Abstract
Voltage-gated calcium channels are multi-subunit protein complexes that specifically allow calcium ions to enter the cell in response to membrane depolarization. But, for many years it seemed that the skeletal muscle calcium channel CaV1.1 is the exception. The classical splice variant CaV1.1a activates slowly, has a very small current amplitude and poor voltage sensitivity. In fact adult muscle fibers work perfectly well even in the absence of calcium influx. Recently a new splice variant of the skeletal muscle calcium channel CaV1.1e has been characterized. The lack of the 19 amino acid exon 29 in this splice variant results in a rapidly activating calcium channel with high current amplitude and good voltage sensitivity. CaV1.1e is the dominant channel in embryonic muscle, where the expression of this high calcium-conducting CaV1.1 isoform readily explains developmental processes depending on L-type calcium currents. Moreover, the availability of these two structurally similar but functionally distinct channel variants facilitates the analysis of the molecular mechanisms underlying the unique current properties of the classical CaV1.1a channel.
Keywords: CaV1.1, Voltage-gated calcium channel, L-type calcium current, Skeletal muscle, Excitation–contraction coupling
Function of voltage-gated calcium channels in skeletal muscle EC coupling
In excitable cells, voltage-gated calcium channels convert membrane depolarization into intracellular calcium signals which regulate contraction, secretion, and transcription. In addition, calcium entering through voltage-gated calcium channels is important for many processes during the development of nerve and muscle cells. Accordingly, altered calcium channel functions cause diseases and voltage-gated calcium channels are prime drugs targets for the treatment of cardio-vascular and neurological dysfunctions.
In skeletal muscle the voltage-gated calcium channel CaV1.1 is the primary voltage-sensor in EC coupling (Melzer et al. 1995). In response to membrane depolarization CaV1.1 activates calcium release from the sarcoplasmic reticulum through the ryanodine receptor (RyR1). This process is independent of calcium influx (Armstrong et al. 1972; Rios and Brum 1987), but requires the exact localization of CaV1.1 and its physical interaction with the calcium release channel (Block et al. 1988; Grabner et al. 1999; Kugler et al. 2004). Conversely, a retrograde interaction between the RyR1 and CaV1.1 results in an amplification of the L-type calcium current (Grabner et al. 1999; Nakai et al. 1996). Nevertheless, the classical skeletal muscle L-type calcium currents are small, activate slowly and open only at 30 mV more positive membrane potentials than EC coupling. Here we contrast the biophysical properties of the adult CaV1.1a (Flucher and Tuluc 2011) and the recently discovered embryonic CaV1.1e (Tuluc et al. 2009; Flucher and Tuluc 2011) isoforms and thus review the evidence for the molecular mechanisms responsible for the specific gating properties of CaV1.1 and their physiological implications.
The specific gating behavior of CaV1.1 is determined by different domains in the channel’s primary structure
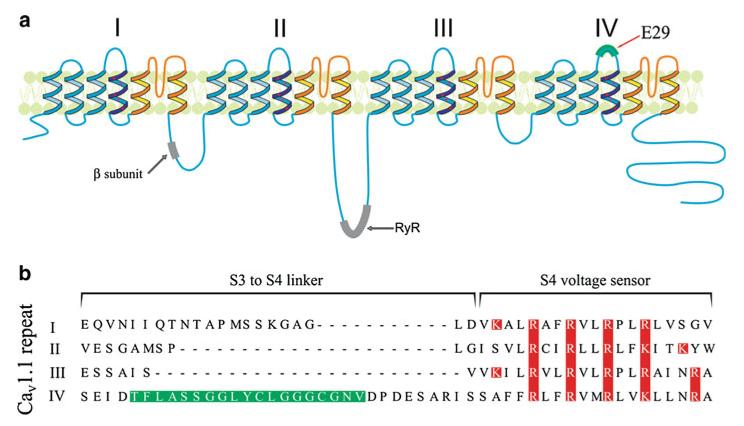
The α1 subunit of voltage-gated calcium channels contains four homologous repeats (I–IV) each formed by six transmembrane segments (S1–S6) (Fig. 1a). Similar to other voltage-gated ion channels, the transmembrane segments S1–S4 (Fig. 1a blue segments) form the voltage sensor with S4 containing positively charged amino-acids every three amino acids, termed gating charges. These charges move in response to depolarization, resulting in the partial outward translocation of the S4 segment through the membrane. The S5 and S6 segments—which flank the pore-forming P-loop—form the actual gating machinery of the channel (Fig. 1a orange segments). The P-loops from all four domains come together and line the pore of the channel. The movement of the S4 segment is transmitted to the neighboring S5 and possible S6 segments (Catterall 2010; Payandeh et al. 2011; Yarov-Yarovoy et al. 2006), resulting in a conformational change that opens the gate. In potassium channels, which form homo- or hetero-tetramers of six transmembrane subunits, the combination of different subunits is a source of high functional heterogeneity. In contrast, the four repeats of the voltage-gated calcium channel are linked together and show considerable structural heterogeneity. While the S4 segments are well conserved between the four repeats, the other transmembrane segments and especially the linkers between them differ greatly both in lengths and amino-acid sequence (see Fig. 1b, Sequence alignment of the S3–S4 linker together with S4 of CaV1.1 fourth repeat) Therefore, it is likely that the four repeats differentially contribute to the gating of the calcium channel.
