Abstract
Background
Endothelial dysfunction contributes to cardiovascular disease in diabetes mellitus. Autophagy is a multistep mechanism for removal of damaged proteins and organelles from the cell. Under diabetic conditions, inadequate autophagy promotes cellular dysfunction and insulin resistance in non-vascular tissue. We hypothesized that impaired autophagy contributes to endothelial dysfunction in diabetes mellitus.
Methods and Results
We measured autophagy markers and endothelial nitric oxide synthase (eNOS) activation in freshly isolated endothelial cells from diabetic subjects (n=45) and non-diabetic controls (n=41). p62 levels were higher in cells from diabetics (34.2±3.6 vs. 20.0±1.6, P=0.001), indicating reduced autophagic flux. Bafilomycin inhibited insulin-induced activation of eNOS (−21±5% vs. 64±22%, P=0.003) in cells from controls, confirming that intact autophagy is necessary for eNOS signaling. In endothelial cells from diabetics, activation of autophagy with spermidine restored eNOS activation, suggesting that impaired autophagy contributes to endothelial dysfunction (P=0.01). Indicators of autophagy initiation including the number of LC3-bound puncta and beclin 1 expression were similar in diabetics and controls, whereas an autophagy terminal phase indicator, the lysosomal protein Lamp2a, was higher in diabetics. In endothelial cells under diabetic conditions, the beneficial effect of spermidine on eNOS activation was blocked by autophagy inhibitors bafilomycin or 3-methyladenine. Blocking the terminal stage of autophagy with bafilomycin increased p62 (P=0.01) in cells from diabetics to a lesser extent than in cells from controls (P=0.04), suggesting ongoing, but inadequate autophagic clearance.
Conclusion
Inadequate autophagy contributes to endothelial dysfunction in patients with diabetes and may be a target for therapy of diabetic vascular disease.
Keywords: autophagy, endothelial cells, diabetes mellitus
Subjects with type 2 diabetes mellitus have increased risk for cardiovascular disease that persists despite aggressive control of glucose, cholesterol, and blood pressure.1, 2 Endothelial dysfunction contributes to the pathogenesis of cardiovascular disease in diabetes, and an improved understanding of the responsible mechanisms could lead to new approaches for therapy.3 Diabetes is associated with excess production of reactive oxygen species by complexes of the mitochondrial electron transport chain and other enzymes in endothelial cells that decrease the bioactivity of nitric oxide, activate pro-inflammatory signaling pathways, and cause damage to cellular proteins and organelles.4 Since damaged mitochondrial enzymes produce more oxidants, failure of quality control mechanisms could exacerbate oxidative stress and cellular dysfunction.5, 6
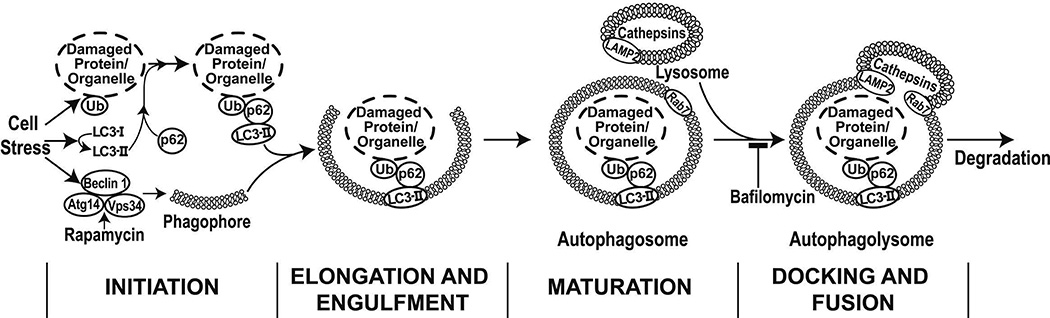
As is illustrated in Figure 1, autophagy is a multistep mechanism for the clearance of damaged proteins and organelles from the cell.7, 8 Components are tagged for degradation through ubiquitination and are linked to LC3 through the adapter protein p62. During initiation of autophagy, LC3-I is lipidated to form LC3-II, which targets the damaged material to the developing double-membrane autophagosome. Beclin 1, the mammalian homolog of yeast Atg6, is part of the lipid-PI3 kinase complex that coordinates autophagosome formation.9, 10 The autophagosome fuses with a lysosome to produce the autophagolysosome, a process that is mediated in part by SNARE proteins, including Rab7 and Lamp2a.11, 12 The contents of the autophagolysosome are degraded by cathepsins. p62 is degraded along with the targeted organelles and proteins, while LC3 may be degraded or recycled back into the cytosol.7, 8
Figure 1.
