Abstract
Objectives
Endothelial dysfunction is linked to insulin resistance, inflammatory activation and increased cardiovascular risk in diabetes mellitus; however the mechanisms remain incompletely understood. Recent studies have identified pro-inflammatory signaling of Wnt5a through JNK as a regulator of metabolic dysfunction with potential relevance to vascular function. We sought to gain evidence that increased activation of Wnt5a-JNK signaling contributes to impaired endothelial function in patients with diabetes mellitus.
Approach
We measured flow-mediated dilation of the brachial artery and characterized freshly isolated endothelial cells by protein expression, eNOS activation, and nitric oxide production in from 85 subjects with Type 2 diabetes mellitus (n=42) and age- and sex-matched non-diabetic controls (n=43) and in human aortic endothelial cells treated with Wnt5a.
Results
Endothelial cells from patients with diabetes displayed 1.3-fold higher Wnt5a levels (P=0.01) along with 1.4-fold higher JNK activation (P<0.01) without a difference in total JNK levels. Higher JNK activation was associated with lower flow-mediated dilation, consistent with endothelial dysfunction (r=0.53, P=0.02). Inhibition of Wnt5a and JNK signaling restored insulin and A23187-mediated eNOS activation and improved nitric oxide production in endothelial cells from patients with diabetes. In endothelial cells from non-diabetic controls, rWnt5a treatment inhibited eNOS activation replicating the diabetic endothelial phenotype. In HAECs, Wnt5a-induced impairment of eNOS activation and nitric oxide production was reversed by Wnt5a and JNK inhibition.
Conclusions
Our findings demonstrate that non-canonical Wnt5a signaling and JNK activity contributes to vascular insulin resistance and endothelial dysfunction and may represent a novel therapeutic opportunity to protect the vasculature in patients with diabetes.
Keywords: diabetes mellitus type 2, endothelium, inflammation
Introduction
Patients with Type 2 diabetes experience high rates of adverse cardiovascular events even with implementation of current risk reduction interventions. A key feature of diabetes is the development of endothelial dysfunction that participates in the clinical development of vascular disease 1. Experimental studies identify altered vascular insulin signaling as a modulator of impaired endothelial function in diabetes models 2, and our recent work has demonstrated endothelial insulin resistance and inflammatory activation as a drivers of endothelial dysfunction in patients with diabetes 3. However, the specific regulators accounting for abnormal endothelial phenotype in human diabetes remain incompletely defined.
Recent work supports the concept that Wnt5a signaling is a novel inflammatory mediator in metabolic diseases. Wnt proteins comprise a large family of secreted glycoproteins that are well-established as regulators of development 4. Emerging evidence links non-canonical Wnt signaling, particularly Wnt5a, to disease processes in metabolic disorders 5, 6. In animal models, enhanced Wnt5a signaling in adipose tissue contributes to obesity-associated insulin resistance and metabolic dysfunction 7, 8. Further, Wnt5a activation impairs angiogenesis in retina 9 and in a model of obesity-linked peripheral artery disease 10. As a non-canonical Wnt, Wnt5a triggers the activation of signaling pathways through JNK (c-jun N-terminal kinase) activation 7, 11. JNK is a stress-activated kinase in the MAP kinase family with heightened activity in metabolic diseases. Activated JNK promotes insulin resistance in non-vascular tissues by phosphorylation of IRS-1 at an inhibitory site 12. There has been considerable interest in pharmacologic inhibitors of JNK as potential therapies for diabetes.13, 14 However, there has been limited translational work investigating the functional impact of of non-canonical Wnt signaling and JNK activation to endothelial function in humans. The present study sought to investigate the involvement of the Wnt5a/JNK axis in vascular endothelial dysfunction in patients with diabetes.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Study subjects and vascular function
We enrolled 42 patients with Type 2 diabetes mellitus and 43 control subjects of similar age and sex. The clinical characteristics and measures of vascular function are shown in the Table. As expected, the patients with diabetes had clinical parameters consistent with metabolic dysfunction including higher fasting glucose, hemoglobin A1C, triglycerides, and body mass index. Likewise, diabetic subjects were taking medications to lower blood glucose, and had lower total and LDL cholesterol levels, likely reflecting the greater use of cholesterol-lowering medications. Endothelium-dependent flow-mediated dilation of the brachial artery was significant lower in the diabetic patients, consistent with the presence of endothelial dysfunction.
Table.
Clinical and Vascular Characteristics
| Nondiabetic (n=43) | Diabetic (n=42) | |
|---|---|---|
| Clinical Characteristics | ||
| Age, y | 50±9 | 54±12 |
| Female sex, % | 42 | 60 |
| Postmenopausal,% women | 50 | 72 |
| Black race, % | 47 | 69* |
| Weight, kg | 85±18 | 105±24* |
| Height, cm | 174±9 | 169±14 |
| Body mass index, kg/m2 | 28.3±5.1 | 36.3±8* |
| Total cholesterol, mg/dL | 197±40 | 184±44 |
| LDL cholesterol, mg/dL | 122±64 | 114±38 |
| HDL cholesterol, mg/dL | 54±16 | 45±10* |
| Triglycerides, mg/dL | 105±59 | 121±50* |
| Fasting glucose, mg/dL | 88±10 | 150±57* |
| Hemoglobin A1c, % | 5.3±1.4 | 7.5±1.9* |
| Systolic blood pressure, mm Hg | 122±15 | 132±26* |
| Diastolic blood pressure, mm Hg | 75±11 | 77±16 |
| Ankle-brachial index | 1.14±0.1 | 1.09±0.1* |
| Antiplatelet therapy, % | 9 | 42* |
| Lipid lowering therapy, % | 12 | 45* |
| ACE inhibitor or ARB therapy, % | 3 | 50* |
| Metformin, % | 0 | 74* |
| Sulfonylureas, % | 0 | 12* |
| Insulin therapy, % | 0 | 48* |
| Peripheral Artery Disease, % | 0 | 7 |
| Coronary Artery Disease, % | 0 | 7 |
| Prior Stroke, % | 2 | 7 |
| Vascular function | ||
| Baseline diameter, mm | 4.12±0.77 | 4.23±0.67 |
| Baseline flow, mL/min | 107±85 | 125±81 |
| Hyperemic flow, mL/min | 953±435 | 762±389 |
| Flow-mediated dilation, % | 9.9±4.1 | 6.2±3.3* |
| Nitroglycerin-mediated dilation | 15.1±8.5 | 9.23±8.3* |
| Baseline flow velocity | 12±6 | 14±6 |
| Hyperemic flow velocity | 116±30 | 88±35* |
Data are expressed as mean±SD. LDL indicates low-density lipoprotein; HDL, high density lipoprotein; ACE, angiotensin-converting enzyme: ARB-angiotensin receptor blocker: PAD, peripheral artery disease: ABI, Ankle-branchial index; CAD, coronary artery disease; CAD, Cardiovascular disease.
