Abstract
Background
Gastric carcinoma is the second leading cause of cancer death. microRNAs play vital roles in regulating expression of related oncogenes. microRNA-25 (miR-25) has been found to be up-regulated in gastric carcinoma. However, its roles in affecting cell apoptosis of gastric carcinoma and the related mechanism remain elusive. This study aimed to uncover the influences of miR-25 on gastric carcinoma cell apoptosis and the possible functional mechanisms involved.
Material/Methods
Human gastric adenocarcinoma cell line AGS was used and transfected with lentivirus containing miR-25-specifc inhibitor sponge or expression vector to analyze the effects of miR-25.
Results
miR-25 had higher expression in AGS than in human gastric epithelial cell line GES-1 (P<0.01). Inhibition of miR-25 by its sponge in AGS cells resulted in suppressed cell viability (P<0.01) and promoted cell apoptosis (P<0.01), while overexpression of miR-25 abrogated these effects (P<0.01 and P<0.05), indicating that miR-25 can promote cell viability and inhibit cell apoptosis in AGS cells. Expression analysis of related factors by Western blot showed that inhibiting miR-25 led to the up-regulation of F-box and WD repeat domain-containing 7 (FBXW7, P<0.01) and the down-regulation of FBXW7 substrates, cyclin E1 (CCNE1, P<0.01), and v-myc avian myelocytomatosis viral oncogene homolog (MYC, P<0.001).
Conclusions
These results indicate that miR-25 has anti-apoptosis roles in AGS cells, possibly via inhibiting FBXW7 and thus promoting oncogenes, such as CCNE1 and MYC. This study provides basic evidence for using miR-25 as a possible therapeutic target in treating gastric carcinoma.
MeSH Keywords: Apoptosis; Cyclin E; Genes, myc; MicroRNAs; Stomach Neoplasms
Background
Gastric carcinoma is the second leading cause of cancer death and the fourth most common cancer worldwide [1]. It can be caused by bacterial infection [2], smoking, and unhealthy diet. Gastric carcinoma is sometimes asymptomatic at earlier stages, adding difficulties to the diagnosis. Most of the cases have developed to advanced stages or spread to other parts of the body by the time of diagnosis, severely impacting the survival rate and treatment success. In Japan and Korea, the survival rate is higher than in Western countries, possibly due to active screening programs. Curative therapy is surgery and adjuvant chemotherapy or radiation therapy, which has advantages over surgery alone [3]. Great efforts have been made to improve diagnostic techniques [4] and reveal intrinsic mechanisms, including the related oncogenes and tumor suppressor genes [5], and DNA methylation [6,7], providing valuable information for treating gastric carcinoma.
microRNAs are small non-coding RNAs regulating gene expression transcriptionally or post-transcriptionally. Their target genes include tumor suppressor genes and oncogenes, and by regulating the expression of these pivotal genes, microRNAs have important roles in the mediation of tumor progression [8]. In gastric carcinoma, microRNAs have been studied for diagnosis and prognosis, with microRNA-181c (miR-181c) associated with the progression and prognosis [9], and miR-421 being a biomarker for screening gastric cancer using gastric juice [10]. In addition, several microRNAs, such as miR-9, miR-433, and miR-200b, were found to be aberrantly expressed in gastric carcinoma, participating in the regulation of proliferation and apoptosis of gastric cancer cells via mediating their target genes [11,12]. Despite these important results, further studies on the regulatory mechanisms of microRNAs remain necessary for a better understanding of the oncogenesis network.
Existing microRNA expression profiles have revealed up-regulation of miR-25 in gastric carcinoma. For example, miR-25, together with another 15 microRNAs, has higher expression in metastatic gastric cancer cells than in normal cells [13], and it is abundant in advanced gastric carcinoma tissues [14]. However, the detailed functions and the regulatory mechanisms of miR-25 in gastric carcinoma cell apoptosis have not been investigated. This study aimed to uncover the roles of miR-25 in modulating gastric carcinoma cell apoptosis and its possible mechanisms. Human gastric adenocarcinoma cell line AGS was used to verify the up-regulated miR-25 compared to human gastric epithelial cell line GES-1. miR-25 was inhibited or overexpressed by transfecting the lentivirus containing its specific inhibitor sponge or expression vector in AGS cells, and its impacts on AGS cells were monitored for cell viability and cell apoptosis. Changes in expression of related factors were detected to reveal the possible functional mechanisms of miR-25. This study sought to provide an overall understanding of miR-25 in regulating AGS cell apoptosis, and to offer evidence for its use as a potential therapeutic target in gastric carcinoma treatment.
