Abstract
Background
Diabetes is a highly prevalent and costly disease. Studies indicate that combined diet and physical activity promotion programs can prevent type 2 diabetes among persons at increased risk.
Purpose
To systematically evaluate the evidence on cost, cost-effectiveness, and cost-benefit estimates of diet and physical activity promotion programs.
Data Sources
Cochrane Library, EMBASE, MEDLINE, PsycINFO, Sociological Abstracts, Web of Science, EconLit, and CINAHL through 7 April 2015.
Study Selection
English-language studies from high-income countries that provided data on cost, cost-effectiveness, or cost-benefit ratios of diet and physical activity promotion programs with at least 2 sessions over at least 3 months delivered to persons at increased risk for type 2 diabetes.
Data Extraction
Dual abstraction and assessment of relevant study details.
Data Synthesis
Twenty-eight studies were included. Costs were expressed in 2013 U.S. dollars. The median program cost per participant was $653. Costs were lower for group-based programs (median, $417) and programs implemented in community or primary care settings (median, $424) than for the U.S. DPP (Diabetes Prevention Program) trial and the DPP Outcomes Study ($5881). Twenty-two studies assessed the incremental cost-effectiveness ratios (ICERs) of the programs. From a health system perspective, 16 studies reported a median ICER of $13 761 per quality-adjusted life-year (QALY) saved. Group-based programs were more cost-effective (median, $1819 per QALY) than those that used individual sessions (median, $15 846 per QALY). No cost-benefit studies were identified.
Limitation
Information on recruitment costs and cost-effectiveness of translational programs implemented in community and primary care settings was limited.
Conclusion
Diet and physical activity promotion programs to prevent type 2 diabetes are cost-effective among persons at increased risk. Costs are lower when programs are delivered to groups in community or primary care settings.
Primary Funding Source
None.
Diabetes is a highly prevalent, severe, and costly disease in the United States. Approximately 29 million Americans (9.3% of the U.S. population) had diabetes in 2012, and that number is projected to increase (1, 2). Diabetes is the leading cause of kidney failure, blindness, and amputation, as well as a major cause of heart disease and stroke (2). In the United States in 2012, the total medical cost of diagnosed diabetes was estimated at $176 billion, and the cost of productivity loss due to diabetes was another $69 billion (3).
Type 2 diabetes accounts for 90% to 95% of all cases of diagnosed diabetes. Common risk factors for type 2 diabetes include obesity, family history of diabetes, physical inactivity, hypertension, hypercholesterolemia, and elevated glucose level. In addition, approximately 37% of the U.S. population aged 20 years or older and 51% of those aged 65 years or older had prediabetes in 2012, meaning that they were at increased risk for type 2 diabetes (2). However, only about 10% of at-risk persons knew their risk status (4).
Randomized clinical trials around the world have shown that combined diet and physical activity promotion programs could prevent or delay progression to type 2 diabetes among persons at increased risk (5–8). Studies have also demonstrated the feasibility and effectiveness of such programs when they are implemented in primary care or community settings (9). In 2014, a systematic review done for the Community Preventive Services Task Force found that programs implemented in health care or community settings effectively reduced the risk for diabetes in persons at increased risk; increased the likelihood of reversion to normoglycemia; and reduced weight and other risk factors for cardiovascular disease, such as elevated blood pressure and lipid levels (10).
Given the potentially large population that is eligible for diet and physical activity promotion programs and the resources needed for implementation, information on program cost and cost-effectiveness is critical for policy decisions, such as benefit coverage for payers, as well as planning for program design and implementation. As a companion to the aforementioned effectiveness review, we did this systematic economic review for the Community Preventive Services Task Force to estimate the cost associated with diet and physical activity promotion programs and the cost-effectiveness and cost-benefit ratios of these programs.
Methods
Data Sources and Searches
We searched the Cochrane Library, EMBASE, MEDLINE, PsycINFO, Sociological Abstracts, Web of Science, EconLit, and CINAHL for English-language articles published between January 1985 and 7 April 2015. Details of the search strategy are available on the Guide to Community Preventive Services (Community Guide) Web site (www.thecommunityguide.org) and in Appendix Table 1 (available at www.annals.org) (11). We also screened reference lists of relevant studies and reviews and considered studies identified by the parallel review of the effectiveness of diet and physical activity promotion programs (10).
Study Selection
We included studies that provided information on program cost; cost-benefit ratio; or incremental cost-effectiveness ratio (ICER), which is measured as dollars per life-year gained (LYG), quality-adjusted life-year (QALY) saved, or disability-adjusted life-year (DALY) averted. Included studies on program cost had to evaluate the actual program implementation cost. Included cost-effectiveness or cost–benefit studies had to meet published criteria for conducting and reporting economic evaluation analysis (12).
We used the same inclusion criteria as the aforementioned effectiveness review for study population, intervention, comparison population, and publication language (10). Criteria included a population at increased risk for type 2 diabetes, based on glycemic measures or risk scores for diabetes, presence of cardiovascular disease, or presence of the metabolic syndrome; intervention with both diet and physical activity components delivered in at least 2 contact sessions over at least 3 months; comparison with a similar population receiving either usual care (standard lifestyle advice) or no intervention for the cost-effectiveness studies; and publication in English. We further restricted our review to studies in high-income countries to provide economic estimates relevant to U.S. settings and populations.
Data Extraction and Quality Assessment
Two authors extracted data from each article according to the Cochrane systematic review protocol (13) and the Community Guide protocol for economic evaluations (14).
Data Synthesis and Analysis
Intervention costs are reported as program costs per participant, including costs to identify eligible participants (through recruitment in the community, referral from providers, or screening and referral in study settings) and to implement the diet and physical activity promotion program (staff time, training materials, and other costs). We also generated program costs per participant per session, calculated by dividing program costs per participant by the total number of core and maintenance sessions delivered. Medians and interquartile intervals (IQIs) of study estimates were reported as summary measures. If there were 4 data points, we reported the range; if there were 3 or fewer data points, all were reported.
Subgroup analyses of intervention costs were done to explore potential factors affecting costs. For delivery setting, we grouped each study into those based on the U.S. DPP (Diabetes Prevention Program) study, in which the intervention was delivered in a clinical trial setting following rigorous procedures as described in study protocols (5), and those done in real-world settings, in which diet and physical activity promotion programs were translated to community or primary care settings, with (translational DPP programs) or without (translational non-DPP programs) explicit adaptation of DPP training materials.
For delivery method, we categorized each study into 1 of the following groups: individual-based programs, in which a participant met 1-on-1 with the program provider at each core session; group-based programs, in which the participants met as a group with the program provider at each core session; or mixed programs, in which the core sessions included both individual and group sessions.
For the type of personnel delivering the program, we grouped each study by whether the program was delivered by health professionals (such as medical staff, physicians, nurses, physiotherapists, case managers, or dietitians), trained laypersons (such as certified diabetes educators, lay health educators, trained community health workers, or trained volunteers with type 2 diabetes), or a mix of health professionals and trained laypersons.
Cost-effectiveness estimates were measured as ICERs, with medians and IQIs provided as summary measures. To improve comparability of ICERs across the studies, we reported them separately by the outcome measures used in different studies: QALYs saved, LYGs, or DALYs averted. For studies found to be cost-saving, we calculated the negative net cost per QALY saved, LYG, or DALY averted whenever possible to calculate the median ICER.
Two economic perspectives were considered: the health system perspective, in which only medical costs and benefits relevant to health systems were considered, and the societal perspective, in which direct non-medical and indirect costs were also considered. When studies provided sufficient data, we calculated ICERs for perspectives beyond those reported.
As with cost estimates, subgroup analysis of ICERs was done by delivery method. We examined cost-effectiveness estimates by type of analysis: within-trial analysis, in which ICERs were calculated from data on actual costs and benefits; modeling of a trial or extension of trials, in which studies used simulation models to estimate program cost and effectiveness during or beyond the trial period; or modeling of the national effect, in which studies estimated ICERs for programs delivered by scaling up programs to the entire country in which the study was conducted.
