Abstract
Summary
Daily teriparatide injections have been shown to reduce vertebral and non-vertebral fractures. Here we demonstrate that the magnitude of fracture risk reduction is independent of baseline fracture probability assessed by FRAX.
Background
Daily administration of 20μg or 40μg teriparatide has been shown to significantly decrease the risk of vertebral and non-vertebral fracture compared with placebo. The aim of the present study was to evaluate fracture risk assessed at baseline using the FRAX® tool and to determine the efficacy of teriparatide as a function of baseline fracture risk.
Methods
1637 postmenopausal women in the pivotal phase 3 trial, randomly assigned to receive placebo (n=544), teriparatide 20 μg per day (n=541) or teriparatide 40 μg per day (n=552), were studied. Baseline clinical risk factors were entered into country-specific FRAX models to compute the 10-year probability of major osteoporotic fractures with or without input of femoral neck BMD. Because there was no difference in effect of 20 and 40μg teriparatide daily on fracture occurrence, the two active groups were merged. The interaction between probability of a major fracture and treatment efficacy was examined by Poisson regression.
Results
The 10-year probability of major osteoporotic fractures (with BMD) ranged from 2.2-67.2%. Treatment with teriparatide was associated with a 37% decrease in all non-vertebral fractures (95% CI:10-56 %) and a 56% decrease in low energy non-vertebral fractures (95% CI:24-75%) compared with placebo. The risk of morphometric vertebral fractures decreased significantly by 66% (95% CI:50-77%). Hazard ratios for the effect of teriparatide on the fracture outcome did not change significantly with increasing fracture probability (p>0.30). Similar findings were noted for the interaction when BMD was excluded from the FRAX model, or when probability of hip fracture was used as the marker of baseline risk.
Conclusion
We conclude that teriparatide significantly decreases the risk of non-vertebral and morphometric vertebral fractures in women by a similar extent, irrespective of baseline fracture probability.
Keywords: teriparatide, osteoporosis, epidemiology, fracture, interaction, BMD, randomised controlled trial
Background
Over recent years there has been increasing interest in the concept of personalised, or stratified, medicine, in which treatments are selected on the basis of individual patient characteristics which may influence treatment efficacy [1]. The paradigm has most often focussed on drug-genotype interactions [1], but in the case of therapies aimed at fracture prevention in osteoporosis, it may be applied to the assessment of baseline fracture probability. There are now many interventions that have been shown in placebo-controlled trials to decrease the risk of fracture in postmenopausal osteoporosis [2]. One such example is the recombinant analogue of 1-34 parathyroid hormone, teriparatide. Treatment with teriparatide has been shown to significantly reduce the risk of vertebral and non-vertebral fractures [3]. Beneficial effects on non-vertebral fractures with teriparatide have been shown to persist for up to 30 months after stopping treatment [4].
Traditional approaches to stratification by baseline fracture risk using T-score classification, prior fracture etc have the major disadvantage of reducing statistical power through mandating subgroup analysis. FRAX®, a computer based algorithm (http://www.shef.ac.uk/FRAX) that provides models for the assessment of 10-year fracture probability in men and women using easily obtained clinical risk factors for fracture [5,6], provides a continuous value, and thus permits assessment of baseline risk whilst maintaining optimal statistical power. The use of this approach has already been applied to several intervention studies. In one such investigation, women aged 75 years or more living in the general community, identified from general practice registers, were randomised to 800mg oral clodronate or matching placebo daily over three years [7]. Greater fracture reduction was observed at higher fracture probabilities, calculated with or without the inclusion of BMD [8]. Similar findings were reported for bazedoxifene [9] and for denosumab [10]. In contrast, the efficacy of raloxifene and strontium ranelate was similar over a wide range of fracture probabilities [11,12]. It is currently unknown whether the efficacy of teriparatide is dependent on the baseline risk of fracture, and the finding of similar interactions would have important implications for targeting of treatment, reimbursement, product differentiation, market segmentation, meta-analysis and health economic assessment. In this study, therefore, we used existing data from a major trial of teriparatide in postmenopausal women to investigate whether fracture prevention efficacy differed according to baseline 10-year fracture probability, assessed using FRAX.
