Abstract
OBJECTIVES
The choice of valve prosthesis for aortic valve replacement (AVR) in young patients is challenging. Decellularized pulmonary homografts (DPHs) have shown excellent results in pulmonary position. Here, we report our early clinical results using decellularized aortic valve homografts (DAHs) for AVR in children and mainly young adults.
METHODS
This prospective observational study included all 69 patients (44 males) operated from February 2008 to September 2015, with a mean age of 19.7 ± 14.6 years (range 0.2–65.3 years). In 18 patients, a long DAH was used for simultaneous replacement of a dilated ascending aorta as an extended aortic root replacement (EARR). Four patients received simultaneous pulmonary valve replacement with DPH.
RESULTS
Thirty-nine patients (57%) had a total of 62 previous operations. The mean aortic cross-clamp time in isolated cases was 129 ± 41 min. There was 1 conduit-unrelated death. The mean DAH diameter was 22.4 ± 3.7 mm (range, 10–29 mm), the average peak gradient was 14 ± 15 mmHg and the mean aortic regurgitation grade (0.5 = trace, 1 = mild) was 0.6 ± 0.5. The mean effective orifice area (EOA) of 25 mm diameter DAH was 3.07 ± 0.7 cm2. DAH annulus z-values were 1.1 ± 1.1 at implantation and 0.7 ± 1.3 at the last follow-up. The last mean left ventricle ejection fraction and left ventricle end diastolic volume index was 63 ± 7% and 78 ± 16 ml/m2 body surface area, respectively. To date, no dilatation has been observed at any level of the graft during follow-up; however, the observational time is short (140.4 years in total, mean 2.0 ± 1.8 years, maximum 7.6 years). One small DAH (10 mm at implantation) had to be explanted due to subvalvular stenosis and developing regurgitation after 4.5 years and was replaced with a 17 mm DAH without complication. No calcification of the explanted graft was noticed intraoperatively and after histological analysis, which revealed extensive recellularization without inflammation.
CONCLUSIONS
DAHs withstand systemic circulation, provide outstanding EOA and appear as an alternative to conventional grafts for AVR in young patients. EARR using DAH is a further option in aortic valve disease associated with aorta ascendens dilatation as it avoids the use of any prosthetic material.
Keywords: Decellularized homografts, Valve replacement, Aortic valve, Valve prosthesis
INTRODUCTION
Valve replacement remains the last therapeutic option for patients with severe aortic valve dysfunction unsuitable for valve reconstruction. Biological and mechanical prostheses are the most frequently used substitutes in clinical practice [1, 2].
Main concerns for the use of mechanical prostheses are need for life-long anticoagulation therapy, suboptimal survival rates compared with the general population, as well as the constant risk of prosthetic valve reintervention [3]. For children and young adults, the Ross operation, in which the patient’s own pulmonary valve is used as an autograft for the replacement of the diseased aortic valve, is considered a rational alternative. In these patients, pulmonary autografts have been shown to grow along with the patient, which in consequence has reduced the need for reoperations [4–6]. Nevertheless, the surgical complexity of the procedure, the associated perioperative mortality, concerns about the potential late failure of two semilunar valves and limited durability of the biological grafts used for pulmonary valve replacement in children and young adults limits the practice of Ross operations.
Despite technological progress, the durability of conventional biological prostheses in young patients remains debatable. Rapid degeneration and subsequent failure of biological allo- and xenografts is generally attributed to the patient's immunological response to remaining cellular components within the graft tissue. The lower durability of these types of grafts in patients with higher immunological competence, such as children and young adults, supports this assumption [7, 8]. Decellularization of valved allografts has demonstrated a reduced immunological response to the resulting valve scaffold [9–11]. Several clinical studies have revealed higher freedom from graft explantation, lower transvalvular gradients for decellularized pulmonary homografts (DPHs) in comparison with conventional cryopreserved homografts and xenografts, when used for pulmonary valve replacement in children and young adults [12–14].
In the animal model, we have demonstrated competence of decellularized aortic allografts in the systemic position, and lesser degeneration and calcification when compared with conventional cryopreserved allografts [15].
However, to date, there are only a few reports on the use of decellularized aortic valve homografts (DAHs) in humans [16, 17]. In the present study, we therefore report our early clinical results using DAH for aortic valve replacement (AVR) in children and young adults.
PATIENTS AND METHODS
The study included all consecutive DAH patients operated in two academic cardiac surgery centres, Hannover Medical School, Germany (n = 66) and State University of Medicine and Pharmacy, Chisinau, Moldova (n = 3), in the period between February 2008 and September 2015.
The study was approved by the institutional Ethics Review Board at Hannover Medical School and by the Ministry of Health of Moldova. Patients or, where relevant, parents provided written informed consent for inclusion in the prospective study.
Perioperative surgical findings, echocardiographic and clinical follow-up data of all patients were collected in the department database (FileMaker 13, FileMaker Intl., Santa Clara, CA, USA) at Hannover Medical School.
Study population
Each DAH candidate underwent thorough outpatient evaluation of the indication for AVR followed by detailed discussion with the patient and/or parents regarding conventional AVR options and this new therapeutic alternative. Once the patient/parents consented for implantation of a DAH, search for a suitable homograft started. Patients were informed when an appropriate homograft was available for processing and after microbiological testing of the DAH was negative, they were admitted to the hospital shortly before operation.
Decellularized aortic homografts
Aortic valve homografts were harvested under sterile conditions from cadavers, brain dead multiorgan donors or transplant patients (‘domino’ hearts). All donors were screened for transmissible diseases, such as HIV, syphilis and hepatitis.
Decellularization was achieved by the detergent treatment of the homografts using a solution of 0.5% sodium deoxycholate (Sigma) and 0.5% sodium dodecylsulphate (Carl Roth, Karlsruhe, Germany) for 48 h, followed by two washing cycles (12 h each) in distilled water, and eight washing cycles with Ringer’s lactate solution. All decellularization and washing steps were performed under continuous shaking at room temperature. To control valve sterility, 10 ml of the last washing solution and three tissue samples of the DAH were incubated for 14 days. DAHs were then stored until implantation in Ringer’s lactate solution at 5°C, but no longer than 60 days from donation.