Because time horizon is important in program planning and budget allocation, we reported ICERs by length of follow-up (short-term [<10 years] or long-term [≥10 years]). In addition, we reported ICERs stratified by country setting (U.S.- or non–U.S.-based) to better inform programs in the United States.
All costs were adjusted to 2013 U.S. dollars by using the Consumer Price Index for medical care services (15) and annual foreign exchange rates from the Federal Reserve Bank for conversion of other currencies (16). If a study did not mention the year used in cost calculations, we assumed costs to be as of 1 year before the study publication year. Interventions were considered cost-effective if the ICER was less than $50 000 per QALY saved, less than $50 000 per LYG (17), or less than the per capita gross domestic product of the relevant country for cost per DALY averted, as recommended by the World Health Organization (18).
Role of the Funding Source
This study was done by employees of the U.S. government as part of their official duties and received no external funding.
Results
After screening, 28 studies met our inclusion criteria and were included in our final review (Figure 1) (19–46). Of these, 6 cost-only studies (20–23, 26, 27) and 6 cost-effectiveness studies (19, 24, 25, 28–30) provided information on the actual cost of diet and physical activity promotion programs, and 22 contributed cost-effectiveness estimates of the programs (19, 24, 25, 28–46). Fourteen studies were U.S.-based (19–24, 26, 27, 31, 35–38, 46). No cost–benefit studies were identified.
Figure 1.
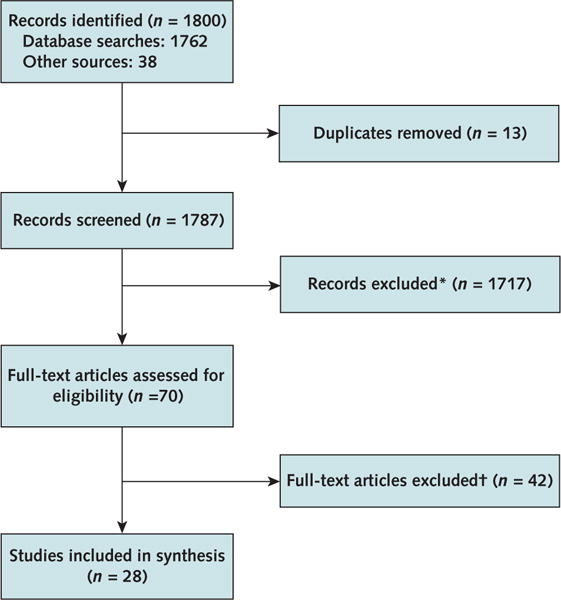
Summary of evidence search and selection.
*Studies had abstracts only, were irrelevant, or did not meet inclusion criteria.
†Did not meet inclusion criteria (for example, included persons with diabetes or had physical activity or diet component but not both). Two studies were conducted in low- or middle-income countries, and 1 did not follow a rigorous cost–benefit analysis.
Intervention Costs
Of the 12 studies that reported the actual costs of implementing the program (20–31), only 4 included costs for identifying persons at increased risk (22, 24, 27, 29). The major cost driver was staff time to deliver the intervention. Most studies provided program cost information embedded in an evaluation of program effectiveness or cost-effectiveness without doing a formal cost analysis (Appendix Table 2, available at www.annals.org).
Program costs per participant ranged from $191 to $5881 (median, $653 [IQI, $383 to $1160]). The most expensive program was the 10-year DPP/DPPOS (Diabetes Prevention Program Outcomes Study), which cost $5881 per participant (19). The cost from the first 3 years (the trial period for DPP, which was based on individual sessions delivered by health professionals) was $4687; the remaining maintenance and follow-up period, called the DPPOS period, was group-based and accounted for only $1194. The translational programs were less intense than the DPP trial and usually had fewer sessions and shorter duration. Most of them were group-based or had a mixture of group and individual sessions and were delivered by either trained laypersons or a mix of health professionals and trained laypersons (Appendix Table 2). They were also less costly than the DPP trial. The median program cost per participant was $424 (IQI, $340 to $793) for the 8 translational DPP programs (20–27) and $1160 (range, $427 to $1416; 4 data points) for the 3 translational non-DPP programs (28–30) (Table 1).
Table 1.
Comparison of Program Costs, by Program Delivery Setting, Method, and Personnel
| Group | Studies, n | Median Total Cost per Participant (IQI or Range), 2013 U.S. $* | Median Cost per Participant per Session (IQI or Range), 2013 U.S. $* |
|---|---|---|---|
| Setting | |||
|
| |||
| DPP/DPPOS† | 1 | 5881 | 102 |
|
| |||
| Translational DPP | 8 | 424 (IQI, 340–793) | 25 (IQI, 16–48) |
|
| |||
| Translational non-DPP‡ | 3 | 1160 (range, 427–1416) | 27 (range, 4–64) |
|
| |||
| Delivery method§ | |||
| Individual-based | 2 | 5881 and 1242 | 102 and 44 |
|
| |||
| Group-based | 8 | 417 (IQI, 341–600) | 17 (IQI, 12–33) |
|
| |||
| Mixed | 3 | 839, 918, and 1416 | 8, 20, and 53 |
| Personnel | |||
|
| |||
| Health professionals‖ | 4 | 1077 (IQI, 381–1329) | 16 (IQI, 7–54) |
|
| |||
| Trained laypersons | 3 | 191, 357, and 839 | 16, 17, and 53 |
|
| |||
| Mixed | 4 | 548 (range, 407–918) | 31 (IQI, 20–55) |
DPP = Diabetes Prevention Program; DPPOS = Diabetes Prevention Program Outcomes Study; IQI = interquartile interval.
Range is provided if there were 4 data points; values from individual studies are provided if there were ≤3 data points.
Cost per participant was calculated for the DPP/DPPOS. Cost per participant per session was calculated for DPP core sessions.
4 data points; 1 study reported data points from 2 groups.
1 study reported data points from individual- and group-based groups.
Includes only translational studies, not the DPP trial; 5 data points; 1 study reported data points from 2 groups.
The median cost per participant per session was $30. The cost per session of the DPP/DPPOS was $102. The median costs per participant per session for the 8 translational DPP programs and the 3 translational non-DPP programs were $25 (IQI, $16 to $48) and $27 (range, $4 to $64), respectively (Table 1).
The median cost per participant was lower in the group-based programs ($417 [IQI, $341 to $600]) (20– 25, 28, 29) than in the DPP/DPPOS ($5881) (19) and the translational non-DPP program ($1242) (29) (Appendix Table 2), both of which used individual sessions. It was also lower than the median cost of programs with a mix of individual and group sessions (median, $918 [range, $839 to $1416]) (26, 27, 30) (Table 1). The median cost per participant for translational programs delivered by trained laypersons (median, $357 [range, $191 to $839]) (21, 22, 26) was lower than that for those delivered by health professionals (median, $1077 [IQI, $381 to $1329]; 4 programs; 5 data points) (20, 28–30); however, there was large variation within personnel type, possibly due to a mixture of delivery settings and methods (Table 1).
Cost-Effectiveness of the Programs
Of 22 studies reporting the cost-effectiveness of the programs, 8 were U.S.-based (19, 24, 31, 35–38, 46). Seventeen studies reported the outcome measure as cost per QALY saved (19, 24, 25, 28–31, 35–40, 42– 44, 46), 6 reported cost per LYG (32–34, 39, 40, 43), and 2 reported cost per DALY averted (41, 45). All studies except 1 (42) reported ICERs from a health system perspective. Eight studies (19, 28, 31, 36, 38, 39, 42, 44) reported ICERs from a societal perspective, and 7 (19, 28, 31, 36, 38, 39, 44) reported both health system and societal perspectives. However, only 1 study included all of the costs and benefits from society as a whole (44). Eighteen studies used modeling techniques (24, 28, 30, 32–46), 2 of which modeled the cost-effectiveness of nationwide community-based programs (45, 46). Fourteen studies were based on data from the DPP trial or the Finnish Diabetes Prevention Study, which used individual sessions (19, 31, 33–41, 43, 44, 46). Most modeling studies considered the health and cost consequences of the program for at least 10 years (28, 30, 32–43, 45, 46). Appendix Table 3 (available at www.annals.org) provides estimates of cost-effectiveness or cost–utility ratios from individual studies, which served as the basis for the summary measure of ICERs.
