Abstract
OBJECTIVES
Potassium-based depolarizing St Thomas' Hospital cardioplegic solution No 2 administered as intermittent, oxygenated blood is considered as a gold standard for myocardial protection during cardiac surgery. However, the alternative concept of polarizing arrest may have beneficial protective effects. We hypothesize that polarized arrest with esmolol/adenosine/magnesium (St Thomas' Hospital Polarizing cardioplegic solution) in cold, intermittent oxygenated blood offers comparable myocardial protection in a clinically relevant animal model.
METHODS
Twenty anaesthetized young pigs, 42 ± 2 (standard deviation) kg on standardized tepid cardiopulmonary bypass (CPB) were randomized (10 per group) to depolarizing or polarizing cardiac arrest for 60 min with cardioplegia administered in the aortic root every 20 min as freshly mixed cold, intermittent, oxygenated blood. Global and local baseline and postoperative cardiac function 60, 120 and 180 min after myocardial reperfusion was evaluated with pressure–conductance catheter and strain by Tissue Doppler Imaging. Regional tissue blood flow, cleaved caspase-3 activity, GRK2 phosphorylation and mitochondrial function and ultrastructure were evaluated in myocardial tissue samples.
RESULTS
Left ventricular function and general haemodynamics did not differ between groups before CPB. Cardiac asystole was obtained and maintained during aortic cross-clamping. Compared with baseline, heart rate was increased and left ventricular end-systolic and end-diastolic pressures decreased in both groups after weaning. Cardiac index, systolic pressure and radial peak systolic strain did not differ between groups. Contractility, evaluated as dP/dtmax, gradually increased from 120 to 180 min after declamping in animals with polarizing cardioplegia and was significantly higher, 1871 ± 160 (standard error) mmHg/s, compared with standard potassium-based cardioplegic arrest, 1351 ± 70 mmHg/s, after 180 min of reperfusion (P = 0.008). Radial peak ejection strain rate increased and the load-independent variable preload recruitable stroke work was increased with polarizing cardioplegia after 180 min, 64 ± 3 vs 54 ± 2 mmHg (P = 0.018), indicating better preserved left ventricular contractility with polarizing cardioplegia. Phosphorylation of GRK2 in myocardial tissue did not differ between groups. Fractional cytoplasmic volume in myocytes was reduced in hearts arrested with polarizing cardioplegia, indicating reduction of cytoplasmic oedema.
CONCLUSIONS
Polarizing oxygenated blood cardioplegia with esmolol/adenosine/magnesium offers comparable myocardial protection and improves contractility compared with the standard potassium-based depolarizing blood cardioplegia.
Keywords: Cardioplegia, Myocardial protection, Cardiac function, Animal model
INTRODUCTION
Over the past two decades, surgical treatment for cardiac disease has occurred in patients that are more elderly and have more complex disease. As a consequence, there is an increasing demand for new perfusion strategies associated with prolonged duration of cardiopulmonary bypass (CPB) and cardiac arrest that will provide improved myocardial protection. Potassium-based depolarizing cardioplegia, delivered as oxygenated cold blood, is a dominant routine used worldwide [1]. A number of different techniques for optimizing myocardial protection have been studied, both experimentally and clinically. Polarizing cardioplegic protocols have also been extensively studied [2]; the concept of polarized arrest has shown that enhanced protection can be achieved [3]. A new polarizing cardioplegic solution, the St Thomas' Hospital Polarizing cardioplegic solution (STH-POL), containing a mixture of the short-acting β-adrenergic receptor blocker esmolol, adenosine and Mg2+, has recently been suggested. The solution combines the inhibition of fast Na+- and L-type Ca2+-channels of esmolol, the KATP-channel opener effect of adenosine and the reduced myocardial Ca2+ load caused by magnesium [3, 4]. Before implementation of a new technique for myocardial protection in clinical studies and practice, it must be compared and found to be non-inferior or superior to the current technique in a translational large animal in vivo model.
In a standardized clinically relevant porcine model involving CPB, we have investigated whether cardioplegic arrest with STH-POL offers myocardial protection comparable with a standard depolarizing potassium-based cardioplegic solution; both delivered as repeated, cold, oxygenated blood cardioplegia. The main focus for the evaluation was to compare the left ventricular global and local function up to 3 h after aortic declamping, weaning and decannulation.
MATERIALS AND METHODS
Animals and anaesthesia
The experimental protocol was approved by the Norwegian State Commission for Laboratory Animals (Project 20135835), and conducted in accordance with the European Communities Council Directive of 2010 (63/EU). The animals were brought to the animal facility about 1 week in advance for acclimatization. Twenty-four young pigs (Norwegian land race) of either gender, weighing 42 ± 2 [standard deviation (SD)] kg were used. After premedication with intramuscular injection of a mixture of ketamine (20 mg/kg), diazepam (10 mg) and atropine (1 mg), the pigs were ventilated spontaneously on mask for a short period with oxygen and 3% isoflurane (Rhodia, Bristol, UK) allowing intravenous access through two ear veins. Before tracheotomy and intubation, loading doses of intravenous fentanyl (0.02 mg/kg), midazolam (0.3 mg/kg), pancuronium (0.063 mg/kg) and pentobarbital (15 mg/kg) were given, and continuous infusions of fentanyl (0.02 mg/kg per h), midazolam (0.3 mg/kg per h), pancuronium (0.2 mg/kg per h) and pentobarbital (4 mg/kg per h) were initialized. The animals were ventilated (Julian, Drägerwerk, Lübeck, Germany) with a mixture of nitrous oxide (56–58%) and oxygen. Prophylactic antibiotic therapy with cefalotin 1 g (in 50 ml 0.9% NaCl) was given IV initially, followed by 0.5 g during CPB and 1.0 g after weaning. Fluid substitution, Ringer’s acetate 15 ml/kg per h with 20 mmol/l KCl added, was given throughout the experiment. In addition, Ringer’s acetate 5 ml/kg per h was provided after weaning from CPB. The anaesthetic protocol has been thoroughly evaluated, allowing the use of neuromuscular blocking agents in young pigs [5].
Surgical protocol and instrumentation
The right femoral artery and vein were cannulated for blood sampling and infusion by surgical cut-down in the groin. An early arterial blood gas analysis determined the need for ventilator adjustments. An open suprapubic cystotomy with insertion of a catheter drained the urine bladder. Rectal temperature was monitored. Midline sternotomy and pericardiotomy was performed and heparin (125 IU/kg) given IV to prevent clotting of catheters. A continuous cardiac output catheter (CCO/EDV 177HF 75, Edwards Lifesciences, Inc., Irvine, CA, USA) was advanced from the left internal mammary vein into the pulmonary artery for monitoring cardiac output, right ventricular end-diastolic volume, central venous pressure (CVP) and pulmonary artery pressure (PAP) (Vigilance II® and TruWave® transducers, Edwards Lifescience, Inc.). The aorta was cannulated proximally with a Millar microtip pressure catheter (Millar MPC-500, Houston, TX, USA) through the left internal mammary artery. The haemodynamic parameters were sampled in real time by a 16-channel Ponemah (ACQ-7700; Data Sciences International, St Paul, MN, USA), digitized and later analysed (Ponemah Physiology Platform v. 5.2, Data Sciences International). A dual-field conductance-pressure catheter (CA71083-PL, CDLeycom, Hengelo, Netherlands) was inserted through the apex and into the left ventricle with the distal part in position just above the aortic valve, verified by epicardial echocardiography (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). The catheter was connected to a Sigma-M signal conditioner (CDLeycom). The left atrium was cannulated by an infant feeding tube for microsphere injections and a tape placed around the inferior vena cava for brief intermittent dynamic preload reductions.
