Abstract
Osteoporosis is a major healthcare problem which is conventionally assessed by dual energy x-ray absorptiometry (DXA). New technologies such as high resolution peripheral quantitative computed tomography (HRpQCT) also predict fracture risk. HRpQCT measures a number of bone characteristics that may inform specific patterns of bone deficits. We used cluster analysis to define different bone phenotypes and their relationships to fracture prevalence and areal bone mineral density (BMD). 177 men and 159 women, in whom fracture history was determined by self-report and vertebral fracture assessment, underwent HRpQCT of the distal radius and femoral neck DXA. Five clusters were derived with two clusters associated with elevated fracture risk. “Cluster 1” contained 26 women (50.0% fractured) and 30 men (50.0% fractured) with a lower mean cortical thickness and cortical volumetric BMD, and in men only, a mean total and trabecular area more than the sex-specific cohort mean. “Cluster 2” contained 20 women (50.0% fractured) and 14 men (35.7% fractured) with a lower mean trabecular density and trabecular number than the sex-specific cohort mean. Logistic regression showed fracture rates in these clusters to be significantly higher than the lowest fracture risk cluster (5) (p<0.05). Mean femoral neck areal BMD was significantly lower than cluster 5 in women in cluster 1 and 2 (p<0.001 for both), and in men, in cluster 2 (p<0.001) but not 1 (p=0.220). In conclusion, this study demonstrates two distinct high risk clusters in both men and women which may differ in etiology and response to treatment. As cluster 1 in men does not have low areal BMD, these men may not be identified as high risk by conventional DXA alone.
Keywords: Bone QCT, DXA, Osteoporosis, Epidemiology, Fracture risk assessment
1.0. Introduction
Osteoporosis is a significant global health problem with around one in two women and one in five men over the age of 50 expected to experience an osteoporotic fracture in their lifetime (1). These can cause significant disability, morbidity and even mortality along with a considerable economic cost of both inpatient and community care services (2). In clinical practice, osteoporosis is diagnosed using dual energy x-ray absorptiometry (DXA) of the hip and lumbar spine. This also has a role in fracture prediction as it has been shown that there is an approximate doubling of risk for every one standard deviation (SD) reduction in areal bone mineral density (BMD) (3). However, it is recognized that the basis of bone fragility is heterogeneous. To group individuals into one seemingly homogeneous group because of a T score below -2.5, one or more spine fractures, or a low trauma hip fracture, would obscure the heterogeneity in structural, cellular, and biomechanical basis of bone fragility (4).
Assessment by DXA does not measure volumetric BMD, does not differentiate between cortical and trabecular compartments, and does not provide measures of bone geometry, all of which might contribute to fracture risk. Recently, cross-sectional imaging techniques, including high resolution peripheral quantitative computed tomography (HRpQCT), have been developed and utilized in a research setting to differentiate fracture cases from those without (5–9). To date, most of these studies have been completed in women. So far, they suggest that specific components of bone structure, such as cortical thickness and trabecular microarchitecture, are deficient in fracture cases. It may, however, be more appropriate to explore different bone phenotypes, combining multiple outcomes related to bone strength, and their relationships to fracture.
In this study we aimed to use statistical cluster analysis, based upon mathematical, rather than pre-defined clinical, assumptions to define bone phenotypes for men and women taking into account all parameters measured by HRpQCT. We then determined whether the data-driven clusters were associated with different rates of fracture occurrence. Additionally, we assessed whether cluster phenotypes with high fracture prevalence had a corresponding low areal BMD as assessed by DXA.
2.0. Materials and Methods
2.1. Study Participants
The Hertfordshire Cohort Study (HCS) is a population-based study in the UK which was designed to examine the relationships between growth in infancy and the subsequent risk of adult diseases, such as osteoporosis. Study design and recruitment have been described in detail previously (10). In brief, in conjunction with the National Health Service Central Registry and the Hertfordshire Family Health Service Association, we traced men and women who were born between 1931 and 1939 in Hertfordshire and still lived there during the period 1998–2003. In 2011-2012, 570 men and women from the geographical area of East Hertfordshire were invited for a follow up study which included HRpQCT. Of these, 376 (66%) agreed to participate. In 344 (91.5%) of those participants scanned, data were also available on fracture status. This group did not differ significantly from the overall recruited cohort (n=376) in terms of demographic and lifestyle factors.
