Abstract
Atherosclerosis is a chronic inflammatory disease of the arterial wall characterized by lipid deposition, plaque formation, and immune cell infiltration. Innate and adaptive immune cells infiltrate the artery during development of the disease. Moreover, advanced disease leads to formation of artery tertiary lymphoid organs in the adventitia (Grabner et al., 2009; Hu et al., 2015). Various and diverse types of immune cells have been identified in the aorta adventitia vs atherosclerotic plaques (Elewa et al., 2016; Galkina et al., 2006; Lotzer et al., 2010; Mohanta et al., 2016; Mohanta et al., 2014; Moos et al., 2005; Srikakulapu et al., 2016; Zhao et al., 2004). There are conflicting reports on the number and subtypes of immune cells in the aorta depending on the age of the animals, the protocol that is used to obtain single cell suspensions, and the dietary conditions of the mice (Campbell et al., 2012; Clement et al., 2015; Galkina et al., 2006; Kyaw et al., 2012). The number of immune cells in the aorta differs as much as tenfold using different protocols (Butcher et al., 2012; Galkina et al., 2006; Gjurich et al., 2015; Grabner et al., 2009; Hu et al., 2015). These discrepant results call for a protocol that robustly documents bona fide aorta cells rather than those in the surrounding tissues or blood. Critical methodological hurdles include the removal of adjacent adipose tissue and small paraaortic lymph nodes lining the entire aortic tree that are not visible by the naked eye. A dissection microscope is therefore recommended. Moreover protocols of aorta preparations should ascertain that lymphocyte aggregates referred to as fat associated lymphoid clusters (FALCs) (Benezech et al., 2015; Elewa et al., 2015) that are often present at the border between the adipose tissue and the adventitia are removed before enzyme digestion. We propose - besides other approaches (Hu et al., 2015; Mohanta et al., 2014) - a combination of immunohistochemical staining and fluorescence activated cell sorter (FACS) analyses from single cell suspensions to quantify the cells of interest. This protocol describes isolation of single cells from mouse aorta for FACS and other analysis.
Materials and Reagents
50 ml Falcon tube (VWR International, CellStar®, catalog number: 188271)
-
100 μm cell strainer (BD, catalog number: 352360)
Note: Currently, it is “Corning, Falcon®, catalog number: 352360”.
1 ml syringe (Henke-Sass, Wolf GmbH, Soft-JECT®, catalog number: 5010-200V0)
5 ml syringe (BD, catalog number: 309646)
Needle-26G (B. Braun Medical Inc., catalog number: 4657683)
-
6-well plate (BD Falcon, catalog number: 353046)
Note: Currently, it is “Corning, Falcon®, catalog number: 353046”.
1.5 ml Eppendorf tube (Eppendorf AG, catalog number: 0030123328)
Trypan blue solution (Sigma-Aldrich, catalog number: 93595)
Phosphate-buffered saline (PBS), pH 7.4 (Thermo Fisher Scientific, Gibco™, catalog number: 10010023)
Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, Gibco™, catalog number: 14040133)
Fetal bovine serum (FBS) (PAN Biotech UK Ltd., catalog number: P30-1506)
Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758)
Collagenase from Clostridium histolyticum, type I (Sigma-Aldrich, catalog number: C0130)
Collagenase from Clostridium histolyticum, type XI (Sigma-Aldrich, catalog number: C7657)
Hyaluronidase from bovine testes, type I-s (Sigma-Aldrich, catalog number: H3506)
Dnase I (Sigma-Aldrich, catalog number: 11284932001)
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (1 M) (Thermo Fisher Scientific, Gibco™, catalog number: 15630106)
Ethanol solution (Sigma-Aldrich, catalog number: 48075)
Anti-mouse CD45 APC antibody (Thermo Fisher Scientific, eBioscience, catalog number: 17-0451-82)
-
LIVE/DEAD® fixable blue dead cell stain kit (Invitrogen, catalog number: L23105)
Note: Currently, it is “Thermo Fisher Scientific, Molecular Probes™, catalog number: L23105”.