Of the 16 studies that included cost per QALY saved from the health system perspective, all but 1 (35) reported ICERs below the cost-effectiveness threshold of $50 000 per QALY saved (Figure 2). Three studies reported cost savings (36, 43, 46). The median ICER from the 16 studies was $13 761 per QALY saved (IQI, $3067 to $21 899).
Figure 2.
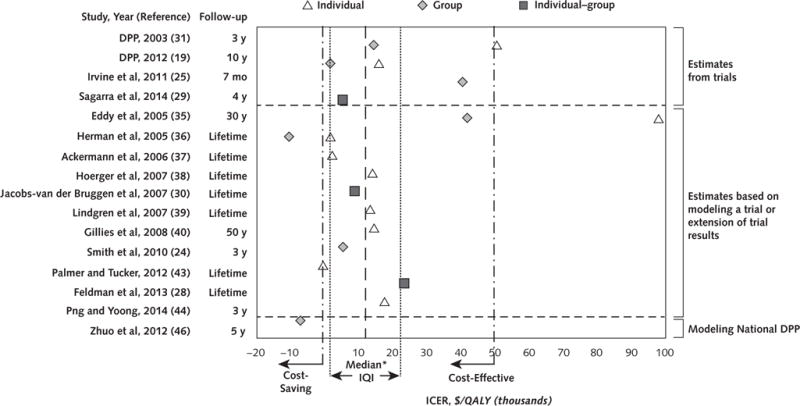
Scatterplot of ICERs from 16 cost-effectiveness or cost–utility analyses that reported cost per QALY saved from the health system perspective.
DPP = Diabetes Prevention Program; ICER = incremental cost-effectiveness ratio; IQI = interquartile interval; QALY = quality-adjusted life-year.
*$13 761 per QALY saved (IQI, $3067 to $21 899).
From the health system perspective, subgroup analyses were done with 5 studies that reported ICERs for both individual- and group-based programs (19, 31, 35, 36, 38). The medians were $15 846 (IQI, $7980 to $72 723) and $1819 (IQI, −$5027 to $16 443) per QALY, respectively. Six studies (24, 25, 28–30, 46) that evaluated the cost-effectiveness of translational programs found a median ICER of $7115 per QALY (IQI, $2252 to $27 582). Two of them were conducted in the United States (24, 46); 1 reported an ICER of $5494 per QALY, and the other reported cost savings.
Studies in the United States reported a median ICER of $9824 per QALY (IQI, $1930 to $41 982; 8 studies), and non-U.S. studies reported a median ICER of $13 860 per QALY (IQI, $6203 to $21 899; 8 studies). By method, the median ICER of the 4 within-trial analyses was $28 097 per QALY (range, $5359 to $50 694) (19, 25, 29, 31). Twelve modeling studies reported a median ICER of $13 367 per QALY (IQI, $2303 to $17 614). By time horizon, the median ICERs were $17 614 per QALY (IQI, $5427 to $45 521; 5 studies) for studies that considered the benefits and costs of the program over less than 10 years and $13 367 per QALY (IQI, $1805 to $15 846; 11 studies) for studies that extended 10 years or beyond (Table 2).
Table 2.
Comparison of Costs per QALY Saved, by Dimension
| Group | Studies, n | Median ICER* (IQI or Range), $/QALY† |
|---|---|---|
| Study perspective‡ | ||
| Societal perspective includes only indirect cost | ||
|
| ||
| Health system | 2 | 13 367 and 23 327 |
|
| ||
| Societal | 2 | 6080 and 22 647 |
| Societal perspective includes only direct nonmedical cost | ||
|
| ||
| Health system | 4 | 15 000 (range, 1805 to 50 694) |
|
| ||
| Societal | 4 | 26 611 (range, 13 574 to 83 310) |
| Societal perspective includes direct nonmedical and indirect costs | ||
|
| ||
| Health system | 1 | 17 614 |
|
| ||
| Societal | 1 | 37 580 |
| Delivery method‡ | ||
|
| ||
| Individual-based | 5 | 15 846 (IQI, 7980 to 72 723) |
|
| ||
| Group-based | 5 | 1819 (IQI, −5027 to 16 443) |
| Setting | ||
|
| ||
| United States | 8 | 9824 (IQI, 1930 to 41 982) |
|
| ||
| Other | 8 | 13 860 (IQI, 6203 to 21 899) |
| Method | ||
|
| ||
| Within-trial | 4 | 28 097 (range, 5359 to 50 694) |
|
| ||
| Modeling extension of trials | 11 | 13 367 (IQI, 2303 to 17 614) |
|
| ||
| Modeling on nationwide, community-based DPP | 1 | −7069 |
|
| ||
| Time horizon | ||
| Short-term (<10 y) | 5 | 17 614 (IQI, 5427 to 45 521) |
|
| ||
| Long-term (≥10 y) | 11 | 13 367 (IQI, 1805 to 15 846) |
DPP = Diabetes Prevention Program; ICER = incremental cost-effectiveness ratio; IQI = interquartile interval; QALY = quality-adjusted life-year.
From health system perspective unless otherwise indicated.
Range is provided if there were 4 data points; values from individual studies are provided if there were ≤3 data points. Costs are in 2013 U.S. dollars.
Data are from the same studies (i.e., the studies reported ICERs from both societal and health system perspectives or from both individual and group delivery methods).
Two studies conducted in Australia (41, 45) reported cost per DALY averted from the health system perspective and used the Australian 2013 per capita gross domestic product of $67 468 as the cost-effectiveness threshold (47). Both studies found the programs to be cost-effective ($21 195 and $50 707 per DALY).
Six other studies reported ICERs as cost per LYG (32–34, 39, 40, 43); all were below the $50 000 threshold. Two studies showed negative costs per LYG, which indicated cost savings (34, 43). The median ICER was $2684 per LYG (IQI, −$2444 to $17 410).
Discussion
Our review found a median ICER for diet and physical activity promotion programs of $13 761 per QALY saved. The 25th and 75th percentiles of the ICERs from the 16 studies that reported cost per QALY saved from the health system perspective were both under $50 000 per QALY, which is a conventional cost-effectiveness threshold (17). The ICERs of diet and physical activity promotion programs measured by cost per LYG or DALY averted were also all under commonly used cost-effectiveness thresholds (18). Thus, we conclude that diet and physical activity promotion programs are cost-effective and involve an efficient use of health care resources.
Our evidence search identified 4 pertinent systematic or narrative reviews evaluating the evidence on cost-effectiveness of diet and physical activity promotion programs for participants at increased risk for type 2 diabetes (48–51). Results from these reviews also suggested that such programs were either cost-effective or cost-saving, independent of country or delivery setting. Previous reviews did not synthesize evidence on costs of diet and physical activity promotion programs. Our systematic review includes 18 additional studies; supports the overall finding of cost-effectiveness; and provides comparative economic estimates by delivery method, setting, and staffing to inform program planning and implementation.
Given the current evidence base, we cannot definitively conclude that the programs are cost-saving. Only 3 studies that reported cost per QALY saved found the program to be cost-saving (36, 43, 46). For the 2 U.S. studies, 1 (36) reported that the DPP program was cost-saving over a lifetime horizon when delivered in group sessions, and the other (46) reported that a nationwide diabetes prevention program became cost-saving in its 11th year, implying that the programs may not save costs in the short term. However, few health care interventions have been found to be cost-saving, and many medical services that are typically covered by insurance have much higher ICERs than the diet and physical activity promotion programs (52). In a 2010 review of the cost-effectiveness of interventions for diabetes prevention and control, the median ICER for lifestyle interventions was at the low end of the spectrum, and the interventions were much more cost-effective than many diabetes treatment interventions, such as intensive glycemic control (48).