Material and Methods
Cells
The human gastric adenocarcinoma cell line AGS and the human gastric epithelial cell line GES-1 were purchased from Cobioer (Nanjing, China). Cells were cultured in F12 (AGS) or Roswell Park Memorial Institute (RPMI)-1640 (GES-1) medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 μg/mL streptomycin, and 100 U/mL penicillin, in a humid atmosphere containing 5% CO2 at 37°C. Cells were passaged when the confluency reached 70%.
Lentivirus transfection
The lentivirus containing miR-25-specific inhibitor sponge or pre-miR-25 expression vector was constructed by GenePharma (Shanghai, China). The AGS cells were seeded on 24-well plates (1×105/well) and cultured for another 24 h. Then, the medium was changed with fresh medium containing 6 μg/mL Polybrene (Sigma-Aldrich, Shanghai, China) and 25 mM lentivirus suspension (lentivirus containing miR-25 sponge or expression vector for experimental groups, and blank lentivirus for the control group), and the cells were incubated at 37°C. After 24 h of incubation, the medium was replaced and the cells were collected for further analyses.
Cell viability analysis
AGS cells at the logarithmic phase were collected and the concentration was adjusted to 5×104/mL. Cell suspension of 100 μL was added to each well of a 96-well plate and cultured for 24, 48, 72, and 96 h. We added 10 μL of methyl thiazolyl tetrazolium (MTT, 5 mg/mL, Beyotime, Shanghai, China) to each well for another 4-h incubation. After removing the medium, 150 μL dimethylsulfoxide was added and the plate was shaken for 10 min to terminate the reaction. A SpectraMax i3x microplate reader (Molecular Devices, Silicon Valley, CA) was used to detect the optical density at 570 nm.
Cell apoptosis analysis
Cell apoptosis was measured with the annexin V: fluorescein isothiocyanate (FITC) and Prodium iodide (PI) dual-staining method using Annexin V: FITC Apoptosis Detection Kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Briefly, the AGS cells were collected and adjusted to the concentration of 1×106/mL at 72 h post-transfection. Cell suspension of 200 μL was assigned for each sample, washed with phosphate-buffered saline (PBS), and resuspended in 100 μL binding buffer. Then, 2 μL Annexin-V-FITC (20 μg/mL) was added and the cells were incubated on ice in the dark for 15 min. Before detection, 400 μL PBS and 1 μL PI (50 μg/mL) were added, and the detection was performed using BD FACSCanto II (BD Biosciences).
Real-time quantitative PCR (qPCR)
AGS cells and GES-1 cells were collected and lysed in RNAiso for Small RNA (TaKaRa, Dalian, China) for miRNA extraction. The One-Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa) was used for reverse transcription. qPCR was conducted on a LightCycler 480 (Roche, Basel, Switzerland) to detect the expression of miR-25 (Fw: 5′-CGG CGG CAT TGC ACT TGG TCT C-3′ and Rv: 5′-GTG CAG GGT CCG AGG T-3′) standardized by U6 reference (Fw: 5′-CTC GCT TCG GCA GCA CAT ATA CT-3′ and Rv: 5′-ACG CTT CAC GAA TTT GCG TGT C-3′). Detection was conducted in triplicate for each sample and data were calculated with the 2−ΔΔCt method.
Western blot
Protein samples of the 3 transfected AGS cell groups were extracted with RIPA lysis buffer (Beyotime). We loaded 20 μg of protein in each lane of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then the bands on the gel were transferred to a polyvinylidene fluoride membrane (Roche). The membrane was blocked in 5% skim milk for 2 h at room temperature and then incubated in specific primary antibodies (Abcam, Cambridge, UK) overnight at 4°C. After incubation in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, the signals were developed using ECL Plus Western Substrate (Piece, Carlsbad, CA), and the relative density was calculated by comparing it to GAPDH using ImageJ software version 1.49 (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were conducted in triplicate and results are presented as the mean ± standard deviation. Statistical analysis was performed using 1-way analysis of variance in SPSS 19.0 (IBM, New York, USA). Differences were considered significant if P<0.05.