Methods
Study population
The analysis population comprised participants from the pivotal global, phase 3, multicentre, double-blind, calcium- and vitamin D-controlled, randomized study of teriparatide, the methods of which have been published previously [3,4]. The study was undertaken with full IRB approval and all participants gave their written informed consent. A total of 9347 ambulatory postmenopausal women were screened for the study, and of these 1637 eligible women were randomly assigned to receive placebo (544 women) or teriparatide (recombinant parathyroid hormone, 1-34) at a dose of 20 μg per day (541 women) or 40 μg per day (552 women). The mean (±SD) durations of treatment in the three groups were 18±5, 18±6, and 17±6 months, respectively.
FRAX
For the purpose of the present analysis, country-specific 10-year fracture probability was used to determine whether absolute and relative risk reductions for fracture outcomes changed as a function of baseline FRAX score. The whole continuous range of 10-year probabilities was used thereby optimising the statistical power by avoiding subgroup analysis. Baseline risk factors, as characterised below, were combined with age and BMI to compute the 10-year probability of a major osteoporotic fracture. Femoral neck BMD was also measured at entry. Using FRAX version 3.8 (www.shef.ac.uk/FRAX), the 10-year probability of a major osteoporotic fracture constituted the primary risk variable. Femoral neck BMD was included in the model for the primary analysis; additionally probabilities were computed without the use of BMD. In a supplementary analysis, the interaction of efficacy with hip fracture probability was examined.
Characterisation of risk factors
Previous fracture
A history of a previous morphometric vertebral fracture of any grade was reported in 1412 patients. Information on other previous fractures was not available. Previous vertebral fracture was assessed from lateral x-rays of the thoracic and lumbar spine by semi-quantitative (SQ) visual assessment of each vertebra, from T4 to L4 [13,14]. The database provided the maximum SQ grade of each enrolled patient. For example, the allocation of a score of 2 indicated that at least one moderate fracture was detected at screening. Maximum scores were given as 0, 1, 2 or 3. The presence of a mild vertebral fracture has been shown to be of no significant prognostic value for non-vertebral fracture outcomes [15]. Although this was also true in the current study, mild vertebral fracture at baseline was associated with a 3-to 4- fold increased risk of incident vertebral fracture, albeit the relationship not achieving statistical significance (p=0.079). Moderate or severe vertebral fractures at baseline were associated with a significant (p=0.012) and much higher relative risk, approximately 6-fold. In contrast, the risk of non-vertebral fractures was not significantly increased irrespective of the grade of vertebral fracture. However, for both mild and moderate/severe grades, the point estimates were above unity and thus mild vertebral fracture was retained as an input risk factor for the computation of FRAX probabilities.
Glucocorticoid use and rheumatoid arthritis
Participants were categorised as users/ non-users of glucocorticoids (users: n=64), and 3 participants were recorded as having a diagnosis of rheumatoid arthritis.
Parental history of hip fracture
No information was available from the dataset and this variable was set as “no” for all women.
Secondary osteoporosis
The following conditions were considered as secondary causes of osteoporosis: premature menopause (n=360 patients), hyperthyroidism (n=4), diabetes mellitus (n=2) and hepatitis (n=33).
Smoking, and alcohol intake
279 participants were current smokers and alcohol intake was classified as nil, or 3 units or more per day (n=5). Alcohol abuse was an exclusion criterion.
Femoral neck BMD
This was measured using equipment from three different manufacturers. After standardisation [16], BMD T-score was calculated using the National Health and Nutrition Examination Survey (NHANES) III young women as a reference value [17].
Country specificity
Probabilities were computed according to the country where the patient was recruited for study using FRAX version 3.8. Where a country was not represented (because of the lack of epidemiological data) a surrogate was chosen. The FRAX® models used comprised: Argentina (n=179); Australia (n=22); Austria (n=17); Belgium (n=44); Canada (n=118); Czech Republic (n=43), Denmark (n=90); Finland (n=146); Hungary (n=100); Israel (n=50); Italy (n=59); New Zealand (n=16); Norway (n=162); Poland (n=137); Sweden (n=47); The Netherlands (n=42), and the US, by ethnicity [Asian (n=1); Black (n=1); Caucasian (n=349); Hispanic (n=14)]. The Lebanese FRAX model was used for Israel as a country-specific model is not currently available.