Most cost-effectiveness studies in our review were model-based because most trials lasted 3 years or less, but both the health and economic effects of the program were expected to last beyond the trial period. Estimated long-term ICERs of the programs from those modeling studies provided valuable information for decision makers in forecasting the health and economic effects of the program. One common critique of model-based studies is a lack of transparency of the models. To ensure the validity of the estimates, we explicitly abstracted studies in which information on program cost and effectiveness was clearly described in the model. Most studies used either a previously validated model or a model used in previous peer-reviewed publications, and all studies explicitly stated important assumptions used to predict future health and economic outcomes of the program. Model-based ICER estimates varied widely, which could have been due to different model structures and health assumptions, such as the rates of progression of diabetes and its complications beyond the trial period. Despite this variation in the derivation of ICERs with the use of modeling, all but 1 study showed that the ICERs of the programs were far below conventional cost-effectiveness thresholds. The 1 study that reported a much higher ICER used a model with a structure that differed greatly from the other studies and assumed a much slower rate of progression to diabetes in the model (35). However, even for this study, when the intervention was delivered in a group setting, the ICER was below the threshold of $50 000 per QALY.
Our findings have several important implications for programs implemented in the field, such as the National Diabetes Prevention Program, a public–private partnership led by the Centers for Disease Control and Prevention to implement a low-cost intervention adapted from the DPP in communities across the country (53). Group-based programs were less costly and more cost-effective than individual-based programs. In group-based programs, several participants could be counseled in the same session; thus, the cost per participant was lower. Evidence also showed that group-based programs may achieve effectiveness similar to that for individual-based programs (10). To reduce cost and achieve higher cost-effectiveness of diet and physical activity promotion programs, it seems that group-based programs should be used when the programs are implemented in real-world settings.
The cost of these programs may present a barrier to implementation despite the evidence on program cost-effectiveness. The original DPP trial was individual-based and resource-intensive. However, the program cost was much lower when it was implemented in a group format in primary care clinics and communities or translational DPP programs and was lower than or similar to currently reimbursable medical practices. For example, the annual per capita expenditure (in 2012 U.S. dollars) on prescription medications for persons with diabetes was $1423 (3), and Medicare currently pays $25.52 per counseling session for weight-loss programs (54). Further, program scale-up is expected to create economies of scale, further reducing the cost. Programs were found to be more cost-effective in longer-term follow-up studies, given that health benefits often last beyond the program period. In addition, many diabetes-related complications do not appear immediately after a person develops diabetes, which limits the ability of short-term studies to capture the full range of health benefits and medical costs avoided by the intervention.
We identified several limitations of the evidence base that future research should address. First, few studies estimated the cost associated with recruiting and engaging eligible persons to participate in the programs, which may generate additional costs when the programs are scaled up. Second, only 2 studies provided a rigorous cost analysis, and there is a lack of information to better understand the cost of scaling up the programs, such as the cost of programs delivered by trained laypersons (27). Third, only 2 studies evaluated the cost-effectiveness of programs implemented in primary care and community settings in the United States. Fourth, although the societal perspective is often preferred, of the 22 cost-effectiveness studies identified, only 8 reported this perspective and only 1 included all cost and benefit components (12). In addition, 1 study reported an ICER from a health plan (payer) perspective. Fifth, no cost-benefit analyses were identified in the review. Finally, although we attempted to stratify ICERs by program features, these characteristics were so intertwined that formal statistical testing of the effect of a single feature was not feasible.
In summary, the available economic evidence indicates that combined diet and physical activity promotion programs are cost-effective when delivered to persons at increased risk for type 2 diabetes. Evidence further suggests that programs using group sessions delivered by trained diabetes educators or laypersons are an economically efficient approach for communities and health care systems, especially those faced with limited resources and an increasing demand for services.
Health care providers have an essential role in the prevention of type 2 diabetes among patients at increased risk. In most cases, clinicians will be involved in identifying at-risk patients, delivering initial or ongoing behavioral counseling (55), and arranging referrals to available services. Our findings, combined with the findings from the concurrent effectiveness review (10), add to the growing body of evidence that diet and physical activity promotion programs using group sessions delivered by trained personnel are both effective and cost-effective. As national, state, and local efforts to implement evidence-based programs expand, health care providers will have additional, effective intervention options for patients identified as being at increased risk for type 2 diabetes.
Acknowledgments
The authors thank William Thomas, MLIS, from the Library Science Branch at the Centers for Disease Control and Prevention for doing the literature search; Verughese Jacob, PhD, from the Community Guide Branch at the Centers for Disease Control and Prevention for his assistance in the study design, data abstraction, and graphical support; and Kate W. Harris, BA, for her help in editing the manuscript. They also thank Elizabeth Luman, PhD, from the Division of Diabetes Translation; Lawrence E. Barker, PhD, from the Division of Community Health; and the other internal reviewers from the Center for Surveillance, Epidemiology and Laboratory Services for their insightful comments on revising the manuscripts, as well as Tao Ran, PhD, from the Community Guide Branch for graphical support. In addition, the authors thank the Community Preventive Services Task Force for its contributions to this evidence review.
Appendix Table 1.
Search Strategy: Combined Diet and Physical Activity Promotion Programs Among Persons at Increased Risk—Economic Review*
| Number | Searches |
|---|---|
| Terms defining diabetes | |
|
| |
| 1 | exp diabetes mellitus/ |
|
| |
| 2 | diabet$.tw. |
|
| |
| 3 | IDDM.tw. |
|
| |
| 4 | NIDDM.tw. |
|
| |
| 5 | MODY.tw. |
|
| |
| 6 | (late onset adj diabet$).tw. |
|
| |
| 7 | (maturity onset adj diabet$).tw. |
|
| |
| 8 | (juvenil adj diabet$).tw. |
|
| |
| 9 | (syndrome X and diabet$).tw. |
|
| |
| 10 | hyperinsulin$.tw. |
|
| |
| 11 | insulin sensitiv$.tw. |
|
| |
| 12 | insulin$ secret$ dysfunc$.tw. |
|
| |
| 13 | impaired glucose toleran$.tw. |
|
| |
| 14 | exp glucose intolerance/ |
|
| |
| 15 | glucose intoleran$.tw. |
|
| |
| 16 | exp insulin resistance/ |
|
| |
| 17 | insulin$ resist$.tw. |
|
| |
| 18 | (non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).tw. |
|
| |
| 19 | (insulin$ depend$ or insulin?depend$).tw. |
|
| |
| 20 | metabolic$ syndrom$.tw. |
|
| |
| 21 | (pluri metabolic$ syndrom$ or plurimetabolic$ syndrom$).tw. |
|
| |
| 22 | ((typ$ 1 or typ$ 2) and diabet$).tw. |
|
| |
| 23 | ((typ I or typ$ II) and diabet$).tw. |
|
| |
| 24 | or/1–23 |
|
| |
| 25 | exp diabetes insipidus/ |
|
| |
| 26 | diabet$ insipidus.tw. |
|
| |
| 27 | 25 or 26 |
|
| |
| 28 | 24 not 27 |
| Terms defining cost | |
|
| |
| 29 | exp “costs and cost analysis”/ |
|
| |
| 30 | exp health care costs/ |
|
| |
| 31 | exp “cost of illness”/ |
|
| |
| 32 | * ECONOMICS/ |
|
| |
| 33 | or/29–32 |
|
| |
| Terms defining benefit | |
| 34 | benefit.mp. |
|
| |
| 35 | (cost$ or expenditure$).mp. |
|
| |
| 36 | Life years.mp. |
|
| |
| 37 | exp Quality-Adjusted Life Years/ |
|
| |
| 38 | Disability adjusted life years.mp. |
|
| |
| 39 | effectiveness.mp. |
|
| |
| 40 | or/34–39 |
| Terms defining both cost and benefit | |
|
| |
| 41 | 33 and 40 |
|
| |
| Additional terms defining cost-effectiveness analysis or cost–benefit analysis | |
| 42 | exp COST-BENEFIT ANALYSIS/ |
|
| |
| 43 | cost-effectiveness analysis.mp. |
|
| |
| 44 | Cost-utility analysis.mp. |
|
| |
| 45 | Economic evaluation.mp. |
|
| |
| 46 | or/42–45 |
|
| |
| 47 | 28 and (41 or 46) |
|
| |
| Terms defining lifestyle interventions preventing diabetes | |
| 48 | primary prevention/ |
|
| |
| 49 | primary prevention.tw. |
|
| |
| 50 | (reduc* adj3 risk).tw. |
|
| |
| 51 | risk reduction behavior/ |
|
| |
| 52 | (prevent* adj3 diabet*).tw. |
|
| |
| 53 | health promotion.mp. |
|
| |
| 54 | diabetes mellitus/pc |
|
| |
| 55 | life style/ |
|
| |
| 56 | lifestyle*.tw. |
|
| |
| 57 | life style*.tw. |
|
| |
| 58 | prediabet*.mp. |
|
| |
| 59 | weight loss/ |
|
| |
| 60 | health education/ |
|
| |
| 61 | health educator*.mp. |
|
| |
| 62 | patient education as topic/ |
|
| |
| 63 | diet/ |
|
| |
| 64 | exp exercise/ |
|
| |
| 65 | motor activity/ |
|
| |
| 66 | physical activity.tw. |
|
| |
| 67 | walking.mp. |
|
| |
| 68 | or/48–67 |
|
| |
| 69 | 47 and 68 |
|
| |
| Defining searching period | |
| 70 | |
| Deduplication of study results | limit 69 to yr=“1985-Current” |
|
| |
| 71 | remove duplicates from 70 |
Databases searched were Cochrane, EMBASE, MEDLINE, PsycINFO, Sociological Abstracts, Web of Science, EconLit, and CINAHL. Searches were done between January 1985 and 7 April 2015. Last run: 7 April 2015.