Cardiopulmonary bypass
CPB (Stöckert SIII, Munich, Germany) was set up with 1200 ml Ringer’s acetate as prime in the circuit. After anticoagulation with heparin 500 IU/kg, the brachiocephalic artery (EOPA 18 Fr, Medtronic, Inc., Minneapolis, MN, USA) and right atrial appendage (MC2 28/36 Fr, Medtronic Inc.) were cannulated, and CPB was established with a flow of 90 ml/min per kg and water temperature of 32°C in the heat exchanger. Aortic cross-clamp time was 60 min and a left heart vent catheter (E061 17 Fr, Edwards Lifesciences, Inc.) was placed through the left atrial appendage into the ventricle. The body temperature was allowed to drift towards 34°C and the CPB flow was reduced to 72 ml/min per kg when the rectal temperature reached 35°C or after 20 min. Rewarming was commenced after 40 min of cross-clamping with reset of CPB flow to 90 ml/min per kg and water temperature at 40°C. Arterial blood gases were obtained before cross-clamping, after 30 min and just before declamping after 60 min. If ventricular fibrillation occurred after declamping, defibrillation was the only allowed antiarrhythmic intervention. All animals were weaned from CPB at 10 min after declamping followed by decannulation. The residual blood in the circuit was returned and protamine sulphate 1 mg/kg was given for heparin reversal.
Cardioplegia
The animals were block-randomized to receive either hyperkalaemic cardioplegia based on a modification of St Thomas' Hospital cardioplegic solution No. 2 (STH-2), or cardioplegia containing the short-acting β-adrenergic blocking agent esmolol, adenosine and magnesium; St Thomas' Hospital polarizing solution (STH-POL) (Table 1). Both solutions were pre-prepared as concentrate and administered as cold (12°C), oxygenated, blood cardioplegia, freshly mixed and delivered by a dual-pump with separate cooling. The cardioplegia was delivered into the aortic root with a flow set to 7% of CPB flow, following a standardized protocol with an initial ‘high-dose’ (1:4 concentrate/blood) for 3 min and 2 min of ‘low-dose’ (1:8 concentrate/blood) given at 20 and 40 min after cross-clamping. The final concentrations of key components in the two cardioplegic solutions are presented in Table 1.
Table 1:
Final molar concentrations in oxygenated blood cardioplegia
| STH-POL |
STH-2 |
|||
|---|---|---|---|---|
| High-dose (3 min) | Low-dose (2 min) | High-dose (3 min) | Low-dose (2 min) | |
| Esmolol (mM) | 1.35 | 0.68 | – | – |
| Adenosine (mM) | 0.50 | 0.25 | – | – |
| Mg2+ (mM) | 20 | 10 | 16 | 9 |
| K+ (mM) | 4.3 | 4.3 | 22 | 14 |
| Cl− (mM) | 106 | 106 | 134 | 120 |
| Procaine HCl (mM) | 0.8 | 0.4 | 0.8 | 0.4 |
STH-POL: St Thomas' Hospital Polarizing cardioplegic solution; STH-2: Modified St Thomas' Hospital cardioplegic solution No. 2.
Experimental protocol
After 10 min of stabilization, baseline variables were obtained; arterial blood gases (ABL80 FLEX COOX, Radiometer Medical ApS, Brønshøj, Denmark), s-troponin-T (Troponin-T hs®, Roche Diagnostics, GmbH, Mannheim, Germany), haemodynamic variables and the first injection of 15-µm microspheres (Dye-Trak ‘F’; Triton Technology, Inc., San Diego, CA, USA). After a short period of ventilation with 100% oxygen, left ventricular pressure–volume loops were registered with respirator shut-off in end-expirium before and during a brief period of inferior vena cava occlusion. A bolus of 5 ml hypertonic saline (10%) was injected into the pulmonary artery and pressure volume loops were recorded in a stable situation three times for estimation of parallel conductance [6, 7]. Epicardial echocardiography was performed with a soft silicone pad placed between the probe (6 MHz sector probe, GE Vingmed Ultrasound) and the epicardium. During brief periods of respiratory shut-off, pulsed wave Doppler (PWD) velocity spectrum recordings in the left aortic outflow tract were noted for timing of end-systole and end-diastole. Tissue Doppler images (TDIs) of the anterior left ventricular wall in short axis view were recorded for radial strain and strain rate measurements.
At full CPB flow, an arterial blood gas was sampled followed by aortic cross-clamping and cardioplegic arrest with STH-POL or STH-2 given as cold blood cardioplegia. During CPB, the ventilator volume was reduced to 50% and with passive drainage of the heart. Additional heparin (250 IU/kg) was given at 30 min of cross-clamping. After 60 min of cardioplegic arrest, the aortic clamp was removed. The animals were weaned from CPB after 10 min of myocardial reperfusion. General haemodynamics were continuously recorded for 180 min. As for baseline, measurements of left ventricular pressures, volumes, contractility, systolic and diastolic function, regional tissue blood flow and echocardiographic recordings were repeated at 60, 120 and 180 min after aortic declamping. Finally, the animal was euthanized with intracardiac injection of high-dose potassium chloride, the heart removed and samples were obtained for regional tissue blood flow measurements, analysis of mitochondrial ultrastructure and mitochondrial fatty acid oxidation capacity, phosphorylated GRK2, Caspase-3 activity and tissue water content.
Pressure–conductance catheter
Data from the pressure-conductance catheter were analysed with a custom-made programme. The mean of 5–8 cardiac cycles during the stable condition and load-independent variables obtained during the dynamic preload reduction were calculated. Absolute volumes were estimated by correcting for parallel conductance and cardiac output. Volumes were indexed by body surface area (BSA) calculated as BSA (m2) = (BW2/3 × k)/100 where BW is body weight in kg and k for pigs is 9 m2/kg−2/3 [8].
Epicardial echocardiography
Local myocardial function (TDI strain and strain rate) in the anterior wall was recorded. Peak systolic strain and peak ejection strain rate were measured with commercial software (EchoPack BT12, GE Vingmed Ultrasound). The size of the region of interest (ROI) was set to 6 × 6 mm and the strain length (SL) to 6 mm. Care was taken not to place the ROIs within a distance of ½SL from the epicardium and ½SL from the endocardium [9]. End-diastole was defined as the first deflection in the QRS complex in the ECG, and start of ejection was defined at the aortic valve opening and end-systole was defined as the time of aortic valve closure from the PWD recording. The mean frame rate for the TDI recordings was 544 ± 78 (SD) frames/s (range 249–696).
