrome [15–17]. Furthermore, the biological effects of IR are tissue-specific [18]. In fat and muscle cells, IR results in reduced uptake of glucose leading to higher glucose levels in blood [19,20]. In liver cells, the IR effects can be observed in reduced synthesis and storage of glycogen, and a failure to repress glucose production, which also results in glucose release into the blood [10]. In several reports, IR has been described as an important adverse effect of GCs [21–23]. However, in human subcutaneous adipose tissue, a study also reported that GCs had no influence on IR [24]. Therefore, we analyzed published studies to understand the correlation between GCs and IR in healthy individuals by performing a meta-analysis.
Material and Methods
Search strategy
To identify relevant published studies, a computerized search was conducted based on the English electronic databases PubMed, EBSCO, Ovid, SpringerLink, Wiley Online Library, Web of Science, and the search engine Google Scholar, as well as the Chinese databases Wanfang Data, China National Knowledge Infrastructure (CNKI), and VIP Information for peer-reviewed studies published until September 2014. Additionally, a manual search of English studies was performed through cross-reference checks of selected articles. The following subject terms and key words were utilized to maximize search sensitivity and specificity: glucocorticoids, glucocorticoid effect, inhaled corticosteroids (ICS), insulin resistance, and insulin sensitivity.
Selection criteria
Studies were selected if they met these criteria: (1) the study designs were randomized controlled trials (RCTs); (2) the study subjects were healthy individuals with normal glucose tolerance; (3) the intervention was achieved mainly by injection of normal saline or GC drugs; (4) the outcomes were changes in fasting plasma glucose (FPG), fasting insulin (FINS), and homeostasis model assessment of insulin resistance (HOMA-IR) for evaluation of GC effects on IR; (5) research in enrolled trials had to be permitted by relevant ethics committees. Studies were excluded if: (1) they were reviews or retrospective studies; (2) they could not meet data integrity; (3) they were unpublished or repeat-published studies.
Methodological quality
Based on the criteria of the Delphi list, the quality of all selected trials was evaluated by 2 reviewers independently [25]. The assessed components included: (1) whether the studies were described as randomized and the methods described for concealing the intervention assignment schedule from clinicians and participants until recruitment were complete and irrevocable (DL01); (2) whether the groups were similar at baseline concerning the indicators most important for prognosis (DL02); (3) whether the inclusion and exclusion criteria were operationalized (DL03); (4) whether the outcome assessors were blinded (DL04); (5) whether the therapist cares/providers were blinded (DL05); (6) whether the patients were blinded (DL06); (7) whether point estimates and measures of variability were presented for the measures of primary outcomes (DL07); (8) whether the analyses included intention-to-treat analyses (DL08).
Statistical analysis
We conducted all meta-analyses with Stata software, version 12.0 (Stata Corporation, College Station, Texas, USA). For continuous outcomes, the means and standard deviations were pooled to a standardized mean difference (SMD) and 95% confidence interval (CI) using a fixed-effects or random-effects model, with the difference of degree of IR triggered by GCs in healthy individuals. Fisher’s z test was performed for measuring the significance of pooled odds ratio (OR). We implemented heterogeneity test using Cochran’s Q-statistics (Cochran, 1954) and quantified the influence of heterogeneity with I2=100% × (Q – DF)/Q [26]. A significant Q-statistic (P<0.05) indicated heterogeneity across studies, and the I2, whose values ranging between 0% and 100%, implied high estimates with higher values. When P<0.05 or I2>50%, a random-effects model was applied and, if not, a fixed-effects model was used. The sensitivity analysis was conducted via deleting studies with higher methodological heterogeneity. Further, the study publication bias was assessed through funnel plots and Egger’s linear regression method to measure the reliability of original results [27].