Operative technique
Operations were performed under general, combined intravenous anaesthesia via median sternotomy on cardiopulmonary bypass. For myocardial protection, intermittent antegrade cold blood or crystalloid cardioplegia was used. DAHs were implanted using a root replacement technique with reimplantation of coronary ostia. In patients with concomitant ascending aortic aneurysms, long DAHs, when available, were used to avoid interposition of vascular prostheses.
The muscle cuff of the DAHs was reinforced with a strip of autologous pericardium and proximal anastomosis was performed using either interrupted 4-0 polypropylene sutures or three running sutures between two commissures. The coronary buttons were anastomosed using continuous 6-0 polypropylene sutures. A running 4-0 polypropylene suture was used for distal anastomosis. At the end of the operation, transoesophageal echocardiography was routinely performed to evaluate DAH function. In paediatric patients, aspirin was administered 3–6 months, and in adult patients warfarin was recommended for 2 months followed by continued aspirin medication.
Postoperative evaluation
All patients were investigated after surgery, at 6 and 12 months, and then every 12 months, including clinical and functional examinations [echocardiography, ECG and cardiac magnetic resonance (CMR) investigation]. Clinical follow-up included a regular physical examination of the patients (physical status, measurements of body height and weight and systemic blood pressure, ECG and New York Heart Association classification). Echocardiographic evaluation (M-mode, two-dimensional, colour flow, pulsatile and continuous wave Doppler) was performed according to the current guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Cardiac MRI was performed on standard 1.5-T MRI systems (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) by vector ECG-gated balanced gradient-echo sequences (steady-state free precession) for volumetric analysis, morphology of the left ventricular outflow tract (LVOT) and the aortic valve, phase-contrast flow measurements and contrast-enhanced angiography where needed. Standard volumetric analysis for end-diastolic and end-systolic left ventricular size, ejection fraction and aortic flow measurements were performed using the dedicated customized software as published [18].
Annulus size, as well as the dimensions of the aorta (sinus of Valsava, sinotubular junction, at the level of the main pulmonary artery, at the level of the truncus brachiocephalicus and at level of the left subclavian artery), was measured on standardized coronary CMR images by a single experienced observer (Samir Sarikouch).
DAH degeneration
According to the latest guidelines of the Society of Thoracic Surgeons for the management of aortic valve and ascending aortic disease [19], degeneration was defined as peak gradient over any part of the conduit of 49 mmHg or more and/or at least moderate valve regurgitation. Regurgitation was graded 0 = none, 0.5 = trace, 1 = mild, 1.5 = mild to moderate, 2 = moderate, 2.5 = moderate to severe and 3 = severe.
Statistical analysis
SPSS 23 (IBM Corporation, Somer, NY, USA) was used for the analyses. To illustrate the portion of explanted, well-functioning and dysfunctional grafts at the various examination intervals, we modified the statistical techniques recommended by Akins et al. [20]. Aortic valve annulus measurements were converted to age- and body size-dependent Z-scores for DPH and bovine jugular vein patients according to a thesis of W. Berdau (Medical University of Kiel, Germany, 1989). Interpolation lines (Lowess fit) ease the assignment according to the z-value at implantation for subgroups.
RESULTS
A total of 69 patients underwent AVR using a DAH in the period between February 2008 and September 2015. The mean time from allograft harvesting until implantation was 41 ± 11 days. Postoperative follow-up was complete, except for 3 Moldavian patients, where only clinical follow-up was available. However, CMR imaging has been initiated for these patients. The observation time was 140.4 years in total, mean 2.0 ± 1.8 years, maximum 7.6 years. The demographic data of the patients stratified by age subgroups are presented in Table 1.
Table 1:
Patient cohort description according to age groups
| Patient age group DAH diameter |
All | <10 years <10 years >10 years |
||
|---|---|---|---|---|
| <15 mm | ≥15 mm | ≥19 mm | ||
| Number of patients | 69 | 4 | 12 | 53 |
| Mean age at implantation (years) | 19.7 ± 14.6 | 1.4 ± 1.2 | 6.4 ± 2.1 | 24.1 ± 13.8 |
| Mean follow-up (years) | 2.0 ± 1.8 | 3.3 ± 1.1 | 1.9 ± 2.2 | 2.0 ± 1.7 |
| Total follow-up (years) | 140.4 | 13.0 | 22.6 | 104.8 |
| Sex, male (%) | 64 | 50 | 75 | 62 |
| Congenital AS/AI/AS + AI | 24/2/5 | 3/0/0 | 6/1/0 | 15/1/5 |
| Acquired AS/AI/AS + AI | 10/5/13 | 0/0/0 | 0/0/0 | 10/5/3 |
| Previous procedures | ||||
| Commissurotomy | 7 | 2 | 1 | 4 |
| Aortic valve reconstruction | 12 | 0 | 4 | 8 |
| Ascending aortic aneurysm | 1 | 0 | 0 | 3 |
| Mitral valve disease | 3 | 1 | 0 | 2 |
| Subvalvular aortic stenosis | 7 | 0 | 3 | 4 |
| Pulmonary valve disease | 3 | 0 | 0 | 3 |
| Ross | 2 | 0 | 0 | 2 |
| No. of previous cardiac surgeries/with ECC/with AVR | ||||
| 1 | 25/25/12 | 3/3/0 | 4/4/2 | 18/17/10 |
| 2 | 5/5/4 | 0/0/0 | 1/1/0 | 4/4/4 |
| 3 and more | 9/7/0 | 0/0/0 | 2/2/0 | 7/5/0 |
| Previous catheter interventions | ||||
| Balloon dilatation | 18 | 2 | 5 | 11 |
| DAH diameter (mm) | ||||
| 10–18 | 7 | 4 | 3 | 0 |
| 19–22 | 22 | 0 | 8 | 14 |
| 23–29 | 40 | 0 | 1 | 39 |
| Operation time (min) | ||||
| Mean OP time | 359.2 ± 101.3 | 417.3 ± 108.6 | 387.6 ± 72.8 | 348.5 ± 104.9 |
| Extracorporeal circulation | 220.8 ± 74.6 | 266.3 ± 103.1 | 234.7 ± 58.7 | 214.0 ± 75.3 |
| Aortic cross-clamp time | 139.0 ± 45.5 | 138.3 ± 52.5 | 147.9 ± 40.9 | 137.1 ± 46.6 |
| At last follow-up | ||||
| Mean DAH diameter (mm) | 21.8 ± 4.4 | 12.5 ± 1.7 | 17.3 ± 3.2 | 23.4 ± 3.2 |
| Mean peak gradient (mmHg) | 13.9 ± 15.3 | 58.0 ± 33.4 | 9.5 ± 6.8 | 11.5 ± 8.4 |
| Mean regurgitation, grade | 0.6 ± 0.5 | 1.4 ± 1.1 | 0.7 ± 0.4 | 0.5 ± 0.4 |
| Mean LVEF (%) | 62.8 ± 7.2 | 65.1 ± 6.5 | 68.0 ± 0.0 | 62.3 ± 7.5 |
| Mean LV EDVi (ml/m2) | 77.8 ± 15.7 | 69.0 ± 18.4 | − | 78.8 ± 15.5 |
| Aortic valve z-values | 0.7 ± 1.3 | −0.4 ± 0.7 | 0.3 ± 1.3 | 0.63 ± 1.2 |
AVR: aortic valve replacement; AS: aortic stenosis; AI: aortic insufficiency; ECC: extracorporeal circulation; OP: operation; LVEF: left ventricle ejection fraction; LV EDVi: left ventricle end diastolic volume index.