Results
miR-25 is up-regulated in AGS cells
As mentioned above, miR-25 is abundant in gastric carcinoma cells, so in this study the up-regulated miR-25 in AGS cells was verified by qPCR (Figure 1). Results showed significantly greater promotion of miR-25 expression in AGS cells than in the normal GES-1 cells (P<0.01), which is consistent with previous studies, implying that miR-25 might be related to the progression of gastric carcinoma. Therefore, we analyzed its roles in AGS cells in the following experiments.
Figure 1.

miR-25 is expressed at higher levels in AGS cells compared to normal gastric epithelial cells GES-1. ** P<0.01. miR-25, microRNA-25. GES-1, human gastric epithelial cell line. AGS, human gastric adenocarcinoma cell line.
Inhibiting miR-25 leads to suppressed cell viability and promotes cell apoptosis
The miR-25-specific inhibitor sponge and expression vector were used to inhibit and overexpress, respectively, miR-25. The altered expression was tested by qPCR and the expected results were obtained (Figure 2A): miR-25 sponge significantly reduced the level of miR-25 compared to cells transfected with blank lentivirus (P<0.001) compared to the level recovered after co-transfection with miR-25 expression vector (P<0.01). Therefore, the 3 transfected AGS cell groups were used to analyze changes in viability and apoptosis.
Figure 2.
miR-25 promotes viability and inhibits apoptosis of AGS cells. Control, AGS cells transfected with blank lentivirus. miR-25 sponge, AGS cells transfected with lentivirus containing miR-25 sponge. miR-25 sponge + miR-25, AGS cells co-transfected with miR-25 sponge lentivirus and miR-25 expression vector lentivirus. ***, P<0.001. **, P<0.01. *, P<0.05. (A) qPCR detecting the effects of miR-25 sponge and miR-25 expression vector transfection. miR-25 is inhibited by its specific inhibitor sponge, and the effects are abrogated by co-transfecting its expression vector. (B) Cell viability of 3 transfected AGS cell groups detected by MTT assay. Detection was performed at 24, 48, 72, and 96 h post-transfection. Significant differences were found between Group miR-25 sponge and the other groups at 72 and 96 h post-transfection. (C) Cell apoptosis of 3 transfected AGS cell groups detected by flow cytometry at 72 h post-transfection. AGS cell apoptosis was promoted by inhibiting miR-25. (D) Histogram indicating the percent of FITC-positive and PI-negative apoptotic AGS cells based on triplicate detections. Cell apoptosis was significantly promoted by miR-25 sponge.
Cell viability detected with MTT method was compared at 24, 48, 72, and 96 h post-transfection (Figure 2B). Generally, the viability of AGS cells transfected with miR-25 sponge gradually decreased, while that of the other 2 cell groups increased as time progressed. Significant differences were found at 72 h post-transfection, when the viability of miR-25 sponge-transfected AGS cells was obviously lower than in the other 2 groups (P<0.01). These results indicated that inhibiting miR-25 leads to suppressed AGS cell viability, and overexpressing miR-25 could again promote cell viability.
Next, we examined cell apoptosis using the annexin V-FITC/PI staining method, detected by flow cytometry (Figure 2C). Results showed an obvious promotion of cell apoptosis when AGS cells were transfected with miR-25 sponge, which was also indicated from the histogram (Figure 2D) based on the percent of FITC-positive and PI-negative cells. Inhibiting miR-25 significantly promoted cell apoptosis (P<0.01) and the effect was reversed by miR-25 co-transfection (P<0.05). Taken together, this evidence shows that miR-25 can promote cell viability and inhibit cell apoptosis, serving as an anti-apoptosis factor in AGS cells.