Fracture outcomes
The documented fractures comprised morphometric vertebral fractures and non-vertebral fractures (validated by radiograph or radiographic report, with the non-vertebral category comprising any non-vertebral fracture). The summary effects of teriparatide (20 and 40μg together) on morphometric vertebral fractures alone and on all non-vertebral fractures were studied. In an additional analysis, the effects of teriparatide on non-vertebral fractures that were coded as being associated with low energy injury were also examined.
Statistical methods
The general approach of the present analysis was to apply models to the entire study population (patients randomised to teriparatide 20 or 40μg daily or placebo) to assess the efficacy of teriparatide in relation to 10-year probability of fracture. Because there was no difference in effect of 20 and 40μg teriparatide daily [3], illustrated in supplementary table 1, the two active groups were merged in order to maximise the power of the analytic approach. Follow-up was stopped when a fracture occurred at the relevant site. For the overall effects of teriparatide (20 and 40μg together) on fracture outcomes an extension of Poisson regression model was used [18]. In contrast to logistic regression, the Poisson regression utilises the length of each individual’s follow-up period and the hazard function is assumed to be exp(β0 + β1 · time from baseline + β2 · current age + β3 · current variable of interest). The observation period of each participant was divided in intervals of one month. For each outcome, one fracture per person was counted.
Poisson regression model A
1. constant, 2. current time, 3. current age, 4. treatment (teriparatide 20 and 40μg versus placebo, where 1 = teriparatide and 0 = placebo)
The interaction between treatment and 10 year probability was examined with the following model.
Poisson regression model B
1. constant, 2. current time, 3. current age, 4. treatment (teriparatide 20 and 40μg versus placebo), 5. 10 year probability, 6. treatment × 10 year probability
Two-sided p-value were used for all analyses and p<0.05 considered to be significant.
Results
Clinical risk factors
A total number of 1637 women were studied of whom 1537 [mean (SD) age 69.5 (7.0 years] had information on the clinical risk factors (Table 1). Of these, 1476 had additional information on femoral neck BMD. Only 9 hip fractures occurred so hip fracture was not separately assessed in relation to FRAX. 105 patients experienced a new vertebral fracture identified by vertebral morphometry, and 119 patients sustained one or more non-vertebral fracture. Table 1 also summarises the FRAX 10-year probability of a major osteoporotic fracture (clinical spine, hip, forearm and humerus fracture) and hip fracture with and without BMD. The maximum follow-up time to any non-vertebral fracture was 2.1 years with a mean of 1.5 years. For morphometric vertebral fracture the maximum follow up was 2.4 years with mean of 1.7 years.
Table 1.
Baseline characteristics of participants
| All evaluable women, n= 1537 | Evaluable women with a BMD test, n=1476 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n (%) | Mean | SD | Range | n (%) | Mean | SD | Range |
| Age (years) | 69.5 | 7.0 | 42-86 | 69.5 | 6.9 | 42-86 | ||
| BMI (kg/m2) | 26.6 | 4.4 | 12-51 | 26.5 | 4.3 | 12-51 | ||
| Previous fracture | 1388 (90) | 1332 (90) | ||||||
| Parental history | Not reported | Not reported | ||||||
| Current smoker | 261 (17) | 250 (17) | ||||||
| Glucocorticoids | 57 (4) | 56 (4) | ||||||
| RA | 3 (0) | 3 (0) | ||||||
| Secondary osteoporosis | 366 (24) | 352 (24) | ||||||
| Alcohol | 4 (0) | 4 (0) | ||||||
| Femoral neck BMD T-score | -2.4 | 0.8 | -4.9-0.9 | |||||
| FRAX probability of major osteoporotic fracture | 19.3 | 10.0 | 1.3-65.6 | 19.4a | 9.9 a | 2.2-67.2 a | ||
| FRAX probability of hip fracture | 7.5 | 6.4 | 0.1-55.4 | 7.6 a | 7.0 a | 0.2-53.2 a | ||
including BMD in FRAX assessment
Overall effects of treatment
Overall, teriparatide treatment was associated with a statistically significant 66% decrease in morphometric vertebral fractures (HR = 0.34; 95%CI: 0.23-0.50). For any non-vertebral fractures, teriparatide treatment based on the pooled doses was associated with a significant 37% decrease in fractures (Table 2).
Table 2.