Appendix Table 2.
Summary Evidence Table of Included Studies Providing Cost of Combined Diet and Physical Activity Promotion Programs to Reduce Type 2 Diabetes Among Persons at Increased Risk
| Study, Year (Reference); Location | Population Size, n | Population Characteristics | Intervention Setting/Intervention Format | Duration | Intervention Intensity (Number of Sessions) | Method | Type of Personnel | Cost Valuation for Identifying Clients (Recruitment; Screening) | Cost Valuation for Implementing the Intervention | Total Program Costs per Person | Cost per Person per Session |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPP/DPPOS | |||||||||||
|
| |||||||||||
| DPP Research Group, 2012 (19); United States | 3,243 | Participants with IGT and fasting hyperglycemia, aged ≥25y, BMI ≥24 kg/m2, 68% women, 45% minority | Clinical trial Intensive lifestyle modification |
10 y | Year 1–3: Same as DPP Year 4–10: 4 quarterly group visits, with the option of 2 additional sessions each year |
Individual | Health professionals: Case managers Medical staff |
– | Staff time: Questionnaire Training materials: Questionnaire Other components: Questionnaire |
Year 1: $2,469 Year 2: $1,090 Year 3: $1,127 Year 4: $214 Year 5: $150 Year 6: $134 Year 7: $167 Year 8: $171 Year 9: $157 Year 10: $201 Total: $5,881 |
$102 |
|
| |||||||||||
| Translational DPP | |||||||||||
| Kramer et al, 2009 (20); United States |
42 | Adults with prediabetes and/or metabolic syndrome | Community setting Modified DPP (group lifestyle balance program) |
1 y | Core: 12 group sessions Maintenance: 9 group sessions Total: 21 sessions |
Group | Health professionals: Trained prevention professionals |
– | Staff time: Staff report Training materials: NR Other components: NR |
$335 | $16 |
|
| |||||||||||
| Kramer et al, 2011 (21); United States | 81 | Adults with prediabetes and/or metabolic syndrome | Community setting Modified DPP (group lifestyle balance program) |
1 y | Core: 12 group sessions Maintenance: 9 group sessions Total: 21 sessions |
Group | Trained laypeople: Diabetes educators |
– | Staff time: NR Training materials: NR Other components: NR |
$357 | $17 |
|
| |||||||||||
| Krukowski et al, 2013 (22); United States | 116 | Older adults (aged ≥60 y) who were obese (BMI ≥30 kg/m2) and who had no significant memory problems | 12-session translational DPP per reference 20 | 1 y | Core: 12 weekly group sessions Total: 12 sessions |
Group | Trained laypeople: Trained lay health educator |
Recruitment: Staff compilation Screening: - |
Staff time: Staff compilation Training materials: Staff compilation Other components: Staff compilation |
$191 | $16 |
|
| |||||||||||
| Vadheim et al, 2010(23); United States | 84 | Adults at high risk for both diabetes and CVD | Community setting Adapted DPP |
10 mo | Core: 1 6 weekly group sessions Maintenance: 6 monthly group sessions Total: 22 sessions |
Group | Mixed health professional and trained
laypeople: Diabetes educator, nurse |
– | Staff time: NR Training materials: NR Other components: NR |
$652 | $30 |
|
| |||||||||||
| Smith et al, 2010 (24); United States | NR | BMI ≥25 kg/m2 and metabolic syndrome | 2 urban and 2 rural medical practices in
Pennsylvania Modified DPP To help patients with metabolic syndrome lose weight and improve at least 1 metabolic syndrome component |
3 mo | 12 group sessions Total: 12 sessions |
Group | Mixed health professional and trained
laypeople: Trained health professional and lay health workers |
Recruitment: - Screening: NR |
Staff time: NR Training materials: NR Other components: NR |
$407 | $34 |
|
| |||||||||||
| Irvine et al, 2011 (25); United Kingdom | 3,887 | At-risk individuals with diabetes (aged 45–70 y) | Community setting Delivered by Diabetes Prevention Facilitators Promote a 7% weight loss within 6 mo using both diet and exercise |
7 mo | Core: 4 group educational sessions in 3
mo Maintenance: 4 monthly group sessions Total: 8 sessions |
Group | Mixed health professional and trained
laypeople: Diabetes prevention facilitators Physicotherapist Volunteers with diabetes |
Recruitment: - Screening: NR |
Staff time: Questionnaire Training materials: Questionnaire Other components: Questionnaire |
$443 | $55 |
|
| |||||||||||
| Ockene et al, 2012 (26); United States | 312 | Participants who were at high risk for type 2 diabetes | Community setting LLDPP between 2004 and 2007 Healthy food choices, walking 4000 steps per day |
1 y | 3 individual and 13 group sessions Total: 16 sessions |
Mixed group and individual | Trained laypeople: Trained community health workers |
– | Staff time: NR Training materials: NR Other components: NR |
$839 | $53 |
|
| |||||||||||
| Lawlor et al, 2013 (27); United States | 301 | Overweight or obese participants (BMI 25–39 kg/m2) with elevated fasting blood glucose indicating prediabetes | Community setting HELP PD trial A DPP-based lifestyle weight-loss group |
2 y | Core: 26 weekly group sessions and 3 individual
sessions in 6 mo Maintenance: 18 monthly group sessions Total: 47 sessions |
Mixed group and individual | Mixed health professional and trained
laypeople: Trained community health workers and dietician |
Recruitment: - Screening: NR |
Staff time: Questionnaire Training materials: Questionnaire Other components: Questionnaire |
Year 1:$613 Year 2: $305 Total: $918 |
$20 |
|
| |||||||||||
| Translational non-DPP | |||||||||||
| Feldman et al, 2013 (28); Sweden | 142 | KMSP in primary care, diagnosed with metabolic syndrome | Primary care Promote healthy lifestyles, in particular changes in dietary and physical activity habits |
1 y | Core: 26 group lifestyle counseling and support
sessions twice a week for 3 mo Maintenance: 18 biweekly group counseling and support sessions for 9 mo Total: 44 sessions |
Group | Health professional: Practice nurses Health coordinator |
Recruitment: Program documentation Screening: - |
Staff time: Program documentation Training materials: Program documentation Other components: Program documentation |
$427* | $10* |
|
| |||||||||||
| Sagarra et al, 2014 (29); Spain | 552 | Aged 45–75 y at risk for diabetes with IGT and/or IFG | Primary care setting DE-PLAN project 6-h structured lifestyle intervention (diet and physical activity) similar to Finnish DPS using specific teaching techniques Individual or group format |
4.2 y | Year 1: 4 sessions (6 h) Years 2–4: Continuous intervention through telephone calls, text message, letters, and interviews, scheduled for every 6–8 wk |
Group or individual (2 groups) | Health professional: Physicians, nurses, and dieticians |
Recruitment: Forms Screening: Forms |
Staff time: Forms Training materials: Forms Other components: Forms |
$1,133 for the whole intensive intervention
group $1,077 for the group format $1,242 for the individual format |
$4 for the group format* $43 for individual format* |
|
| |||||||||||
| Jacobs-van der Bruggen, 2007 (30); Netherlands | NR | Adults with moderate risks for diabetes, obese adults aged 30–70 y | Community setting Nutrition and exercise for adults with moderate risks for diabetes |
3 y | Year 1: 4 individual and 1 group session; 1 individual
advice by a researcher; 52 weekly fitness programs Years 2–3: 3 individual and 1 group session; 52 biweekly fitness programs Total: 114 sessions Nutrition: 9 sessions Fitness: 105 sessions |
Mixed group and individual | Health professionals: Dietitian, not clear who delivered the fitness program |
– | Staff time: Questionnaire Training materials: Questionnaire Other components: Questionnaire |
$1,416 | Regular session: $64 Fitness: $8 |
BMI = body mass index; CVD = cardiovascular disease; DE-PLAN = Diabetes in Europe: Prevention Using Lifestyle, Physical Activity, and Nutritional Intervention; DPP = Diabetes Prevention Program; DPPOS = Diabetes Prevention Program Outcomes Study; DPS = Diabetes Prevention Study; HELP PD = Healthy Living Partnerships to Prevent Diabetes; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; KMSP = Kalmar Metabolic Syndrome Program; LLDPP = Lawrence Latino Diabetes Prevention Project; NR = not reported.