2.2. High resolution peripheral quantitative computed tomography (HRpQCT)
Each participant had measurements of the non-dominant distal radius using HRpQCT (XtremeCT, Scanco Medical AG, Switzerland) except when the non-dominant limb had previously fractured in which case the dominant side was scanned. This allowed acquisition of a stack of parallel CT slices using a two-dimensional detector array. A total of 110 slices were obtained which represented a volume of bone 9mm in axial length with a nominal resolution (voxel size) of 82μm. The scanned limb was immobilized during the examination in a carbon fibre cast. Antero-posterior 2D scout views were performed to determine the region to be scanned. Positioning was in keeping with the manufacturer’s guidelines and as described by Boutroy et al (5). All scans were acquired by one of two trained technicians using standard positioning techniques. Each scan was assessed for motion artefact, and if present a second scan was performed. A total of 8 radial images were excluded due to excessive motion artefact. Image analysis was carried out using the standard manufacturer’s method which has been described in detail previously (11, 12). In brief, we used a semi-automated, hand-drawn contouring system to delineate the periosteal surface. This process was always completed by the same trained operator. A threshold-based algorithm then separated cortical from trabecular compartments. Standard morphologic analysis produced total and trabecular BMD. Trabecular number was determined using the ridge-extraction methods (13). Trabecular thickness and separation were calculated from trabecular density and trabecular number according to standard morphologic relationships (14). Each measure has been validated against micro-CT imaging (15).
Further analysis was performed using an automated segmentation algorithm (16). Assessments were made of total cross-sectional area, cortical area, and cortical density. Cortical density was determined as the average mineral density in the region of interest defined by the autosegmentation cortical bone mask. Using Image Processing Language (IPL, Version 6.1, ScancoMedical), cortical porosity was estimated from the number of void voxels in each thresholded cortex image divided by the number of voxels in the cortex (17). Cortical thickness was determined from the threshold cortex image using a distance transform after removal of intracortical pores (18).
2.3. Dual Energy X-ray Absorptiometry (DXA)
Measurement of aBMD was performed at both femoral necks using a Lunar Prodigy Advance densitometer (GE Medical Systems Lunar). The lowest of the two readings was used in analyses. Morphometric vertebral fractures were diagnosed from a lateral spine view imaged using the same machine and graded based on the Genant semi-quantitative method of vertebral fracture assessment (19).
2.4. Anthropometry and Structured Interviews
Height was measured to the nearest 0.1 cm using a wall-mounted SECA stadiometer on the day of scanning. Weight was measured to the nearest 0.1kg using calibrated SECA 770 digital floor scales (SECA Ltd, Hamburg, Germany).
Details regarding physical activity, dietary calcium intake, smoking status, alcohol consumption, socioeconomic status, bisphosphonate therapy and, in women, years since menopause and use of estrogen replacement therapy, had been collected previously from researcher-administered questionnaires. Physical activity was calculated as a standardised score ranging from 0–100 derived from frequency of gardening, housework, climbing stairs and carrying loads in a typical week (20). Higher scores indicated greater levels of activity. Dietary calcium intake was assessed using a food frequency questionnaire (21). Socioeconomic status was determined using occupation based on the Office of Population Censuses and Surveys Standard Occupational Classification Scheme for occupation and social class (22). Using this system, social class could be classified from highest to lowest as I, II, III non-manual, III manual, IV and V. In men and single women the assessment was based on the current or most recent occupation of the participant and in ever married women the current or most recent occupation of the husband was used instead.
Smoking status was categorised as never smoker, ex-smoker or current smoker by participant self-report at the time questionnaire administration. Alcohol intake was assessed by detailed questions ascertaining the frequency and amount of different forms of alcohol consumed. This was converted into units per week and then categorised into those drinking no alcohol, those drinking some alcohol but less than or equal to the recommended weekly intake (14 units for women, 21 units for men), and those drinking more than the recommended weekly intake. Bisphosphonate use was defined based on whether the participant was currently or had ever taken the medication, as bisphosphonates and their effects can persist after they are ceased.
Positive fracture status was defined as a self-reported fracture since the age of 45 years, assessed by means of validated researcher-administered questionnaires at 3 separate time points (23) and/or evidence of vertebral fracture on vertebral fracture assessment as described above. The East and North Hertfordshire Ethical Committees granted ethical approval for the study and all participants gave written informed consent in accordance with the Declaration of Helsinki (24).
2.5. Statistical analysis
To test for sex-specific differences between fracture and non-fracture participants, ANOVA was used for continuous variables and Chi squared or Fisher’s exact test for categorical variables. Logistic regression was then carried out on all 10 variables, both unadjusted and adjusted for age, height, weight, daily calcium intake, physical activity, smoker status, alcohol consumption, social status, bisphosphonate use, and in women, time since menopause and estrogen replacement therapy, to assess relationships between individual radial HRpQCT parameters and fracture status. Once the HRpQCT variables had been checked and standardised, the k-means partitioning method of cluster analysis was used to produce clusters in men and women separately. With five different clusters, we produced a distinct series of contrasting phenotypes as has been shown in other uses of cluster analyses in the literature (25, 26). The derived clusters were subsequently numbered in order of fracture prevalence. The means and standard deviations (SD) of the unstandardized HRpQCT parameters and femoral neck aBMD, and fracture proportion were then determined for each cluster. Logistic regression was used to determine the likelihood of fracture in each cluster compared to the lowest risk cluster. Statistical significance was defined as a p value of <0.05. Data were analysed using STATA 13.