Results
Baseline characteristics of included studies
A total of 496 citations which studied the correlations between GCs and IR were initially retrieved, with 63 in Chinese and 433 in English. After excluding duplicates (n=208), animal-based subjects (n=143), letters, reviews, and meta-analysis (n=40), and unrelated topic (n=22), 83 full-text articles remained. Six English trials ultimately satisfied selection criteria after we excluded studies with no case-controls or that were not a cohort study (n=25), studies not relevant to GCs (n=24), studies unrelated to IR (n=22), and not enough information in studies (n=6). In total, 201 healthy individuals (105 in GC group and 96 in control group) were included in the 6 studies, which were published between 2002 and 2013 [14,28–32]. Individuals in the control group were treated with placebo while individuals in the GC group received treatment with GCs. All included studies used the GC drugs dexamethasone or prednisolone. The Delphi list is shown in Figure 1. Baseline characteristics of all included studies are shown in Table 1.
Figure 1.
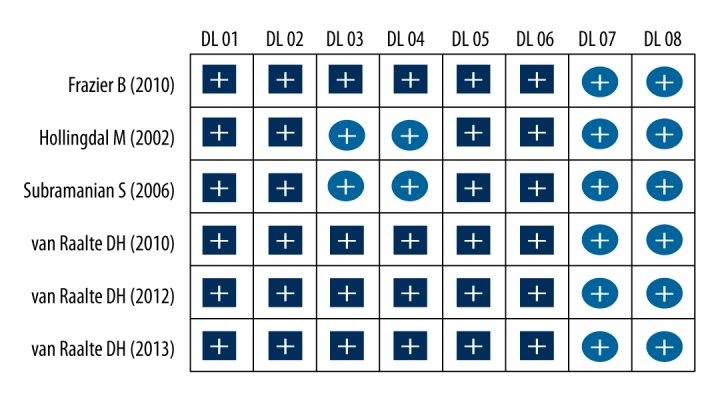
The Delphi list score of included studies.
Table 1.
Baseline characteristics of the included studies.
| First author | Year | Country | Ethnicity | Number | Gender (M/F) | Age (years) | Interventions | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Control | GC | Control | GC | Control | GC | Control | GC | ||||
| van Raalte DH [13] | 2013 | Netherlands | Caucasian | 20 | 8 | 12 | 8/0 | 12/0 | 21±3 | 21±2 | Placebo | Prednisolone (30 mg) |
| van Raalte DH [27] | 2010 | Netherlands | Caucasian | 23 | 11 | 12 | 23/0 | 20–45 | Placebo | Prednisolone (30 mg) | ||
| van Raalte DH [31] | 2012 | Netherlands | Caucasian | 20 | 8 | 12 | 8/0 | 12/0 | 22±3 | 22±3 | Placebo | Prednisolone (30 mg) |
| Subramanian S [28] | 2006 | USA | Caucasian | 36 | 18 | 18 | 7/11 | 33.1±8 | Placebo | Dexamethasone (8 mg) | ||
| Hollingdal M [30] | 2002 | Denmark | Caucasian | 16 | 8 | 8 | - | 24.4±0.5 | Placebo | Prednisolone (30 mg) | ||
| Frazier B [29] | 2010 | USA | Caucasian | 86 | 43 | 43 | 0.419 | 28±5.5 | Placebo | Dexamethasone | ||
M – male; F – female; GC – glucocorticoids.