Myocardial tissue samples and analyses
Multiple biopsies were obtained from the LV endo-, mid- and epicardium, snap-frozen in liquid nitrogen and stored at −80°C for later analysis. Caspase-3 activity was determined using the Caspase-3 Colorimetric Assay Kit (BioVision, Inc., Milpitas, CA, USA). Tissue was homogenized and lysis performed according to the manufacturer's instructions, and triplet samples containing 400 µg total protein, measured by the Quick Start™ Bradford Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA), were incubated for 2 h before fluorometric readings. The activation of G-protein-coupled receptor kinase 2 (GRK2) in the myocardium was assessed with immunoblotting using antibodies against phosphorylated GRK2 (LS-C199027, LifeSpan BioSciences Inc., Seattle, WA, USA) and total GRK2 (CST3982, Cell Signaling, Boston, MA, USA) according to manufacturer's instructions. The blots were quantified using scanning densitometry. Mitochondrial carnitine palmitoyltransferase I and II (CPT-I, CPT-II) activity in the outer and inner mitochondrial membranes were measured in frozen myocardial tissue samples. Each sample was homogenized after adding 500 µl of ice-cold sucrose (0.25 M sucrose, 5 mM HEPES-buffer, 0.2 mM ethylene glycol tetraacetic acid, pH 7.4) and centrifuged at 4°C, 600 g for 12 min. The protein concentration in the supernatant (post-nuclear fraction) was measured (Bio-Rad Protein Assay, Bio-Rad Laboratories, Inc.). In 150 µg of protein, CPT-I and CPT-II were measured as earlier described [10]. In addition, corresponding biopsies were obtained and fixed overnight in 0.1 M Na-cacodylate buffer with 2.5% glutaraldehyde, washed, post-fixated with 1% OsO4, dehydrated and stored in 70% ethanol, sectioned and stained with uranyl acetate for the analysis of myocardial ultrastructure with electron microscopy. From six randomly selected sections, images (×15 000) were obtained from the mid-myocardium, myocyte volume fractions of mitochondria, myofibrils and sarcoplasma were calculated by point counting. The mitochondrial surface density and surface-to-volume ratio were calculated by a Merz grid using the ImageJ freeware [11, 12]. Tissues obtained from the left and the right ventricle were weighed, hydrolysed, microspheres isolated by filtration, colours dissolved and quantified by fluorospectrophotometry together with the reference blood samples. Blood flow rate was calculated as earlier described [13]. Myocardial tissue water content was calculated as a fraction of wet weight after drying samples for 3 weeks at 60°C.
Statistical analysis
Data were analysed by using SPSS v. 22 (SPSS, Inc., Chicago, IL, USA) and values given as mean ± (SE) or median (25% quartile; 75% quartile) unless otherwise noted. Baseline variables were compared by two-sample Student's t-test on data with normal distribution and with Wilcoxon–Mann–Whitney U-test on ranks if the Kolmogorov–Smirnov test or the Levene equal variance tests were significant. Variables obtained during and after CPB were analysed separately by two-way analysis of variance (ANOVA) for repeated measurement (RM-ANOVA) with time as within factor (Pw) and cardioplegia with STH-POL or STH-2 as grouping factor (Pg) and post hoc Bonferroni contrasts between individual group means. If Mauchly's test of sphericity was significant (P < 0.05), the Greenhouse–Geisser adjustment of degrees of freedom was selected for the evaluation of main effects. For Caspase-3 activity and estimation of GRK2, wall layers were set as related variables. If a significant interaction (Pi < 0.10) effect was found, new ANOVAs for simple main effect were performed with adjustment of degrees of freedom. Cell means were finally compared with Neumann–Keuls multiple contrast tests when justified by the preceding ANOVA. The morphological variables were analysed with a nested ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Group characteristics at baseline and during cardiopulmonary bypass
Four animals were excluded due to reasons other than technical failure: two in the STH-POL group developed severe pulmonary hypertension and arterial hypoxia with heart failure during baseline measurements before allocation to treatment groups; two in the STH-2 group, one due to severe pulmonary hypertension during instrumentation before CPB and one due to persistent ventricular fibrillation after declamping. Excluded animals were replaced by consecutive experiments and the results are given for 10 animals in each group. Baseline variables describing left and right ventricular function, general haemodynamics, tissue blood flow rate, arterial blood gases and serum troponin-T did not differ between groups (Table 2, Figs 1–3).
Table 2:
Baseline variables before cardiac arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia
| Variable | STH-POL | STH-2 | Statistics |
|---|---|---|---|
| LV-EDVi (ml/m2) | 83 ± 5 | 78 ± 4 | P = 0.50 |
| LV-ESVi (ml/m2) | 41 ± 4 | 35 ± 4 | P = 0.28 |
| LV-SVi (ml/m2) | 50 ± 3 | 51 ± 2 | P = 0.72 |
| RV-EDVi (ml/m2) | 136 ± 4 | 148 ± 7 | P = 0.16 |
| RV-EF (%) | 24 ± 1 | 26 ± 1 | P = 0.28 |
| MAP (mmHg) | 95 ± 4 | 96 ± 3 | P = 0.77 |
| CVPmean (mmHg) | 5.8 ± 0.5 | 6.2 ± 0.5 | P = 0.58 |
| PAPmean (mmHg) | 18.8 (15.5; 21.8) | 19.6 (18.3; 20.4) | P = 0.47 |
| LV blood flow (ml/min per g) | 0.92 ± 0.05 | 1.04 ± 0.06 | P = 0.16 |
| RV blood flow (ml/min per g) | 0.68 ± 0.07 | 0.83 ± 0.05 | P = 0.14 |
| Arterial blood gases | |||
| pH | 7.51 (7.49; 7.52) | 7.52 (7.51; 7.53) | P = 0.25 |
| pCO2 (kPa) | 5.2 ± 0.1 | 5.02 ± 0.1 | P = 0.24 |
| HCO3− (mmol/l) | 31.2 ± 0.2 | 31.2 ± 0.4 | P = 0.95 |
| BE (mmol/l) | 7.3 ± 0.2 | 7.2 ± 0.4 | P = 0.89 |
| pO2 (kPa) | 25.8 ± 0.8 | 25.6 ± 0.5 | P = 0.88 |
| Hb (g/dl) | 8.2 ± 0.2 | 8.1 ± 0.2 | P = 0.64 |
| Hct (%) | 25.6 ± 0.5 | 25.2 ± 0.6 | P = 0.64 |
| s-Na+ (mmol/l) | 142 (140; 143) | 142 (141; 143) | P = 0.94 |
| s-K+ (mmol/l) | 3.7 ± 0.1 | 3.7 ± 0.1 | P = 0.59 |
| s-Cl− (mmol/l) | 103.1 ± 0.9 | 103.4 ± 0.6 | P = 0.78 |
| s-Troponin-T (µmol/l) | 47.6 ± 4.7 | 53.4 ± 6.5 | P = 0.48 |
Values are mean ± SE or median (25-percentile; 75-percentile), n = 10.
LV and RV: left and right ventricle; i: value indexed for body surface area; EDV: end-diastolic volume; ESV: end-systolic volume; SV: stroke volume; MAP: mean arterial pressure; CVPmean: mean central venous pressure; PAPmean: mean pulmonary artery pressure; STH-POL: St Thomas' Hospital Polarizing cardioplegic solution; STH-2: St Thomas' Hospital cardioplegic solution No. 2; SE: standard error.
P-values from two-sample t-tests or Mann–Whitney rank sum tests.