The mean aortic cross-clamp time for isolated AVR was 129 ± 41 min and the mean cardiopulmonary bypass time was 200 ± 68 min when used as the first aortic valve prosthesis. More information about operation times for subgroups is provided in Tables 1 and 2. There was no operative mortality.
Table 2:
Characteristics of patients who underwent extended aortic root replacement
| Patient group | EARR |
|---|---|
| Number of patients | 18 |
| Mean age at implantation (years) | 29.1 ± 15.7 |
| Mean follow-up (years) | 1.9 ± 1.2 |
| Total follow-up (years) | 34.1 |
| Sex, male (%) | 83 |
| Congenital AS/AI/AS + AI | 5/0/3 |
| Acquired AS/AI/AS + AI | 3/2/0 |
| Previous procedures | |
| Commissurotomy | 0 |
| Aortic valve reconstruction | 3 |
| Ascending aortic aneurysm | 2 |
| Mitral valve disease | 2 |
| Subvalvular aortic stenosis | 0 |
| Pulmonary valve disease | 2 |
| Ross | 1 |
| No. of previous cardiac surgeries/with ECC/with AVR | |
| 1 | 6/6/4 |
| 2 | 2/2/2 |
| 3 and more | 1/0/0 |
| Previous catheter interventions | |
| Balloon dilatation | 4 |
| DAH diameter (mm) | |
| 10–18 | 0 |
| 19–22 | 1 |
| 23–29 | 17 |
| Operation time (min) | |
| Mean OP time | 306.9 ± 68.0 |
| Extracorporeal circulation | 195.1 ± 48.6 |
| Aortic cross-clamp time | 125.3 ± 31.7 |
| At last follow-up | |
| Mean DAH diameter (mm) | 24.9 ± 2.9 |
| Mean EOA (cm2) | 3.2 ± 0.6 |
| Mean peak gradient (mmHg) | 9.4 ± 4.9 |
| Mean regurgitation, grade | 0.6 ± 0.3 |
| Mean LVEF (%) | 61.7 ± 7.3 |
| Mean LV EDVi (ml/m2) | 80.9 ± 16.1 |
| Aortic valve z-values | 0.9 ± 0.8 |
EARR: extended aortic root replacement; EOA: effective orifice area; AVR: aortic valve replacement; AS: aortic stenosis; AI: aortic insufficiency; ECC: extracorporeal circulation; OP: operation; LVEF: left ventricle ejection fraction; LV EDVi: left ventricle end diastolic volume index.
Perioperative complications
In 2 patients (2.9%), a coronary bypass surgery was required as a result of intraoperative (n = 1) or postoperative (n = 1) myocardial ischaemia. In 1 patient, a single coronary artery bypass graft to the right coronary artery was performed as preoperatively intended due to coronary artery anomaly.
Intraoperative ischaemia was suspected in 1 patient due to ECG changes and right ventricular dysfunction during weaning from cardiopulmonary bypass (CBP). The patient received a venous aorto-coronary bypass graft to the right coronary artery in the first segment and had an uneventful recovery after the operation.
One other patient showed a progressive increase in cardiac enzymes and ECG changes during the first postoperative day. An urgent coronary angiography revealed a stenosis of the common trunk of the left coronary artery, which was difficult to prepare and reimplant following a previous aortic valve-sparing procedure. The left internal mammary artery on left anterior descending artery and a venous graft on a postero-lateral branch were used as left coronary artery bypasses for myocardial revascularization. The patient's subsequent course was uneventful and he was discharged on the 12th postoperative day.
In the third patient with truncus arteriosus communis type I and origin anomalies of both coronary arteries, a venous aorto-coronary bypass to the proximal right coronary artery was performed before CBP weaning due to poor right ventricular contractility, the further intra- and postoperative course was uneventful.
Postoperative endocarditis
One patient with hypoplastic left heart syndrome, discharged on the 7th and readmitted on the 18th postoperative day for cardiac tamponade, required emergency surgical intervention. Intraoperatively, severe infective endocarditis of the native ascending aorta with a secondary rupture of the wall 3 cm above the distal DAH anastomosis was identified. The DAH with superficial endocarditic vegetations was explanted and replaced by a biological valved conduit. The patient died in septic multiorgan failure on the third postoperative day. Microbiological investigation revealed invasive aspergillosis (Aspergillus flavus) of the native aorta within the area of previous aortic reconstructive surgery (Norwood repair) and superficial DAH infection.
The infection was classified as sporadic as preoperative sterility testing of the DAH was inconspicuous and no other aspergillus infections were observed during that period. In addition, a DPH of the same donor showed no signs of aspergillosis in another patient 2 years after implantation.
Haemodynamic performance of DAH
The mean diameter of implanted DAHs was 22.4 ± 3.7 mm (range, 10–29 mm), the average peak gradient was 14 ± 15 mmHg and the mean aortic regurgitation grade (0.5 = trace and 1 = mild) was 0.6 ± 0.5. The last mean left ventricle ejection fraction (LVEF) and left ventricle end diastolic volume index (LV EDVi) was 63 ± 7% and 78 ± 16 ml/m2 body surface area (BSA), respectively. The mean effective orifice area (EOA) of a 25-mm diameter DAH was 3.07 ± 0.7 cm2.