miR-25 regulates FBXW7, CCNE1 and MYC
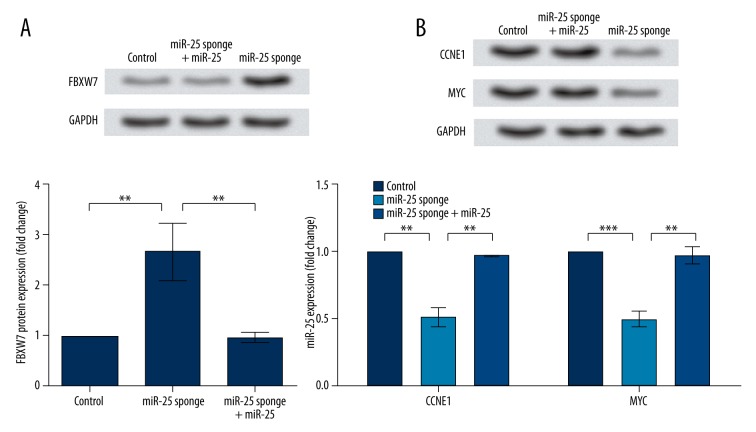
F-box and WD repeat domain-containing 7 (FBXW7) was selected to analyze the functional mechanism of miR-25 in AGS cells, because previous studies have proved that miR-25 expression is negatively related to FBXW7 in prostatic small cell neuroendocrine carcinoma [15]. Western blot results showed that the expression of PBXW7 protein was significantly up-regulated by miR-25 sponge (P<0.01) and decreased after co-transfection with miR-25 expression vector (P<0.01, Figure 3A), showing that miR-25 can affect FBXW7 expression in AGS cells.
Figure 3.
miR-25 regulates expression of FBXW7, CCNE1, and MYC. Control, AGS cells transfected with blank lentivirus. miR-25 sponge, AGS cells transfected with lentivirus containing miR-25 sponge. miR-25 sponge + miR-25, AGS cells co-transfected with miR-25 sponge lentivirus and miR-25 expression vector lentivirus. The density of bands is normalized by GAPDH before comparison. ***, P<0.001. **, P<0.01. (A) FBXW7 protein expression is inhibited by miR-25 as is shown by Western blot and histogram generated from triplicate experiments. FBXW7, F-box, and WD repeat domain-containing 7. (B) CCNE1 and MYC protein expression was promoted by miR-25, as shown by Western blot and a histogram generated from triplicate experiments. CCNE1, cyclin E1. MTC, v-myc avian myelocytomatosis viral oncogene homolog.
Expression changes of cyclin E1 (CCNE1) and v-myc avian myelocytomatosis viral oncogene homolog (MYC) were analyzed by Western blot. Both of these 2 factors are critical targets of FBXW7, and are negatively correlated with FBXW7 expression in hepatocellular carcinoma [16]. Western blot analysis showed that these 2 factors were also affected by miR-25, with their expression was significantly down-regulated by miR-25 sponge (P<0.01 or P<0.001, Figure 3B). In AGS cell co-transfected with miR-25 sponge and expression vector, expression levels of the 2 factors were up-regulated (P<0.01). Taken together, this indicates that miR-25 can inhibit FBXW7 and promote CCNE1 and MYC in AGS cells, implying its potential functional mechanism in gastric carcinoma.
Discussion
In this study, miR-25 was found to be up-regulated in human gastric adenocarcinoma cell line AGS, and its altered expression by sponge or expression vector indicates that miR-25 can promote viability and inhibit apoptosis of AGS cells. The protein expression of FBXW7, a target of miR-25, is suppressed, and the expression of CCNE1 and MYC is promoted by up-regulated miR-25, implying the possible regulatory mechanism of miR-25 in AGS cells.
Cyclins are fundamental regulators of the cell cycle, playing an important role in tumorigenesis. CCNE1 has been shown by earlier studies to contribute to the progression of gastric carcinoma and to possess prognostic value [17,18]. MYC is a proto-oncogene associated with the regulation of cell cycle and cell growth [19]. Its gene amplification and overexpression have been observed in the carcinogenesis process of gastric carcinoma patients [20], as well as in other human cancers. Both CCNE1 and MYC are regulated by FBXW7, an ubiquitin ligase that degrades substrates like CCNE1 and MYC through ubiquitin-dependent proteolysis to modulate cell cycle progression [21]. Mutations in FBXW7 lead to increased stabilization of MYC [22], and down-regulation of FBXW7 generates synergistic accumulation of cellular and active chromatin-bound MYC [23]. Colorectal cancer cells possess the characteristic of CCNE1 and MYC accumulation caused by decreased FBXW7-mediated ubiquitination [24]. FBXW7 can be regulated by miR-25 during the reprogramming of mouse fibroblast cells to induced pluripotent stem cells [25]. Therefore, it is reasonable to speculate that the down-regulation of CCNE1 and MYC detected in this study was caused by the promotion of FBXW7 when miR-25 was inhibited. The up-regulated miR-25 likely inhibits FBXW7, which further promotes the expression of cell cycle regulator CCNE1 and MYC, thus modulating the viability and apoptosis of AGS cells.