Fig. 1.
Structure of the skeletal muscle calcium channel α1S subunit. a Predicted transmembrane topology of CaV1.1 α1S subunit. The regions of the cytoplasmic loops interacting with the β subunit and the RyR1 are emphasized in gray, while the location of exon 29, which is missing in the CaV1.1e isoform is highlighted in green. b Sequence alignment of the extracellular S3–S4 linkers together with the S4 transmembrane segment from repeats one to four of the skeletal muscle calcium channel. The amino acid sequence encoded by the exon 29 in IVS3–S4 linker is highlighted in green, while red letters emphasize the positively charged residues responsible for the detection of the membrane depolarization. (Color figure online)
In fact, a striking example of the heterogeneous contribution of the four repeats to channel gating comes from the skeletal muscle CaV1.1a calcium channel, which activates two distinct voltage-dependent processes. As stated above, CaV1.1a rapidly activates EC-coupling at 30 mV lower voltages compared to gating of the channel itself. Moreover, compared to ECC coupling and to the activation kinetics of other voltage-gated calcium channels CaV1.1a currents activate very slowly. In the early 1990s several studies elucidated the mechanism and the molecular determinants of CaV1.1 specific gating properties. Using skeletal CaV1.1a and cardiac CaV1.2 chimeras two consecutive studies from the Beam laboratory proposed that the first repeat, in particular the transmembrane segment IS3 together with IS3–IS4 linker represent the molecular entity responsible for the specific gating behavior of CaV1.1a and CaV1.2 calcium channels (Tanabe et al. 1991; Nakai et al. 1994). Earlier (Feldmeyer et al. 1990) elegantly showed that in frog muscle the CaV1.1a slow activation kinetics can be accelerated using a depolarization pre-pulse. Although, this experiment could never be reproduced in mammalian muscle, these studies suggested an attractive mechanistic model explaining the peculiar gating behavior of the skeletal muscle calcium channel (Melzer et al. 1995). According to this model up to three of the repeats undergo a fast voltage-dependent transition that is sufficient to gate the opening of the RyR1, but not the opening of the CaV1.1a channel pore. As it has been demonstrated that the loop connecting repeats II and III represents the link between CaV1.1 α1 subunit and RyR1 (Grabner et al. 1999; Kugler et al. 2004; Tanabe et al. 1990), it is reasonable to assume that a conformational change of the voltage sensors in the adjacent repeats II and III, or perhaps only one of them, may be sufficient to open the RyR1 (see Fig. 1). The gating of the channel pore requires the translocation of all four voltage sensors through the membrane. In the classical CaV1.1a α1 subunit this transition is slow and appears to depend on higher voltages than those of the other repeats. According to the work by Nakai et al. (1996) the slowly responding S4 is located in the first repeat. However, the same study indicates that the slow activation kinetics of repeat I is not necessarily coupled to the poor voltage sensitivity of the transition limiting channel opening.
Recently we identified a new splice variant of the skeletal muscle CaV1.1 calcium channel, which shed light on the molecular entity responsible for the poor voltage sensitivity and small amplitude of CaV1.1 (Tuluc et al. 2009). This new splice variant, lacks exon 29 which encodes for 19 amino-acids in the extracellular loop between S3 and S4 in the fourth repeat. The CaV1.1e splice variant has an eightfold higher current amplitude compared to the full lengths CaV1.1a due to an increased open probability and better voltage sensitivity. Very important for the issue at hand is the fact that its voltage dependence of activation is shifted by 30 mV to the left, while the voltage dependence of EC-coupling (when measured in the absence of calcium influx) was not affected. Because in this splice variant lacking exon 29 EC-coupling and calcium currents activate at the same potential, we concluded that in CaV1.1e the S4 voltage sensors from all four domains respond equally to the membrane depolarization.
Therefore, the fourth domain and especially the IVS3–IVS4 linker containing exon 29 is the molecular determinant of the specific voltage dependence and amplitude (Tuluc et al. 2009), whereas the first domain (IS3 + IS3–IS4 linker) is mainly responsible for the kinetic properties (Nakai et al. 1994). Apparently, these two voltage-sensors differentially determine the characteristic gating behavior (slow activation kinetics and poor voltage sensitivity, respectively) of the skeletal muscle calcium channel. Because ECC couples fast and at low voltages, it is unlikely that the full conformational transition of either one of these voltage-sensors is required for activating the RyR1. The exact mechanism how these structures modulate CaV1.1 calcium current properties and, whether repeats II and III are sufficient for EC-coupling need to be further investigated.