3.0. Results
3.1. Fracture sites
Forty four men and 48 women reported a fracture since the age of 45 years. Table 1 shows that a total of 55 fractures occurred in men and 88 in women. The most common fracture site was the spine; 14 vertebral fractures were reported in men and 23 in women. There were a total of 19 fractures of the distal radius and ulna and 15 of the distal tibia and fibula. Only one man and one woman reported a prior hip fracture.
Table 1.
Fracture sites in men and women
| Fracture Site | Men | Women | Total |
|---|---|---|---|
| Vertebrae | 14 | 23 | 37 |
| Distal Radius / Ulna | 5 | 14 | 19 |
| Distal Tibia / Fibula | 6 | 9 | 15 |
| Humerus | 7 | 4 | 11 |
| Hand | 4 | 8 | 12 |
| Foot | 7 | 12 | 19 |
| Hip | 1 | 1 | 2 |
| Other | 11 | 17 | 28 |
| Total | 55 | 88 | 143 |
3.2. Demographic and Lifestyle Characteristics by Fracture Status
The mean ages of men with and without a prevalent fracture were not significantly different at 75.7 and 76.1 years respectively (table 2). Women with a prevalent fracture were on average 1.2 years older than their non-fractured counterparts at 77.2 years of age (p=0.011). They were also 3.1 years further from the menopause (p=0.006). Height, weight, BMI, calcium intake, and levels of physical activity did not differ by fracture status in either men or women. Similarly, alcohol consumption, smoking status, and social class were comparable in those that had fractured to those that had not.
Table 2.
Participant characteristics by fracture status in men and women
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Fracture (n=44) | No Fracture (n=133) | Fracture (n=48) | No Fracture (n=111) | |||
| Mean (SD) | Mean (SD) | p value | Mean (SD) | Mean (SD) | p value | |
| Age (years) | 75.7 (2.4) | 76.1 (2.5) | 0.255 | 77.2 (2.4) | 76.0 (2.6) | 0.011 |
| Height (cm) | 174.9 (7.4) | 173.2 (6.1) | 0.125 | 160.2 (8.3) | 160.1 (5.3) | 0.932 |
| Weight (kg) | 81.5 (11.6) | 83.1 (12.5) | 0.466 | 69.0 (14.1) | 72.5 (12.2) | 0.114 |
| BMI (kg/m2) | 26.7 (3.5) | 27.7 (3.8) | 0.117 | 26.9 (5.0) | 28.3 (4.6) | 0.080 |
| Daily Ca2* (mg) | 1176 (373) | 1249 (283) | 0.178 | 1087 (399) | 1147 (386) | 0.371 |
| Physical Activity | 67.0 (13.6) | 64.9 (13.3) | 0.350 | 62.8 (11.5) | 62.3 (14.3) | 0.829 |
| Time since Menopause (yrs) | NA | NA | NA | 29.9 (6.2) | 26.8 (6.54) | 0.006 |
| n(%) | n(%) | p-value | n(%) | n(%) | p-value | |
| Alcohol | ||||||
| None | 1 (2.3) | 7 (5.3) | 10 (20.8) | 22 (20.0) | ||
| ≤ Recommendeda | 33 (75.0) | 106 (79.7) | 38 (79.2) | 85 (77.3) | ||
| > Recommended | 10 (22.7) | 20 (15.0) | 0.424 | 0 (0.0) | 3 (2.73) | 0.727 |
| Smoking | ||||||
| Never | 21 (47.7) | 59 (39.1) | 28 (58.3) | 72 (64.9) | ||
| Ex | 22 (50.0) | 75 (56.4) | 17 (35.4) | 37 (33.3) | ||
| Current | 1 (2.3) | 6 (4.5) | 0.604 | 3 (6.3) | 2 (1.8) | 0.313 |
| Social Statusb | ||||||
| I – IIINM | 18 (42.9) | 56 (44.1) | 22 (45.8) | 48 (43.2) | ||
| IIIM – V | 24 (57.1) | 71 (55.9) | 0.889 | 26 (54.2) | 63 (56.8) | 0.763 |
| Bisphosphonate (ever use) | ||||||
| Yes | 1 (2.3) | 1 (0.7) | 6 (12.5) | 6 (5.4) | ||
| No | 43 (97.7) | 132 (99.3) | 0.436 | 42 (87.5) | 105 (94.6) | 0.187 |
| HRTc (ever use) | ||||||
| Yes | NA | NA | NA | 30 (62.5) | 57 (51.4) | |
| No | 18 (37.5) | 54 (48.6) | 0.195 | |||
Key: Recommended maximum weekly consumption of alcohol (14 units for women, 21 units for men);
I-IIINM (I to III non-manual), IIIM-V (III manual to V);
Hormone Replacement Therapy.