The present study shows that miR-25 inhibits cell apoptosis and promotes cell viability in the AGS cell line, consistent with that detected in other cancer cells. miR-25 is expressed at higher levels in ovarian cancer, promoting cell proliferation via directly modulating the apoptosis facilitator BCL2L11 [26]. It inhibits cell apoptosis in cholangiocarcinoma through regulating TRAIL death receptor-4 [27], and increases proliferation of non-small cell lung cancer cells via the pathway involving cell division cycle 42 [28]. Another study of gastric carcinoma reported reversion-inducing cysteine-rich protein with Kazal motifs as a target of miR-25, taking part in the regulation of cell growth and motility [29]. Despite its multiple target genes, miR-25 has conserved pro-growth and anti-apoptosis functions in various human cancer cells. The results of this study offer new evidence for the anti-apoptosis action of miR-25 in gastric carcinoma, suggesting its potential as a therapeutic target for treating gastric carcinoma.
A very recent study has found miR-25 is capable of directly targeting FBXW7 and promoting proliferation, invasion, and migration of gastric carcinoma cells in tissues and cell lines [30]. Because it has already been shown that FBXW7 is a direct target of mir-25 by comparing the luciferase activity of the wild-type and mutant 3′ untranslated region of FBXW7, a similar verification was not repeated in this study. Instead, this study investigated the roles of miR-25 in gastric carcinoma from another perspective, revealing its anti-apoptosis function in AGS cells. In addition, the possible regulatory pathway was found by analyzing the changes in expression of CCNE1 and MYC. These results, together with the abovementioned study, provide a more integrated elucidation of miR-25 functioning in gastric carcinoma cells via regulating FBXW7 and, further downstream, CCNE1 and MYC.
Conclusions
We have verified the up-regulated expression and the anti-apoptosis role of miR-25 in human gastric adenocarcinoma cell line AGS. FBXW7 and its downstream factors, CCNE1 and MYC, are involved in the execution of miR-25 roles. This study provides evidence for using miR-25 as a potential therapeutic target in treating gastric carcinoma.
Footnotes
Conflict of interests
No conflict of interest exists.
Source of support: This study was supported by the National Natural Science Foundation of China (No. 81272698)
References
- 1.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–49. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau TO, Tang CM, Yu J. Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: disease and treatments. World J Gastroenterol. 2014;20:6448–56. doi: 10.3748/wjg.v20.i21.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev. 2007;33:729–40. doi: 10.1016/j.ctrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Cui DX, Zhang L, Yan XJ, et al. A microarray-based gastric carcinoma prewarning system. World J Gastroenterol. 2005;11:1273–82. doi: 10.3748/wjg.v11.i9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–10. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 6.Hibi K, Kitamura YH, Mizukami H, et al. Frequent CDH3 demethylation in advanced gastric carcinoma. Anticancer Res. 2009;29:3945–47. [PubMed] [Google Scholar]
- 7.Sakata M, Kitamura YH, Sakuraba K, et al. Methylation of HACE1 in gastric carcinoma. Anticancer Res. 2009;29:2231–33. [PubMed] [Google Scholar]
- 8.Drakaki A, Iliopoulos D. MicroRNA gene networks in oncogenesis. Curr Genomics. 2009;10:35–41. doi: 10.2174/138920209787581299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui M, Yue L, Fu Y, et al. Association of microRNA-181c expression with the progression and prognosis of human gastric carcinoma. Hepatogastroenterology. 2013;60:961–64. doi: 10.5754/hge121333. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Cui L, Ye G, et al. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 2012;33:2349–55. doi: 10.1007/s13277-012-0497-x. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Zhang H, Zhang Z, et al. Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp Clin Cancer Res. 2009;28:82–90. doi: 10.1186/1756-9966-28-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurashige J, Kamohara H, Watanabe M, et al. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19(Suppl 3):S656–64. doi: 10.1245/s10434-012-2217-6. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhang Y, Zhang H, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–33. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 14.Kim BH, Hong SW, Kim A, et al. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol. 2013;107:505–10. doi: 10.1002/jso.23271. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Sun Y, Chen X, et al. p53 mutation directs AURKA overexpression via miR-25 and FBXW7 in prostatic small cell neuroendocrine carcinoma. Mol Cancer Res. 2015;13:584–91. doi: 10.1158/1541-7786.MCR-14-0277-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu K, Zheng X, Zan X, et al. Evaluation of Fbxw7 expression and its correlation with the expression of c-Myc, cyclin E and p53 in human hepatocellular carcinoma. Hepatol Res. 2012;42:904–10. doi: 10.1111/j.1872-034X.2012.01005.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi T, Watanabe A, Sawada H, et al. Prognostic value of cyclin E and p53 expression in gastric carcinoma. Cancer. 1998;82:1238–43. doi: 10.1002/(sici)1097-0142(19980401)82:7<1238::aid-cncr5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Xiangming C, Natsugoe S, Takao S, et al. The cooperative role of p27 with cyclin E in the prognosis of advanced gastric carcinoma. Cancer. 2000;89:1214–19. [PubMed] [Google Scholar]
- 19.Calcagno D-Q. MYC and gastric adenocarcinoma carcinogenesis. World J Gastroenterol. 2008;14:5962–68. doi: 10.3748/wjg.14.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calcagno DQ, Leal MF, Demachki S, et al. MYC in gastric carcinoma and intestinal metaplasia of young adults. Cancer Genet Cytogenet. 2010;202:63–66. doi: 10.1016/j.cancergencyto.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Li DC, Li ZF, et al. Upregulation of miR-27a contributes to the malignant transformation of human bronchial epithelial cells induced by SV40 small T antigen. Oncogene. 2011;30:3875–86. doi: 10.1038/onc.2011.103. [DOI] [PubMed] [Google Scholar]
- 22.King B, Trimarchi T, Reavie L, et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell. 2013;153:1552–66. doi: 10.1016/j.cell.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Rodriguez-Barrueco R, Yu J, et al. MYC is a critical target of FBXW7. Oncotarget. 2014;6:3292–305. doi: 10.18632/oncotarget.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z, Zhou Y, Evers BM, Wang Q. Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. Biochem Biophys Res Commun. 2012;418:426–32. doi: 10.1016/j.bbrc.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D, Davis MP, Abreu-Goodger C, et al. MiR-25 regulates Wwp2 and Fbxw7 and promotes reprogramming of mouse fibroblast cells to iPSCs. PLoS One. 2012;7:e40938. doi: 10.1371/journal.pone.0040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Zuo Z, Lu X, et al. MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol Rep. 2012;27:594–98. doi: 10.3892/or.2011.1530. [DOI] [PubMed] [Google Scholar]
- 27.Razumilava N, Bronk SF, Smoot RL, et al. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;55:465–75. doi: 10.1002/hep.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang T, Chen T, Li Y, et al. Downregulation of miR-25 modulates non-small cell lung cancer cells by targeting CDC42. Tumour Biol. 2015;36:1903–11. doi: 10.1007/s13277-014-2793-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Wang Y, Yang L, et al. MiR-25 promotes gastric cancer cells growth and motility by targeting RECK. Mol Cell Biochem. 2014;385:207–13. doi: 10.1007/s11010-013-1829-x. [DOI] [PubMed] [Google Scholar]
- 30.Gong J, Cui Z, Li L, et al. MicroRNA-25 promotes gastric cancer proliferation, invasion, and migration by directly targeting F-box and WD-40 domain protein 7, FBXW7. Tumour Biol. 2015;36(10):7831–40. doi: 10.1007/s13277-015-3510-3. [DOI] [PubMed] [Google Scholar]