Only included cost to deliver lifestyle intervention.
Appendix Table 3.
Summary Evidence Table of Included Studies Providing Cost-Effectiveness of Combined Diet and Physical Activity Promotion Programs to Reduce Type 2 Diabetes Among Persons at Increased Risk
| Study, Year (Reference); Country | Population Characteristics | Duration of Intervention/Follow-up | Cost Data Source | Benefit Data Source | Effectiveness Outcome | Model | QALY/DALY/LYG | ICER Health System | ICER Society |
|---|---|---|---|---|---|---|---|---|---|
| Within-trial analysis (n=4) | |||||||||
|
| |||||||||
| DPP Research Group, 2003 (31); United States* | IGT | 3 y/3 y | Real DPP cost data | Survey, CMS fee schedule | Reduce incidence by 58% | Within trial | 0.072 additional QALY | Individual: $50,694/QALY Group: $14,476/QALY |
Individual: $83,130/QALY Group: $46,820/QALY |
|
| |||||||||
| DPP Research Group, 2012 (19); United States* | Participants with IGT and fasting hyperglycemia, ≥25 y, BMI ≥24 kg/m2, 68% women, 45% minority | 10 y/10 y | Real DPP cost data | Survey | DPPOS trial 0.12 additional QALY |
Within trial | 0.12 additional QALY | Individual $15,846/QALY Group: $1,819/QALY |
Individual $24,373/QALY Group: $10,351/QALY |
|
| |||||||||
| Irvine et al, 2011 (25); United Kingdom | At-risk individuals with diabetes (aged 45–70 y) | 7 mo/7 mo | Real cost data | Survey, NHS reference cost, drug formulary | 0.012 additional QALY | Within trial | 0.012 additional QALY | $40,347/QALY | † |
|
| |||||||||
| Sagarra et al, 2014 (29); Spain | Aged 45–75 y, at risk for diabetes with IGT and/or IFG | 4.2 y/4.2 y | Real cost data | Forms | Reduce incidence by 36.5% 0.012 additional QALY |
Within trial | 0.012 additional QALY | $5,359/QALY | † |
| Modeling the trial or extension of trials (n = 16) | |||||||||
|
| |||||||||
| Segal et al, 1998 (32); Australia | Seriously obese Seriously obese with IGT or NGT |
2–3 y/25 y | Based on literature | Survey, insurance scheme | Reducing incidence from 70% to 30% | Markov model | 1 additional LYG | $4,561/LYG | † |
|
| |||||||||
| Caro et al, 2004 (33); Canada | Overweight or obese with IGT | 5 y/10 y | Based on Finnish DPS | Literature, fee schedule, formularies | Based on DPP, Finnish DPS At 5th year, incidence −58% At 10th year, incidence −22% |
Markov model | 0.31 additional LYG | $806/LYG | † |
|
| |||||||||
| Palmer et al, 2004 (34); Australia, France, Germany, Switzerland, United Kingdom | IGT | 3 y/lifetime | DPP apply to fee schedule | Claims | Based on DPP, assuming the effect would not persist beyond the 3rd year | Markov model | 0.08 (Australia) 0.07 (France) 0.07 (Germany) 0.06 (Switzerland) 0.16 (United Kingdom) |
−$8.176/LYG
(Australia) −$11,682/LYG (France) −$15.018/LYG (Germany) −$19,029/LYG (Switzerland) $8.565/LYG (United Kingdom) Mean: −$9.073/LYG |
† |
|
| |||||||||
| Eddy et al, 2005 (35); United States* | IGT | Until diabetes onset/30 y | Year 1 to 3: DPP cost Year 4 and beyond: DPP year 3 cost |
Accounting data | Effect of DPP persists as long as receiving the
intervention At end of 30 y, incidence −15% |
Archimedes Diabetes Model | 0.159 additional QALY | Individual $94,752/QALY Group: $18,409/QALY Individual: $221,549/QALY (HMO perspective) Group: $41,879/QALY (HMO perspective) |
– |
|
| |||||||||
| Herman et al, 2005 (36); United States | IGT | Until diabetes onset/lifetime | Year 1 to 3: DPP cost Year 4 and beyond: DPP year 3 cost |
Claims | The effect of DPP persists as long as receiving the
intervention At the end of lifetime, incidence −24% |
Markov model | 0.57 additional QALY | Individual: $1,805/QALY Group: −$10,450/QALY |
Individual: $13,574/QALY |
|
| |||||||||
| Ackerman et al, 2006 (37); United States | Overweight or obese 50-year-old adults with IGT | Until diabetes onset/lifetime | Year 1 to 3: DPP cost Year 4 and beyond: DPP year 3 cost |
Claims | Based on DPP The DPP effect will continue as long as receiving intervention |
Markov model | Age 50 y: 0.59 additional QALY Age 65 y: 0.27 additional QALY |
Age 50 y: $2,070/QALY Age 65 y: $2,536/QALY |
† |
|
| |||||||||
| Hoerger et al, 2007 (38); United States* | Aged 45–74 y, overweight and obese (BMI
≥25 kg/m2) Groups |
Until diabetes onset/lifetime | Year 1 to 3: DPP cost Year 4 and beyond: DPP year 3 cost |
Claims | The effect of DPP persists as long as receiving the intervention | Markov model | 0.040 additional QALY | Individual: $14,154/QALY Group: $396/QALY |
Individual: $28,849/QALY |
|
| |||||||||
| Jacobs-van der Bruggen et al, 2007 (30); Netherlands | Adults with moderate risks for diabetes, obese adults aged 30–70 y | 3 y/lifetime | 2 published Dutch trials | Literature | BMI: −0.3 to −1.5
kg/m2 Physical activity: 50%−75% more from inactive to moderately active, 20% more from moderately to active |
Markov model | 1.17 additional QALY | $8,735/QALY | † |
|
| |||||||||
| Lindaren et al, 2007 (39); Sweden | IGT Age 60 y BMI >25 kg/m2, FPG >6.1 |
6 y/lifetime | Finnish DPS | Literature | Based on Finnish DPS; no lasting effect if the intervention stops | Markov model | 0.2 additional QALY | $14,852/LYG $13,367/QALY |
$6,756/LYG $6,080/QALY |
|
| |||||||||
| Gillies et al, 2008 (40); United Kingdom | NR | Until diabetes onset/50 y | A systematic review of weight loss programs | Literature, such as UKPDS | Hazard ratio, −0.649 from review | Markov model | 0.05 additional LYG 0.09 additional QALY |
$25,083/LYG $14,352/QALY |
† |
|
| |||||||||
| Bertram et al, 2010(41); Australia | Age >55 y, or age >45 y plus high BMI, family history of type 2 diabetes mellitus or hypertension; people from “high-risk” groups | Average trial period/lifetime | A systematic review and meta-analysis of lifestyle interventions | Benefit schedule | Based on meta-analysis Relative risk: 0.49 Assuming 10% decay of effect after the intervention |
Microsimulation model | 0.05 additional DALY averted | $21,195/DALY | † |
|
| |||||||||
| Smith et al, 2010 (24); United States | BMI ≥25 kg/m2 and metabolic syndrome | 3 mo/3 y | A community-based DPP in Pennsylvania, United States | Literature (DPP, UKPDS, Framingham Heart Study) | By 1 y, metabolic risk:
−16.2% By 3 y, risk:−19% |
Markov model | 0.01 QALY | $5,494/QALY | † |
|
| |||||||||
| Neumann et al, 2011 (42); Germany | FINDRISC between 11–20, or FINDRISC ≥21 and without diagnosis of diabetes | 5 y/lifetime | SDPP | CODE-2 study calculation of average annual direct health care costs of persons with NGT, IGT, and type 2 diabetes | Based on literature, such as PREDIAS and SDPP in
Germany Assuming the effectiveness of the intervention lasts only for 1 y after the intervention (disappears at 7th year) |
Markov model | 0.02–0.03 QALY depending on sex and age | † | Age 30 y: −$41,772/QALY for men,