Figures 1 and 2 show the development of mean gradients and regurgitation in the DAH over time.
Figure 1:
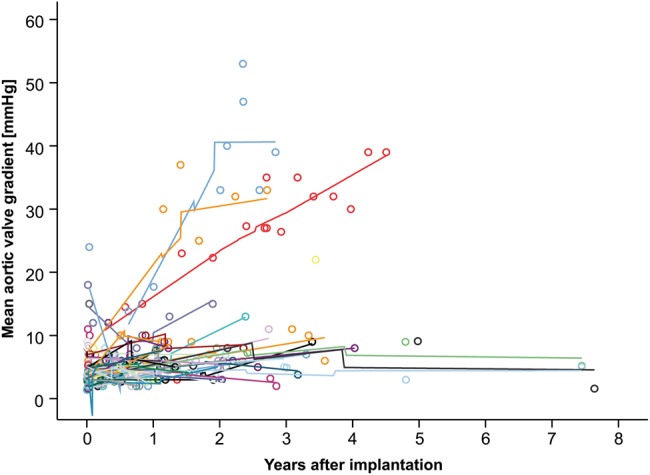
Echocardiographic mean gradient over time in the DAH. Different colours represent different patients; loess-smoothed lines are interpolated between the measurements for each individual. Some individuals show gradients that decrease over time.
Figure 2:
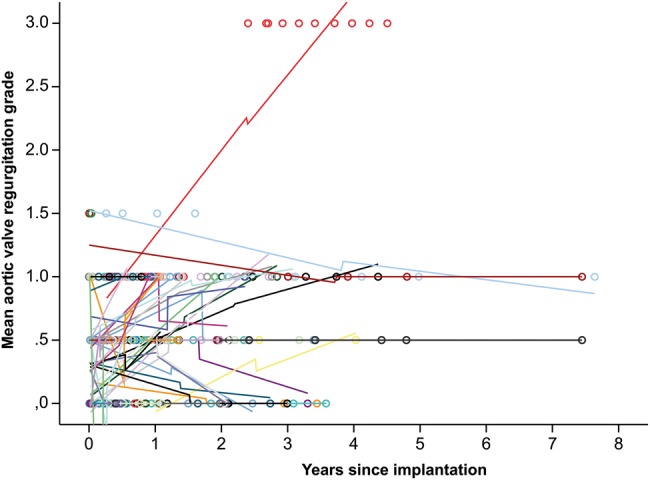
Valvular regurgitation over time in DAH (0 = none, 0.5 = trace, 1 = mild, 1.5 = mild to moderate, 2 = moderate, 2.5 = moderate to severe and 3 = severe). This figure shows the individual aortic valve insufficiency development and loess-smoothed interpolation lines. The decrease of insufficiency is not uncommon.
Figure 3 summarizes the portion of explanted, well-functioning and dysfunctional grafts (>49 mm Hg peak gradient and/or at least moderate regurgitation) at the various examination intervals. For 3 Moldavian patients, only clinical follow-up was available.
Figure 3:
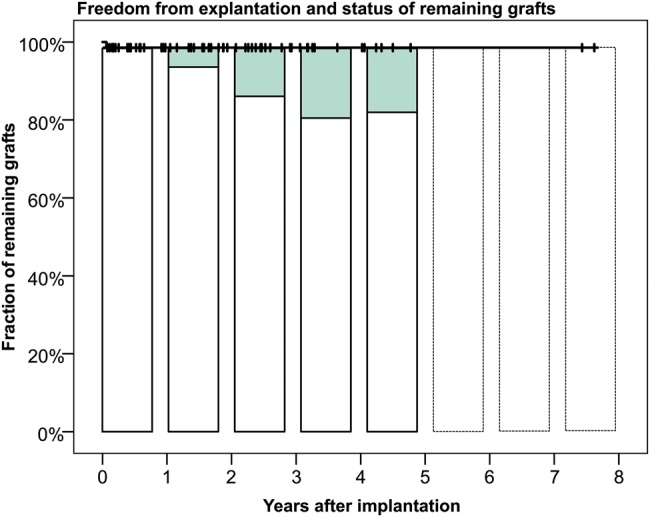
Freedom from explantation including the percentage of conduits with degeneration signs for the DAH (peak gradient >49 mmHg and/or at least moderate regurgitation). For 3 Moldavian patients, only clinical follow-up was available.
DAH in very young patients
To determine growth potential of the DAH, we divided patients younger than 10 years in two subgroups, in patients having received a small DAH (<15 mm; n = 4, mean age 1.4 ± 1.2 years) and in patients with larger DAH diameters (valve diameter >15 mm; n = 12, mean age 6.4 ± 2.1 years).
The first subgroup includes the patients with the smallest grafts of our study with diameters of 10, 11, 12 and 14 mm implanted at the respective ages of 0.2, 0.8, 2.9 and 1.7 years. In all 4 patients, after a follow-up of 4.5, 1.8, 2.9 and 2.6 years and an increase of body surface area from 0.25–0.55, 0.35–0.4, 0.52–0.63 and 0.5–0.62 m2, respectively, no corresponding increase in valve annulus diameter could be observed so far (Fig. 4, black lines).
Figure 4:
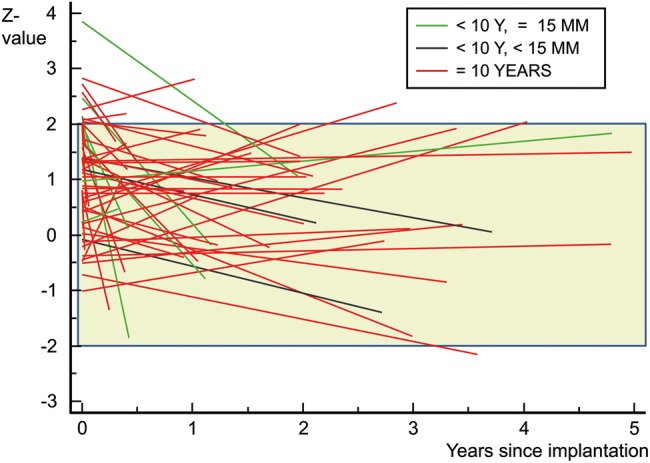
z-Value development of DAH annulus size for subgroups over time. Black lines delineate patients younger than 10 years with a DAH smaller than 15 mm at implantation, green—patients younger than 10 years with a DAH bigger than 15 mm and red—patients older than 10 years. z-Value development of DAH annulus size over time. Package labelled annulus diameter was rounded to z-value integers, and each postoperative measurement is again expressed as a z-value according to the actual height and weight of the patient. A loess fit curve was then drawn for each implant size group. The green area shows the normal range in the middle of which the lines should converge.