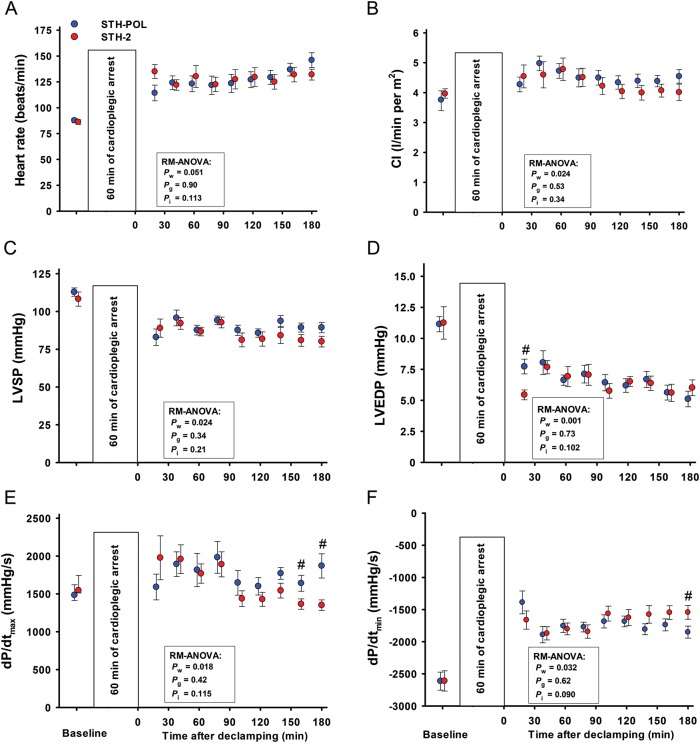
Figure 1:
Baseline left ventricular haemodynamic variables mean (SE) or median (25%; 75%), and values obtained after CPB and 60 min of cardiac arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia. Bars represent SE. Pw, Pg and Pi: P-values for within subjects, between groups and interaction from two-way RM-ANOVA, respectively; #Significantly different from STH-POL at 180 min of reperfusion with Bonferroni post hoc test. SE: standard error of the mean; CPB: cardiopulmonary bypass; RM-ANOVA: analysis of variance for repeated measurement; CI: Cardiac Index; LVSP: left ventricular peak systolic pressure; LVEDP: left ventricular peak end-diastolic pressures; dP/dtmax and dP/dtmin: peak positive and peak negative of the first derivative of left ventricular pressure.
Figure 3:
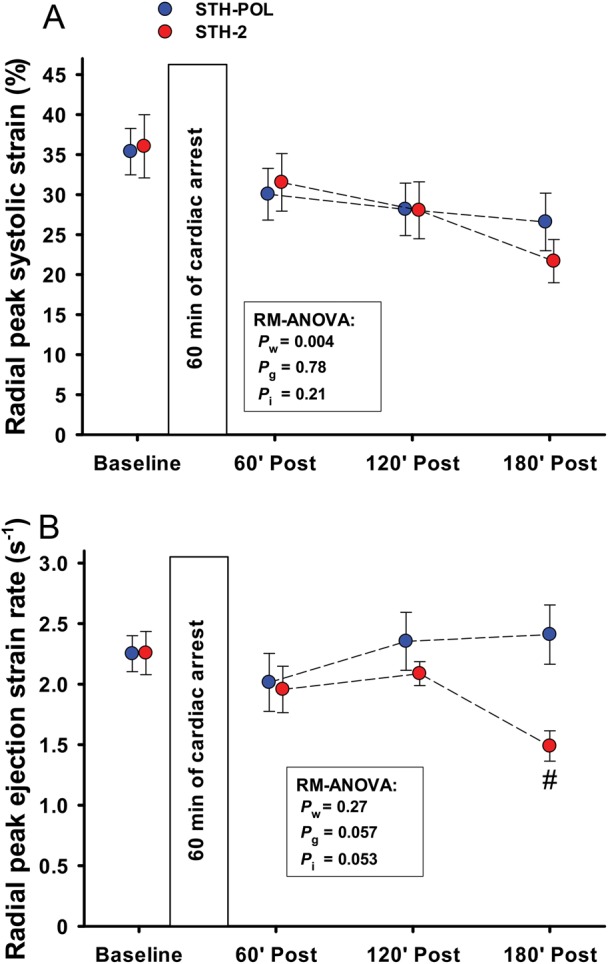
(A) Radial peak systolic TDI strain and (B) peak ejection strain rate at baseline and 60, 120 and 180 min after CPB and aortic declamping in the left ventricular anterior wall following cardiac arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia. Mean values, bars are SE. #Significantly different from STH-POL at 180 min of reperfusion. Statistics as in Fig. 1. SE: standard error; RM-ANOVA: analysis of variance for repeated measurements.
The myocardial contraction rapidly ceased after aortic cross-clamping and start of the cardioplegic infusion, with the median time being 28 (11; 43) s for STH-POL and 22 (18; 27) s for STH-2 group (P = 0.79). During CPB, haemoglobin levels and serum-potassium were moderately, but significantly increased in the STH-2 group compared with the STH-POL group at 30 and 60 min (see Supplementary Material). There were no differences between the groups regarding mean arterial pressure, temperature and arterial blood gases during CPB.
Cardiac and haemodynamic variables after declamping
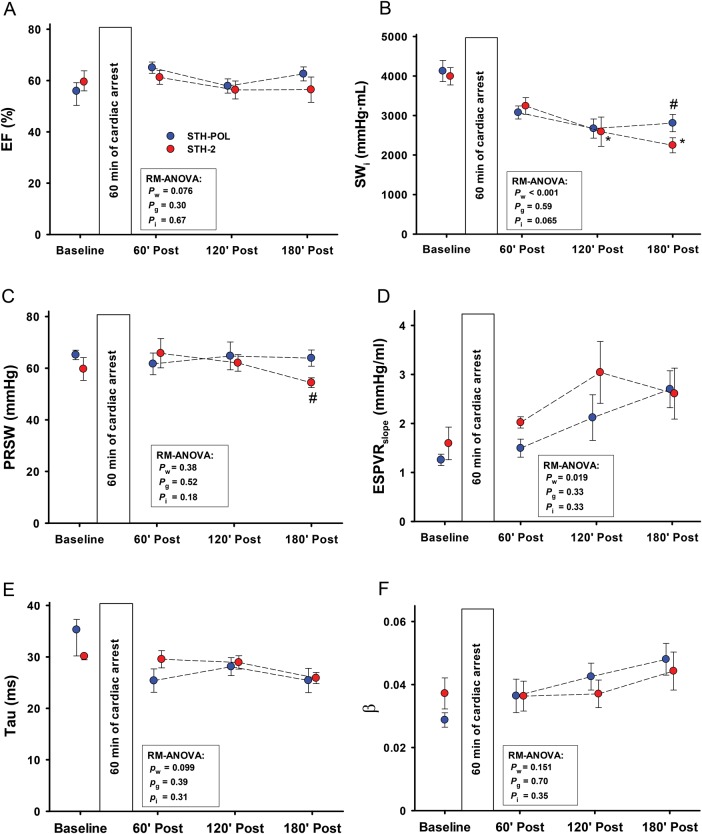
Heart rate was increased and left ventricular systolic (LVSP) and diastolic (LVEDP) pressures decreased with time in both groups after weaning from CPB (Fig. 1). Cardiac index (CI) increased during the first hour after declamping in both groups, but then decreased slightly but staying above baseline levels during the remaining 120 min of reperfusion (Pw = 0.024). There were no significant differences between the groups. In the STH-POL group, the first derivative of the left ventricular pressure (LV-dP/dtmax) increased from 120 to 180 min of reperfusion, reaching values significantly higher than the STH-2 group after 165 and 180 min of reperfusion. LV-dP/dtmin was more negative in the STH-POL group after 180 min. There was a significant difference between groups for indexed stroke work (SWi) and for the load-independent contractility variable preload recruitable stroke work (PRSW) at 180 min after declamping (Fig. 2). Ejection fraction and the slope of the end-systolic pressure–volume relationship (ESPVRslope) did not differ between groups, neither did the isovolumic relaxation constant Tau nor the left ventricular compliance β. Whereas radial peak systolic strain in the anterior left ventricular wall decreased significantly over time in both groups (Pw = 0.004), radial peak ejection strain rate decreased in the STH-2 group to values significantly lower than in the STH-POL group at 180 min (Fig. 3). There was a significant decrease over time in indexed left ventricular stroke volume (LV-SVi) and a decrease in right ventricular end-diastolic volume (RV-EDVi) for both groups (Table 3). The mean aortic blood pressure (MAP) was unchanged in the STH-POL group and decreased over time in the STH-2 group with a significant difference between groups at 180 min of reperfusion. The mean PAP was unchanged and CVP slightly increased over time in both groups (Pw = 0.003). Regional tissue blood flow rate in the left and the right ventricular wall was unchanged from 60 to 180 min of reperfusion. Left ventricular tissue water content averaged 80.1 ± 0.1% (n = 20), with no group difference. Serum troponin-T levels did not differ between groups at 180 min after declamping; median values were 504 (328; 886) µmol/l in the STH-POL group and 580 (401; 717) µmol/l in the STH-2 group (P = 0.73).