Auxiliary channel subunits as modulator of L-type calcium currents properties
The voltage gated calcium channel is a complex formed by multiple subunits. Besides the major voltage-sensing and pore-forming α1S subunit, skeletal muscle calcium channels contain three auxiliary subunits (Catterall 2000); the extracellular α2δ-1 (Davies et al. 2010), the intracellular β1a, and the integral membrane γ-1 subunit. The role of the calcium channel subunits has been extensively explored using either heterologous or homologous expression systems and knock-out mouse models (Striessnig and Koschak 2008).
By heterologous coexpression of calcium channel subunits in Xenopus oocytes and mammalian cells it has been shown that the α2δ-1 subunit is an important determinant of membrane incorporation and calcium current properties. In contrast, in skeletal myotubes we could show that the α2δ-1 subunit has no role in membrane incorporation or triad targeting of either the skeletal CaV1.1 or the cardiac CaV1.2 calcium channels. Instead in muscle cells it functions primarily as an important modulator of the current properties (Flucher et al. 2005; Obermair et al. 2005, 2008; Tuluc et al. 2007). In dysgenic myotubes reconstituted with the endogenous CaV1.1a, the knock-down of α2δ-1 subunit using shRNA resulted in calcium currents with altered kinetic properties, while the voltage dependence and amplitude were not affected. The lack of α2δ-1 subunit strongly accelerated the activation and inactivation kinetics compared to the very slow activating and inactivating currents of dysgenic myotubes expressing the full CaV1.1a channel complex (Obermair et al. 2005). Even though the CaV1.1e splice variant intrinsically has faster activation and inactivation kinetics, the depletion of α2δ-1 subunit further accelerated its gating properties (Tuluc et al. 2009), indicating that it is modulated by the α2δ-1 subunit in essentially the same way as the full-length CaV1.1a. Interestingly, in dysgenic myotubes reconstituted with cardiac CaV1.2 the depletion of α2δ-1 also altered the calcium current kinetic properties but in the opposite direction (Tuluc et al. 2007). Whereas the CaV1.2 calcium channel complex containing α2δ-1 has fast activation kinetics, the lack of α2δ-1 reduced both the activation and the inactivation rates. Computer modeling of cardiac myocytes demonstrated that particularly the delayed inactivation prolongs the cardiac action potential similarly to long QT syndromes and Timothy disease (Splawski et al. 2004, 2005; Tuluc et al. 2007). The lack of α2δ-1 subunit also shifted the voltage dependence of activation towards more positive potentials and reduced the calcium current amplitude. These results were fully confirmed in vivo in a α2δ-1 knockout mouse model (Fuller-Bicer et al. 2009). The genetic ablation of α2δ-1 subunit resulted in cardiac CaV1.2 calcium currents with slower activation and inactivation kinetics, shifted voltage dependence of activation towards more positive potentials and reduced L-type calcium currents amplitude. The changes in CaV1.2 calcium current properties resulted in decreased basal myocardial contractibility and relaxation. Thus, the function of α2δ-1 in muscle cells is to stabilize the kinetic properties intrinsically determined by the α1 subunit; α2δ-1 makes the skeletal currents slow and the cardiac currents fast. Importantly, its action on the α1 subunit is independent of the presence of exon 29 in the voltage sensor of the fourth repeat.
The β subunits are necessary for the efficient expression of functional channels in the membrane. In addition, the β4 subunit seems to have functions independent of calcium channel complex (Subramanyam et al. 2009). The over-expression of β1–β4 subunits in adult rat cardiac myocytes increased the amplitude of the gating charge currents and L-type calcium currents by augmenting the number of channels functionally incorporated in the membrane. Furthermore, the different β subunits differentially altered the CaV1.2 inactivation kinetics (Colecraft et al. 2002). In skeletal muscle β1a is the only β subunit expressed and the genetic ablation of β1 subunit reduces substantially the number of channels expressed in the membrane and completely abolishes the gating charge currents (Gregg et al. 1996; Schredelseker et al. 2005). Interestingly, reconstitution of β1-null skeletal myotubes with different β isoforms showed that only β1a is capable of restoring the proper interaction and structural organization of the CaV1.1 and RyR1 and therefore proper skeletal muscle EC-coupling (Schredelseker et al. 2009; Schuhmeier et al. 2005; Sheridan et al. 2004). The β3 subunit fails to promote membrane incorporation, whereas the β2 subunit (Sheridan et al. 2004) is capable of promoting CaV1.1 membrane incorporation but fails to restore the characteristic skeletal muscle EC-coupling. In contrast to heterologous cells it seems that in skeletal muscle β subunits do not affect calcium current voltage dependence or kinetic properties (Sheridan et al. 2004). Thus, the central role of β subunits in skeletal muscle calcium channel complex is promotion of membrane expression, with an exclusive role of β1a in coupling of CaV1.1 to the RyR1.