Comparison of FPG levels
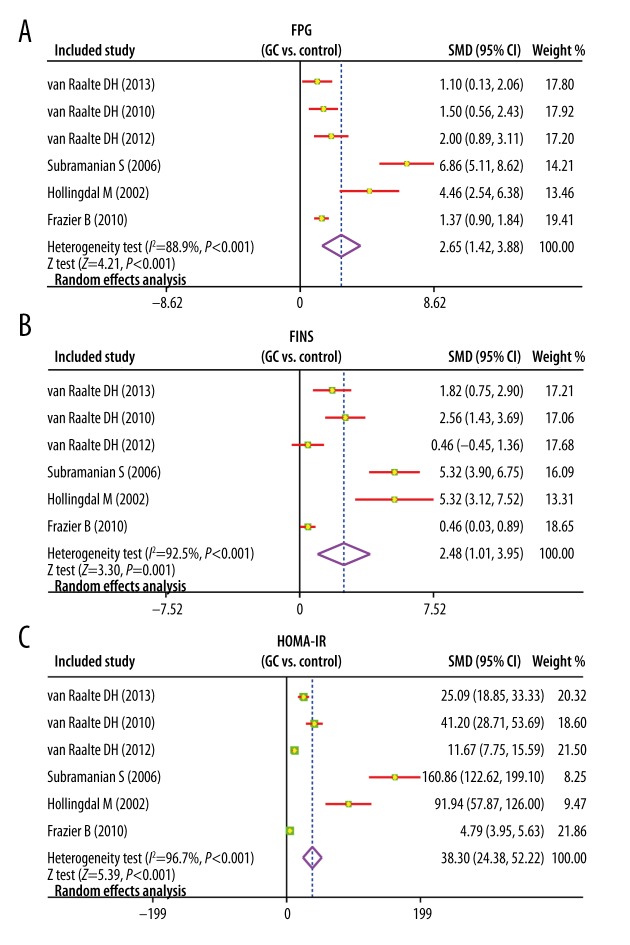
Heterogeneity existed across studies (I2=88.9%, P<0.001), and thus a random-effects model was used. The meta-analysis revealed that the FPG levels in GC group was evidently higher compared with the control group (SMD=2.65; 95%CI=1.42~3.88; P<0.001), suggesting that there was a significant difference between the 2 groups (Figure 2). The subgroup analysis based on intervention drug revealed that with prednisolone, the FPG levels in GC group was evidently higher compared with that in the control group (SMD=2.01; 95%CI=0.96~3.05; P<0.001). However, after using dexamethasone, compared with the control group, the FPG levels in GC group had no marked change (SMD=4.05; 95%CI=−1.33~9.43; P=0.140) (Figure 3).
Figure 2.
(A–C) Forest plots of comparisons of fasting plasma glucose, fasting insulin, and homeostasis model assessment of insulin resistance levels between the glucocorticoid group and the control group.
Figure 3.
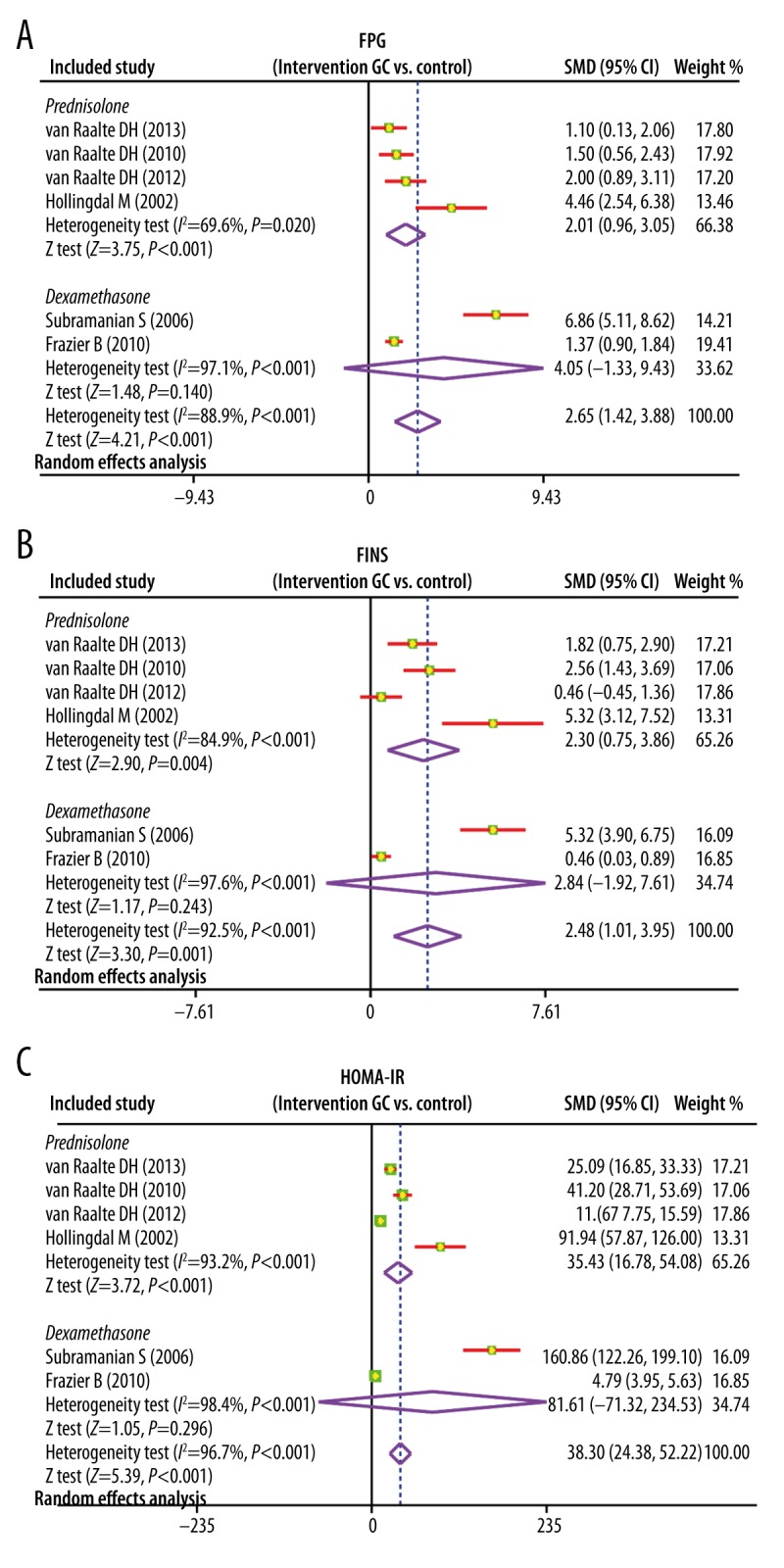
(A–C) Forest plots on subgroup analysis by intervention drug of comparison of fasting plasma glucose, fasting insulin, and homeostasis model assessment of insulin resistance levels between the glucocorticoid group and the control group.
Comparison of FINS levels