3.3. Individual HRpQCT predictors of fracture status
In both men and women, the odds of fracture were significantly greater in those with a lower cortical thickness in the unadjusted analyses [OR(95% CI) per SD reduction: 1.65(1.13,2.42) and 1.63(1.06,2.52) in men and women respectively] (table 3). There was a tendency towards a greater odds of prevalent fracture with lower trabecular density, number and thickness. However, this only reached statistical significance in women (p<0.05 for all). These significant associations were attenuated by adjustment for age, height, weight, calcium intake, physical activity, smoker status, alcohol consumption, social status, bisphosphonate use, time since menopause and hormone replacement therapy. In men, there was a reduction in the odds of prevalent fracture for every one SD reduction in total area and trabecular area (OR(95%CI) 0.50(0.31,0.78) and 0.52(0.34,0.78) respectively, p<0.01 for both). This relationship was not shown in women.
Table 3.
Standardised odds ratios for prevalent fracture for a one standard deviation reduction in each HRpQCT parameter
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Total area | 0.50 (0.31,0.78) | 0.003 | 0.46 (0.27,0.80) | 0.006 | 1.07 (0.52, 2.20) | 0.859 | 1.49 (0.57, 3.85) | 0.416 |
| Trabecular area | 0.52 (0.34, 0.78) | 0.002 | 0.50 (0.31, 0.80) | 0.004 | 0.90 (0.48, 1.72) | 0.758 | 1.13 (0.49, 2.60) | 0.766 |
| Cortical area | 1.36 (0.88, 2.10) | 0.164 | 1.38 (0.83,2.29) | 0.209 | 2.71 (1.28, 5.72) | 0.009 | 3.03 (1.25, 7.31) | 0.014 |
| Cortical thickness | 1.65 (1.13, 2.42) | 0.010 | 1.63 (1.07, 2.49) | 0.022 | 1.63 (1.06, 2.52) | 0.027 | 1.61 (0.98, 2.64) | 0.058 |
| Cortical density | 1.36 (0.96, 1.94) | 0.085 | 1.48 (0.99, 2.21) | 0.058 | 1.24 (0.89, 1.71) | 0.200 | 1.20 (0.83, 1.74) | 0.340 |
| Cortical porosity | 1.08 (0.77, 1.53) | 0.649 | 1.08 (0.71, 1.64) | 0.730 | 1.49 (1.01, 2.19) | 0.046 | 1.68 (1.06, 2.66) | 0.026 |
| Trabecular density | 1.32 (0.87, 2.00) | 0.186 | 1.50 (0.93, 2.40) | 0.093 | 1.92 (1.31, 2.82) | 0.001 | 1.83 (1.15, 2.91) | 0.011 |
| Trabecular number | 1.42 (0.88, 2.28) | 0.148 | 1.56 (0.91, 2.66) | 0.103 | 1.38 (1.03, 1.85) | 0.033 | 1.27 (0.88, 1.83) | 0.208 |
| Trabecular thickness | 1.20 (0.83, 1.73) | 0.334 | 1.31 (0.86, 1.97) | 0.205 | 2.05 (1.36, 3.09) | 0.001 | 1.92 (1.22, 3.02) | 0.005 |
| Trabecular separation | 0.61 (0.33, 1.11) | 0.107 | 0.55 (0.26, 1.14) | 0.108 | 0.74 (0.57, 0.97) | 0.028 | 0.80 (0.58, 1.11) | 0.182 |
Key: Adjusted for age, height, weight, calcium intake, physical activity, smoker status, alcohol consumption, social status, bisphosphonate use, and in women, time since menopause and hormone replacement therapy.
3.4. DXA and Fracture Characteristics of Clusters
The statistical cluster analysis derived 5 stable clusters in men and women (tables 4 and 5). In 2 of the clusters, clear associations with fracture risk were identified whereas in the remaining clusters no elevation of risk was shown. In men, the OR (95%CI) for having a prevalent fracture was 10.33 (2.59,41.26) in cluster 1 and 5.74 (1.14,28.78) in cluster 2. The magnitude of this relationship was similar in women. In women, femoral neck areal BMD was significantly lower in clusters 1 to 4 when compared to cluster 5 (table 5). However, in men, it was lower in clusters 2 and 3, higher in cluster 4, and did not differ significantly in cluster 1 (table 4). A total of 28.6% of men and 35.0% of women in cluster 2 were osteoporotic using DXA areal BMD criteria. By contrast, only 10.0% and 11.5% of men and women respectively in cluster 1 were osteoporotic using DXA areal BMD criteria, although they demonstrated a similar or higher proportion of fractures.
Table 4.