Fc block (anti-CD16/32) (Thermo Fisher Scientific, eBioscience, catalog number: 16-0161-82)
FACS buffer (see Recipes)
EDTA buffer (see Recipes)
Enzyme cocktail (see Recipes)
Equipment
Dissecting scissors (Fine Science Tools, catalog number: 91460-11)
Curved forceps (Fine Science Tools, catalog number: 11073-10)
CO2 supply machine (Next Advance, model: Quietek CO2 induction system)
Neubauer cell counting chamber (Marienfeld-Superior)
Microscope (Carl Zeiss Microscopy, model: Axiovert 40C)
Dissecting microscope equipped with cold light (Carl Zeiss Microscopy, model: Stemi2000)
Water bath (Thomas Scientific, model: 1196x11)
BD LSRFortessa (BD Bioscience)
Procedure
-
A.Isolation of mouse aorta
- Euthanize mouse by CO2 as approved by the appropriate Animal Care and Use Committee.
- Place the mouse in supine position and fix arms and legs onto foam plate with needles.
- Spray 75% ethanol onto abdominal fur, and draw blood by cardiac puncture using 26G needle fixed to a 1 ml syringe.
- Cut skin, subcutaneous tissue, and open peritoneal cavity from abdomen to thorax along the middle line; fix skin/subcutaneous tissue onto a foam plate with needles.
- Open the mediastinum and cut off ribs.
- Puncture the right atrium using a 26G needle.
- Use a 5 ml syringe with a 26G needle attached, perfuse the remaining blood in the aorta from left ventricle with 5 ml 2 mM EDTA buffer, 10 ml PBS, and 10 ml FACS buffer, respectively. To avoid blood contamination, additional 3 perfusions using 5 ml FACS buffer each are needed during dissection steps. Following this, the aorta should be white.
- Remove intestine, spleen, liver, lung, and leave heart, kidneys, and aorta in situ.
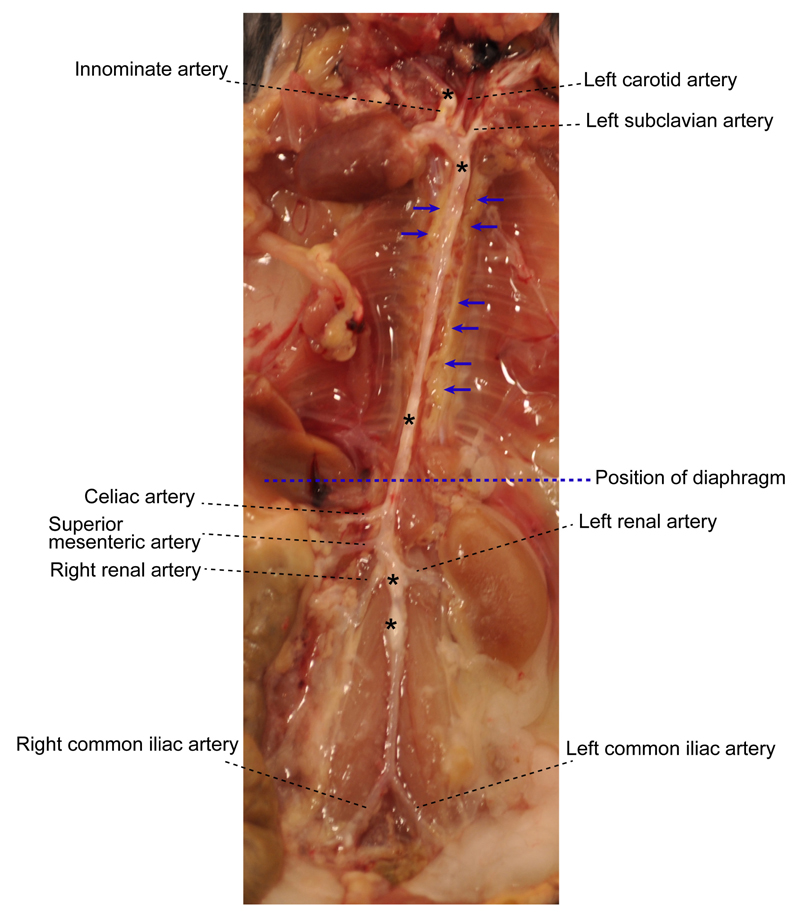
- Under a dissecting microscope equipped with a cold light source (magnification, 2.5x), carefully dissect and remove adipose tissue that is located adjacent to the adventitia while leaving the aorta adventitia intact. At this step, there is no morphologically definable tissue identifier to distinguish adventitia and adjacent adipose tissue (Figure 1). However the adipose tissue is soft and can be removed easily; aorta adventitia is tight.