P<0.05
Impaired endothelial activation and function in patients with diabetes
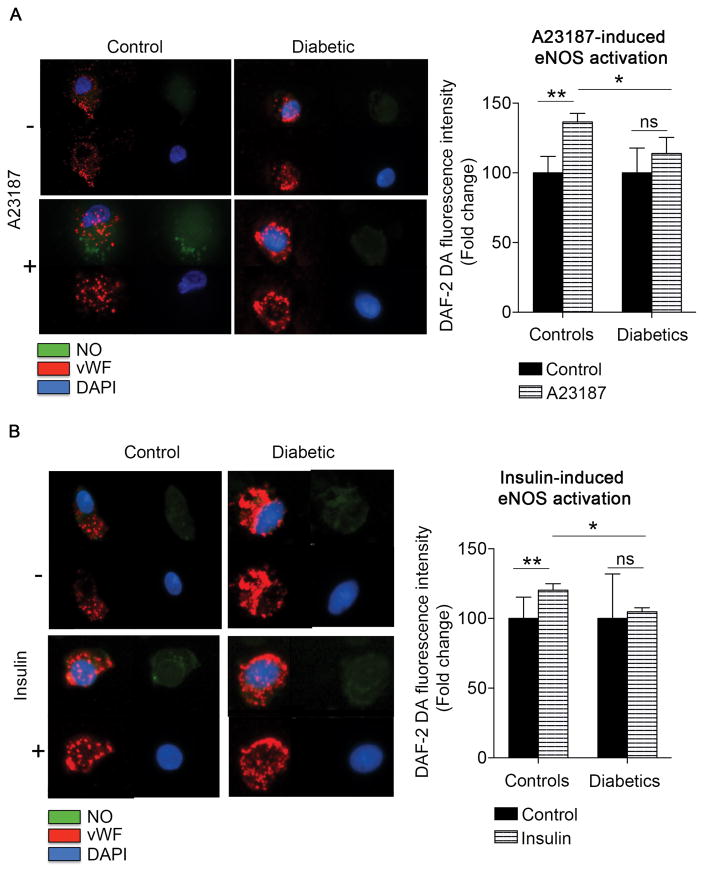
To study endothelial function we evaluate activation of eNOS in endothelial cells isolated from diabetic patients and non-diabetic controls. As shown in Figure SIA, and consistent with our prior reports, eNOS activation by insulin was lower in freshly isolated endothelial cells from patients with diabetes (P<0.001)3. In addition, activation of eNOS by the calcium ionophore A23187 was impaired in patients with diabetes consistent with a multifaceted impairment of eNOS signaling in diabetes (P=0.02) (Figure SIB). Further, we now show reduced insulin- and A23186-induced nitric oxide production in endothelial cells from patients with diabetes consistent with endothelial dysfunction (Figure 1). The specificity of the nitric oxide probe was corroborated by using the nitric oxide synthase inhibitor L-NAME in the presence or absence of A23187. As expected, L-NAME treatment significantly limited the A23187-induced stimulation of nitric oxide in control patients (n=8, **P=0.003) (Figure SII).
Figure 1. Diabetes is associated with altered nitric oxide production.
Venous endothelial cells from diabetic and non-diabetic patients were freshly isolated and loaded with nitric oxide specific probe DAF-2 DA fluorescence probe, treated with eNOS agonist and fluorescence intensity was quantified. A, Representative cells from nondiabetic and diabetic patients treated with A23187 (1 μM, 20 min) are shown. Pooled data demonstrate that A23187 increased 36±6% NO production in endothelial cells from nondiabetic controls (n=5, **P=0.003) but not in endothelial cells from patients with diabetes (n=7). There was a significant difference in the response to A23187 between control and diabetic groups (*P=0.01) B, Representative cells from nondiabetic and diabetic patients incubated with 0 nmol/L or 10 nmol/L of insulin for 30 min are shown. Pooled data demonstrate that insulin increased NO production in endothelial cells from nondiabetic controls (n=7, **P=0.0016) but not in endothelial cells from patients with diabetes (n=5). There was a significant difference in the response to insulin between control and diabetic groups (*P=0.028).
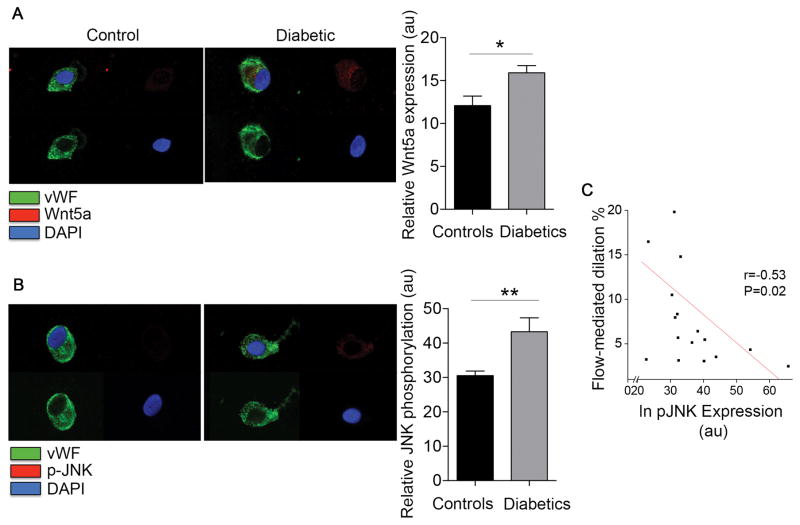
Wnt5a expression and JNK activation in freshly isolated endothelial cells from patients with diabetes
To investigate the potential role of elevated Wnt5a expression in endothelial dysfunction associated with diabetes, we first evaluated Wnt5a protein levels in endothelial cells by quantitative immunofluorescence staining. As shown in Figure 2A, endothelial cells from diabetic patients had higher Wnt5a expression compared with non-diabetic controls (P=0.01). A compelling body of evidence supports that Wnt5a is predominantly involved in β-catenin-independent signaling with JNK, its main downstream target 15, 16. To evaluate JNK activation in, we performed quantitative immunofluorescence to measure JNK phosphorylation at the activation sites (Threonine 183/Tyrosine 185). As shown in Figure 2B, diabetic patients showed higher levels of activated JNK compared with controls (43±4.3 vs. 31±1.3, **P=0.003). Levels of total JNK expression were similar in both groups (P=0.17) (Figure SIII), suggesting that higher levels of activated JNK in cells from diabetics was not attributable to altered total protein expression. Notably, higher levels of activated JNK were associated with lower flow-mediated dilation consistent with a link between JNK activation and impaired endothelial vasodilator function (Figure 2C).