Cluster analysis of bone microarchitectural parameters in men
| Cluster 1 (n=30) | Cluster 2 (n=14) | Cluster 3 (n=47) | Cluster 4 (n=52) | Cluster 5 (n=34) | |
|---|---|---|---|---|---|
| HRpQCT | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Total area (mm2) | 530.4 (45.8)↑ | 425.7 (56.6) | 405.1 (40.0) | 423.1 (52.5) | 364.1 (49.1) |
| Trabecular area (mm2) | 466.0 (41.7)↑ | 359.4 (51.5) | 341.0 (37.6) | 338.5 (53.5) | 284.5 (46.5) |
| Cortical area (mm2) | 62.8 (11.0) | 63.1 (11.0) | 62.2 (7.2) | 82.5 (10.3) | 75.9 (12.4) |
| Cortical thickness (µm) | 621.9 (120.9)↓ | 747.3 (139.0) | 729.3 (78.0) | 951.0 (133.1) | 984.0 (154.8) |
| Cortical density (mg/cm3) | 849.9 (44.8)↓ | 917.3 (54.2) | 882.3 (32.7) | 915.5 (31.8) | 963.9 (21.3)↑ |
| Cortical porosity (%) | 4.5 (1.5) | 3.0 (1.1) | 4.4 (1.3) | 4.9 (1.4) | 3.0 (0.8) |
| Trabecular density (mg/cm3) | 162.6 (29.6) | 123.1 (34.3)↓ | 173.1 (22.2) | 208.9 (26.7) | 179.1 (24.6) |
| Trabecular number (cm-1) | 23.6 (2.0) | 18.1 (2.3)↓ | 23.7 (1.8) | 25.3 (1.2) | 22.5 (1.5) |
| Trabecular thickness (µm) | 57.3 (8.3) | 56.4 (15.4) | 61.0 (6.6) | 69.0 (8.5) | 66.3 (9.0) |
| Trabecular separation (µm) | 369.6 (36.9) | 508.9 (111.4)↑ | 364.3 (34.9) | 327.9 (20.1) | 379.5 (30.6) |
| DXA | |||||
| Femoral neck aBMD (g/cm2)a | 0.91 | 0.83*** | 0.89* | 1.03*** | 0.94 |
| Normalb | 14 (46.7) | 2 (14.3) | 16 (34.0) | 38 (73.1) | 15 (44.1) |
| Osteopenic | 13 (43.3) | 8 (57.1) | 27 (57.5) | 14 (26.9) | 18 (52.9) |
| Osteoporotic | 3 (10.0) | 4 (28.6) | 4 (8.5) | 0 (0.0) | 1 (2.9) |
| Fracture | |||||
| Proportion with prevalent fracture | 50.0% | 35.7% | 21.3% | 21.2% | 8.8% |
| OR (95% CI) of fracturea | 10.33*** (2.59,41.26) | 5.74* (1.14,28.78) | 2.79 (0.71,11.05) | 2.77 (0.71,10.79) | 1.0 Reference |
Key: p value for difference between clusters 0.003.
p value for difference when compared to lowest risk cluster (cluster 5);
Count (percentage).
p<0.05,
p<0.01,
p<0.001.
Bold if mean is >1SD from sex-specific mean;
indicates mean is >1SD above the sex-specific mean;
indicates mean is >1SD below the sex-specific mean.
Table 5.
Cluster analysis of bone microarchitectural parameters in women
| Cluster 1 (n=26) | Cluster 2 (n=20) | Cluster 3 (n=39) | Cluster 4 (n=39) | Cluster 5 (n=35) | |
|---|---|---|---|---|---|
| HRpQCT | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Total area (mm2) | 307.0 (37.7) | 280.4 (34.8) | 255.1 (28.6) | 326.8 (36.6) | 262.2 (23.3) |
| Trabecular area (mm2) | 267.7 (36.3) | 232.7 (33.3) | 205.7 (26.6) | 277.9 (34.6) | 205.6 (23.5) |
| Cortical area (mm2) | 38.3 (4.6)↓ | 46.5 (8.1) | 47.3 (6.2) | 47.6 (7.3) | 54.8 (7.1) |
| Cortical thickness (µm) | 481.7 (60.3)↓ | 680.8 (129.6) | 732.3 (105.4) | 627.3 (99.2) | 855.7 (133.9)↑ |
| Cortical density (mg/cm3) | 834.9 (46.7)↓ | 919.1 (42.9) | 947.7 (29.8) | 882.3 (40.4) | 929.5 (40.1) |
| Cortical porosity (%) | 3.4 (1.1) | 2.7 (1.4) | 2.8 (1.0) | 3.9 (1.2) | 4.6 (1.2) |
| Trabecular density (mg/cm3) | 110.0 (19.9) | 76.9 (17.9)↓ | 145.3 (23.9) | 151.9 (20.2) | 192.5 (24.7)↑ |
| Trabecular number (cm−1) | 19.3 (3.2) | 13.4 (2.5)↓ | 20.5 (2.4) | 22.7 (2.1) | 24.4 (1.7) |
| Trabecular thickness (µm) | 47.9 (6.5) | 48.0 (10.4) | 59.3 (8.6) | 56.1 (7.1) | 65.9 (6.9) |
| Trabecular separation (µm) | 485.9 (97.8) | 722.2 (146.0)↑ | 434.8 (61.1) | 388.8 (41.2) | 346.6 (30.6) |
| DXA | |||||
| Femoral neck aBMD (g/cm2)a | 0.75*** | 0.73*** | 0.85* | 0.85* | 0.94 |
| Normalb | 2 (7.7) | 5 (25.0) | 16 (41.0) | 17 (43.6) | 24 (68.6) |
| Osteopenic | 21 (80.8) | 8 (40.0) | 21 (55.9) | 20 (51.3) | 10 (28.6) |
| Osteoporotic | 3 (11.5) | 7 (35.0) | 2 (5.1) | 2 (5.1) | 1 (2.8) |
| Fracture | |||||
| Proportion with prevalent fracture | 50.0% | 50.0% | 30.8% | 20.5% | 14.3% |
| OR (95% CI) of fracturea | 6.0* (1.77,20.31) | 6.0* (1.65,21.80) | 2.67 (0.83,8.55) | 1.55 (0.45,5.27) | 1.0 Reference |
Key: p value for difference between clusters 0.006.