Figure 2:
Left ventricle functional variables at baseline and 60, 120 and 180 min after CPB and aortic declamping following cardiac arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia. Mean or median values, bars are SE or 25 and 75 percentiles. Statistics as in Fig. 1. #Significantly different from STH-POL at 180 min of reperfusion, *Significantly different from 60 min of reperfusion within STH-2 group. EF: ejection fraction; SWi: indexed stroke work; PRSW: slope of preload recruitable stroke work; ESPVRslope: slope of the end-systolic pressure volume relationship; Tau: isovolumic relaxation constant; β: the logarithmic end-diastolic pressure volume relationship; RM-ANOVA: analysis of variance for repeated measurements.
Table 3:
Cardiac and haemodynamic variables and tissue blood flow 60, 120 and 180 min after aortic declamping following 60 min of polarizing (STH-POL) or depolarizing (STH-2) cardioplegic arrest
| Variable | 60 min (A) | 120 min (B) | 180 min (C) | RM-ANOVA statistics |
|---|---|---|---|---|
| LV-EDVi (ml/m2) | ||||
| STH-POL | 60 ± 3 | 59 ± 4 | 54 ± 2 | Pw = 0.26, Pg = 0.55, Pi = 0.92 |
| STH-2 | 64 ± 4 | 60 ± 6 | 58 ± 4 | |
| LV-ESVi (ml/m2) | ||||
| STH-POL | 23 ± 2 | 28 ± 4 | 22 ± 2 | Pw = 0.54, Pg = 0.36, Pi = 0.53 |
| STH-2 | 27 ± 3 | 29 ± 4 | 30 ± 7 | |
| LV-SVi (ml/m2) | ||||
| STH-POL | 42 ± 2 | 37 ± 2 | 37 ± 1 | Pw < 0.001, Pg = 0.22, Pi = 0.51 |
| STH-2 | 42 ± 3 | 37 ± 4 | 34 ± 3 | |
| RV-EDVi (ml/m2) | ||||
| STH-POL | 134 ± 5 | 128 ± 4 | 123 ± 3 | Pw = 0.006, Pg = 0.55, Pi = 0.73 |
| STH-2 | 142 ± 6 | 132 ± 4 | 130 ± 5 | |
| RV-EF (%) | ||||
| STH-POL | 30 ± 2 | 28 ± 1 | 31 ± 2 | Pw = 0.162, Pg = 0.32, Pi = 0.35 |
| STH-2 | 30 ± 2 | 26 ± 1 | 27 ± 2 | |
| MAP (mmHg) | ||||
| STH-POL | 72 ± 4 | 71 ± 4 | 74 ± 4 | Pw = 0.127, Pg = 0.31, Pi = 0.046 |
| STH-2 | 73 ± 3 | 67 ± 4 | 63 ± 3a,# | |
| CVP (mmHg) | ||||
| STH-POL | 8.0 ± 0.7 | 8.1 ± 0.8 | 9.3 ± 0.9 | Pw = 0.003, Pg = 0.57, Pi = 0.85 |
| STH-2 | 8.4 ± 0.7 | 8.9 ± 0.8 | 10.1 ± 1.1 | |
| PAP (mmHg) | ||||
| STH-POL | 24 ± 1 | 24 ± 2 | 25 ± 1 | Pw = 0.30, Pg = 0.12, Pi = 0.63 |
| STH-2 | 26 ± 2 | 28 ± 2 | 28 ± 2 | |
| LV blood flow (ml/min per g) | ||||
| STH-POL | 1.39 ± 0.15 | 1.24 ± 0.10 | 1.36 ± 0.10 | Pw = 0.16, Pg = 0.46, Pi = 0.69 |
| STH-2 | 1.32 ± 0.08 | 1.19 ± 0.06 | 1.21 ± 0.09 | |
| RV blood flow (ml/min per g) | ||||
| STH-POL | 1.53 ± 0.21 | 1.27 ± 0.10 | 1.30 ± 0.09 | Pw = 0.073, Pg = 0.58, Pi = 0.92 |
| STH-2 | 1.60 ± 0.17 | 1.34 ± 0.08 | 1.42 ± 0.17 | |
Values are mean ± SE for 10 animals in each group.
LV and RV: left and right ventricle; i: value indexed for body surface area; EDV: end-diastolic volume; ESV: end-systolic volume; SV: stroke volume; MAP: mean arterial pressure; CVP: mean central venous pressure; PAP: mean pulmonary artery pressure; STH-POL: St Thomas' Hospital polarizing cardioplegic solution; STH-2: St Thomas' Hospital cardioplegic solution No. 2; RM-ANOVA: analysis of variance for repeated measurement.
Pw, Pg and Pi: P-values for within subjects, between groups and interaction from two-way RM-ANOVA, respectively.
aSignificant difference within group means from value(s) in columns with corresponding capital letters.
#Significantly different from STH-POL at 180 min of reperfusion.
Myocardial apoptosis and β-receptor activity
There was no difference between groups in myocardial apoptotic activation evaluated as cleaved caspase-3 activity (Pg = 0.84) (Fig. 4). In the subepicardium, cleaved caspase-3 activity was increased in both groups compared with that in the mid-myocardial and subendocardial wall layers (Player = 0.024). Phosphorylation of GRK2 (relative to tissue from control heart) in the mid-myocardial wall layers was increased in both groups compared with levels in the subendo- and subepicardial wall layers (Player = 0.001). The total GRK2 content did not differ between wall layers or between groups.