−$52,136/QALY for women Age 50 y: −$25,079/QALY for men, −$35,217/QALY for women Age 70 y: $39,666/QALY for men, $32,259/QALY for women |
|
| |||||||||
| Palmer et al, 2012 (43); Australia | NR | 10 y/lifetime | DPPOS, using medical benefits schedule in Australia | Survey, unit cost data in Australia | Based on DPPOS trial 0.12 additional QALY | Semi-Markov simulation | 0.3 LYG 0.12 QALY |
−$234/LYG −$411/QALY |
† |
|
| |||||||||
| Feldman et al, 2013 (28); Sweden | NR | 1 y/lifetime | Based on a lifestyle trial in Sweden | Swedish previously published studies | Based on the KMSP in Sweden Assuming effect continued at year 2 then gradually decreased, reaching the level at the start in year 5 and beyond (e.g., −0.4 to −1.1) in BMI in different risk groups +2 to −7 in waist circumference +0.2 to −0.6 in fasting glucose |
Markov model | 0.05–0.14 additional QALY | $4,104/QALY for men with high
risk $23,327/QALY for women with high risk |
Cost-saving for men with high
risk $22,647/QALY for women with high risk |
|
| |||||||||
| Png and Yoong, 2014 (44); Singapore | IGT | 3 y/3 y | DPP, applying unit cost obtained from the Singapore
National University Hospital cost repository Singapore Household Expenditure Survey |
Singapore National University Hospital cost repository | Based on 3-y DPP trial, not explicitly reporting the risk reduction | Markov model | 0.05 QALY | $17,614/QALY | $37,580/QALY |
| Modeling nationwide diabetes prevention programs (n = 2) | |||||||||
|
| |||||||||
| Colagiuri and Walker, 2008 (45); Australia | Australians aged 45–74 y | 10 y/1 0 y | An unspecified “lifestyle program” at Australia, $500 per person per year | Literature, such as UKPDS | Diabetes incidence in IGT:
−60% In IFG: −30% |
Markov model | 36,009 additional DALY averted in the whole nation | $50,707/DALY | † |
|
| |||||||||
| Zhuo et al, 2012 (46); United States | 18–64 y and 65–84 y U.S. population | Until diabetes onset/25 y | Year 1: Based on YMCA-DPP Year beyond: Based on DPPOS maintenance period |
Claims | Year 1: Diabetes incidence: −40% to
−50% Year 2: Diabetes incidence: −40% to −50% Year 3 and beyond: −10 to −15% |
Markov model | 0.04 additional LYG 0.03 additional QALY |
16–64 y:
−$8,378/QALY 65–84 y: −$5,760/QALY |
† |
BMI = body mass index; CMS = Centers for Medicare & Medicaid Services; CODE-2 = Cost of Diabetes in Europe–Type 2; DALY = disability-adjusted life-year; DPP = Diabetes Prevention Program; DPPOS = Diabetes Prevention Program Outcomes Study; DPS = Diabetes Prevention Study; FINDRISC = Finnish Type 2 Diabetes Risk Score; FPG = fasting plasma glucose; ICER = incremental cost-effectiveness ratio; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; KMSP = Kalmar Metabolic Syndrome Program; LYG = life-year gained; NGT = normal glucose tolerance; NHS = National Health Service; NR = not reported; PREDIAS = Prevention of Diabetes Self-management Program; QALY = quality-adjusted life-year; SDPP = Saxon Diabetes Prevention Programme; UKPDS = United Kingdom Prospective Diabetes Study; YMCA = Young Men’s Christian Association.
Study reported from “societal perspective”; however, it was actually from “health system perspective” because only costs to the health system were included.
Study did not include or report the cost or cost-effectiveness for the category.
Footnotes
From Centers for Disease Control and Prevention, Atlanta, Georgia, and HealthPartners Research Foundation, Minneapolis, Minnesota.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-0469.
Author Contributions: Conception and design: R. Li, S. Qu, P. Zhang, S. Chattopadhyay, D. Hopkins, N.P. Pronk.
Analysis and interpretation of the data: R. Li, S. Qu, P. Zhang, S. Chattopadhyay, A. Albright, D. Hopkins, N.P. Pronk. Drafting of the article: R. Li, S. Qu, S. Chattopadhyay, A. Albright.
Critical revision of the article for important intellectual content: R. Li, S. Qu, P. Zhang, S. Chattopadhyay, E.W. Gregg, A. Albright, D. Hopkins, N.P. Pronk.
Final approval of the article: R. Li, S. Qu, P. Zhang, S. Chat-topadhyay, E.W. Gregg, A. Albright, D. Hopkins, N.P. Pronk. Provision of study materials or patients: R. Li.
Statistical expertise: R. Li, S. Qu, S. Chattopadhyay. Administrative, technical, or logistic support: R. Li, S. Qu, S. Chattopadhyay, E.W. Gregg, D. Hopkins.
Collection and assembly of data: R. Li.
Contributor Information
Rui Li, Centers for Disease Control and Prevention, 4770 Bu-ford Highway Northeast, MS F-75, Atlanta, GA 30341.
Shuli Qu, Centers for Disease Control and Prevention, 4770 Buford Highway Northeast, MS E-69, Atlanta, GA 30341.
Ping Zhang, Centers for Disease Control and Prevention, 4770 Bu-ford Highway Northeast, MS F-75, Atlanta, GA 30341.
Sajal Chattopadhyay, Centers for Disease Control and Prevention, 4770 Buford Highway Northeast, MS E-69, Atlanta, GA 30341.
Edward W. Gregg, Centers for Disease Control and Prevention, 4770 Bu-ford Highway Northeast, MS F-75, Atlanta, GA 30341.
Ann Albright, Centers for Disease Control and Prevention, 4770 Bu-ford Highway Northeast, MS F-75, Atlanta, GA 30341.
David Hopkins, Centers for Disease Control and Prevention, 4770 Buford Highway Northeast, MS E-69, Atlanta, GA 30341.
Nicolaas P. Pronk, HealthPartners Research Foundation, 70 33rd Avenue South, Mailstop HBG/21111H, Minneapolis, MN 55425.
References
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2014 Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf on 19 April 2015.
- 3.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Awareness of prediabetes—United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2013;62:209–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–6. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 7.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Preventiaon Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 9.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 10.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163:437–51. doi: 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Community Guide. Atlanta, GA: Community Preventive Services Task Force; 2014. Diabetes Prevention and Control: Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among People at Increased Risk. Accessed at www.thecommunityguide.org/diabetes/supportingmaterials/SScombineddietandpa-econ.html on 19 April 2015. [Google Scholar]
- 12.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alderson P, Green S, Higgins JPT, editors. The Cochrane Library. Chichester, United Kingdom: J Wiley; 2004. Cochrane Reviewers’ Handbook 4.2.2. [Google Scholar]
- 14.Economic Evaluation Abstraction Form. 2010 Accessed at www.thecommunityguide.org/about/EconAbstraction_v5.pdf on 19 April 2015.