p value for difference when compared to lowest risk cluster (cluster 5);
Count (percentage).
p<0.05,
p<0.01,
p<0.001.
Bold if mean is >1SD from sex-specific mean;
indicates mean is >1SD above the sex-specific mean;
indicates mean is >1SD below the sex-specific mean.
3.5. HRpQCT Cluster Descriptions
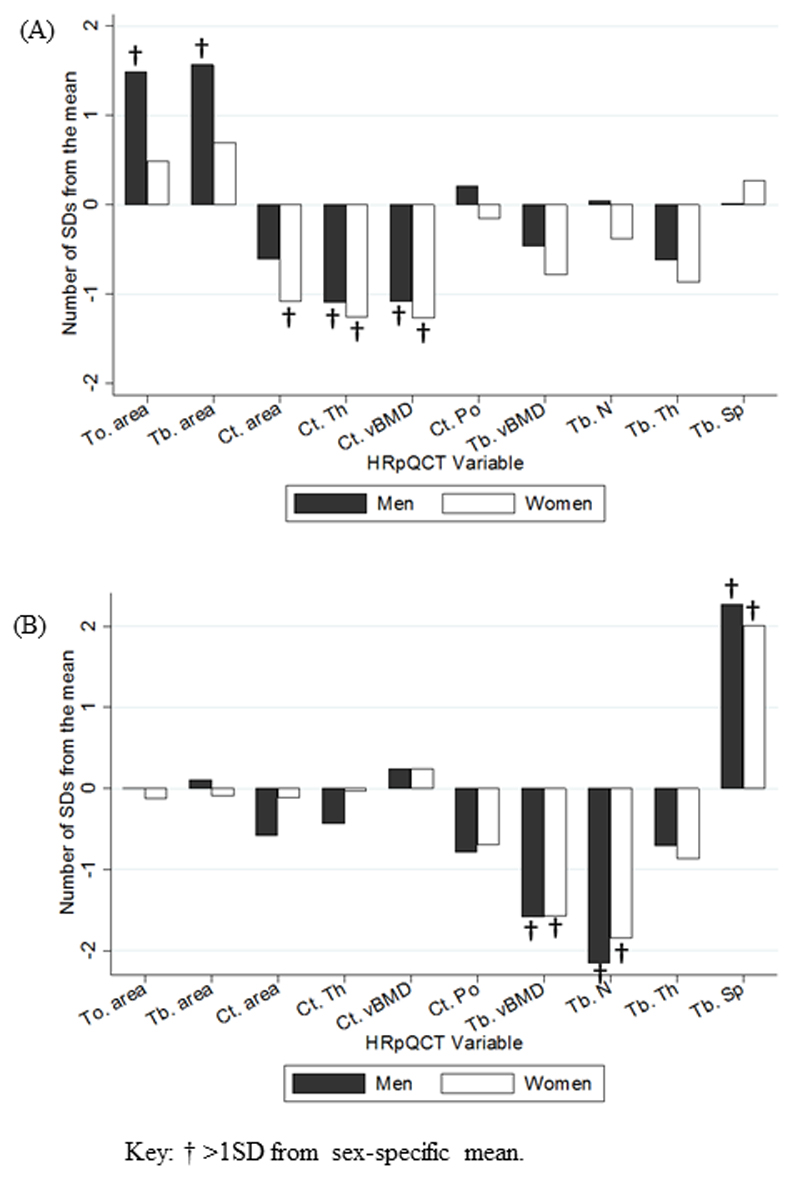
The HRpQCT phenotypes of clusters 1 and 2 were similar in men and women. In both sexes, cluster 1 had a phenotype of “cortical deficiency” with a mean cortical thickness and cortical density of more than one SD below the sex-specific mean. In men, this cluster also demonstrated a mean total and trabecular area of more than one SD above the sex-specific mean. This feature was not, however, found in women (figure 1).