Figure 4:
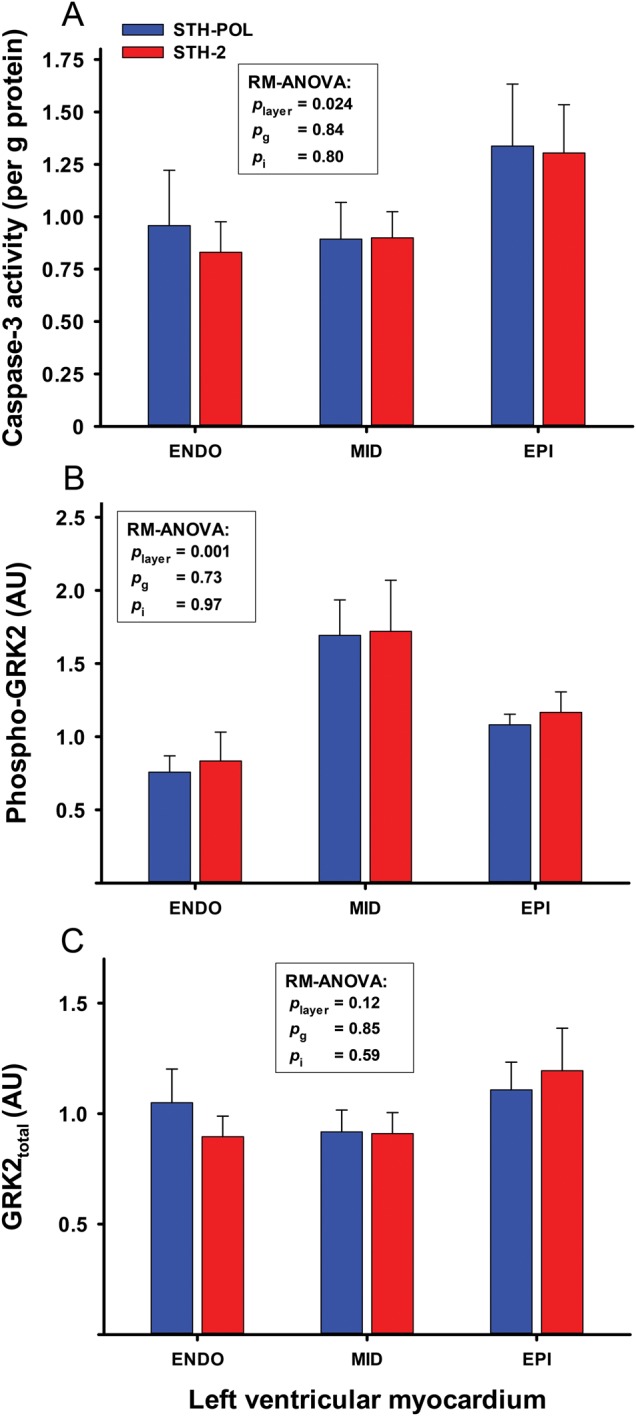
(A) Caspase-3 activity (n = 10 + 9), (B) phosphorylated (Phospho-GRK2) and (C) total (GRK2total) G protein-coupled receptor kinase-2) (n = 9 + 9) in myocardial tissue samples obtained 180 min after reperfusion following CPB and 60 min of cardioplegic arrest with polarizing (STH-POL) and depolarizing (STH-2) cardioplegia. Bars are mean + SE. Statistics as in Fig. 1. SE: standard error; RM-ANOVA: analysis of variance for repeated measurements.
Mitochondrial ultrastructure and metabolism
In the mid-myocardium, there was a significant increase in the volume fraction of the sarcoplasma resulting in a concomitant decrease in the volume fraction of myofibrils in the STH-2 group (Fig. 5). The volume fraction of mitochondria did not differ significantly. Furthermore, the surface density of the mitochondria did not differ between groups. The surface-to-volume ratio was slightly decreased for the STH-POL group, indicating mitochondrial swelling. The carnitine transport systems for long chain fatty acids, evaluated as CPT-I and CPT-II activity, were not affected by the selection of the cardioplegic protocol.
Figure 5:
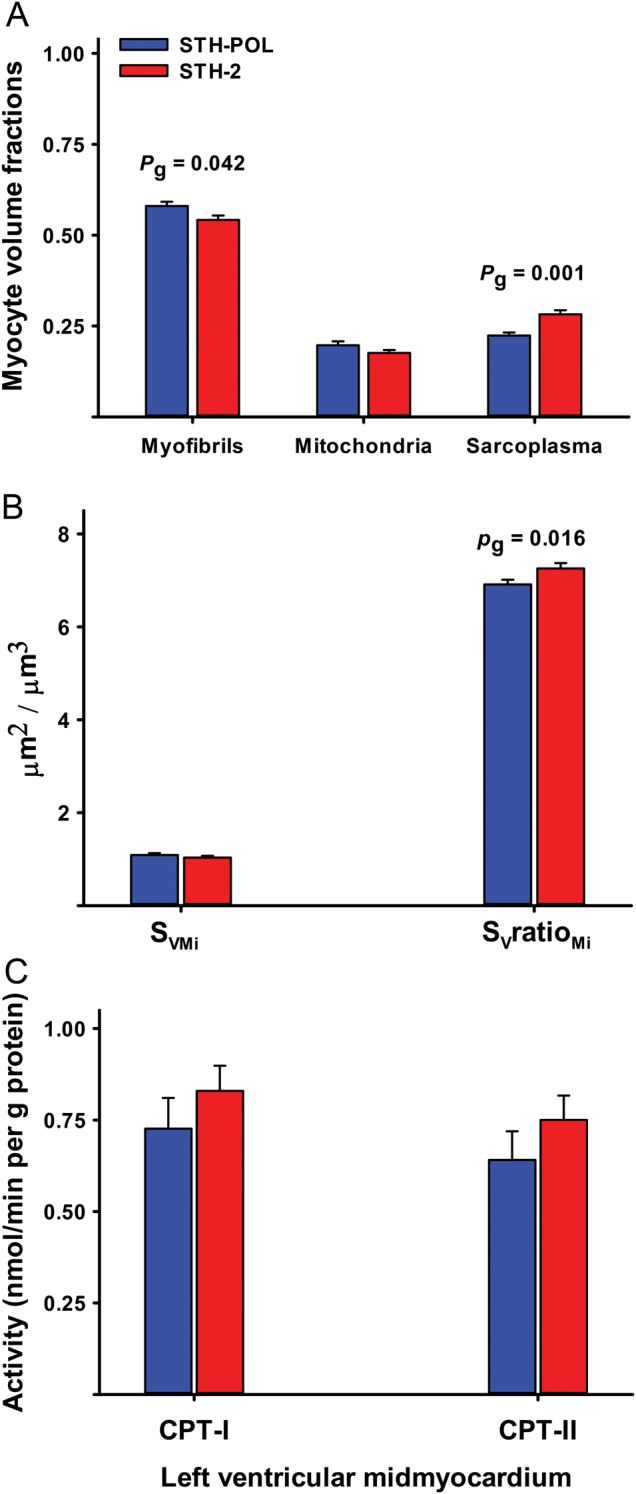
(A) Mid-myocardial myocyte volume fractions, (B) mitochondrial surface density (SVMi), surface-to-volume ratio (SVratioMi) and (C) carnitine palmitoyltransferase activity in the outer (CPT-I) and inner (CPT-II) mitochondrial membrane. Bars are mean + SE. Pg is the probability by two-sample t-tests. SE: standard error.