In 2 of these patients, the gradient was caused by inadequate growth of the subvalvular area, a common issue in severe congenital aortic stenosis.
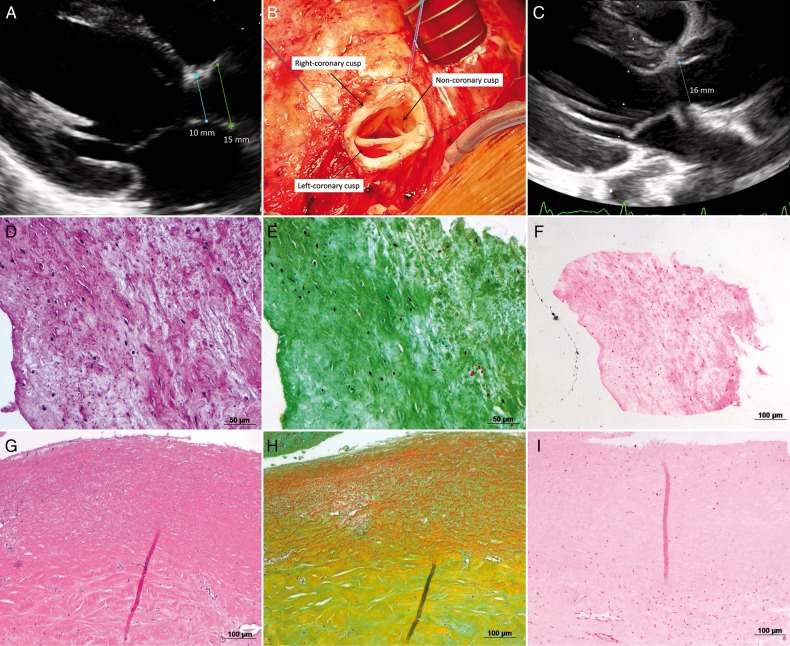
One of these 2 patients, with the smallest implanted DAH (10 mm), developed progressive LVOT obstruction at subvalvular level within the first year after operation with a constant increase in the gradient across the LVOT, which caused progressing valvular regurgitation (Fig. 5A). This patient underwent reoperation 4.5 years after DAH implantation. Intraoperatively, only slight adhesions around the DAH were observed that permitted easy graft preparation and explantation. No macroscopic calcification was noticed. Jet-lesion destruction of the right coronary cusp was the reason for the regurgitation. After LVOT enlargement by resection of subvalvular tissue, a 17-mm DAH was implanted without complications (Fig. 5C) and the patient was discharged 7 days after the operation. Histological analysis revealed good recellularization of the graft with lesser cells in the non-coronary cusp. Cells found were mainly fibroblasts, few myofibroblasts and only rarely inflammatory cells. No calcification was found microscopically (Fig. 5E and H).
Figure 5:
(A) Preoperative echocardiography demonstration subvalvular stenosis and sufficient annulus size; (B) intraoperative aspect of the DAH before explantation (explantation performed after completion of the patients enrolment in the study); (C) postoperative echocardiography after implantation of a 17-mm DAH and resection of subvalvular stenosis; (D) HE staining of the non-coronary cusp; (E) Pentachrom staining of the non-coronary cusp; (F) Van Kossa staining of the non-coronary cusp; (G) HE staining of the non-coronary sinus; (H) Pentachrom staining of the non-coronary sinus; (I) Van Kossa staining of the non-coronary sinus.
One of the 4 smallest patients showed a supravalvular stenosis at the distal anastomosis after previous aorta ascendens and aortic arch procedures.
The fourth patient, with a Konno enlargement of the LVOT, had normal DAH function with stable trivial valve regurgitation and no stenosis 1 year after implantation.
In the second subgroup, 12 patients with a mean age of age 6.4 ± 2.1 years received grafts with diameters of 15–25 mm. At a mean follow-up of 1.9 ± 2.2 years for this subgroup, none of the patients in this subgroup showed an increase in transvalvular gradients and the grafts have not revealed significant regurgitation during this time. Echocardiographic and CMR examination of DAHs did not reveal any signs of structural graft degeneration either.
Figure 4 shows the z-value development of DAH annulus size in subgroups over time. Black lines delineate patients younger than 10 years with a DAH smaller than 15 mm at implantation, green lines delineate patients younger than 10 years with a DAH bigger than 15 mm and red lines patients older than 10 years.
Extended aortic root replacement with a long DAH
In 18 patients, with associated dilatation of the ascending aorta, extended aortic root replacement (EARR) with a long DAH was performed to avoid the use of any vascular prosthesis. Characteristics are summarized in Table 2.
In this subgroup, the mean diameter of the DAH at implantation was 25.8 ± 1.8 mm resulting in a mean EOA of 3.2 ± 0.6 cm2, an average peak gradient at the latest follow-up (mean: 1.9 ± 1.2 years, total 31.1 years) of 9.4 ± 4.9 mmHg and a mean aortic regurgitation grade of 0.6 ± 0.3 (0 = none, 0.5 = trace and 1 = mild). The last mean LVEF was 61.7 ± 7.3% and the LV EDVi was 81 ± 16 ml/m2 BSA. Aortic valve z-values were 1.1 ± 0.8 at implantation and 0.9 ± 0.8 at the last follow-up. No dilatation was observed at any level of the graft (annulus, sinus of Valsava, sinotubular junction or at the level of the main pulmonary artery) during follow-up so far.