Overall effects of PTH on fracture outcomes
| Outcome | n with fracture | Total n | HR | 95% CI |
|---|---|---|---|---|
| Morphometric vertebral fracture | 105 | 1326 | 0.34 | 0.23-0.50 |
| Any non-vertebral fracture | 119 | 1637 | 0.63 | 0.44-0.90 |
| Low energy non-vertebral fracture | 58 | 1637 | 0.44 | 0.25-0.76 |
| Hip fracture | 9 | 1637 | 0.60 | 0.16-2.24 |
Interaction between treatment and fracture probability
Probability of a major osteoporotic fracture calculated with BMD
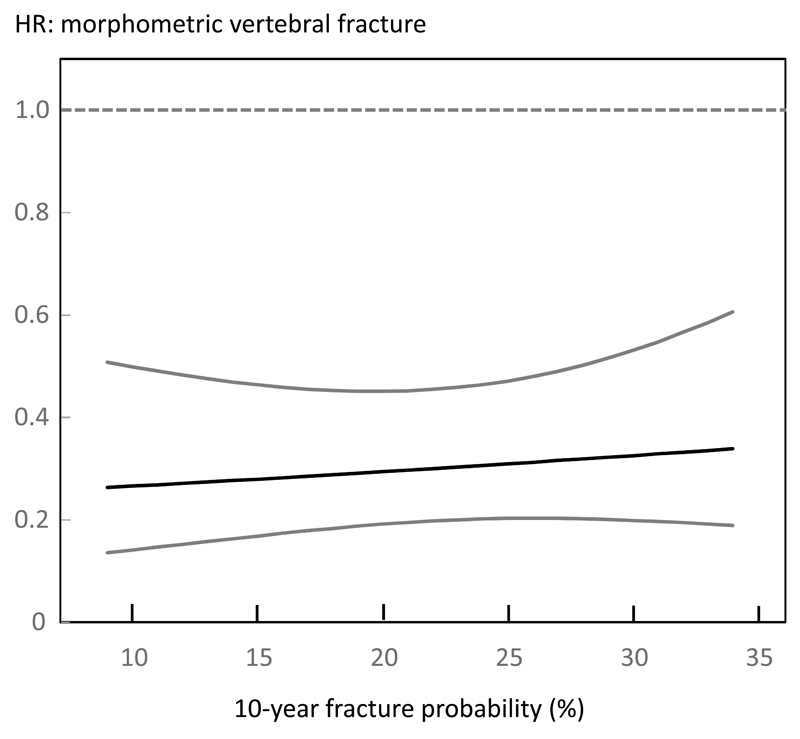
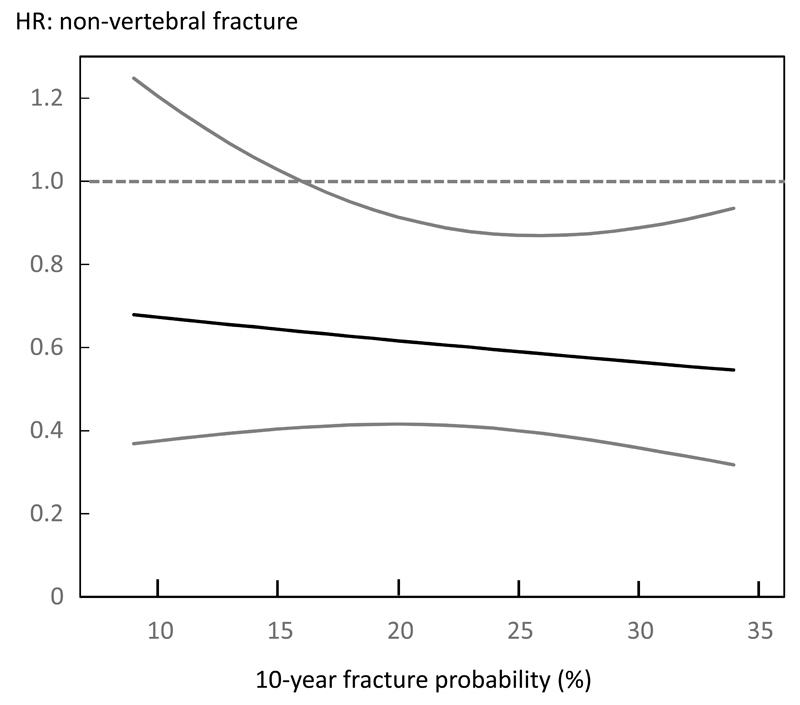
Table 3 shows the effects of teriparatide treatment at different percentiles of FRAX probabilities for a major osteoporotic fracture calculated with BMD (n=1476). Note that the model uses probability as a continuous function and the percentiles shown are for illustrative purposes only. Teriparatide reduced morphometric vertebral fractures across the full range of baseline probabilities. For non-vertebral fractures, there was a non-significant trend for a slightly greater reduction in risk at higher baseline probabilities but there was no interaction between FRAX probability and treatment efficacy for either fracture outcome (p>0.30 for both). The efficacy of teriparatide on morphometric vertebral fracture and non-vertebral fracture risk over the range of probabilities estimated with BMD is shown in Figures 1 and 2 respectively.