DISCUSSION
This study provides evidence that ‘polarised’ arrest with a solution containing a mixture of esmolol, adenosine and magnesium in oxygenated blood offers myocardial protection that is comparable with standard potassium-based (depolarised) cold, intermittent oxygenated blood cardioplegia in a clinically relevant porcine model. At 180 min after aortic declamping, left ventricular contractile function evaluated as LV-dP/dtmax, PRSW and peak ejection strain rate was better maintained in animals with polarizing compared with depolarizing cardioplegic arrest for 60 min. Furthermore, the hearts arrested with polarizing cardioplegia generate an increased systemic blood pressure (MAP) 180 min after declamping. This could be a result of reduced development of left ventricular myocardial stunning in the STH-POL group 3 h after declamping. Troponin-T release and the degree of apoptosis did not differ and contradict differences in irreversible myocardial damage between groups. The increase in variables describing contractility such as PRSW, LV-dP/dtmax and strain rate in the STH-POL group 180 min after declamping is not a result of activation of the Frank–Starling mechanism in the left ventricle. The left ventricular end-diastolic volume was unchanged and did not differ between groups (Table 3). Similarly, the load-dependent variables of left ventricular EF (Fig. 2) and radial TDI strain (Fig. 3) were not different between groups during the 180 min of observation after declamping. Group differences in LV-dP/dtmax and strain rate could be affected by the differences in heart rate. However, heart rate did not differ between groups after declamping (Fig. 1). The increased contractility did not improve cardiac function evaluated by LV-EF and Cardiac Index. The increased indexed stroke works at 180 min in the STH-POL group, potentially resulting from slight, but non-significant increases in LVSP and CI, indicate that left ventricle performance was improved following polarizing cardioplegic arrest in the present study. This increase in left ventricular workload was not reflected in a substantial increase in left ventricular tissue blood flow rate. We interpret these results due to an improvement in myocardial protective efficacy with polarised cardioplegic arrest, possibly due to a reduced ionic inhomogeneity and consequently reduced energy utilization [3].
In chronic heart failure, increased catecholamine release with impaired signalling and desensitization of β-adrenergic receptors (β-AR) contributes to myocardial dysfunction [14]. During CPB and cardioplegic arrest, serum catecholamines increase and norepinephrine is released locally from the hypothermic and anoxic myocardium [15, 16]. This can lead to β-AR desensitization and is associated with increased GRK2 activity. Hence, β-AR desensitization and increased GRK2 activity may explain several aspects of the myocardial stunning observed after cardiac surgery [17]. The addition of esmolol systemically during CPB in dog hearts subsequently arrested with potassium-based crystalloid cardioplegia demonstrated enhanced β-adrenergic receptor signalling with improved postischaemic function [18]. Similarly, the addition of esmolol before CPB in pigs arrested with cold potassium-based blood cardioplegia improved late contractile function [19]. Interestingly, the addition of esmolol to cold potassium-based blood cardioplegia was also able to enhance late myocardial recovery of function in pigs subjected to CPB and 100 min of ischaemic arrest [20]. As indicated by the tissue levels of phosphorylated GRK2 in the present study, there is no evidence for altered GRK2 activity in either group (Fig. 4). Since neither adenylate cyclase activity nor GRK2 activity was measured directly, the prevention of β-receptor desensitization could not be completely ruled out as an explanation for the improved contractile function observed in animals with polarizing cardioplegia (Figs 1–3).
The administration of repeated oxygenated blood cardioplegia during cardiac surgery implies multiple episodes of ischaemia and re-oxygenation before the final declamping and reperfusion. Beta-receptor blockade during cardioplegic arrest is of potential interest for reducing both ischaemic- and lethal reperfusion injury. However, the unwanted negative inotropic effect during reperfusion and weaning could be potentially harmful, especially if the β-blocking agent has a prolonged half-life. Esmolol is rapidly metabolized by blood esterase and has a half-life of ∼9 min [21]. Esmolol has been found beneficial as an alternative to cardioplegia by inducing ‘minimal myocardial contraction’ [22], or as a supplement to standard cardioplegic regimes [19, 20]. In the present study, there was a trend towards an early but transient reduction in heart rate as well as a significantly higher LVEDP at 20 min after declamping (Fig. 1), indicating a residual negative chronotropic and inotropic effect of esmolol early after declamping. However, by 30 min after declamping, these negative effects were abolished.
Myocardial ischaemic injury and lethal reperfusion injury are reduced by β-adrenergic blockers and the modulation of apoptotic cell death is also linked to β-receptor adrenergic signalling and GRK2 activation [23]. In the present study, neither release of troponin-T at 180 min after aortic declamping nor activation of apoptotic activity (judged by tissue cleaved caspase-3 activity) differed between treatment groups.
In research and in clinical practice, different forms of cardioplegic solutions delivered as crystalloid or as blood-based cardioplegia have been studied and are in use. Both intracellular solutions with amino acids as buffer and low levels of Na+ and Ca2+, and extracellular solutions with normal levels of Na+ and Ca2+ rely on moderately increased concentrations of potassium to obtain cardiac arrest [1]. Potassium-based depolarizing cardioplegia inactivates the fast Na+ channel in the myocyte membrane, by inducing re-equilibration of the membrane potential to a more positive level and thereby preventing the voltage-activated Na+ spike of the action potential and the triggering of contraction [3]. This, together with myocardial cooling, reduces energy consumption and oxygen demands during cardiac arrest. At this re-equilibrated membrane potential, the non-inactivating Na+ current leads to increases in intracellular Na+, with subsequent reversal of the Na+/Ca2+-exchanger; the resultant Na+ and Ca2+ overload is potentially harmful. Increased tissue oedema in hearts arrested with the hyperkalaemic STH-2 cardioplegia was found compared with the normokalaemic STH-POL cardioplegia, and this is consistent with previous findings [22]. In contrast, esmolol inhibits the fast Na+-channels and the L-type Ca2+-channels [4]; adenosine is a KATP-channel opener and magnesium is an endogenous Ca2+-channel blocker. These pharmacological effects will reduce the ionic heterogeneity that occurs during ischaemia and will concomitantly reduce high-energy phosphate utilization. Adenosine induces hyperpolarized cardiac arrest in normokalaemic cardioplegic solutions, and also in a clinical setting [24]. Magnesium is a component of STH-2 cardioplegia, exerting an anti-ischaemic protective effect. However, the efficacy of magnesium as adjuvant to cardioplegic solutions has been questioned [25]. One could therefore speculate if esmolol and adenosine alone could be an alternative polarizing cardioplegic solution.
Limitations
In addition to the obvious possibility of species differences, the present study is performed in healthy young hearts without coronary pathology where the conditions for myocardial protection are optimized. In addition, the limited cross-clamp time and the total potassium load in the present study could obscure possible advantages with polarizing cardioplegic arrest. Furthermore, an increase in both ischaemic time connected to more complex surgical procedures and prolonged observation time after declamping could more precisely clarify such differences. Also, intermittent STH-POL cardioplegia should be compared with other routines, for instance with solutions designed for single-shot administration.