Cellular stress induces autophagy, a multi-step process that serves to remove damaged and dysfunctional cellular components and organelles.
The pattern of autophagy proteins in cells reflects the state of autophagy. Activation of autophagy results in the accumulation of LC3-bound puncta, consistent with conversion of LC3-I to LC3-II, and decreased p62 reflecting degradation in the autophagolysosome. Impairment of autophagy at the initiation stage is characterized by a loss of LC-3-bound puncta, decreased LC3-II, and increased p62. Failure of the terminal phases of autophagy, including autophagosome-lysosome fusion or cargo degradation, is characterized by a normal or increased number of puncta, increased LC3-II, and increased p62 in the cell, reflecting an inability to clear autophagosomes and degrade p62.
Recent studies link autophagy and diabetes mellitus. Activators of autophagy such as exercise and calorie restriction improve insulin sensitivity.7, 8 Reduced autophagy contributes to insulin resistance in traditional insulin-responsive tissues such as liver, skeletal muscle, and adipose tissue.13, 14 In pancreatic beta cells, diabetic conditions are associated with inadequate autophagic clearance and lysosome function leading to impaired insulin secretion.15 Little is known, however, about autophagy in vascular tissue or its contribution to diabetic vascular disease, particularly in human subjects. In the present study, we hypothesized that impaired autophagy contributes to endothelial dysfunction in diabetes. We sought to determine the state of autophagy in freshly isolated endothelial cells from patients with autophagy and to evaluate whether autophagy contributes to endothelial insulin signaling and nitric oxide production.
METHODS
Study Subjects
We enrolled adults with type 2 diabetes mellitus, defined as fasting glucose ≥126 mg/dL, hemoglobin A1C≥6.5% or ongoing treatment for diabetes. Similarly-aged non-diabetic adults with fasting glucose below 100 mg/dL served as controls. Each subject made a study visit for collection of blood and endothelial cells and non-invasive testing of endothelial function. Subjects fasted overnight prior to the visit. Vasoactive medications were withheld for 24 hours prior to study. Blood glucose and lipid levels were measured in the Boston Medical Center Clinical Laboratory. The protocol was approved by the Boston Medical Center Institutional Review Board and all study subjects provided written informed consent.
Vascular Function Testing
Brachial artery flow-mediated vasodilation was measured as previously described.16 In brief, vascular ultrasound was used to measure brachial artery diameter and blood flow velocity before and one minute following induction of reactive hyperemia by five minutes of upper arm occlusion with a narrow gauge blood pressure cuff. Hyperemic flow increases shear stress at the endothelial surface, activates endothelial nitric oxide synthase (eNOS), and induces dilation of the conduit brachial artery. We also assessed non-endothelium dependent dilation by measuring brachial diameter before and three minutes after sublingual nitroglycerin (0.4 mg).
Venous Endothelial Cell Biopsy and Cell Activation Experiments
Peripheral vein endothelial cells were collected as previously described.17–20 Briefly, a 0.018 inch J-wire (Arrow International, Reading, PA) was inserted into a forearm vein through a 20 or 22 gauge intravenous catheter and used to gently rub the endothelial surface. Endothelial cells were recovered from the wire in red blood cell lysis/dissociation buffer, centrifuged, and fixed onto poly-L-lysine coated slides (Sigma, St. Louis, MO) using 4% paraformaldehyde. Slides were dried, and frozen at −80°C prior to staining and immunofluorescence imaging to measure protein expression as described below. Under all conditions with the exception of spermidine treated cells, freshly isolated venous endothelial cells from the human subjects were fixed within two hours following biopsy. For the spermidine experiment, endothelial cells were treated with 3 mM spermidine or vehicle (endothelial growth medium-2 without growth factors) for 6 hours following which the cells were incubated an additional 18 hours in media with 5% serum.