Figure 1.
Mean difference from the population mean of bone microarchitectural parameters in (A) clusters 1 and (B) cluster 2.
Cluster 2 showed a phenotype of “trabecular deficiency” with mean trabecular density and number of more than one SD below the sex-specific mean in both sexes. Consequently, trabecular separation was more than one SD above the sex-specific mean in men and women. Cluster 3 and 4 did not differ by more than one SD in any parameter. Cortical density in men, and cortical thickness and trabecular density in women were more than one SD above the sex-specific mean in cluster 5.
4.0. Discussion
This study demonstrates two high risk bone phenotypes using HRpQCT in both men and women. The first was characterised by low cortical thickness and density and, in men only, a higher total and trabecular area whereas the second showed low trabecular density and number. Interestingly, although fracture rates were higher in all of these groups, in men the first cluster was not associated with low femoral neck areal BMD and therefore would not have been identified by conventional DXA techniques.
In women, we showed in univariate analyses that lower cortical area and thickness and trabecular density, number, and thickness were associated with a greater odds ratio of prevalent fracture. This is consistent with previous HRpQCT studies (5–8) and is likely to reflect specific components that, when deficient, lead to a weakening of bone structure. Although these associations were attenuated by adjustment for demographic and lifestyle factors, relationships were maintained suggesting the findings are not purely due to confounding, for example by age, as women who had fractured were on average older than those that had not.
Bone microarchitecture by fracture status has been examined to a far lesser extent in men. One study did explore the differences in radial bone microarchitecture in men with idiopathic osteoporosis (9). In keeping with the current study, they also showed that men with fractures tended to have lower cortical thickness but larger bones in cross-section, specifically total and trabecular area. One possible explanation for this finding in men and not women is that although men have larger bones, their cortical thickness is not proportionally increased. Consequently, they will inherently have higher buckling ratios on average potentially elevating their risk of buckling. This may increase their sensitivity to increases in total bone area in conjunction with reduced cortical thickness. Ostertag and colleagues (9) also found trabecular density to be significantly lower in those that had fractured but this difference did not reach statistical significance in the current study.
When all available parameters of bone microarchitecture were explored using cluster analysis, the high-risk clusters identified were similar in men and women. The second cluster is akin to a predictable phenotype of high turnover leading to predominant deterioration in the metabolically-active trabecular bone with a high surface area to volume ratio (27). In keeping with this, the cluster was demonstrated to have a low femoral neck areal BMD when compared to the reference cluster. A considerable proportion of individuals in this cluster also fell into the osteoporotic range as defined by DXA. It would be of interest to compare bone turnover markers in these cluster groups.
In contrast, the first cluster, with overall lower cortical thickness and density, contained a much smaller proportion of osteoporotic individuals as defined by DXA despite having a similar, or in men higher, proportion with prevalent fractures. Furthermore, men in the first cluster did not differ significantly in areal BMD from those in cluster 5 (referent). As men in this cluster tend to have larger bones in cross-section, this is likely to falsely elevate measures of aBMD which does not assess true volumetric density (28). Consequently, these men at high risk of fracture might not be identified by routine DXA scanning. Interestingly, although larger bones in younger individuals tend to be associated with greater bone strength, they also tend to be associated with a thinner cortex (5). In older individuals, this phenotype becomes more pronounced due to normal age-related changes and may lead to a structure at risk of buckling (29, 30).
This study does have limitations. Firstly, the study is not prospective. As the fractures occurred before the radial scans, it is more difficult to imply that the bone deficits led to the development of fractures. However, biologically this would seem the most likely explanation for the associations shown. Secondly, the ascertainment of non-vertebral fractures was retrospective and occurred through self-report. However, the questionnaires used have been validated (23). Thirdly, in 22 men and 19 women the dominant limb was scanned (as the non-dominant limb had previously fractured). However, we did not identify any significant differences in radial bone microarchitecture when these individuals were compared to those in which the non-dominant limb was scanned (results not shown). Fourthly, all participants were older Caucasian men and women recruited from the HCS. This may limit generalizability to other regions, ages and ethnic groups, however, the cohort has been shown to be fairly representative of the UK population (10). Lastly, k-means cluster analysis models can be very unstable which significantly affects the generalizability of the findings in this study. The results are, however, certainly hypothesis generating and it is clearly important that these evaluations are repeated in different population samples
In conclusion, this study has pointed to two high fracture risk bone phenotypes in both men and women using HRpQCT. These findings not only highlight a group which may be underdiagnosed by DXA alone but may also demonstrate distinct phenotypes of bone fragility with differing risk factors, aetiologies, patterns of fracture site, and responses to pharmacological therapy. Further research is required to confirm whether individuals in these “high risk” clusters do have a higher risk of fracture prospectively and in which other ways clusters 1 and 2 differ. This may have significant implications for prevention and management of osteoporosis in the future.