Figure 2. Diabetes is associated with an enhanced Wnt5a expression and JNK phosphorylation in endothelial cells.
Venous endothelial cells from diabetic and non-diabetic patients were freshly isolated as described in methods. Endothelial cells were identified by von Willebrand factor (vWF) staining and nuclear morphology. A, Representative cell from a diabetic patient (right) shows higher Wnt5a expression compared with a cell from a non-diabetic control (left). Pooled data show that Wnt5a levels were higher in diabetic patients (n=9) compared with the non-diabetic controls (n=12, *P=0.01). B, JNK activation was evaluated as JNK phosphorylation at Thr183/Tyr185 (red). Representative cell from a patient with diabetes (right) show higher basal JNK phosphorylation at Thr183/Tyr185 compared with the nondiabetic control (left). Pooled data demonstrated an enhanced JNK activation in endothelial cells from diabetic patients (n=8) compared with nondiabetic controls (n=11, **P=0.003). C, Higher JNK activation in freshly isolated venous endothelial cells from patients was associated with lower flow-mediated dilation (r=0.53, *P=0.02).
Wnt5a activation impairs endothelial function
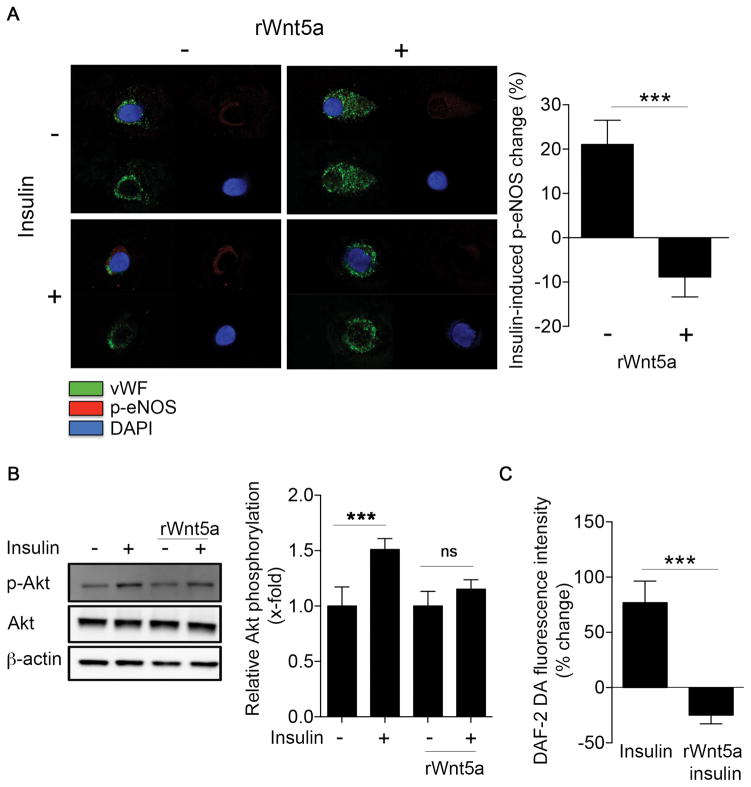
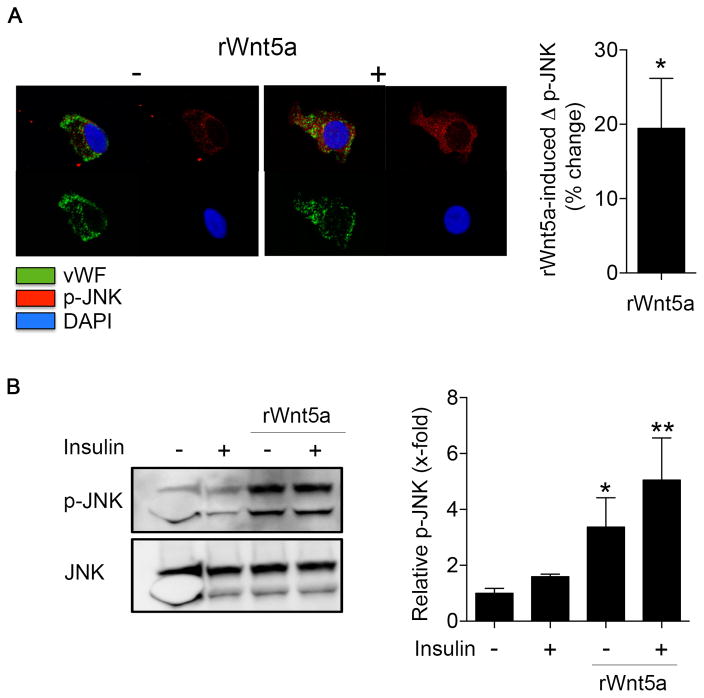
To evaluate the effects of exogenous Wnt5a on endothelial function, we incubated freshly isolated endothelial cells from non-diabetic controls with recombinant Wnt5a protein and measure its impact on insulin-stimulated eNOS activation by quantitative immunofluorescence. Treatment of endothelial cells isolated from control patients with Wnt5a (100 ng/ml, 30 min) impaired insulin-stimulatd eNOS phosphorylation at Serine 1177 (−9±5% compared to 21±6%, P<0.001, Figure 3A).
Figure 3. Wnt5a promotes insulin resistance in endothelial cells.