There was heterogeneity among studies (I2=92.5%, P<0.001), and thus a random-effects model was applied. The results of present meta-analysis suggested that there was significant difference in the FINS levels between the 2 groups and FINS levels in GC group was significantly higher than in the control group (SMD=2.48; 95%CI=1.01~3.95; P=0.001) (Figure 2). Subgroup analysis by intervention drug indicated that, with prednisolone, the FINS levels was markedly higher compared with the control group (SMD=2.30; 95%CI=0.75~3.86; P=0.004), while with dexamethasone there was no significant change of FINS levels (SMD=2.84; 95%CI=−1.92~7.61; P=0.243) (Figure 3).
Comparison of HOMA-IR levels
According to our analysis, heterogeneity existed among studies (I2=96.7%, P<0.001), and therefore a random-effects model was performed. This meta-analysis indicated that the HOMA-IR levels in GC group was distinctly higher than that in the control group (SMD=38.30; 95%CI=24.38~52.22; P<0.001); therefore, there was significant difference between the 2 groups (Figure 2). Subgroup analysis by intervention drug (Figure 3) showed that HOMA-IR levels in the prednisolone group were significantly higher than that in the control group (SMD=35.43; 95%CI=16.78~54.08; P<0.001); while after dexamethasone treatment, the HOMA-IR levels showed no change (SMD=81.61; 95%CI=−71.32~234.53; P=0.296).