Table 3.
Hazard ratio between treatments (teriparatide versus placebo) for all fractures at different values of 10-year probability of a major osteoporotic fracture calculated with BMD.
| Percentile | 10 year probability | Morphometric vertebral fracture | Any non-vertebral fracture | Low energy non-vertebral fracture | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| 10th | 8.5% | 0.26 | 0.14-0.50 | 0.68 | 0.37-1.23 | 0.47 | 0.20-1.12 |
| 25th | 12.2% | 0.27 | 0.16-0.47 | 0.65 | 0.39-1.09 | 0.46 | 0.22-0.96 |
| 50th | 17.6% | 0.29 | 0.19-0.45 | 0.62 | 0.41-0.94 | 0.45 | 0.25-0.81 |
| 75th | 24.4% | 0.31 | 0.20-0.47 | 0.59 | 0.40-0.87 | 0.43 | 0.25-0.76 |
| 90th | 32.6% | 0.34 | 0.19-0.60 | 0.55 | 0.32-0.93 | 0.41 | 0.19-0.87 |
Figure 1.
Hazard ratio (HR) between treatments (teriparatide versus placebo) for morphometric vertebral fractures according to baseline 10-year probability of a major osteoporotic fracture calculated with inclusion of BMD.
Figure 2.
Hazard ratio (HR) between treatments (teriparatide versus placebo) for non-vertebral fracture according to baseline 10-year probability of a major osteoporotic fracture calculated with inclusion of BMD.
Probability of a major osteoporotic fracture calculated without BMD
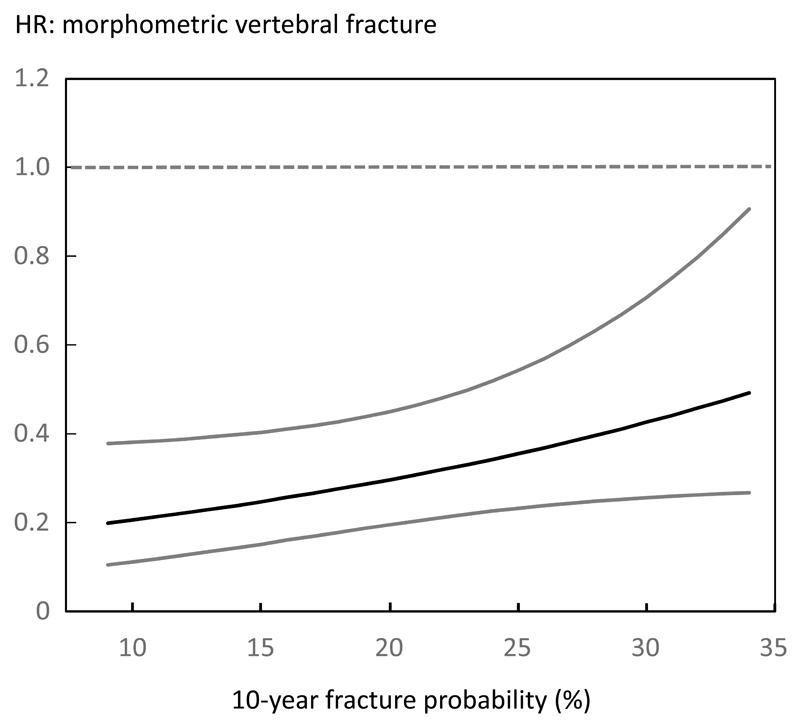
Table 4 shows the effects of teriparatide at different percentiles of FRAX probabilities for a major osteoporotic fracture calculated without BMD. Again the percentiles shown are for illustrative purposes as the model uses the continuous distribution of baseline probabilities. There was no statistically significant interaction between FRAX probability and the efficacy (p>0.30 for non-vertebral fracture outcome and p=0.061 for vertebral fracture outcome). For non-vertebral fracture outcomes the point estimates at each percentile of probability were near identical to those computed with BMD in the model. For vertebral fracture, treatment was again effective across the full range of baseline probabilities though there was a trend, albeit not achieving statistical significance, for efficacy to be greater at lower fracture probabilities when BMD was not included in the FRAX model (Figure 3). This phenomenon was attributable to a significant interaction between age and efficacy (p=0.037) for the outcome of morphometric vertebral fracture (HR = 0.15; 95%CI: 0.06-0.36 for the age of 60 and HR = 0.40; 95%CI: 0.26-0.61 for the age of 75 years).