So far only skeletal muscle calcium channel complexes have been demonstrated to contain a γ subunit, and other γ isoforms (stargazing) have been shown to be associated with glutamate receptors in the brain (Kato et al. 2007, 2008; Vandenberghe et al. 2005). In heterologous expression systems, coexpression of the skeletal muscle γ1 subunit enhances the activation and inactivation kinetics, and shifts the voltage dependence of inactivation of cardiac CaV1.2 calcium channel (Singer et al. 1991; Sipos et al. 2000). However, this γ1 subunit is exclusively expressed in skeletal muscle and therefore the physiological role of γ1 subunit could be tested only in γ1-null mice (Ahern et al. 2001; Freise et al. 2000). Functional analysis of γ1-null myotubes showed that γ1 subunit accelerates the activation and inactivation kinetics of CaV1.1 calcium currents (Ahern et al. 2001; Ursu et al. 2001). Interestingly, in γ1-null muscle fibers, the CaV1.1 calcium current kinetics are similar to calcium currents recorded from control fibers (Ursu et al. 2004). But, the γ1 subunit shifts the voltage dependence of calcium current inactivation and voltage dependence of inactivation of calcium release from RyR towards more depolarizing potentials in both myotubes and fibers (Ursu et al. 2004). Therefore the γ1 subunit has been suggested to act as an endogenous CaV1.1 calcium channel antagonist (Andronache et al. 2007) and, in concert with the α2δ-1 subunit (Obermair et al. 2005), limits the L-type calcium current in skeletal muscle, although by a different mechanism.
Together this evidence shows that in skeletal muscle auxiliary calcium channel subunits are capable of modifying channel targeting (β1a) and the calcium channel gating and current properties (α2δ-1, γ1). However, the mechanisms by which they exert their effect on the CaV α1 subunit is largely unknown. So far only the interaction site between the β subunits and the α1 subunit has been characterized (Chen et al. 2004; De Waard et al. 1996; Pragnell et al. 1994). In contrast there is comparably little evidence for the molecular mechanism responsible for β subunits modulatory effects (Barrett and Tsien 2008). As stated above in skeletal muscle the strongest modulator of CaV1.1 calcium current properties is the α2δ-1 subunit. Attempts have been made to identify the interaction site between the CaV α1 subunit and the α2δ-1 subunit (Felix et al. 1997; Gurnett et al. 1997) but so far the exact sequence involved in this interaction and the modulatory mechanism are still elusive. Consistent with its extracellular localization, the α2δ-1 subunit exerts its modulatory functions extracellularly, possibly via single or multiple interactions. The extracellular loops between IS3-S4 and IVS3-S4 are critical determinants of calcium current kinetics and voltage dependence, respectively. As the CaV1.1e isoform is still modulated by α2δ-1, we ruled out the possibility that the α2δ-1 subunit interacts with CaV1.1 α1 subunit at the IVS3-S4 linker (Tuluc et al. 2009). Interestingly, the lack of the α2δ-1 subunit (Gach et al. 2008; Obermair et al. 2005) results in CaV1.1 calcium currents with fast activation and inactivation kinetics, with characteristics identical to calcium currents recorded earlier from chimeric channels in which the first repeat had been replaced with the corresponding domain from the cardiac CaV1.2 calcium channel (Tanabe et al. 1991). Future research needs to examine whether this is the amino acid sequence through which α2δ-1 subunit exerts its modulatory function.
Physiological role of L-type calcium currents in skeletal muscle
The contribution of L-type calcium currents to skeletal muscle EC coupling is negligible and therapeutic use of l-channel blockers does not affect mature skeletal muscle function (Melzer et al. 1995). Therefore L-type calcium currents were never considered to be of substantial importance for mature muscle function and the question as to the role of L-type calcium currents in mammalian skeletal muscle functions remained unanswered. Nevertheless, the growing number of channelopathies associated with CaV1.1 (Jurkat-Rott and Lehmann-Horn 2005; Jurkat-Rott et al. 2000; Pirone et al. 2010; Striessnig et al. 2010) indicates that the L-type calcium currents are essential for muscle function, but the mechanism how the CaV1.1 calcium channel mutations affect the skeletal muscle EC-coupling is still elusive. In fact, considering that during the brief depolarization of an action potential the channel may not open at all, it is difficult to conceive how altered current properties should lead to muscle defects. However, the short splice variant lacking exon 29 has a very high current amplitude and in myotubes expressing CaV1.1e EC-coupling has a cardiac-like calcium-dependent calcium release component (Tuluc et al. 2009). The sizeable calcium currents through CaV1.1e contribute directly to the myoplasmic calcium concentration and possibly also indirectly by augmenting RyR1 calcium release via calcium-induced calcium release. Whereas adult muscles express very small amounts of the short splice variant, in developing myotubes CaV1.1e is the dominant splice variant (Flucher and Tuluc 2011). One possibility how this channel might nevertheless contribute to the development of progressive myopathies were, if morphological defects of the myofibers went hand in hand with a shift in expression patterns from adult to embryonic splice variants. Ectopic expression of CaV1.1e in adult fibers would be expected to lead to calcium overload and thus might activate disease pathways or cause cell damage. Future research needs to determine whether CaV1.1e is up-regulated in diseased muscles or in myopathy animal models and whether block of L-type calcium currents counteracts the disease progression.