Sensitivity analysis and publication bias
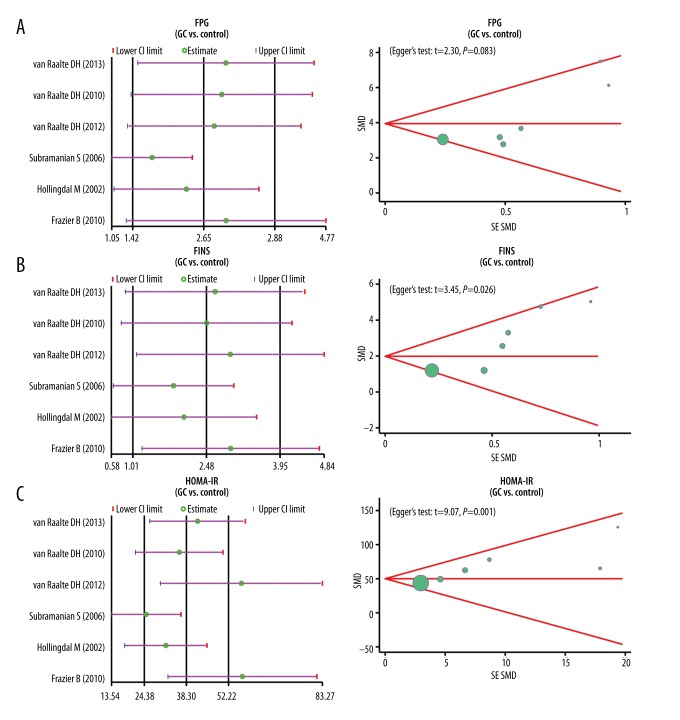
Based on the sensitivity analysis, we found that the 4 included studies had no marked influence on the pooled SMD of FPG, FINS, and HOMA-IR levels. However, the CI values of the 2 articles published by van Raalte DH and Frazier B et al. exceeded the upper CI limit. The funnel plots of FPG showed symmetry, which indicated that there were no publication bias (P=0.083); while the funnel plots of FINS and HOMR-IR showed asymmetry, indicating for the presence of publication bias (both P<0.05) (Figure 4).
Figure 4.
(A–C) Sensitivity analyses, funnel plots, and Egger’s linear regression analyses of fasting plasma glucose, fasting insulin, and homeostasis model assessment of insulin resistance levels to evaluate publication bias.
Discussion
GCs are the most frequently prescribed class of anti-inflammatory drugs in treatment of multiple chronic inflammatory diseases, but carry warnings about adverse effects, among which IR is the most common [6]. Nonetheless, there was also a study that failed to find a correlation between GCs and IR in human subcutaneous adipose tissue [24]. To explore whether there is a correlation between GCs and IR, we conducted this systematic meta-analysis.
The main result of this meta-analysis was that GCs use was associated with marked increases in FPG, FINS, and HOMA-IR levels, suggesting GCs use may correlate with IR. Homeostasis model assessment was first illustrated by Matthews et al., and was a method for evaluating IR; this model is based on the theory of a feedback loop between β cells and the liver [33]. HOMA-IR has been broadly used in multiple epidemiologic studies in assessment of IR because it can reflect IR more correctly than FINS levels alone [34]. The HOMA-IR level was significantly increased with the usage of GCs in our study, indicating that there might be association between GCs and IR. Our results showed that while the FINS level were elevated after the usage of GCs, the FPG level was instead increased, indicating use of GCs may have a profound IR effect, consistent with previous studies that showed IR was a predominant adverse effect of GCs [21,22]. GCs can promote an increase in adiposity together with an increase in lipolysis, resulting in elevated free fatty acids (FFA) in circulation [35]. FFA in turn leads to the inhibition on the insulin secretion from pancreatic β cells through obstructing insulin signaling via the activation of serine-kinases and consequently induces the occurrence of IR [36].
The subgroup analysis on the basis of intervention drug revealed that after using prednisolone, the FPG, FINS, and HOMA-IR levels were markedly increased, while there were no marked changes with dexamethasone. Prednisolone and dexamethasone are synthetic GCs that have been used in clinical medical practice because of their antiallergic, anti-inflammatory, and immunosuppressive functions [37]. Even though the synthetic GCs are widely applied in various pathological conditions, they also have adverse metabolic influences [38]. Based on this meta-analysis, there is a significant difference between the effects of prednisolone and dexamethasone, suggesting that dexamethasone might be a better clinical choice compared with prednisolone.