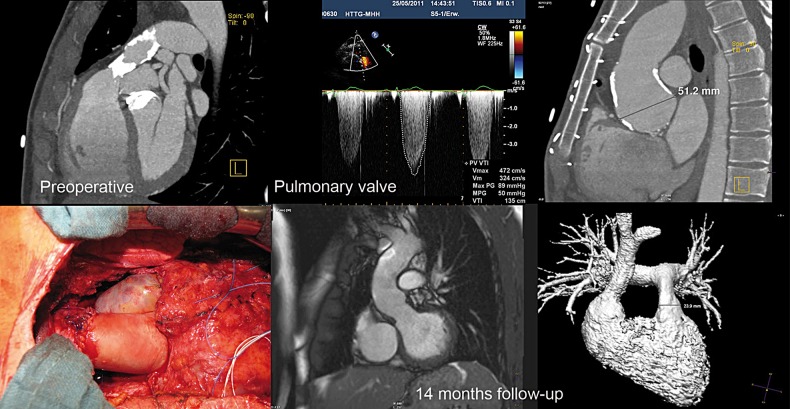
One of these was a patient who had a Ross procedure, who experienced a rapidly failing autograft and therefore underwent secondary autograft replacement using a mechanical valve in combination with aortic root repair using a conventional homograft in an overseas institution. Ten years later, he developed bacterial endocarditis with critical stenosis of the homograft used for pulmonary valve replacement during the initial Ross procedure. Because of the concomitant severe calcification of the aortic allograft used during the second operation to replace the ascending aorta and the patient's wish to cease anticoagulation therapy, the pulmonary valve, the aortic valve and the ascending aorta were replaced by decellularized allografts in one operation (Fig. 6). Three years after the operation both grafts show no signs of degeneration, there is no dilatation of the ascending aorta and the patient is free from anticoagulation therapy.
Figure 6:
Preoperative aspects of the severely calcified and stenotic conventional pulmonary homograft and calcified dilated aortic bulbus in a Ross patient are shown in the first row. The second row shows the intraoperative aspect after double valve replacement with decellularized homografts including EARR by a long DAH and CMR images after 14 months.
In 4 patients, double valve replacement with DAH and DPH was performed in total.
Pregnancy with a DAH
A DAH was implanted in a 35-year old female patient suffering from severe aortic regurgitation after repair of a ventricular septal defect in childhood with subsequent AV block necessitating permanent pacemaker stimulation. Owing to severe histamine intolerance and the wish for a second child, she opted for AVR with a DAH. She became pregnant 3 months after the AVR and gave birth to a healthy girl. DAH function and subsequently LV function were regular during and after pregnancy up to 28 months after DAH implantation.
DISCUSSION
Our data in the largest published cohort to date demonstrate that AVR using DAH is a safe and reproducible surgical procedure in children and adults. A CABG rate of 2.9% is in line with the literature for redo operations, e.g. after initial valve-sparing procedures [21].
Haemodynamic performance of the DAH was excellent up to 7.6 years of follow-up. We observed stable valvular gradients and no relevant valvular regurgitation and consecutively excellent myocardial function, as determined by CMR. No dilatation was observed at any level, including in patients where long DAHs were used for EARR.
This correlates with data published by Zehr et al. [16] and da Costa et al. [17], the only two clinical studies published in this field. In these two studies, DAHs have been implanted in patients with a mean age of 34 and 53 years and a mean follow-up of 30.3 and 19 months, respectively. In both studies, DAHs showed stable transvalvular gradients and good valve competence at the last follow-up when compared with values at discharge. However, both studies do not provide data for paediatric use and the decellularization protocols do vary, as we strictly used non-cryopreserved homografts.
When implanted in young adults, DAHs seem to remodel parallel with the physiological development of the body as shown by the z-value development of implanted grafts. However, in patients receiving very small grafts, we observed a tendency towards a gradient increase over time. This to our understanding suggests that, in very young patients, who are rapidly growing, isolated replacement of the aortic valve using DAHs without enlargement of the LVOT might not be adequate. Congenital, multi-level LVOT obstruction, as well as stenosis of the ascending aorta, requires adequate graft diameters and length, but may also necessitate a supplementary surgical approach to enlarge the LVOT. Results after the use of the conventional aortic homograft in this special patient group are scarcely published. Ross or Ross-Konno procedures are commonly used for the treatment of severe anomalies of the aortic valve with LVOT obstruction [22, 23]. However, these procedures harbour several disadvantages, including placing two semilunar valves at risk in an originally single-valve disease, risk of dilatation of the pulmonary autograft and pre-programmed reoperation rates for pulmonary homograft exchange during childhood.
In this context, we think that the use of DAHs in small children has the advantage to avoid addressing two valves during surgery, subsequently shortening the operation time and avoiding the problem of reoperation on the pulmonary homograft. Especially for very sick patients, the reduction of both the operative time and the complexity of the operation may have a favourable influence on the perioperative outcome of the patient.
At present, only experimental data in the growing sheep model have been available to evaluate potential for growth in decellularized aortic allografts. In our study, we present for the first time clinical data of DAH implantation in 16 patients younger than 10 years. Although our mean follow-up is very short in this group of patients, limiting meaningful discussion on the subject of graft degeneration, the issue of adaptive growth of the decellularized grafts can be cautiously addressed. In our youngest patient with a DAH of 10 mm that required LVOT enlargement 4.5 years postoperatively, the subvalvular problem appeared intraoperatively as a combination of LVOT obstruction, commonly associated with congenital aortic stenosis, and proximal DAH anastomosis. Despite the subvalvular stenosis and development of clinically relevant regurgitation, severe LV hypertrophy and impaired LV function recovered postoperatively to normal values and allowed the physiological development of the child over a 4.5-year postoperative period. Implantation of a 17-mm DAH including coronary reimplantation was performed in a routine operation as no calcification was observed macroscopically and microscopically.
Effective orifice area
One very important aspect for the choice of prosthesis for AVR, especially in childhood and in patients with impaired left ventricular function, is the EOA, as larger orifices directly translate into reduced myocardial work, regression of left ventricular hypertrophy and improvement in patient functional status, thereby leading to increased survival.