Endothelial cells from nondiabetic patients and HAECs were treated with rWnt5a protein (100 ng/mL) for 30 minutes at 37°C, before insulin stimulation (10 nM, 30 minutes). A, Freshly isolated endothelial cells from nondiabetic patients were treated with 0 or 10 nmol/L insulin for 30 minutes in the absence or the presence of rWnt5a, and then fixed. eNOS activation was evaluated as eNOS phosphorylation at Ser1177. Representative cells from nondiabetic patient treated with rWnt5a (right) are shown. Insulin increased eNOS phosphorylation at Ser1177 in the nondiabetic patient (left top, red vs. left bottom, red), but treatment with rWnt5 impaired insulin-mediated changed in eNOS phosphorylation at Ser1177 (right top, red vs. right bottom, red). Pooled data demonstrated that eNOS phosphorylation increased 21±6% from baseline with insulin stimulation in the absence of Wnt5a vs. a 9±5% decrease in cells treated with Wnt5a recombinant protein (n=10, ***P<0.001). B, Akt activation was studied by Western blot by evaluating Akt phosphorylation at Ser473. The blots shown are representative of at least three independent experiment that yielded equivalent results. The bar graph represents the mean values ±SEM of at least three independent experiments (n=8, ***P<0.001 Insulin vs control, P=0.06 Insulin rWnt5a vs rWnt5a). C, HAECs were loaded with nitric oxide specific fluorescent probe DAF-2 DA (5 μM, 30 min), then treated with 0 nmol/L or 10 nmol/L insulin for 30 minutes in the absence or the presence of rWnt5a. The bar graph represents the mean values ±SEM independent experiments (n=8, ***P<0.001).
We performed experiments in HAECs to further characterize the impairment of insulin signaling induced by Wnt5a treatment. Incubating HAECs with Wnt5a recombinant protein (100 ng/ml) increased endothelial Wnt5a levels by western blot (P<0.001) and quantitative immunofluorescence (P=0.007) (Figure SIV). As shown in Figure 3B, Wnt5a treatment abrogated the insulin stimulated, activating phosphorylation of Akt at Ser473 as assessed by Western immunoblot. Correspondingly, Wnt5a also reduced insulin-stimulated nitric oxide synthase activity (Figure 3C and Figure SVA). The specificity of the nitric oxide synthase activity assay was tested using the nitric oxide synthase inhibitor L-NAME in the presence or absence of insulin. As shown in Figure SVB, L-NAME treatment completely abolished the increase in nitric oxide induced by insulin (n=8, **P=0.004). In a similar fashion, Wnt5a treatment reduced both A23187-mediated stimulation of eNOS phosphorylation and A23187-mediated stimulation of nitric oxide release (Figure SVI). Collectively, the findings in freshly isolated patient cells and cultured endothelial cells suggest that Wnt5a activation mimics the endothelial dysfunction observed in the endothelial cells from patients with diabetes.
Wnt5a and JNK inhibition restore endothelial nitric oxide synthase activation and endothelial function
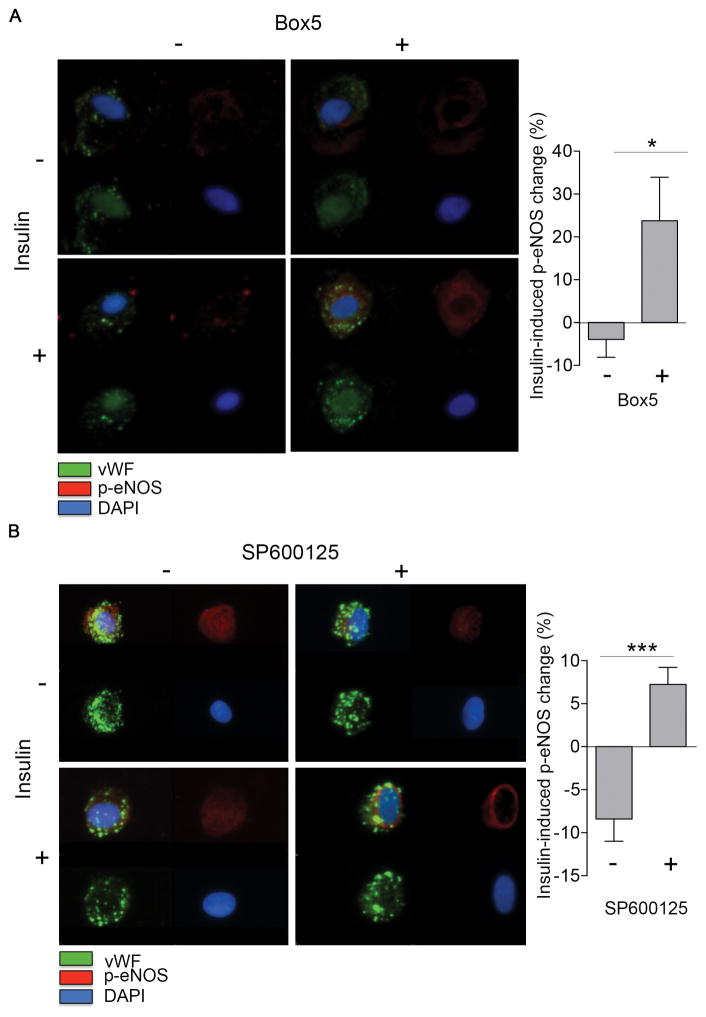
To gain insight into the potential role of Wnt5a signaling in endothelial dysfunction in diabetes, we evaluated the effect of Wnt5a inhibition on eNOS activation in diabetes. We treated freshly isolated endothelial cells from diabetic patients with the Wnt5a competitive antagonist, Box5 (100 μM, 1h) 17, 18. As shown in Figure 4A Wnt5a inhibition by Box5 restored eNOS activation by insulin in endothelial cells from diabetic patients (n=7, P=0.04). In addition, incubation of cultured endothelial cells with Box5 restored insulin-mediated eNOS phosphorylation in the presence of rWnt5a (Figure SVII, n=4, *P=0.05). Then we analyzed the role in endothelial dysfunction of the key Wnt5a downstream modulator, JNK that has previously been described to promote insulin resistance in non-vascular cell types 12, 19, 20. To investigate the importance of JNK to endothelial dysfunction and insulin resistance, we treated freshly isolated endothelial cells from diabetic patients with SP600125, specific inhibitor of JNK 21. Inhibition of JNK with this reagent restored insulin-mediated eNOS phosphorylation in endothelial cells from patients with diabetes (Figure 4B). Further, JNK inhibition restored A23187-induced nitric oxide production in these cells (**P=0.002 vs. control) (Figure SVIII). Collectively, these findings suggest that Wnt5 and JNK activation contributes to endothelial dysfunction in diabetic patients.