Some limitations exist in this meta-analysis. First, because there were few human studies that investigated the effects of GC on healthy individuals, we could only enroll 6 studies. Because of the small number of included studies and small sample size, there was publication bias that affected the results of this meta-analysis. For instance, the main result was that the FPG and FINS levels were significant elevated after receiving GCs, while no significant differences were observed in FPG and FINS levels after use of dexamethasone. This might be attributed to the small number of enrolled trials. Second, the research subjects were all whites, but no subjects were from Asian, African, or and mixed populations. The ethnicity factor may affect the final results because of the differences of diet, climate, and lifestyle. Therefore, our study is not representative of all ethnicities. Second, several included studies had no complete data on sex or age, which would also influence our results to some extent.
Conclusions
GCs use might result in elevated FPG, FINS, and HOMA-IR levels, and increase the risk of IR in healthy individuals.
Acknowledgement
We would like to thank the reviewers for their helpful comments on this manuscript. We are also obliged to colleagues who shared ideas with us during the composition of this article.
Footnotes
Conflicts of interest
The authors declare that there was no competing interest.
Source of support: Departmental sources
References
- 1.Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Recent advances in the molecular mechanisms determining tissue sensitivity to glucocorticoids: novel mutations, circadian rhythm and ligand-induced repression of the human glucocorticoid receptor. BMC Endocr Disord. 2014;14:71. doi: 10.1186/1472-6823-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauner M, Goettsch C, Stein N, et al. Dissociation of osteogenic and immunological effects by the selective glucocorticoid receptor agonist, compound A, in human bone marrow stromal cells. Endocrinology. 2011;152:103–12. doi: 10.1210/en.2010-0456. [DOI] [PubMed] [Google Scholar]
- 3.Lee SR, Kim HK, Youm JB, et al. Non-genomic effect of glucocorticoids on cardiovascular system. Pflugers Arch. 2012;464:549–59. doi: 10.1007/s00424-012-1155-2. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos G. Sensitivity and resistance to glucocorticoids. Pediatric Rheumatology. 2014;12:1. [Google Scholar]
- 5.Vandevyver S, Dejager L, Tuckermann J, Libert C. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154:993–1007. doi: 10.1210/en.2012-2045. [DOI] [PubMed] [Google Scholar]
- 6.Keenan CR, Salem S, Fietz ER, Gualano RC, Stewart AG. Glucocorticoid-resistant asthma and novel anti-inflammatory drugs. Drug Discov Today. 2012;17:1031–38. doi: 10.1016/j.drudis.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Joanny E, Ding Q, Gong L, et al. Anti-inflammatory effects of selective glucocorticoid receptor modulators are partially dependent on up-regulation of dual specificity phosphatase 1. Br J Pharmacol. 2012;165:1124–36. doi: 10.1111/j.1476-5381.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J, Cheng B, Zhu X, Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011;187:942–50. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
- 9.Rose AJ, Herzig S. Metabolic control through glucocorticoid hormones: an update. Mol Cell Endocrinol. 2013;380:65–78. doi: 10.1016/j.mce.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Fleuren WW, Toonen EJ, Verhoeven S, et al. Identification of new biomarker candidates for glucocorticoid induced insulin resistance using literature mining. BioData Min. 2013;6:2. doi: 10.1186/1756-0381-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. J Clin Hypertens (Greenwich) 2011;13:238–43. doi: 10.1111/j.1751-7176.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercurio V, Carlomagno G, Fazio V, Fazio S. Insulin resistance: Is it time for primary prevention? World J Cardiol. 2012;4:1–7. doi: 10.4330/wjc.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Raalte DH, Diamant M, Ouwens DM, et al. Glucocorticoid treatment impairs microvascular function in healthy men in association with its adverse effects on glucose metabolism and blood pressure: a randomised controlled trial. Diabetologia. 2013;56:2383–91. doi: 10.1007/s00125-013-3016-8. [DOI] [PubMed] [Google Scholar]