Table 4.
Hazard ratio between treatments (PTH versus placebo) for all fractures at different values of 10 year probability of a major osteoporotic fracture calculated without BMD.
| Percentile | 10 year probability | Morphometric vertebral fracture | Any non-vertebral fracture | Low energy non-vertebral fracture | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| 10th | 7.9% | 0.20 | 0.10-0.38 | 0.70 | 0.39-1.29 | 0.49 | 0.21-1.18 |
| 25th | 11.8% | 0.23 | 0.13-0.40 | 0.68 | 0.41-1.12 | 0.49 | 0.23-1.01 |
| 50th | 17.3% | 0.28 | 0.18-0.44 | 0.65 | 0.43-0.96 | 0.47 | 0.26-0.85 |
| 75th | 25.2% | 0.37 | 0.24-0.58 | 0.60 | 0.40-0.90 | 0.46 | 0.26-0.81 |
| 90th | 33.5% | 0.50 | 0.27-0.94 | 0.55 | 0.31-0.99 | 0.44 | 0.20-0.96 |
Figure 3.
Hazard ratio (HR) between treatments (teriparatide versus placebo) for morphometric vertebral fractures according to baseline 10-year probability of a major osteoporotic fracture calculated without inclusion of BMD.
FRAX hip fracture probability, and low energy fractures
Similar findings were apparent when baseline probability of hip fracture was used instead of the probability of major osteoporotic fracture, summarised in supplementary tables 2 and 3. There were 58 patients in whom non-vertebral fractures were characterised as low energy fractures. Teriparatide treatment was associated with a significant decrease in non-vertebral fracture risk (HR = 0.44; 95% CI: 0.25-0.76) in these patients. The quantum of effect was somewhat greater than the effect of teriparatide on all non-vertebral fractures (HR = 0.63; 95%CI: 0.44-0.90). Supplementary table 4 shows the effects of teriparatide on this fracture outcome at different percentiles of FRAX probabilities for a major osteoporotic fracture calculated with BMD. There was no interaction between FRAX probability and the efficacy (p>0.30) for non-vertebral fracture outcomes.
Discussion
The present study demonstrated the ability of teriparatide treatment to reduce non-vertebral, low energy non-vertebral and morphometric-vertebral fracture risk with relative risk reductions (37%, 56% and 66%, respectively) comparable to those reported previously [3]. However, the current analysis, found no evidence of a significant interaction between baseline fracture probability and treatment efficacy. These findings suggest that the therapy may be usefully employed across the whole range of fracture probability reported in this study, and that baseline fracture probability alone may not constitute a valid criterion on which to stratify the use of this medication.