Role of L-type calcium currents in excitation-coupled calcium entry
Entry of divalent ions into skeletal muscle in response to sustained depolarization or repetitive stimulation has been termed excitation-coupled calcium entry (ECCE) (Cherednichenko et al. 2004). ECCE is a phenomenon which may be important for maintaining force generation during tetanic stimulation and may contribute to the pathologically increased calcium concentration during episodes of malignant hyperthermia (Cherednichenko et al. 2008; Yang et al. 2007). ECCE requires both the CaV1.1 and the RyR1, but initially was thought to be distinct of the L-type calcium current. Instead, based on its pharmacological profile, transient receptor potential channels or store-operated calcium entry have been suggested to be the basis of ECCE (Cherednichenko et al. 2008; Hurne et al. 2005; Lyfenko and Dirksen 2008). Most recent evidence indicates that the skeletal L-type calcium current is the major contributor to ECCE (Bannister et al. 2009). Actually ECCE can also be reconstituted in dysgenic (CaV1.1-null) myotubes by expression of the cardiac CaV1.2 (Bannister and Beam 2009). This channel isoform has current properties similar to those of CaV1.1e. Whether the adult CaV1.1a isoform is also capable of reconstituting ECCE in dysgenic muscle has not been addressed. Furthermore, the depletion of the auxiliary α2δ-1 calcium channel subunit resulted in a complete loss of ECCE during KCl depolarization and a more rapid decay of calcium transients during bouts of repetitive electrical stimulation like those occurring during normal muscle activation in vivo (Gach et al. 2008). Thus, ECCE requires the L-type calcium current but not skeletal muscle-type EC coupling. Considering the small amplitude and poor voltage sensitivity of the long form CaV1.1a, it seems very unlikely that this channel can supply the calcium current required for ECCE. But, ECCE—just like expression of CaV1.1e—is preferentially found in developing myotubes. Therefore, it is much more likely that the molecular entity responsible for ECCE is the embryonic CaV1.1e isoform.
Role of L-type calcium currents in neuromuscular junction formation
In the early 1980s Pinçon-Raymond, Powell, and Rieger published a series of articles (Pincon-Raymond and Rieger 1982; Powell et al. 1984; Rieger et al. 1984) in which they thoroughly describe the neuromuscular junction (NMJ) phenotype in the dysgenic mouse (mdg). Later this mouse was identified as a spontaneous null-mutant of the skeletal muscle CaV1.1 α1 subunit (Knudson et al. 1989; Tanabe et al. 1988). One of the earliest steps in the development of the NMJ is the regular arrangement of acetylcholine receptors (AChR) in the center of muscle fibers, termed pre-patterning. Using 125I-α-bungarotoxin autoradiography of AChRs and a range of other techniques the authors observed that compared to normal embryonic muscles, dysgenic diaphragm muscles expressed multiple endplates per fiber and increased amounts of extrajunctional AChR (Flucher and Tuluc 2011). The AChR clusters were not concentrated in a single centrally localized band but broadly dispersed over the muscle. Furthermore they found that this aberrant postsynaptic patterning is correlated with increased nerve sprouting, extended innervations territories and ultrastructurally immature features of the NMJs. Recently, the developmental defects in AChR clustering and innervation patterns were found in another calcium channel knock-out mouse model (Chen et al. 2011). The distribution of AChR and branching of the phrenic nerve were studies in diaphragms of embryos of the calcium channel β1 (Cacnb1−/−) knock-out mouse earlier generated by (Gregg et al. 1996). The lack of β1 subunit results in a reduced number of channels functionally incorporated in the membrane and complete loss of L-type calcium currents and EC-coupling. Therefore, the Cacnb1−/− mouse and the dysgenic (Cacna1S−/−) mouse models are functionally equivalent in that both lack L-type calcium currents and EC-coupling; consequently they both die at birth from respiratory failure.