- 15.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 17.Savage DB, Semple RK. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol. 2010;21:329–36. doi: 10.1097/MOL.0b013e32833b7782. [DOI] [PubMed] [Google Scholar]
- 18.Lin HV, Ren H, Samuel VT, et al. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60:700–9. doi: 10.2337/db10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brands M, van Raalte DH, Joao Ferraz M, et al. No difference in glycosphingolipid metabolism and mitochondrial function in glucocorticoid-induced insulin resistance in healthy men. J Clin Endocrinol Metab. 2013;98:1219–25. doi: 10.1210/jc.2012-3266. [DOI] [PubMed] [Google Scholar]
- 22.Pretty C, Chase JG, Lin J, et al. Impact of glucocorticoids on insulin resistance in the critically ill. Comput Methods Programs Biomed. 2011;102:172–80. doi: 10.1016/j.cmpb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887–94. doi: 10.1136/ard.2011.151464. [DOI] [PubMed] [Google Scholar]
- 24.Hazlehurst JM, Gathercole LL, Nasiri M, et al. Glucocorticoids fail to cause insulin resistance in human subcutaneous adipose tissue in vivo. J Clin Endocrinol Metab. 2013;98:1631–40. doi: 10.1210/jc.2012-3523. [DOI] [PubMed] [Google Scholar]
- 25.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 28.van Raalte DH, Nofrate V, Bunck MC, et al. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162:729–35. doi: 10.1530/EJE-09-1034. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, DeRosa MA, Bernal-Mizrachi C, et al. PPARalpha activation elevates blood pressure and does not correct glucocorticoid-induced insulin resistance in humans. Am J Physiol Endocrinol Metab. 2006;291:E1365–71. doi: 10.1152/ajpendo.00230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frazier B, Hsiao CW, Deuster P, Poth M. African Americans and Caucasian Americans: differences in glucocorticoid-induced insulin resistance. Horm Metab Res. 2010;42:887–91. doi: 10.1055/s-0030-1265131. [DOI] [PubMed] [Google Scholar]
- 31.Hollingdal M, Juhl CB, Dall R, et al. Glucocorticoid induced insulin resistance impairs basal but not glucose entrained high-frequency insulin pulsatility in humans. Diabetologia. 2002;45:49–55. doi: 10.1007/s125-002-8244-y. [DOI] [PubMed] [Google Scholar]
- 32.van Raalte DH, Brands M, Serlie MJ, et al. Angiopoietin-like protein 4 is differentially regulated by glucocorticoids and insulin in vitro and in vivo in healthy humans. Exp Clin Endocrinol Diabetes. 2012;120:598–603. doi: 10.1055/s-0032-1321864. [DOI] [PubMed] [Google Scholar]
- 33.Okita K, Iwahashi H, Kozawa J, et al. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J. 2013;60:283–90. doi: 10.1507/endocrj.ej12-0320. [DOI] [PubMed] [Google Scholar]
- 34.Capasso I, Esposito E, Pentimalli F, et al. Homeostasis model assessment to detect insulin resistance and identify patients at high risk of breast cancer development: National Cancer Institute of Naples experience. J Exp Clin Cancer Res. 2013;32:14. doi: 10.1186/1756-9966-32-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest. 2012;122:3854–7. doi: 10.1172/JCI66180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capurso C, Capurso A. From excess adiposity to insulin resistance: the role of free fatty acids. Vascul Pharmacol. 2012;57:91–97. doi: 10.1016/j.vph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Rafacho A, Ortsater H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014;223:R49–62. doi: 10.1530/JOE-14-0373. [DOI] [PubMed] [Google Scholar]
- 38.Urbanska J, Karewicz A, Nowakowska M. Polymeric delivery systems for dexamethasone. Life Sci. 2014;96:1–6. doi: 10.1016/j.lfs.2013.12.020. [DOI] [PubMed] [Google Scholar]