- 15.Bureau of Labor Statistics. Consumer Price Index: All Urban Consumers. 2014 Accessed at http://data.bls.gov/cgi-bin/surveymost?cu on 21 April 2015.
- 16.Board of Governors of the Federal Reserve System. Foreign Exchange Rate. 2015 Accessed at www.federalreserve.gov/releases/g5a/current on 19 April 2015.
- 17.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon OutcomesRes. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization; 2001. Accessed at http://whqlibdoc.who.int/publications/2001/924154550x.pdf on 19 April 2015. [Google Scholar]
- 19.Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–30. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am JPrevMed. 2009;37:505–11. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ. 2011;37:659–68. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 22.Krukowski RA, Pope RA, Love S, Lensing S, Felix HC, Prewitt TE, et al. Examination of costs for a lay health educator-delivered translation of the Diabetes Prevention Program in senior centers. Prev Med. 2013;57:400–2. doi: 10.1016/j.ypmed.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadheim LM, Brewer KA, Kassner DR, Vanderwood KK, Hall TO, Butcher MK, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. 2010;26:266–72. doi: 10.1111/j.1748-0361.2010.00288.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith KJ, Hsu HE, Roberts MS, Kramer MK, Orchard TJ, Piatt GA, et al. Cost-effectiveness analysis of efforts to reduce risk of type 2 diabetes and cardiovascular disease in southwestern Pennsylvania, 2005–2007. Prev Chronic Dis. 2010;7:A109. [PMC free article] [PubMed] [Google Scholar]
- 25.Irvine L, Barton GR, Gasper AV, Murray N, Clark A, Scarpello T, et al. Cost-effectiveness of a lifestyle intervention in preventing type 2 diabetes. Int J Technol Assess Health Care. 2011;27:275–82. doi: 10.1017/S0266462311000365. [DOI] [PubMed] [Google Scholar]
- 26.Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102:336–42. doi: 10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawlor MS, Blackwell CS, Isom SP, Katula JA, Vitolins MZ, Morgan TM, et al. Cost of a group translation of the Diabetes Prevention Program: Healthy Living Partnerships to Prevent Diabetes. Am J Prev Med. 2013;44:S381–9. doi: 10.1016/j.amepre.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman I, Hellström L, Johansson P. Heterogeneity in cost-effectiveness of lifestyle counseling for metabolic syndrome risk groups—primary care patients in Sweden. Cost Eff Resour Alloc. 2013;11:19. doi: 10.1186/1478-7547-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagarra R, Costa B, Cabré JJ, Solà-Morales O, Barrio F, et al. Grupo de Investigación DE-PLAN-CAT/PREDICE Lifestyle interventions for diabetes mellitus type 2 prevention. Rev Clin Esp (Barc) 2014;214:59–68. doi: 10.1016/j.rce.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs-van der Bruggen MA, Bos G, Bemelmans WJ, Hoogenveen RT, Vijgen SM, Baan CA. Lifestyle interventions are cost-effective in people with different levels of diabetes risk: results from a modeling study. Diabetes Care. 2007;30:128–34. doi: 10.2337/dc06-0690. [DOI] [PubMed] [Google Scholar]
- 31.Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–23. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal L, Dalton AC, Richardson J. Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus. Health Promot Int. 1998;13:197–209. [Google Scholar]
- 33.Caro JJ, Getsios D, Caro I, Klittich WS, O’Brien JA. Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med. 2004;21:1229–36. doi: 10.1111/j.1464-5491.2004.01330.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmer AJ, Roze S, Valentine WJ, Spinas GA, Shaw JE, Zimmet PZ. Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther. 2004;26:304–21. doi: 10.1016/s0149-2918(04)90029-x. [DOI] [PubMed] [Google Scholar]
- 35.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251–64. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 36.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. Diabetes Prevention Program Research Group The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–32. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackermann RT, Marrero DG, Hicks KA, Hoerger TJ, Sorensen S, Zhang P, et al. An evaluation of cost sharing to finance a diet and physical activity intervention to prevent diabetes. Diabetes Care. 2006;29:1237–41. doi: 10.2337/dc05-1709. [DOI] [PubMed] [Google Scholar]
- 38.Hoerger TJ, Hicks KA, Sorensen SW, Herman WH, Ratner RE, Ackermann RT, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care. 2007;30:2874–9. doi: 10.2337/dc07-0885. [DOI] [PubMed] [Google Scholar]
- 39.Lindgren P, Lindström J, Tuomilehto J, Uusitupa M, Peltonen M, Jönsson B, et al. DPS Study Group Lifestyle intervention to prevent diabetes in men and women with impaired glucose tolerance is cost-effective. Int J Technol Assess Health Care. 2007;23:177–83. doi: 10.1017/S0266462307070286. [DOI] [PubMed] [Google Scholar]
- 40.Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ. 2008;336:1180–5. doi: 10.1136/bmj.39545.585289.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertram MY, Lim SS, Barendregt JJ, Vos T. Assessing the cost-effectiveness of drug and lifestyle intervention following opportunistic screening for pre-diabetes in primary care. Diabetologia. 2010;53:875–81. doi: 10.1007/s00125-010-1661-8. [DOI] [PubMed] [Google Scholar]
- 42.Neumann A, Schwarz P, Lindholm L. Estimating the cost-effectiveness of lifestyle intervention programmes to prevent diabetes based on an example from Germany: Markov modelling. Cost Eff Resour Alloc. 2011;9:17. doi: 10.1186/1478-7547-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer AJ, Tucker DM. Cost and clinical implications of diabetes prevention in an Australian setting: a long-term modeling analysis. Prim Care Diabetes. 2012;6:109–21. doi: 10.1016/j.pcd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Png ME, Yoong JS. Evaluating the cost-effectiveness of lifestyle modification versus metformin therapy for the prevention of diabetes in Singapore. PLoS One. 2014;9:e107225. doi: 10.1371/journal.pone.0107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colagiuri S, Walker AE. Using an economic model of diabetes to evaluate prevention and care strategies in Australia. Health Aff (Millwood) 2008;27:256–68. doi: 10.1377/hlthaff.27.1.256. [DOI] [PubMed] [Google Scholar]
- 46.Zhuo X, Zhang P, Gregg EW, Barker L, Hoerger TJ, Pearson-Clarke Tony, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff (Millwood) 2012;31:50–60. doi: 10.1377/hlthaff.2011.1115. [DOI] [PubMed] [Google Scholar]
- 47.The World Bank. GDP per capita (current US$) 2014 Accessed at http://data.worldbank.org/indicator/NY.GDP.PCAP.CD on 15 April 2015.
- 48.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–94. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radl K, Ianuale C, Boccia S. A systematic review of the cost-effectiveness of lifestyle modification as primary prevention intervention for diabetes mellitus type 2. Epidemology Biostatistics and Public Health. 2013;10:8. [Google Scholar]
- 50.Vijgen SM, Hoogendoorn M, Baan CA, de Wit GA, Limburg W, Feenstra TL. Cost effectiveness of preventive interventions in type 2 diabetes mellitus: a systematic literature review. Pharmacoeconomics. 2006;24:425–41. doi: 10.2165/00019053-200624050-00002. [DOI] [PubMed] [Google Scholar]
- 51.Wylie-Rosett J, Herman WH, Goldberg RB. Lifestyle intervention to prevent diabetes: intensive and cost effective. Curr Opin Lipidol. 2006;17:37–44. doi: 10.1097/01.mol.0000203890.27267.eb. [DOI] [PubMed] [Google Scholar]
- 52.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med. 2008;358:661–3. doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 53.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J PrevMed. 2013;44:S346–51. doi: 10.1016/j.amepre.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Medicare & Medicaid Services. Physician fee schedule. 2014 Accessed at www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=1&T&4&HT&0&CT&0&H1&G0447&M&1 on 19 April 2015.
- 55.Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral Counseling to Promote a Healthy Lifestyle for Cardiovascular Disease Prevention in Persons With Cardiovascular Risk Factors: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2014. (Evidence synthesis no. 113. AHRQ publication no. 13-05179-EF-1). [PubMed] [Google Scholar]