Clinical implications
On the basis of the present study, polarizing cardioplegia with esmolol, adenosine and magnesium seems non-inferior to standard depolarizing, potassium-based repeated, oxygenated blood cardioplegia. Clinical research protocols could be initiated, at least in patients requiring a short-to-medium time of cardiac arrest during heart surgery.
CONCLUSIONS
This translational large animal study demonstrates that polarizing oxygenated blood cardioplegia with esmolol/adenosine/magnesium offers comparable myocardial protection and improves contractility compared with the standard potassium-based depolarizing blood cardioplegia.
SUPPLEMENTARY MATERIAL
Funding
Pirjo-Riitta Salminen and Geir Olav Dahle were research fellows funded by the Western Norway Regional Health Authority. Financial support was received from the Western Norway Regional Health Authority, the Bergen University Heart Fund, Norwegian Health Association, the Norwegian Research School in Medical Imaging and the Grieg Foundation. Funding to pay the Open Access publication charges for this article was provided by the University of Bergen.
Conflict of interest: none declared.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the supply of the Merz grid developed for the ImageJ and the kind advice by Aleksandr Mironov, University of Manchester. The technical assistance from Lill-Harriet Andreassen, Cato Johnsen, Kjersti Milde, Gry-Hilde Nilsen, Anne Aarsand, Randi Sandvik and the staff at the Vivarium, University of Bergen is greatly appreciated.
REFERENCES
- 1.Habertheuer A, Kocher A, Laufer G, Andreas M, Szeto WY, Petzelbauer P et al. Cardioprotection: a review of current practice in global ischemia and future translational perspective. Biomed Res Int 2014;2014:325725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruyama Y, Chambers DJ, Ochi M. Future perspective of cardioplegic protection in cardiac surgery. J Nippon Med Sch 2013;80:328–41. [DOI] [PubMed] [Google Scholar]
- 3.Chambers DJ, Fallouh HB. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol Ther 2010;127:41–52. [DOI] [PubMed] [Google Scholar]
- 4.Fallouh HB, Bardswell SC, McLatchie LM, Shattock MJ, Chambers DJ, Kentish JC. Esmolol cardioplegia: the cellular mechanism of diastolic arrest. Cardiovasc Res 2010;87:552–60. [DOI] [PubMed] [Google Scholar]
- 5.Fannelop T, Dahle GO, Matre K, Segadal L, Grong K. An anaesthetic protocol in the young domestic pig allowing neuromuscular blockade for studies of cardiac function following cardioplegic arrest and cardiopulmonary bypass. Acta Anaesthesiol Scand 2004;48:1144–54. [DOI] [PubMed] [Google Scholar]
- 6.Steendijk P, Staal E, Jukema JW, Baan J. Hypertonic saline method accurately determines parallel conductance for dual-field conductance catheter. Am J Physiol Heart Circ Physiol 2001;281:H755–63. [DOI] [PubMed] [Google Scholar]
- 7.Szwarc RS, Mickleborough LL, Mizuno S, Wilson GJ, Liu P, Mohamed S. Conductance catheter measurements of left ventricular volume in the intact dog: parallel conductance is independent of left ventricular size. Cardiovasc Res 1994;28:252–8. [DOI] [PubMed] [Google Scholar]
- 8.Hawk C, Leary SL, Morris TH. Formulary for Laboratory Animals. 3rd edn Ames: Blackwell Publishing Professional, 2005. [Google Scholar]
- 9.Matre K, Fannelop T, Dahle GO, Heimdal A, Grong K. Radial strain gradient across the normal myocardial wall in open-chest pigs measured with Doppler strain rate imaging. J Am Soc Echocardiogr 2005;18:1066–73. [DOI] [PubMed] [Google Scholar]
- 10.Froyland L, Madsen L, Eckhoff KM, Lie O, Berge RK. Carnitine palmitoyltransferase I, carnitine palmitoyltransferase II, and acyl-CoA oxidase activities in Atlantic salmon (Salmo salar). Lipids 1998;33:923–30. [DOI] [PubMed] [Google Scholar]
- 11.Howard C, Reed MG. Unbiased Stereology. 2nd edn Oxford: QTP Publications, 2010. [Google Scholar]
- 12.Weibel ER, Kistler GS, Scherle WF. Practical stereological methods for morphometric cytology. J Cell Biol 1966;30:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R et al. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation 1991;83:974–82. [DOI] [PubMed] [Google Scholar]
- 14.Cannavo A, Liccardo D, Koch WJ. Targeting cardiac beta-adrenergic signaling via GRK2 inhibition for heart failure therapy. Front Physiol 2013;4:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reves JG, Karp RB, Buttner EE, Tosone S, Smith LR, Samuelson PN et al. Neuronal and adrenomedullary catecholamine release in response to cardiopulmonary bypass in man. Circulation 1982;66:49–55. [DOI] [PubMed] [Google Scholar]
- 16.Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J 1991;12(Suppl F):38–47. [DOI] [PubMed] [Google Scholar]
- 17.Bulcao CF, Pandalai PK, D'Souza KM, Merrill WH, Akhter SA. Uncoupling of myocardial beta-adrenergic receptor signaling during coronary artery bypass grafting: the role of GRK2. Ann Thorac Surg 2008;86:1189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth JV, Spahn DR, McRae RL, Chesnut LC, El-Moalem H, Atwell DM et al. Esmolol improves left ventricular function via enhanced beta-adrenergic receptor signaling in a canine model of coronary revascularization. Anesthesiology 2002;97:162–9. [DOI] [PubMed] [Google Scholar]
- 19.Fannelop T, Dahle GO, Matre K, Moen CA, Mongstad A, Eliassen F et al. Esmolol before 80 min of cardiac arrest with oxygenated cold blood cardioplegia alleviates systolic dysfunction. An experimental study in pigs. Eur J Cardiothorac Surg 2008;33:9–17. [DOI] [PubMed] [Google Scholar]
- 20.Dahle GO, Salminen PR, Moen CA, Eliassen F, Jonassen AK, Haaverstad R et al. Esmolol added in repeated, cold, oxygenated blood cardioplegia improves myocardial function after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2015;29:684–93. [DOI] [PubMed] [Google Scholar]
- 21.Sum CY, Yacobi A, Kartzinel R, Stampfli H, Davis CS, Lai CM. Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther 1983;34:427–34. [DOI] [PubMed] [Google Scholar]
- 22.Mehlhorn U, Sauer H, Kuhn-Regnier F, Sudkamp M, Dhein S, Eberhardt F et al. Myocardial beta-blockade as an alternative to cardioplegic arrest during coronary artery surgery. Cardiovasc Surg 1999;7:549–57. [DOI] [PubMed] [Google Scholar]
- 23.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW et al. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res 2010;107:1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsen O, Naesheim T, Aas KN, Sorlie D, Steensrud T. Adenosine instead of supranormal potassium in cardioplegia: it is safe, efficient, and reduces the incidence of postoperative atrial fibrillation. A randomized clinical trial. J Thorac Cardiovasc Surg 2013;145:812–8. [DOI] [PubMed] [Google Scholar]
- 25.Duan L, Zhang CF, Luo WJ, Gao Y, Chen R, Hu GH. Does magnesium-supplemented cardioplegia reduce cardiac injury? A meta-analysis of randomized controlled trials. J Card Surg 2015;30:338–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.