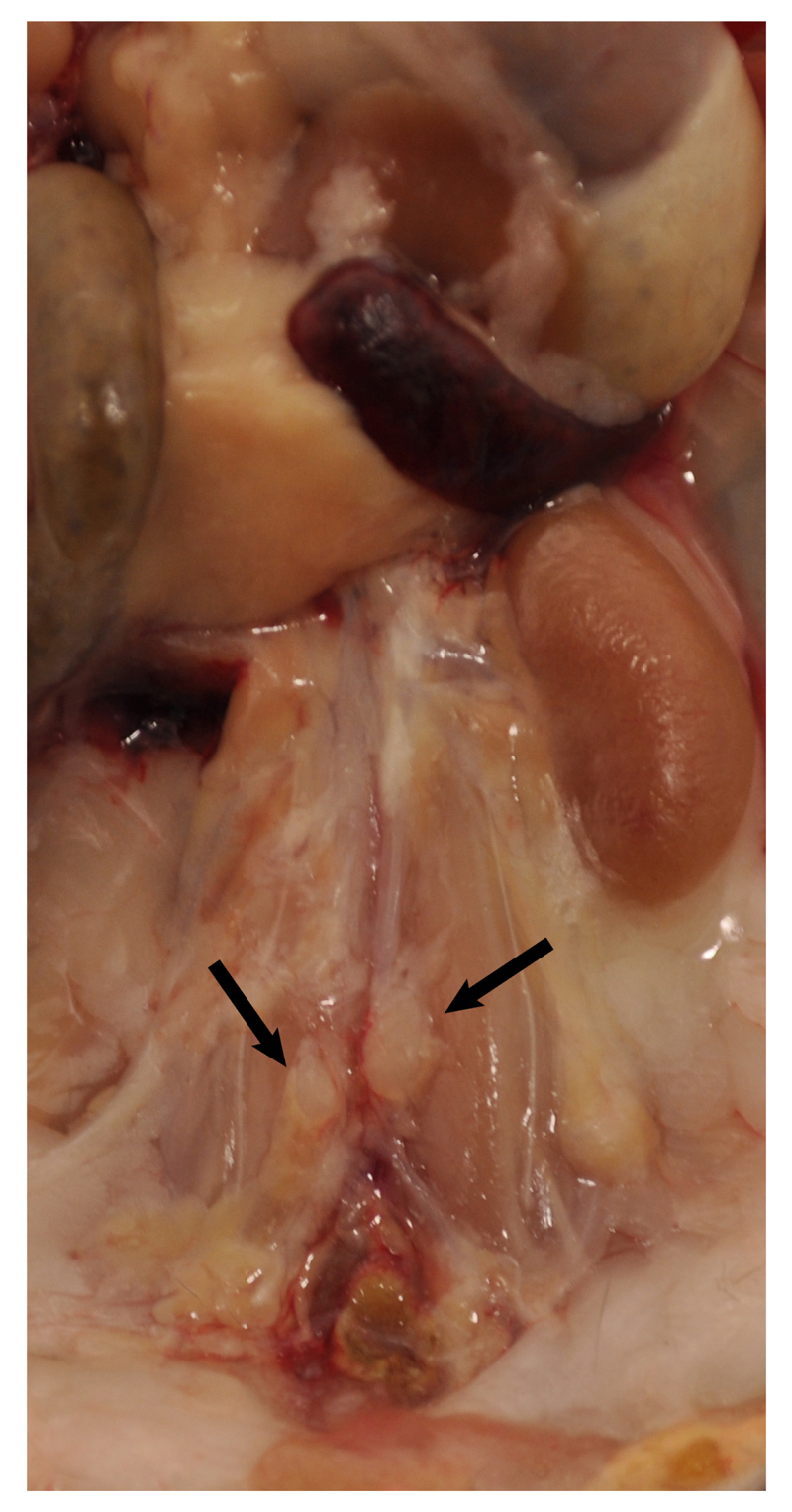
- Carefully remove any lymph nodes that are close to the aorta (Figure 2). Lymph nodes are light brown. There are also some small lymph nodes in adjacent adipose tissue that are not visible with the naked eye requiring a dissection microscope for detection and removal.
- Main artery branches should be left untouched (0.5 cm long from their branching location): Innominate artery, left carotid artery, left subclavian artery, renal arteries, celiac artery, and the common iliac artery (Figure 1).
- Harvest whole aorta and cut it at the level of diaphragm (Figure 1) into two segments: Abdominal aorta and thoracic aorta if analyses of individual aorta segments is needed.