Figure 4. Wnt5a and JNK JNK inhibitors restores endothelial nitric oxide synthase activation and insulin signaling in endothelial cells from diabetic patients.
As described in Methods, freshly isolated endothelial cells from patients with diabetes were isolated. eNOS phosphorylation at Ser1177 was quantified by evaluating 20 cells per patient in each condition. A, Representative cells from a patient with diabetes treated with 0 or 100 μmol/L Box5, Wnt5a competitive antagonist, for 1 hour, along with 0 or 10 nmol/L insulin (30 minutes). Treatment with Box5 improved the insulin-mediated change in eNOS phosphorylation at Ser1177 (right top, red vs. right bottom, red) compared with control condition (left top, red vs. left bottom, red). Pooled data demonstrate that treatment with Box5 restored insulin-mediated eNOS phosphorylation at Ser1177 in endothelial cells from patients with diabetes (n=7, *P=0.04). B, Representative cells from a patient with diabetes treated with 0 or 10 μmol/L SP600125, a chemical inhibitor for JNK, along with 0 or 10 nmol/L insulin for 30 minutes. Treatment with SP600125 improved the insulin-mediated change in eNOS phosphorylation at Ser1177 (right top, red vs. right bottom, red) compared with control condition (left top, red vs. left bottom, red). Pooled data demonstrate that treatment with SP600125 restored insulin-mediated eNOS phosphorylation at Ser1177 in endothelial cells from patients with diabetes (n=7, ***P<0.001).
JNK activation mediates Wnt5a-induced endothelial dysfunction
To determine if Wnt5a was an upstream modulator of JNK in our model, we examined JNK activation after recombinant Wnt5a protein treatment in both freshly isolated endothelial cells from healthy patients and in HAECs. As shown in Figure 5A, Wnt5a treatment in endothelial cells from non-diabetic controls induced a significant increase in JNK phosphorylation at the Thr183/Tyr185 activation site (*P=0.01). Similarly, Wnt5a overexpression activated JNK at these phosphorylation sites in HAECs (*P=0.02 vs control) (Figure 5B). Wnt5a inhibition by Box5 induced a significant decrease in JNK phosphorylation in endothelial cells freshly isolated from diabetic patients (Figure SIX).
Figure 5. Wnt5a induces an increase in JNK activation in endothelial cells.
Venous endothelial cells from non-diabetic patients or HAECs were treated with rWnt5a protein (100 ng/mL, 1h). A, Venous endothelial cells freshly isolated from non-diabetic patients as described in methods and JNK activation was evaluated as JNK phosphorylation at Thr183/Tyr185. Representative cells from a nondiabetic patient are shown. Pooled data demonstrated an enhanced JNK activation in endothelial cells when treated with rWnt5a (n=8, *P=0.01). B, JNK activation was studied by Western blot by evaluating JNK phosphorylation at Thr183/Tyr185 after rWnt5a treatment. The blots shown are representative of at least three independent experiment that yielded equivalent results. The bar graph represents the mean values ±SEM of at least three independent experiments (n=8, *P=0.02 rWnt5a vs control, *P=0.003 rWnt5a insulin vs control).
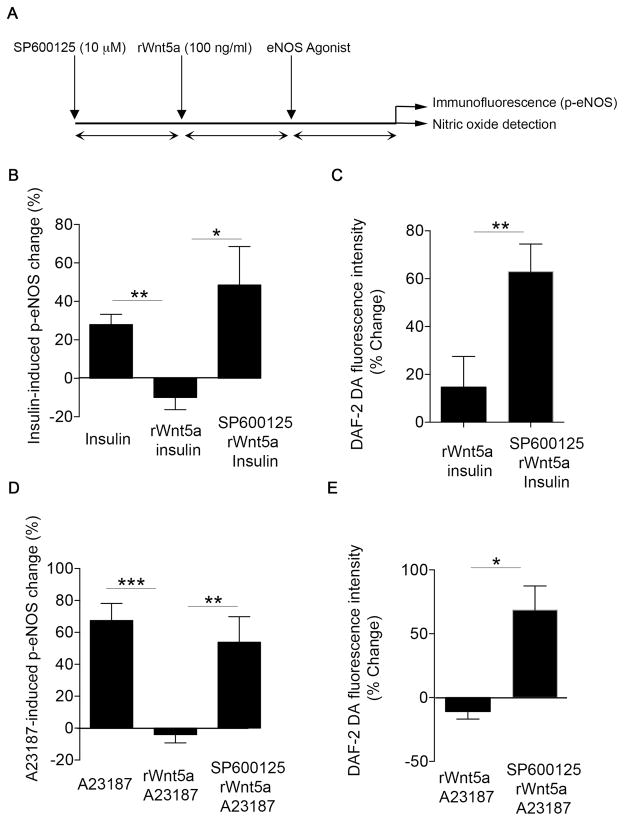
Then, we evaluated whether the endothelial dysfunction induced by Wnt5a was dependent on JNK activation. In cultured cells, Wnt5a-induced JNK activation was completely blocked by pretreatment with the JNK inhibitor SP600125 (Figure SX). As shown in Figure 6, inhibition of JNK with SP600125 prevented the inhibitory effects of Wnt5a treatment insulin- and A23187-stimulated eNOS phosphorylation at Ser1177. The findings were not attributable to differential eNOS expression (Figure SXI). Furthermore, JNK inhibition restored the insulin- and A23187-stimulated nitric oxide production in HAEC’s that were pretreated with Wnt5a (Figure 6C and 6E). Taken together, these results suggest that JNK activation mediates the endothelial dysfunction induced by Wnt5a.
Figure 6. JNK activation is essential for Wnt5a-induced endothelial dysfunction.