In fact, most available valve prostheses exhibit a smaller orifice area than that of a normal aortic valve (in adults, >2.5 cm2). A mismatch between the anatomical ring of the aortic valve and the implanted graft has an important impact on perioperative short- or long-term survival and the postoperative remodelling of the left ventricle [24]. Patient-prosthesis mismatch (PPM), defined in three grades, is calculated by dividing the EOA by the BSA to obtain the EOA index. PPM is defined as none by an EOA index >0.85 cm2/m2, as moderate at 0.65–0.85 cm2/m2 and as severe at <0.65 cm2/m2. Moderate and severe PPM increases operative mortality by 2.1- and 11.4-fold, respectively [25]. Several studies indicate that moderate PPM is found in up to 30% of patients after AVR and, in some cases, complex reoperations are required in order to prevent congestive heart failure. In our study, the mean diameter of implanted DAHs of 25 mm size was 3.07 ± 0.7 cm2. In comparison, the average EOAs of a 25-mm Carpentier-Edwards Magna biological valve and 25-mm SJM Regent mechanical valves are 2.01 and 2.6 cm2, respectively.
The lack of need for anticoagulation therapy, the larger orifice area as well as the expected reduced immunogenicity of DAHs might positively influence a decision for this graft, especially in women of reproductive age. In our cohort of patients with decellularized homografts, 2 patients, 1 with a DPH and 1 with a DAH, have given birth to healthy newborns. Both women remained asymptomatic from a cardiac perspective throughout the pregnancy and did not reveal any decline in graft function after delivery. To the best of our knowledge, this is the first report of successful pregnancy in patients with decellularized homografts. Our data suggest that a DAH may be considered as an option for AVR in women wishing to start a family.
Limitations
The main limitation of this study is the short follow-up and the limited number of implanted small grafts. However, as patients are followed continuously, more data on the mid-term follow-up will be available in the near future. As DAHs have been approved by the German authorities (www.pei.de, ARISE AV PEI.G.11766.01.1), the use of DAHs will increase in the future and allow a matched comparison to conventional biological prostheses, mechanical valves and the Ross procedure in young patients.
CONCLUSION
DAHs may be considered as an alternative option to other conventional grafts for AVR and EARR, particularly for young patients with contraindications for warfarin and patients with impaired left ventricular function, as EOAs are outstanding. In infants, DAH implantation might require a more complex reconstruction of the LVOT to avoid subvalvular stenosis, especially in patients with very small aortic rings. Further prospective clinical controlled trials will be needed to confirm these encouraging early results and have been initiated by us within the HORIZON 2020 programme of the European Union.
Funding
This study was supported by a grant from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 643597.
Conflict of interest: Axel Haverich holds shares of Corlife Ohg, a company for the processing of decellularized allografts, equivalent to those used in this study.
ACKNOWLEDGEMENT
We thank Nina McGuinness for editorial assistance.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Hazekamp (Leiden, Netherlands): Actually, I think that the aortic homograft is underestimated at the moment. A decrease has been shown in the use of homografts, and I think Mr. Pepper will discuss this topic shortly hereafter. Anyway, in Hannover you have shown us that the process of decellularization is technically feasible, without deterioration of the aortic homografts and the pulmonary homografts also, that surgical implantation is feasible, and that there is no later dilatation of these homografts up to 7.5 years. That is something that you should be congratulated on. I am a little bit biased as a participant in ESPOIR and hopefully in the HORIZON study. I hope you forgive me for this.
I am interested in two things. I have two questions. One of the questions is, did you see on CT or on chest X-rays any calcification in the aortic wall, both in the short and in the long homografts?
Dr Tudorache: Our follow-up, in most of the patients is around 2 years, the mean follow-up, and in these patients we didn't see it. Moreover, last week we performed CT scan and MRI in our oldest patients, and even in these patients, 7.5 years after the operation, we didn't see calcification inside. We performed as well some operations in the sheep model and sporadically implanted allografts in the aortic position, and we had some spots inside, but 2.5 years after the implantation. I think it should be enough.
Dr Hazekamp: My other question is, you showed us these histology slides of the explanted homograft, but can you explain a little bit more, go a little bit more into detail about what cells did you see and how they were aligned? For example, did you see a true endothelial lining on this homograft and was it uniformly covering the homograft, and the myofibroblasts, which I think you saw them because you wrote it in the paper, were they scattered or were they in a uniform way introducing themselves in these homografts?
Dr Tudorache: Actually, this is not normal. It has already changed tissue, and of all 3 cusps, only one, the non-coronary cusp, had a normal macroscopic view. The non-coronary cusp already had hypogenesis because of probably turbulence in this graft, because we had progressive aortic valve degeneration in this, with consequent insufficiency, and the insufficiency was actually caused by a missing left coronary cusp. The right coronary cusp had as well some modification and thickening. That's why it is very difficult to say whether the recellularization of this graft after the implantation takes the normal course, as probably in the autograft. Nevertheless, we saw a lot of myofibroblasts. The endothelial lining of the cusps has been seen, as well in the proximal part of the cusps. The tip of the cusp, of the non-coronary cusp, which was macroscopically the normal one, was sporadically covered with some cells, but this was not comparable with a perfect native cusp. But once again, this is not a normal decellularized homograft and we explanted because of another cause.
Dr Hazekamp: No, I agree, I agree. Of course, the key question is do you think that these decellularized homografts, when they get repopulated by the recipient cells, that they will behave like a normal aortic valve? It's speculation, I know, because there are no explants, but what do you think?
Dr Tudorache: That's why we started this multicentre study now.
Dr Hazekamp: Just speculate.
Dr Tudorache: We don't want to speculate. We show now our data. Unfortunately, we don't have the explants, and maybe this is a good answer to your question, but that is why we are trying now to implant more in several centres, and maybe in several years we will have the response to your question.
Dr F. da Costa (Curitiba, Brazil): We have a similar experience; by the way, the longest. We have now 103 decellularized aortic allografts implanted. Follow-up is extending up to 10 years. The mean follow-up is 4.8 years. I don't think those valves will grow, to tell you the truth, but there is one striking feature of those valves, that up to 10 years, and we have several above 7 or 8 years, the CT scan shows no calcification, and the only patient that we had to redo was secondary to patient out growing the valve with subsequent stenosis—and I think you had 2 of those. We implanted a small homograft in a small kid and the kid outgrew the graft—the cusps were absolutely normal, the conduit with no calcification at all, but there was no growth. So I believe decellularized aortic allografts are going to be major advances in aortic valve surgery, but it is not going to be a living graft, as the pulmonary autograft. That is my belief at the moment.