CaV1.1a L-type calcium currents have such a small current amplitude and poor voltage-sensitivity that during 200 ms depolarizations in combined voltage-clamp and calcium imaging experiments their contribution to the myoplasmic calcium transient is not detectible (Flucher and Tuluc 2011; Schuhmeier et al. 2005). During the brief depolarization of a single action potential or during spontaneous electrical activity in developing muscle fibers prior to innervation, it is very unlikely that the pores of these channels open at all. Thus, for all we know about the classical skeletal muscle calcium channel, CaV1.1a cannot be the source of the L-type calcium currents activating NMJ pre-patterning. However, the CaV1.1e splice variant lacking exon 29 has a large current amplitude and left-shifted voltage dependence of activation. In contrast to the full-length CaV1.1a calcium entering through CaV1.1e during 200 ms step depolarization substantially contributes to cytosolic calcium concentration. Furthermore, CaV1.1e is the dominant splice variant (~80%) in both mouse and human myotubes (Tuluc et al. 2009). Therefore the expression pattern and the biophysical properties of CaV1.1e implicate it in the neuromuscular junction formation during embryonic development (Flucher and Tuluc 2011).
Conclusion
For many years it has been accepted that the sole role of the skeletal muscle calcium channels CaV1.1 is to trigger the opening of the sarcoplasmic reticulum calcium release channel RYR1 and thus trigger skeletal muscle EC-contraction. Yet, there is substantial new evidence that implicates the skeletal muscle calcium channel in processes that depend on the influx of calcium through CaV1.1; most prominently ECCE and the formation of the neuromuscular junction. To date we know two splice variants of the CaV1.1 α1S subunit expressed in skeletal muscle. In the classical long isoform (CaV1.1a) the auxiliary α2δ-1 and γ1 subunits as well as unique intrinsic structures in the voltage-sensor of the first and fourth repeat (the latter including exon 29) limit the kinetics and voltage-sensitivity of the calcium current. In contrast, the newly discovered short splice variant (CaV1.1e), which lacks exon 29 and has a high current amplitude, fast activation kinetics and normal voltage sensitivity, is a very efficient calcium channel. The expression of such dramatically different channel variants indicate fundamentally different functions in developing and mature skeletal muscle, respectively. While both splice variants support the direct, calcium independent coupling of the voltage sensor and RyR1, only the embryonic CaV1.1e variant functions as genuine L-type calcium channel and may thus be of physiological and clinical importance.
Acknowledgments
This study was supported by grants from the Austrian Research Fund (FWF) P20059 and P23479 to BEF, and from the Medical University Innsbruck MFI 2007-417 to PT.
Contributor Information
Petronel Tuluc, Department of Physiology and Medical Physics, Medical University Innsbruck, Fritz-Pregl-Str. 3, 6020 Innsbruck, Austria; Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, Peter-Mayr Strasse 1/I, 6020 Innsbruck, Austria.
Bernhard E. Flucher, Department of Physiology and Medical Physics, Medical University Innsbruck, Fritz-Pregl-Str. 3, 6020 Innsbruck, Austria
References
- Ahern CA, Powers PA, Biddlecome GH, Roethe L, Vallejo P, Mortenson L, Strube C, Campbell KP, Coronado R, Gregg RG. Modulation of l-type Ca2+ current but not activation of Ca2+ release by the gamma1 subunit of the dihydropyridine receptor of skeletal muscle. BMC Physiol. 2001;1:8. doi: 10.1186/1472-6793-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronache Z, Ursu D, Lehnert S, Freichel M, Flockerzi V, Melzer W. The auxiliary subunit gamma 1 of the skeletal muscle l-type Ca2+ channel is an endogenous Ca2+ antagonist. Proc Natl Acad Sci USA. 2007;104(45):17885–17890. doi: 10.1073/pnas.0704340104. doi:10.1073/pnas.0704340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N’-tetra-acetic acid. Biochim Biophys Acta. 1972;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Bannister RA, Beam KG. The cardiac alpha (1C) subunit can support excitation-triggered Ca2+ entry in dysgenic and dyspedic myotubes. Channels (Austin) 2009;3(4):268–273. doi: 10.4161/chan.3.4.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister RA, Pessah IN, Beam KG. The skeletal l-type Ca (2+) current is a major contributor to excitation-coupled Ca (2+) entry. J Gen Physiol. 2009;133(1):79–91. doi: 10.1085/jgp.200810105. doi:10.1085/jgp.200810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 l-type calcium channels. Proc Natl Acad Sci USA. 2008;105(6):2157–2162. doi: 10.1073/pnas.0710501105. doi:10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67(6):915–928. doi: 10.1016/j.neuron.2010.08.021. doi:10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429(6992):675–680. doi: 10.1038/nature02641. doi:10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu Y, Sugiura Y, Allen PD, Gregg RG, Lin W. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat Neurosci. 2011;14(5):570–577. doi: 10.1038/nn.2792. doi:10.1038/nn.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA. 2004;101(44):15793–15798. doi: 10.1073/pnas.0403485101. doi:10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, Lopez JR, Allen PD, Pessah IN. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol. 2008;73(4):1203–1212. doi: 10.1124/mol.107.043299. doi:10.1124/mol.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca (2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541(Pt 2):435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci USA. 2010;107(4):1654–1659. doi: 10.1073/pnas.0908735107. doi:10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard M, Scott VE, Pragnell M, Campbell KP. Identification of critical amino acids involved in alpha1-beta interaction in voltage-dependent Ca2+ channels. FEBS Lett. 1996;380(3):272–276. doi: 10.1016/0014-5793(96)00007-5. doi:10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Melzer W, Pohl B, Zollner P. Fast gating kinetics of the slow Ca2+ current in cut skeletal muscle fibres of the frog. J Physiol. 1990;425:347–367. doi: 10.1113/jphysiol.1990.sp018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci. 1997;17(18):6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Tuluc P. A new l-type calcium channel isoform required for normal patterning of the developing neuromuscular junction. Channels (Austin) 2011;5(6) doi: 10.4161/chan.5.6.17951. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Obermair GJ, Tuluc P, Schredelseker J, Kern G, Grabner M. The role of auxiliary dihydropyridine receptor subunits in muscle. J Muscle Res Cell Motil. 2005;26(1):1–6. doi: 10.1007/s10974-005-9000-2. doi:10.1007/s10974-005-9000-2. [DOI] [PubMed] [Google Scholar]
- Freise D, Held B, Wissenbach U, Pfeifer A, Trost C, Himmerkus N, Schweig U, Freichel M, Biel M, Hofmann F, Hoth M, Flockerzi V. Absence of the gamma subunit of the skeletal muscle dihydropyridine receptor increases l-type Ca2+ currents and alters channel inactivation properties. J Biol Chem. 2000;275(19):14476–14481. doi: 10.1074/jbc.275.19.14476. doi:275/19/14476. [DOI] [PubMed] [Google Scholar]
- Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A. Targeted Disruption of the Voltage-Dependent Ca2+ Channel {alpha}2/{delta}-1 Subunit. Am J Physiol. 2009 doi: 10.1152/ajpheart.00122.2009. doi:10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach MP, Cherednichenko G, Haarmann C, Lopez JR, Beam KG, Pessah IN, Franzini-Armstrong C, Allen PD. Alpha2-delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes. Biophys J. 2008;94(8):3023–3034. doi: 10.1529/biophysj.107.118893. doi:10.1529/biophysj.107.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Suda N, Beam KG. The II–III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J Biol Chem. 1999;274(31):21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation–contraction coupling. Proc Natl Acad Sci USA. 1996;93(24):13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel alpha2delta and alpha1 subunits. J Biol Chem. 1997;272(29):18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- Hurne AM, O’Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, Pessah IN. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem. 2005;280(44):36994–37004. doi: 10.1074/jbc.M506441200. doi:10.1074/jbc.M506441200. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lehmann-Horn F. Muscle channelopathies and critical points in functional and genetic studies. J Clin Investig. 2005;115(8):2000–2009. doi: 10.1172/JCI25525. doi:10.1172/JCI25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000;23(1):4–17. doi: 10.1002/(sici)1097-4598(200001)23:1<4::aid-mus3>3.0.co;2-d. doi:10.1002/(SICI)1097-4598(200001)23:1<4:AID-MUS3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27(18):4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. doi:10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59(6):986–996. doi: 10.1016/j.neuron.2008.07.034. doi:10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Chaudhari N, Sharp AH, Powell JA, Beam KG, Campbell KP. Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J Biol Chem. 1989;264(3):1345–1348. [PubMed] [Google Scholar]
- Kugler G, Weiss RG, Flucher BE, Grabner M. Structural requirements of the dihydropyridine receptor alpha1S II-III loop for skeletal-type excitation–contraction coupling. J Biol Chem. 2004;279(6):4721–4728. doi: 10.1074/jbc.M307538200. doi:10.1074/jbc.M307538200. [DOI] [PubMed] [Google Scholar]
- Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol. 2008;586(Pt 20):4815–4824. doi: 10.1113/jphysiol.2008.160481. doi:10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241(1):59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Nakai J, Adams BA, Imoto K, Beam KG. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of l-type calcium channels. Proc Natl Acad Sci USA. 1994;91(3):1014–1018. doi: 10.1073/pnas.91.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380(6569):72–75. doi: 10.1038/380072a0. doi:10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation–contraction coupling. J Biol Chem. 2005;280(3):2229–2237. doi: 10.1074/jbc.M411501200. doi:10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Tuluc P, Flucher BE. Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol. 2008;8(311):318. doi: 10.1016/j.coph.2008.01.008. doi:10.1016/j.coph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–358. doi: 10.1038/nature10238. doi:10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincon-Raymond M, Rieger F. Extensive multiple innervation and abnormal synaptogenesis in muscular dysgenesis (mdg/mdg) in the mouse embryo. Reprod Nutr Dev. 1982;22(1B):217–226. doi: 10.1051/rnd:19820208. [DOI] [PubMed] [Google Scholar]