HAECs were treated with 100 nmol/L rWnt5a for 30 minutes in the absence or the presence of SP600125 (10 μmol/L, 30 minutes) and insulin or A23187 response was evaluated. A, Scheme of experimental design. B, Insulin mediated eNOS activation was evaluated as eNOS phosphorylation at Ser1177. Pooled data demonstrate that insulin increased eNOS phosphorylation at Ser1177, but treatment with rWnt5 impaired insulin-mediated changed in eNOS phosphorylation at Ser1177 (n=4, **P=0.002). Incubation with JNK chemical inhibitor SP600125 restored insulin-mediated eNOS phosphorylation at Ser1177 in HAECs treated with rWnt5a (n=4, *P=0.04). C, HAECs were loaded with nitric oxide specific probe (DAF-2 DA, 5 μM) in the absence or in the presence of SP600125. Then, cells were treated with rWnt5a and nitric oxide production by insulin was quantified by fluorescence microscopy (n=3, **P=0.001). D, A23187 mediated eNOS activation was evaluated as eNOS phosphorylation at Ser1177. Pooled data demonstrate that A23187 increased eNOS phosphorylation at Ser1177, but treatment with rWnt5 impaired eNOS activation (n=11, ***P<0.001). Incubation with JNK chemical inhibitor SP600125 improved A23187-mediated eNOS activation in HAECs treated with rWnt5a (n=11, **P=0.01). E, HAECs were loaded with nitric oxide specific probe (DAF-2 DA, 5 μM) in the absence or in the presence of SP600125. Then, cells were treated with 100 nmol/L rWnt5a and nitric oxide production by A23187 was quantified by fluorescence microscopy (n=3, *P=0.047).
Role of oxidative stress in Wnt5a-induced endothelial dysfunction
Oxidative stress has been implicated as a mediator of endothelial dysfunction in diabetes 22–24. Moreover, reactive oxygen species (ROS) and oxidative stress have been described as signaling regulators relevant to JNK and Wnt5a in other cell types 25–32. In order to evaluate the relevance of ROS to Wnt5a-dependent endothelial dysfunction, we studied the effect of Wnt5a on ROS production in cultured endothelial cells. In HAECs treated with rWnt5a, there was no change in oxidative stress measured by nitrotyrosine levels or DHE fluorescence levels (Figure SXII). We have previously shown that nitrotyrosine levels are higher in endothelial cells from patients with diabetes3. In endothelial cells from patients with diabetes, treatment with the Wnt5a inhibitor Box5 had no effect on nitrotyrosine levels (Figure SXII). Taken together, these findings suggest that Wnt5a-mediated endothelial dysfunction is not primarily induced through ROS generation.
Discussion
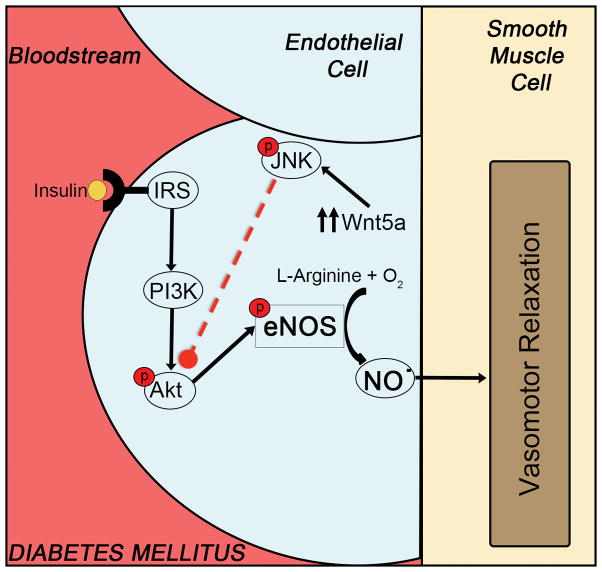
In the present study, we demonstrated the functional relevance of a novel inflammatory pathway to endothelial insulin resistance and dysfunction in human diabetes. Using endothelial cells directly isolated from patients with diabetes, we provide evidence for that Wnt5a signaling and JNK activation mediate impaired endothelial function in patient with diabetes. Impaired endothelial cell signaling and function was associated with higher Wnt5a expression and JNK phosphorylation in diabetes. Wnt5a treatment induced an endothelial phenotype that mimicked diabetes with endothelial insulin resistance and JNK activation. Critically, pharmacological antagonists of Wnt5a and JNK, restored endothelial signaling and increased nitric oxide production in patients with diabetes. In cultured endothelial cells, Wnt5a and JNK inhibition blocked the adverse effects of Wnt5a on eNOS activation and nitric oxide production, indicating that JNK activation is a downstream modulator of the adverse effects of Wnt5a on endothelial function. Taken together, our findings suggest that the Wnt5a-JNK signaling pathway is a contributing mechanism for endothelial dysfunction that may serve as a treatment target to blunt the adverse vascular consequences of diabetes (Figure 7).
Figure 7.
Endothelial cells from diabetic patients showed an enhanced expression of the pro-inflammatory cytokine Wnt5a, accompanied with an increased activation of JNK. Wnt5a/JNK sequential activation contributes to endothelial dysfunction and impairment in eNOS activation and nitric oxide production
Chronic systemic inflammation and nutrient excess promote insulin resistance in diabetes. Pro-inflammatory cytokines and nutrient overabundance converge to activate JNK 33, 34. JNK has been widely demonstrated to be activated in metabolic diseases in animal models 11, 33, 35 and has been described as a crucial mediator of insulin resistance through IRS-1 phosphorylation at Serine 307 35. In preclinical models, JNK deletion protects against diet-induced insulin resistance in diabetes and preserves endothelial function in high-cholesterol fed mice indicating a potential connection to vascular disease 35, 36. Human studies show increased JNK activation in adipose tissue in obesity 37–39; however, the relevance to vascular dysfunction is unknown. JNK activity has been proposed as a potential treatment target for obesity, insulin resistance and type 2 diabetes 13, 40 and several JNK inhibitors have been tested in preclinical models and early stage trials as anti-inflammatory agents 41–43. Our finding that JNK is activated in endothelial cells from diabetic patients and is associated with endothelial dysfunction supports the concept that JNK signaling is upregulated in the diabetic vasculature. Most importantly, our observation that JNK inhibition has the potential to restore insulin action in the endothelium and promote nitric oxide production suggests the possibility that JNK modulation will have beneficial vascular effects in diabetes.
Targeting specific upstream activators of JNK in the endothelium may have additional clinical relevance. In this regard, we focused on non-canonical Wnt5a given our recent work demonstrating relevance to metabolic and vascular dysfunction in animal models, obese humans, and peripheral artery disease. Wnt5a functions as a novel pro-inflammatory mediator of JNK-activation relevant to the vasculature 7, 10, 44–46. In animal models, Wnt5a ablation ameliorates systemic insulin resistance. In adipose tissue, Wnt5a contributes to obesity-associated metabolic dysfunction and inflammation 11. In both animal models and human studies of peripheral artery disease, Wnt5a activation impedes angiogenesis indicating its relevance to vascular function. Thus, prior work suggests the potential for Wnt5a to impact vascular function in diabetes.