Highlights.
-
-
The presence of a prevalent fracture is associated with lower cortical thickness in both men and women
-
-
Two distinct bone phenotypes have been identified with elevated fracture prevalence
-
-
Men with bones with large cross-section and thin cortex are associated with prevalent fracture but may not be identified by DXA as being at risk
5.0. Acknowledgements
All authors have made substantial contributions to conception, design and interpretation of data; participated in drafting or revising the manuscript; approved the final version of the submitted manuscript; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research has been made possible thanks to an unrestricted grant from the International Osteoporosis Foundation and SERVIER and a fellowship grant from Arthritis Research UK (Grant number 19583). The Hertfordshire Cohort Study was supported by the Medical Research Council (MRC) of Great Britain; Arthritis Research UK; and the International Osteoporosis Foundation. The work herein was also supported by the NIHR Nutrition BRC, University of Southampton and the Oxford NIHR Musculoskeletal BRU, University of Oxford. Imaging of participants was performed at the MRC Human Nutrition Research in Cambridge. Kate Ward was funded by the MRC (Programme number U105960371). We thank all of the men and women who took part in the Hertfordshire Cohort Study; the HCS Research Staff; and Jennifer Woolston for her role in image acquisition and scan processing.
References
- 1.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359(9320):1841–50. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 5.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin EndocrinolMetab. 2005;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 6.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone MinerRes. 2010;25(12):2572–81. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilayphiou N, Boutroy S, Sornay-Rendu E, van RB, Munoz F, Delmas PD, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in postmenopausal women. Bone. 2010;46(4):1030–7. doi: 10.1016/j.bone.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone MinerRes. 2007;22(3):425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 9.Ostertag A, Collet C, Chappard C, Fernandez S, Vicaut E, Cohen-Solal M, et al. A case-control study of fractures in men with idiopathic osteoporosis: fractures are associated with older age and low cortical bone density. Bone. 2013;52(1):48–55. doi: 10.1016/j.bone.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Syddall HE, Aihie SA, Dennison EM, Martin HJ, Barker DJ, Cooper C. Cohort profile: the Hertfordshire cohort study. IntJ Epidemiol. 2005;34(6):1234–42. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 11.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. TechnolHealth Care. 1998;6(5–6):329–37. [PubMed] [Google Scholar]
- 12.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone MinerRes. 2006;21(1):124–31. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.H T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- 14.Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72(4):1396–409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. MedEng Phys. 2007;29(10):1096–105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone MinerRes. 2010;25(5):983–93. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genant HK, Wu CY, van KC, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. JBone MinerRes. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 20.Dallosso HM, Morgan K, Bassey EJ, Ebrahim SB, Fentem PH, Arie TH. Levels of customary physical activity among the old and the very old living at home. J EpidemiolCommunity Health. 1988;42(2):121–7. doi: 10.1136/jech.42.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am GeriatrSoc. 2008;56(1):84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standard Occupational Classification. London: HMSO, (OPCS) OoPCaS; 1991. [Google Scholar]
- 23.Ismail AA, O’Neill TW, Cockerill W, Finn JD, Cannata JB, Hoszowski K, et al. Validity of self-report of fractures: results from a prospective study in men and women across Europe. EPOS Study Group. European Prospective Osteoporosis Study Group. Osteoporos Int. 2000;11(3):248–54. doi: 10.1007/s001980050288. [DOI] [PubMed] [Google Scholar]
- 24.Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian MedAssoc. 2009;107(6):403–5. [PubMed] [Google Scholar]
- 25.Vu T, Finch CF, Day L. Patterns of comorbidity in community-dwelling older people hospitalised for fall-related injury: a cluster analysis. BMC geriatrics. 2011;11:45. doi: 10.1186/1471-2318-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang HS, Youn IS, Lee KH, Lim HJ. Classification of facial asymmetry by cluster analysis. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2007;132(3):279.e1–6. doi: 10.1016/j.ajodo.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int. 2003;14(Suppl 3):S2–8. doi: 10.1007/s00198-002-1340-9. [DOI] [PubMed] [Google Scholar]
- 28.Lorente Ramos RM, Azpeitia Arman J, Arevalo Galeano N, Munoz Hernandez A, Garcia Gomez JM, Gredilla Molinero J. Dual energy X-ray absorptimetry: fundamentals, methodology, and clinical applications. Radiologia. 2012;54(5):410–23. doi: 10.1016/j.rx.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Mori S. [Assessment of hip fracture risk related to the structure] Clin Calcium. 2010;20(9):1349–56. [PubMed] [Google Scholar]
- 30.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(6):1108–19. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]