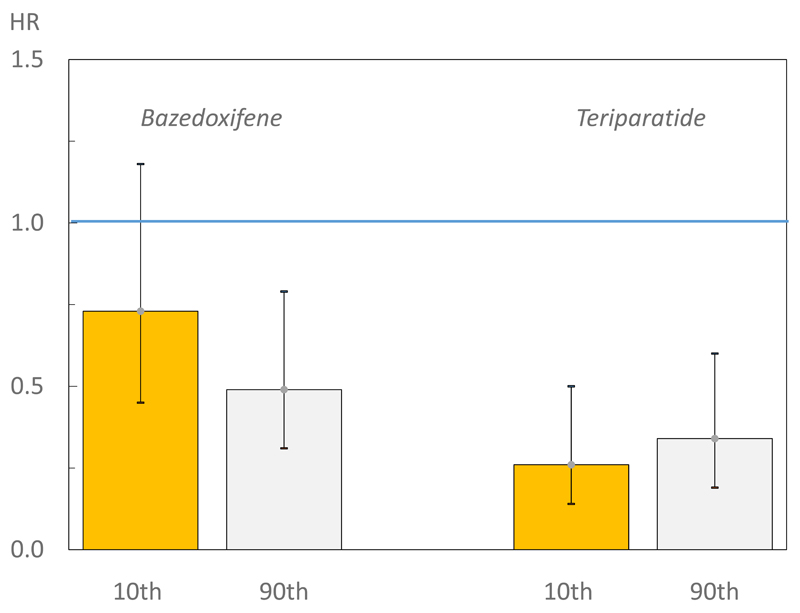
The findings from the present analysis are similar to those described for raloxifene [11,19] and strontium ranelate [12] but contrast with retrospective assessments of two other phase III studies. The first of these was a 3-year prospective, randomized, placebo-controlled trial of oral clodronate in elderly women, identified from general practice registers. A marked trend for a greater fracture reduction at higher fracture probabilities was observed, with or without the use of BMD. The interaction was statistically significant when BMD was excluded from the probability calculation [8] and efficacy was evident at fracture probabilities that exceeded 20%. These data are very similar to the results of a second study in which there was a significant effect of bazedoxifene on clinical fractures with fracture probabilities that exceeded 17% [9]. More recently, similar findings were reported from a pre-planned analysis of denosumab [10]. The contrasting effects of teriparatide and bazedoxifene are shown in figure 4.
Figure 4.
Hazard ratio (HR) between treatments (bazedoxifene versus placebo) for morphometric vertebral fractures according to baseline 10-year probability of a major osteoporotic fracture calculated with inclusion of BMD (left 2 panels). Baseline probability was set at the 10th and 90th percentile of fracture probability. The two right hand panels show the equivalent hazard ratios for teriparatide.
A possible explanation for the lack of a relationship between the anti-fracture efficacy of teriparatide and the probability of fractures might relate to the relative absence of low risk subjects within this population, with the participants having relatively high pre hoc fracture probabilities; only 17% (without BMD), and 15% (with BMD) had a FRAX probability <10%. It is possible, therefore that any attenuation of efficacy with low fracture probabilities might not have been observed. The mean 10-year probability of a major fracture computed with BMD in the FRAX model was 19% in the present study and 21% in the study of raloxifene [11] (in which no probability-efficacy interaction was observed) but only 10.9% in patients studied with bazedoxifene [9] (in which an interaction was found), observations which would be consistent with this hypothesis. However, in women participating in the clodronate study (in which a fracture probability-treatment efficacy interaction was identified) the mean 10-year probability was 18%, a value much closer to that of the present study (but with a wider range of probabilities). It will be important to assess further phase 3 studies in order to shed light on these disparate findings. Another possibility is that risk factors competed in their interaction with efficacy. For example, if advancing age were associated with lower efficacy whereas low BMD were associated with increased efficacy, these effects would mask any overall interaction between effectiveness and fracture probability. Indeed, in this study efficacy tended to be greater in younger individuals who, by virtue of age, have lower fracture probabilities. A similar finding has been reported with the use of teriparatide given weekly [20].
There are a number of limitations to this study, which should be considered in the interpretation of these findings: Firstly, the outcome measures were confined to morphometric vertebral and non-vertebral fractures, together with low energy non-vertebral fractures. Other outcomes of possible interest, such as hip fracture alone, clinical vertebral fractures, and all clinical fractures were not able to be assessed due to low numbers (hip fracture) or lack of documentation (clinical vertebral fractures). However, although it is uncertain whether the same relationship between efficacy and fracture probability would be observed with the inclusion of other fracture outcomes, the similar lack of interaction for both non-vertebral and morphometric vertebral fracture would suggest that differences are unlikely. Secondly, data were not available for parental history of hip fracture and prior non-vertebral fracture. Although the omission of these data may have led to modest underestimation of baseline fracture probability across the whole study, there is no reason to believe that these attributes would be more frequently present in treatment or placebo groups following randomisation. Finally, the probability of a major osteoporotic fracture rather than hip fracture was employed as the index of fracture risk, since the former more closely related to the outcome variable. However a sensitivity analysis using hip fracture probability gave almost identical results, thus validating this approach. Furthermore, the findings persisted when BMD was omitted from the FRAX calculation.