The present study defines the regulatory contribution of Wnt5a signaling to abnormal endothelial signaling in diabetes. Endothelial cells from patients with diabetes displayed higher Wnt5a expression. Activating Wnt5a produced an increase in JNK activity and a marked abnormality of endothelial response to insulin in endothelial cells isolated from non-diabetic subjects. Importantly, Wnt5a inhibition decreased JNK activation and restored endothelial insulin response in endothelial cells from diabetic patients. Wnt5a mediated a decrease in nitric oxide production in cultured endothelial cells similar to the phenotype observed in the endothelial cells from diabetic patients. Moreover, blocking JNK activity prevented the induction of endothelial insulin resistance and dysfunction by Wnt5a in endothelial cells. Collectively, our findings support the concept that JNK activation participates in the development of endothelial dysfunction in diabetes and that Wnt5a is a regulator of JNK activation. Thus, blocking Wnt5a signaling through pharmacologic approaches may serve as a strategy to blunt JNK activation and restore insulin signaling in the vasculature in diabetes.
Under diabetic conditions, ROS levels are enhanced in different tissues, and have been implicated in diabetic complications 23, 47. Previous literature in animal models, indicates that JNK is involved in oxidative stress-mediated induction of insulin resistance48. In the present study, our findings do not suggest that oxidative stress is the primary mediatory of Wnt5a-induced endothelial dysfunction.
Some limitations of our study should be considered. The characterization of endothelial phenotype was performed in venous endothelial cells not arterial endothelial cells, which may be more relevant to vasodilator function. However, both venous and arterial cells are similar exposed to abnormal metabolic conditions such as higher glucose and insulin levels in diabetes, and prior studies have demonstrated associations between endothelial phenotype in venous and arterial cells collected in a similar fashion 49, 50. Flow-mediated endothelium-dependent vasodilation and endothelial function is not only mediated by nitric oxide, but also involves the production of prostacyclin and endothelium-derived hyperpolarizing factor 51, 52. Additional work would be needed to define whether Wnt5a/JNK signaling alters the availability of these vasodilators. Diabetes is accompanied by multiple systemic risk factors that might contribute to the abnormal endothelial phenotype observed in the present study when comparing diabetic versus non-diabetic patients. However, the modest sample size limits multivariable adjustment for confounders. Additional studies will be required to define the relative contribution of each metabolic disturbance in diabetes to altered endothelial phenotype. Further, the studies using JNK inhibitor support the relevance of this pathway in metabolic diseases. It is likely that multiple stress-activated and inflammatory pathways contribute to JNK activation in diabetes 40, 53. Based on previous studies that identify Wnta as a novel mediator of metabolic dysfunction and JNK activation, we focused on the impact of Wnt5a activation. Nevertheless, we do not exclude other potential signaling cascades involved in the JNK pathway. Additional work will be required to determine the complete set of regulators of both Wnt5a and JNK that impact the vascular endothelium in diabetes.
Conclusions
Our study may have important clinical implications for diabetic vascular disease. There is a critical need for new approaches to protect the vasculature from the adverse effects of nutrient overabundance, insulin resistance, and inflammation in diabetes. The clinical relevance of endothelial dysfunction to the heightened atherosclerotic risk in diabetes is well-established. However, the pathways leading from inflammation and nutrient excess in diabetes to abnormal vascular function remain incompletely elucidated. Pharmacologic inhibitors of Wnt5a-JNK signaling are under development and identification of the functional importance of Wnt5a-JNK signaling to endothelial dysfunction in human diabetes indicates that these drugs may serve as agents to mitigate cardiovascular risk.
Supplementary Material
Significance.
Type 2 Diabetes is associated with endothelial dysfunction that contributes to the development of cardiovascular diseases. Experimental studies link Wnt5a as a pro-inflammatory mediator in metabolic dysfunction acting through JNK activation. We found evidence that elevated Wnt5a levels in endothelial cells from diabetic patients were associated with increased JNK activation and endothelial dysfunction. We demonstrated that treatment with a Wnt5a and JNK inhibitors improved insulin signaling and eNOS activation in endothelial cells from diabetic patients. This study provides evidence that the Wnt5a/JNK signaling pathway contributes to endothelial dysfunction in diabetes and supports the possibility that treatments with Wnt5a inhibitors may be a novel strategy to restore endothelial function in diabetes.
Acknowledgments
Sources of Funding: The project was supported by National Heart, Lung, and Blood Institute grants HL081587, HL115391, HL102299. Dr. Fetterman was supported by the NIH-sponsored Boston University Medical Center Multidisciplinary Training Program in Cardiovascular Research (T32 HL007224). Dr. Gokce is supported by NIH grants HL081587 and HL115775. Dr. Farb is supported by an American Heart Association Postdoctoral Fellowship 12POST11780028. Dr. Inagaki is supported by the NIH-sponsored Immunobiology of Trauma Training Grant T32 GM86308.