- To study cells from the adventitia vs plaques, detachment of plaque (Figure 1) from the intima is needed: Open aorta longitudinally, fix it with needles, and place the intima side en face; carefully remove the plaque with a curved forceps. Plaque is white, easy to distinguish, and removed without effort.
- Keep aorta segments in ice-cold FACS buffer in 6-well plates until enzyme digestion.
Figure 1. Aorta anatomy in situ.
Aorta was dissected from a 78-week old Apoe-/- mouse. Artery branches are indicated. Blue dashed line indicates position of diaphragm. The plaques in aorta segments are white indicated by asterisks. The adjacent adipose tissue is indicated by blue arrows.
Figure 2. Lymph nodes adjacent to aorta.
Two lumbar lymph nodes adjacent to the abdominal aorta adventitia are indicated by black arrows. There are also invisible small lymph nodes close to or within the paraaortic adipose tissue that line the entire paraaortic connective tissue.
-
B.Enzyme digestion (Grabner et al., 2009; Hu et al., 2015)
- Enzyme cocktail: 400 U/ml collagenase type I, 120 U/ml collagenase type XI, 60 U/ml hyaluronidase and 60 U/ml DNase1, 20 mM HEPES in Dulbecco’s phosphate buffered saline (DPBS) containing calcium.
- Transfer aorta from the 6-well plate to a 1.5 ml Eppendorf tube containing 0.5 ml enzyme cocktail. Cut aorta tissue into small pieces using scissors.
- Transfer enzyme cocktail with aorta tissue to a 50 ml Falcon tube. Add additional 2 ml enzyme cocktail (2.5 ml enzyme cocktail for each thoracic and abdominal aorta total).
- Transfer tube containing aorta tissue pieces to a water bath for 50 min digestion at 37 °C with slow shaking.
-
C.Prepare immune cell suspension from aorta
- After 50 min, pour the digestion solution onto a 100 μm cell strainer which is placed on the top of a new 50 ml Falcon tube.
- Mash remaining aorta tissue with syringe plunger and rinse cell strainer with 5 ml FACS buffer.
- Collect flowthrough from steps C1-2 and centrifuge at 300 x g, 4 °C for 10 min.
- Carefully remove supernatant after centrifugation, and resuspend cell pellet in 2 ml FACS buffer.
- Count aortic cells after mixing with trypan blue under a light microscope.
-
D.Aorta cell staining and flow cytometry
- Centrifuge cell suspension at 300 x g, 4 °C for 5 min.
- To block Fc receptors, incubate cell pellet in 100 μl 1:100 diluted anti-CD16/32 mAb in FACS buffer (1 μg/ml final concentration) at 4 °C for 20 min.
- Fill the staining tube with 300 μl FACS buffer and centrifuge as step D1.
- Discard the supernatant and incubate the cell pellet with 100 μl 1:200 diluted anti-mouse CD45 mAb in FACS buffer (1 μ/ml final concentration) at 4 °C for 20 min.
- Fill the staining tube with 300 μl FACS buffer and centrifuge as in step D1.
- Resuspend the cell pellet with 200 μl FACS buffer and keep the sample on ice until FACS.
- To exclude dead cells, DAPI, PI or LIVE/DEAD cell viability assay dye, like Indo-1, is needed to stain with the cells before measurement.
Notes
To avoid cell contamination from blood or tissues adjacent to the aorta, the perfusion steps and removal of associated adipose tissue as well as lymph nodes should be done very carefully under the microscope. IF staining showed that FALCs are present in the paraaortic adipose tissue sometimes close to the adventitia.
Blood should be completely flushed out of the aorta before digestion. EDTA buffer prevents blood clotting, and it is initially used for perfusing the aorta. However, EDTA can inhibit the activity of digestion enzymes that require Ca++; EDTA needs to be flushed out before digestion is initiated.
During dissection of the aorta, intermittently spray FACS buffer onto the aorta to prevent drying.
Calcium is required for collagenase activity. Therefore, DPBS with calcium is recommended for preparing the enzyme cocktail.
The total number of aortic cells after digestion varies depending on age, sex, diet, and mouse genotype.
Enzyme activity may differ from batch to batch. Enzyme activity tests of each batch are needed.
Enzyme digestion can remove some surface antigens. Therefore, it is necessary to test each surface marker after enzyme digestion. For example, CD138 detection will be low after digestion.
During digestion, some cells will undergo apoptosis. Therefore, flow cytometry staining of live/dead cells is strongly recommended using DAPI, PI or other dye.