Dr Tudorache: I can't answer this question as well. We performed some operations in growing sheep and compared the Ross procedure with implantation of decellularized aortic homografts, and we saw in decellularized homografts the increase of the diameter of the grafts from 18 to 22 mm without loss of the functionality. This data will be published in the journal, I think next month. Also comparing the homograft, we have less of an increase in diameter as compared with the autograft in these sheep. Nevertheless, the increase in the diameter was more physiological in the decellularized group when compared with the autograft group. In normal sheep, the increase was comparable with the decellularized graft and the autograft was a little bit bigger. This is the answer to your question.
REFERENCES
- 1.Siregar S, de Heer F, Groenwold RH, Versteegh MI, Bekkers JA, Brinkman ES et al. Trends and outcomes of valve surgery: 16-year results of The Netherlands Cardiac Surgery National Database. Eur J Cardiothorac Surg 2014;46:386–97. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann A, Funkat AK, Lewandowski J, Frie M, Schiller W, Hekmat K et al. Cardiac surgery in Germany during 2012: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg 2014;62:5–17. [DOI] [PubMed] [Google Scholar]
- 3.Bouhout I, Stevens LM, Mazine A, Poirier N, Cartier R, Demers P et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg 2014;148:1341–6. e1. [DOI] [PubMed] [Google Scholar]
- 4.Kalfa D, Mohammadi S, Kalavrouziotis D, Kharroubi M, Doyle D, Marzouk M et al. Long-term outcomes of the Ross procedure in adults with severe aortic stenosis: single-centre experience with 20 years of follow-up. Eur J Cardiothoracic Surg 2015;47:159–67. [DOI] [PubMed] [Google Scholar]
- 5.Andreas M, Wiedemann D, Seebacher G, Rath C, Aref T, Rosenhek R et al. The Ross procedure offers excellent survival compared with mechanical aortic valve replacement in a real-world setting. Eur J Cardiothoracic Surg 2014;46:409–13. [DOI] [PubMed] [Google Scholar]
- 6.Luciani GB, Lucchese G, Carotti A, Brancaccio G, Abbruzzese P, Caianiello G et al. Two decades of experience with the Ross operation in neonates, infants and children from the Italian Paediatric Ross Registry. Heart 2014;100:1954–9. [DOI] [PubMed] [Google Scholar]
- 7.Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg 2006;131:558–64. e4. [DOI] [PubMed] [Google Scholar]
- 8.El-Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet 2010;376:524–31. [DOI] [PubMed] [Google Scholar]
- 9.Baraki H, Tudorache I, Braun M, Hoffler K, Gorler A, Lichtenberg A et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009;30:6240–6. [DOI] [PubMed] [Google Scholar]
- 10.Neumann A, Sarikouch S, Breymann T, Cebotari S, Boethig D, Horke A et al. Early systemic cellular immune response in children and young adults receiving decellularized fresh allografts for pulmonary valve replacement. Tissue Eng Part A 2014;20:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boer U, Schridde A, Anssar M, Klingenberg M, Sarikouch S, Dellmann A et al. The immune response to crosslinked tissue is reduced in decellularized xenogeneic and absent in decellularized allogeneic heart valves. Int J Artif Organs 2015;38:199–209. [DOI] [PubMed] [Google Scholar]
- 12.Ruzmetov M, Shah JJ, Geiss DM, Fortuna RS. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg 2012;143:543–9. [DOI] [PubMed] [Google Scholar]
- 13.Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 2011;124:S115–23. [DOI] [PubMed] [Google Scholar]
- 14.da Costa FD, Takkenberg JJ, Fornazari D, Balbi Filho EM, Colatusso C, Mokhles MM et al. Long-term results of the Ross operation: an 18-year single institutional experience. Eur J Cardiothoracic Surg 2014;46:415–22. [DOI] [PubMed] [Google Scholar]
- 15.Tudorache I, Calistru A, Baraki H, Meyer T, Hoffler K, Sarikouch S et al. Orthotopic replacement of aortic heart valves with tissue-engineered grafts. Tissue Eng Part A 2013;19:1686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zehr KJ, Yagubyan M, Connolly HM, Nelson SM, Schaff HV. Aortic root replacement with a novel decellularized cryopreserved aortic homograft: postoperative immunoreactivity and early results. J Thorac Cardiovasc Surg 2005;130:1010–5. [DOI] [PubMed] [Google Scholar]
- 17.da Costa FD, Costa AC, Prestes R, Domanski AC, Balbi EM, Ferreira AD et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 2010;90:1854–60. [DOI] [PubMed] [Google Scholar]
- 18.Sarikouch S, Peters B, Gutberlet M, Leismann B, Kelter-Kloepping A, Koerperich H et al. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging 2010;3:65–76. [DOI] [PubMed] [Google Scholar]
- 19.Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O'Gara PT et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg 2013;95:S1–66. [DOI] [PubMed] [Google Scholar]
- 20.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732–8. [DOI] [PubMed] [Google Scholar]
- 21.El-Hamamsy I, Ibrahim M, Stevens LM, Witzke H, Clark L, Yacoub MH. Early and long-term results of reoperative total aortic root replacement with reimplantation of the coronary arteries. J Thorac Cardiovasc Surg 2011;142:1473–7. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K, Rizal RE, Lavrsen M, Malhotra SP, Akram SA, Davies R et al. Midterm results of the modified Ross/Konno procedure in neonates and infants. Ann Thorac Surg 2012;94:156–62. [DOI] [PubMed] [Google Scholar]
- 23.Shinkawa T, Bove EL, Hirsch JC, Devaney EJ, Ohye RG. Intermediate-term results of the Ross procedure in neonates and infants. Ann Thorac Surg 2010;89:1827–32. [DOI] [PubMed] [Google Scholar]
- 24.Walther T, Rastan A, Falk V, Lehmann S, Garbade J, Funkat AK et al. Patient prosthesis mismatch affects short- and long-term outcomes after aortic valve replacement. Eur J Cardiothoracic Surg 2006;30:15–9. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Vaquero D, Llosa JC, Diaz R, Khalpey Z, Morales C, Alvarez R et al. Impact of patient-prosthesis mismatch on 30-day outcomes in young and middle-aged patients undergoing aortic valve replacement. J Cardiothoracic Surg 2012;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]