ABBREVIATIONS
- eNOS
endothelial nitric oxide
- HAEC
human aortic endothelial cells
- JNK
c-jun N-terminal kinase
- Wnt
wingless-type family member
Footnotes
Disclosures: None
References
- 1.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: Studies in mammalian models. Exp Physiol. 2008;93:158–163. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 3.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase c-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt PM, Malgor R. Wnt5a: A player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis. 2014;237:155–162. doi: 10.1016/j.atherosclerosis.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halleskog C, Schulte G. Wnt-3a and wnt-5a counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. Journal of neurochemistry. 2013;125:803–808. doi: 10.1111/jnc.12250. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalan V, Gomez-Ambrosi J, Rodriguez A, Perez-Hernandez AI, Gurbindo J, Ramirez B, Mendez-Gimenez L, Rotellar F, Valenti V, Moncada R, Marti P, Sola I, Silva C, Salvador J, Fruhbeck G. Activation of noncanonical wnt signaling through wnt5a in visceral adipose tissue of obese subjects is related to inflammation. The Journal of clinical endocrinology and metabolism. 2014;99:E1407–1417. doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 9.Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. Endothelial cell-derived non-canonical wnt ligands control vascular pruning in angiogenesis. Development. 2014;141:1757–1766. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of vegf-a contributes to impaired vascularization in peripheral artery disease. Nature medicine. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Noncanonical wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–1248. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for jnk in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 13.Bennett BL, Satoh Y, Lewis AJ. Jnk: A new therapeutic target for diabetes. Current opinion in pharmacology. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 14.Graczyk PP. Jnk inhibitors as anti-inflammatory and neuroprotective agents. Future Med Chem. 2013;5:539–551. doi: 10.4155/fmc.13.34. [DOI] [PubMed] [Google Scholar]
- 15.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase ror2 is involved in non-canonical wnt5a/jnk signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. Jnk functions in the non-canonical wnt pathway to regulate convergent extension movements in vertebrates. EMBO reports. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdoch CE, Shuler M, Haeussler DJ, Kikuchi R, Bearelly P, Han J, Watanabe Y, Fuster JJ, Walsh K, Ho YS, Bachschmid MM, Cohen RA, Matsui R. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. The Journal of biological chemistry. 2014;289:8633–8644. doi: 10.1074/jbc.M113.517219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, Axelsson L, Andersson T. A t-butyloxycarbonyl-modified wnt5a-derived hexapeptide functions as a potent antagonist of wnt5a-dependent melanoma cell invasion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19473–19478. doi: 10.1073/pnas.0909409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-jun nh(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 20.Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the jnk pathway in liver affects insulin resistance status. The Journal of biological chemistry. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 21.Cui J, Zhang M, Zhang YQ, Xu ZH. Jnk pathway: Diseases and therapeutic potential. Acta pharmacologica Sinica. 2007;28:601–608. doi: 10.1111/j.1745-7254.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 24.Hartman ML, Shirihai OS, Holbrook M, Xu G, Kocherla M, Shah A, Fetterman JL, Kluge MA, Frame AA, Hamburg NM, Vita JA. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vascular medicine. 2014;19:67–74. doi: 10.1177/1358863X14521315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the jnk pathway in diabetes. Current diabetes reviews. 2005;1:65–72. doi: 10.2174/1573399052952613. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Guan Y, Chen Y, Zhang C, Shi C, Zhou F, Yu L, Juan J, Wang X. Expression of wnt5a and its receptor fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. International journal of clinical and experimental pathology. 2013;6:1245–1260. [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka A, Kizuka F, Lee L, Tamura I, Taniguchi K, Asada H, Taketani T, Tamura H, Sugino N. Progesterone increases manganese superoxide dismutase expression via a camp-dependent signaling mediated by noncanonical wnt5a pathway in human endometrial stromal cells. The Journal of clinical endocrinology and metabolism. 2010;95:E291–299. doi: 10.1210/jc.2010-0619. [DOI] [PubMed] [Google Scholar]
- 28.Shen HM, Liu ZG. Jnk signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free radical biology & medicine. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of map kinases. Methods in enzymology. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes mellitus-induced apoptosis and heart defects through restoration of impaired wnt signaling. Circulation Cardiovascular genetics. 2015;8:665–676. doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hii HP, Liao MH, Chen SJ, Wu CC, Shih CC. Distinct patterns of wnt3a and wnt5a signaling pathway in the lung from rats with endotoxic shock. PloS one. 2015;10:e0134492. doi: 10.1371/journal.pone.0134492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CL, Wang JY, Ko JY, Surendran K, Huang YT, Kuo YH, Wang FS. Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology. 2008;149:2934–2942. doi: 10.1210/en.2007-1372. [DOI] [PubMed] [Google Scholar]
- 33.Vallerie SN, Hotamisligil GS. The role of jnk proteins in metabolism. Sci Transl Med. 2010;2:60rv65. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 34.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for jnk in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 36.Osto E, Matter CM, Kouroedov A, Malinski T, Bachschmid M, Camici GG, Kilic U, Stallmach T, Boren J, Iliceto S, Luscher TF, Cosentino F. C-jun n-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation. 2008;118:2073–2080. doi: 10.1161/CIRCULATIONAHA.108.765032. [DOI] [PubMed] [Google Scholar]
- 37.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sourris KC, Lyons JG, de Courten MP, Dougherty SL, Henstridge DC, Cooper ME, Hage M, Dart A, Kingwell BA, Forbes JM, de Courten B. C-jun nh2-terminal kinase activity in subcutaneous adipose tissue but not nuclear factor-kappab activity in peripheral blood mononuclear cells is an independent determinant of insulin resistance in healthy individuals. Diabetes. 2009;58:1259–1265. doi: 10.2337/db08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabio G, Davis RJ. Cjun nh2-terminal kinase 1 (jnk1): Roles in metabolic regulation of insulin resistance. Trends in biochemical sciences. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho H, Black SC, Looper D, Shi M, Kelly-Sullivan D, Timofeevski S, Siegel K, Yu XH, McDonnell SR, Chen P, Yie J, Ogilvie KM, Fraser J, Briscoe CP. Pharmacological characterization of a small molecule inhibitor of c-jun kinase. Am J Physiol Endocrinol Metab. 2008;295:E1142–1151. doi: 10.1152/ajpendo.90298.2008. [DOI] [PubMed] [Google Scholar]
- 42.Liu G, Rondinone CM. Jnk: Bridging the insulin signaling and inflammatory pathway. Curr Opin Investig Drugs. 2005;6:979–987. [PubMed] [Google Scholar]
- 43.Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, Chen LH, Cellitti JF, Riel-Mehan M, Emdadi A, Solinas G, Karin M, Pellecchia M. Identification of a new jnk inhibitor targeting the jnk-jip interaction site. Proc Natl Acad Sci U S A. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: Its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 45.Gustafson B, Smith U. The wnt inhibitor dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61:1217–1224. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grunberg J, Gustafson B, Klimcakova E, Stich V, Langin D, Laakso M, Smith U. Wisp2 regulates preadipocyte commitment and ppargamma activation by bmp4. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2563–2568. doi: 10.1073/pnas.1211255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Current vascular pharmacology. 2006;4:215–227. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- 48.Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-jun n-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. The Journal of biological chemistry. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 49.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: A new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 50.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic gmp. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 52.Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney international. 2006;70:840–853. doi: 10.1038/sj.ki.5001680. [DOI] [PubMed] [Google Scholar]
- 53.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators of inflammation. 2010;2010:359732. doi: 10.1155/2010/359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.