- Pirone A, Schredelseker J, Tuluc P, Gravino E, Fortunato G, Flucher BE, Carsana A, Salvatore F, Grabner M. Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit. Am J Physiol. 2010;299(6):C1345–C1354. doi: 10.1152/ajpcell.00008.2010. doi:10.1152/ajpcell.00008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JA, Rieger F, Blondet B, Dreyfus P, Pincon-Raymond M. Distribution and quantification of ACh receptors and innervation in diaphragm muscle of normal and mdg mouse embryos. Dev Biol. 1984;101(1):168–180. doi: 10.1016/0012-1606(84)90127-1. doi:10.1016/0012-1606(84)90127-1. [DOI] [PubMed] [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368(6466):67–70. doi: 10.1038/368067a0. doi:10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Rieger F, Powell JA, Pincon-Raymond M. Extensive nerve overgrowth and paucity of the tailed asymmetric form (16 S) of acetylcholinesterase in the developing skeletal neuromuscular system of the dysgenic (mdg/mdg) mouse. Dev Biol. 1984;101(1):181–191. doi: 10.1016/0012-1606(84)90128-3. doi:10.1016/0012-1606(84)90128-3. [DOI] [PubMed] [Google Scholar]
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation–contraction coupling in skeletal muscle. Nature. 1987;325(6106):717–720. doi: 10.1038/325717a0. doi:10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA. 2005;102(47):17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation–contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit. J Biol Chem. 2009;284(2):1242–1251. doi: 10.1074/jbc.M807767200. doi:10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmeier RP, Gouadon E, Ursu D, Kasielke N, Flucher BE, Grabner M, Melzer W. Functional interaction of CaV channel isoforms with ryanodine receptors studied in dysgenic myotubes. Biophys J. 2005;88(3):1765–1777. doi: 10.1529/biophysj.104.051318. doi:10.1529/biophysj.104.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan DC, Cheng W, Carbonneau L, Ahern CA, Coronado R. Involvement of a heptad repeat in the carboxyl terminus of the dihydropyridine receptor beta1a subunit in the mechanism of excitation–contraction coupling in skeletal muscle. Biophys J. 2004;87(2):929–942. doi: 10.1529/biophysj.104.043810. doi:10.1529/biophysj.104.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Sipos I, Pika-Hartlaub U, Hofmann F, Flucher BE, Melzer W. Effects of the dihydropyridine receptor subunits gamma and alpha2delta on the kinetics of heterologously expressed l-type Ca2+ channels. Pflugers Arch. 2000;439(6):691–699. doi: 10.1007/s004249900201. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119(1):19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac l-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102(23):8089–8096. doi: 10.1073/pnas.0502506102. doi:10.1073/pnas.0502506102 discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels (Austin) 2008;2(4):233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated l-type Ca2+ channels. Pflugers Arch. 2010;460(2):361–374. doi: 10.1007/s00424-010-0800-x. doi:10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, Geley S, Flucher BE. Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels (Austin) 2009;3(5):343–355. doi: 10.4161/chan.3.5.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation–contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336(6195):134–139. doi: 10.1038/336134a0. doi:10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation–contraction coupling. Nature. 1990;346(6284):567–569. doi: 10.1038/346567a0. doi:10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Adams BA, Numa S, Beam KG. Repeat I of the dihydropyridine receptor is critical in determining calcium channel activation kinetics. Nature. 1991;352(6338):800–803. doi: 10.1038/352800a0. doi:10.1038/352800a0. [DOI] [PubMed] [Google Scholar]
- Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel {alpha}2{delta}-1 subunit in cardiac excitation–contraction coupling. Proc Natl Acad Sci USA. 2007;104(26):11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. A Ca(V)1.1 Ca(2+) channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J. 2009;96(1):35–44. doi: 10.1016/j.bpj.2008.09.027. doi:10.1016/j.bpj.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu D, Schuhmeier RP, Freichel M, Flockerzi V, Melzer W. Altered inactivation of Ca2+ current and Ca2+ release in mouse muscle fibers deficient in the DHP receptor gamma1 subunit. J Gen Physiol. 2004;124(5):605–618. doi: 10.1085/jgp.200409168. doi:10.1085/jgp.200409168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu D, Sebille S, Dietze B, Freise D, Flockerzi V, Melzer W. Excitation–contraction coupling in skeletal muscle of a mouse lacking the dihydropyridine receptor subunit gamma1. J Physiol. 2001;533(Pt 2):367–377. doi: 10.1111/j.1469-7793.2001.0367a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA. 2005;102(2):485–490. doi: 10.1073/pnas.0408269102. doi:10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Allen PD, Pessah IN, Lopez JR. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282(52):37471–37478. doi: 10.1074/jbc.M701379200. doi:10.1074/jbc.M701379200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K(+) channels. Proc Natl Acad Sci USA. 2006;103(19):7292–7297. doi: 10.1073/pnas.0602350103. doi:10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]