In conclusion, this post hoc analysis of pivotal trial data, whilst demonstrating benefits for reduction of non-vertebral, low energy non-vertebral and morphometric-vertebral fractures consistent with the original study, found no evidence of an interaction between treatment efficacy and baseline 10-year fracture probability derived from FRAX. These data suggest, therefore, that the efficacy of teriparatide for reduction of morphometric vertebral fractures and non-vertebral fractures, including those low-energy fractures, is comparable over a broad range of FRAX probabilities, and that stratification of patients by fracture probability is unlikely to provide additional benefits over current approaches to allocation of teriparatide treatment.
Supplementary Material
Acknowledgements
This manuscript is based on a report commissioned and funded by Eli Lilly. The funders did not contribute to analysis. The authors were granted full access to all data necessary for this work.
Footnotes
Disclosures
NH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma. JA Kanis has received consulting fees, advisory board fees, lecture fees, and/or grant support from the majority of companies concerned with skeletal metabolism. EVM has received consultancy, lecture fees, research grant support and/or honoraria from ActiveSignal, Alliance for Better Bone Health, AMGEN, Bayer, Consilient Healthcare, GE Lunar, Hologic, Internis Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Servier, Tethys, UCB and Univadis. RTB and BHM are employees and shareholders of Eli Lilly and Company.
References
- 1.Hingorani AD, Windt DA, Riley RD, et al. PROGRESS Group Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013 Feb 5;346:e5793. doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 4.Prince R, Sipos A, Hossain A, et al. Sustained non vertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20:1507–1513. doi: 10.1359/JBMR.050501. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, on behalf of the World Health Organization Scientific Group . Assessment of osteoporosis at the primary health-care level Technical Report. WHO Collaborating Centre, University of Sheffield; UK: 2008. [Google Scholar]
- 6.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCloskey EV, Beneton M, Charlesworth D, et al. Clodronate reduces the incidence of fractures in community dwelling elderly women unselected for osteoporosis: results of a double-blind, placebo-controlled randomized study. J Bone Miner Res. 2007;22:135–141. doi: 10.1359/jbmr.061008. [DOI] [PubMed] [Google Scholar]
- 8.McCloskey EV, Johansson H, Oden A, et al. Ten-year fracture probability identifies women who will benefit from clodronate therapy – additional results from a double blind, placebo controlled randomised study. Osteoporos Int. 2009;20:811–818. doi: 10.1007/s00198-008-0786-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johansson H, Odén A, McCloskey EV. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX®. Bone. 2009;44:49–54. doi: 10.1016/j.bone.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 10.McCloskey EV, Johansson H, Odén A, et al. Denosumab reduces the risk of all osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX®. J Bone Miner Res. 2012;27(7):1480–6. doi: 10.1002/jbmr.1606. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johansson H, Odén A, et al. A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone. 2010;47:729–735. doi: 10.1016/j.bone.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Jönsson B, Odén A, McCloskey EV. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX®. Osteoporos Int. 2011;22:2347-2355 with erratum. Osteoporos Int. 2011;22:2347–2355. doi: 10.1007/s00198-010-1474-0. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 14.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–96. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 15.Johansson H, Odén A, McCloskey EV, Kanis JA. Mild morphometric vertebral fractures predict vertebral fractures but not non-vertebral fractures. Osteoporos Int. 2014;25:235–41. doi: 10.1007/s00198-013-2460-0. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 17.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 18.Breslow NE, Day NE. Statistical Methods in Cancer Research Volume II, IARC Scientific Publications No 32. Lyon. 1987:131–135. [PubMed] [Google Scholar]
- 19.Kim K, Svedbom A, Luo X, Sutradhar S, Kanis JA. Comparative cost-effectiveness of bazedoxifene and raloxifene in the treatment of postmenopausal osteoporosis in Europe using the FRAX algorithm. Osteoporos Int. 2014;25:325–37. doi: 10.1007/s00198-013-2521-4. [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, Shiraki M, Sugimoto T, et al. Once-weekly teriparatide reduces the risk of vertebral fracture in patients with various fracture risks: subgroup analysis of the Teriparatide Once-Weekly Efficacy Research (TOWER) trial. J Bone Miner Metab. 2014;32(4):441–6. doi: 10.1007/s00774-013-0505-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.