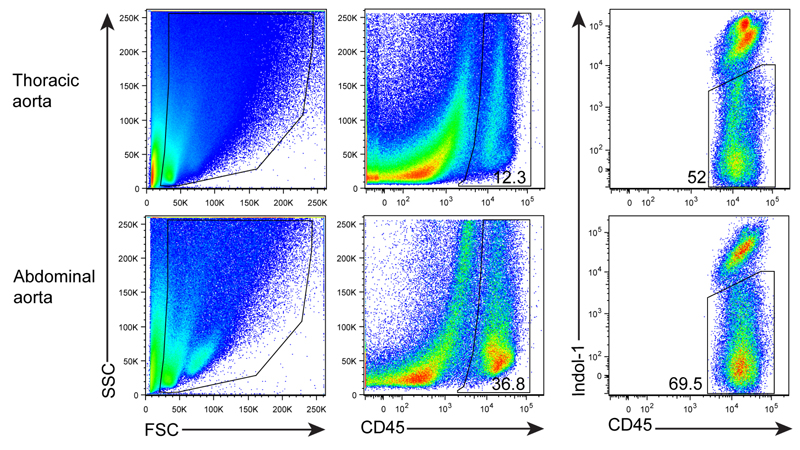
After digestion, tissue fragments will be generated. In order to get ‘clean’ FACS plots, anti-mouse CD45 mAb (a panleukocyte marker) and Indo-1 (a marker for dead cell staining) are added and gated (Figure 3).
Figure 3. FACS profile and gating for living CD45+ cells.
Aorta single cell suspensions were prepared from a 78 week-old Apoe-/- mouse. Cells were stained with anti-CD45 mAb and dead cells using Indo-1.
Recipes
-
FACS buffer
PBS + 2% FBS
-
EDTA buffer
PBS + 2 mM EDTA
-
Enzyme cocktail
400 U/ml collagenase type I
120 U/ml collagenase type XI
60 U/ml hyaluronidase and 60 U/ml DNase1
20 mM HEPES in Dulbecco’s phosphate buffered saline (DPBS) containing calcium
Acknowledgments
This work is supported by the German Research Council (HA 1083/15-4 to A.J.R.H.; MO 3052/1-1 to S.M., and YI 133/2-1 to C.Y.) and the European Research Council (AdG 249929 to C.W.).
References
- 1.Benezech C, Luu NT, Walker JA, Kruglov AA, Loo Y, Nakamura K, Zhang Y, Nayar S, Jones LH, Flores-Langarica A, McIntosh A, et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol. 2015;16(8):819–828. doi: 10.1038/ni.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110(5):675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012;110(6):889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston AT, Delbosc S, Alsac JM, Bruneval P, Deschildre C, et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131(6):560–570. doi: 10.1161/CIRCULATIONAHA.114.010988. [DOI] [PubMed] [Google Scholar]
- 5.Elewa YH, Ichii O, Kon Y. Comparative analysis of mediastinal fat-associated lymphoid cluster development and lung cellular infiltration in murine autoimmune disease models and the corresponding normal control strains. Immunology. 2016;147(1):30–40. doi: 10.1111/imm.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjurich BN, Taghavie-Moghadam PL, Galkina EV. Flow cytometric analysis of immune cells within murine aorta. Methods Mol Biol. 2015;1339:161–175. doi: 10.1007/978-1-4939-2929-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J Exp Med. 2009;206(1):233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity. 2015;42(6):1100–1115. doi: 10.1016/j.immuni.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7(1):e29371. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotzer K, Dopping S, Connert S, Grabner R, Spanbroek R, Lemser B, Beer M, Hildner M, Hehlgans T, van der Wall M, Mebius RE, et al. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling. Arterioscler Thromb Vasc Biol. 2010;30(3):395–402. doi: 10.1161/ATVBAHA.109.191395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanta S, Yin C, Weber C, Hu D, Habenicht AJR. Aorta atherosclerosis lesion analysis in hyperlipidemic mice. Bio-protocol. 2016;6(11):e1833. doi: 10.21769/bioprotoc.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, Weih F, Weber C, Gerdes N, Habenicht AJ. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res. 2014;114(11):1772–1787. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 14.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(11):2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 15.Srikakulapu P, Hu D, Yin C, Mohanta SK, Vineela Bontha S, Peng L, Beer M, Weber C, McNamara CA, Grassia G, Maffia P, et al. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-Cell responses in aged ApoE ITALIC! -/- mice. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.115.306983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10(9):966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]