For some experiments, insulin-mediated eNOS activation was examined by measuring the levels of phosphorylated eNOS (serine 1177) in cells treated with 10 nM insulin (Sigma Aldrich, St. Louis, MO) or vehicle (endothelial growth medium-2 without growth factors, Lonza Inc, Walkersville, MD) for 30 minutes prior to fixation. We also performed insulin stimulation experiments and measured expression of autophagy proteins after incubation with 10 nM bafilomycin A1 (LC Laboratories, Woburn, MA) for 60 minutes to inhibit autophagy by blocking autophagolysosome acidification, or 18 hours after 6 hours of spermidine (3 mM) treatment to globally activate autophagy. Finally, we activated eNOS with 10 nM insulin for 30 minutes and measured nitric oxide production using the fluorescent probe DAF-2DA (EMD Millipore, Billerica, MA) as previously described.21
Quantification of Protein Levels in Freshly Isolated Endothelial Cells
Protein levels in freshly isolated endothelial cells were quantified using immunofluorescence microscopy.18–20 Slides were stained with one of the following primary antibodies for one hour, unless otherwise indicated: p62 (1:100 dilution; Abnova, Cambridge, MA), beclin 1 (1:300 dilution; Novus Biologicals, Littleton, CO), LC3B (1:100; Abcam, Cambridge, MA), phosphorylated Ser1177 eNOS (1:200 dilution; Millipore), eNOS (1:100 dilution; BD Transduction Laboratories, San Diego, CA), Rab7 (1:100 dilution for 4 hours; Abcam), Lamp2a (1:100 dilution; Abcam), Atg7 (1:200 dilution; Sigma Aldrich, St. Louis, MO) and acetylated Lys9 histone 3 (1:100 dilution for 4 hours; Cell Signaling, Beverly, MA). The fluorescence intensity was quantified in 20 endothelial cells and averaged for each condition. To control for batch-to-batch variability, fluorescence intensity was normalized to the intensity in human aortic endothelial cells (HAECs), which were stained simultaneously. Final intensity was calculated by dividing the average fluorescence intensity for the subject sample by the average fluorescence intensity of the HAEC sample and multiplying by 100. The intensity is expressed in arbitrary units (AU). All quantification was performed blinded to subject identity and diabetes status. Confocal images of selected proteins assessed by immunofluorescence were taken for illustrative purposes using a Leica SP5 microscope (Solms, Germany).
Western Blots
Following lysis of HAECs in RIPA buffer containing proteinase and phosphatase inhibitors, protein levels were quantified utilizing BioRad’s (Hercules, CA) Dc Protein assay, per manufacturer instructions. For all Western blots, 30 µg of protein was loaded onto 4–20% gradient gels and run at 100 mV, transferred to PVDF membranes, and blocked in 3% BSA/TBS-T for 30 minutes. The blots were probed against LC3A/B (1:1000 dilution; Abcam, Cambridge, MA) and β-actin (1:1000 dilution; Cell Signaling, Danvers, MA) overnight in 3% BSA/ TBS-T at 4°C. All blots were imaged using a LAS-4000 Luminescent Image Analyzer by Fuji (Tokyo, Japan) and data was normalized to β-actin.
Cell Culture
To gain further insight into the links between altered autophagy and eNOS signaling in diabetes, we used HAECs exposed to physiological (5 mM) and elevated (30 mM) glucose concentrations for 24 hours. HAECs were purchased from Lonza, Inc and maintained with the EGM-2 bullet kit containing 5 mM glucose. Expression of autophagy proteins and insulin-mediated activation of eNOS was assessed by immunofluorescence, as described above. To block autophagy and to probe the level of autophagic flux, HAECs were incubated for 60 min with 10 nM bafilomycin A1.22, 23 To determine whether activating autophagy would reverse glucose-induced endothelial dysfunction, HAECs were incubated with 30 mM glucose in EGM-2 media for 24 hours and then treated with 3 mM spermidine (Sigma Aldrich) for six hours followed by 18 additional hours of 30 mM glucose exposure before fixing cells for protein expression or examining insulin-induced eNOS activation as described above. In addition, insulin-induced eNOS activation was assessed following treatment with autophagy inhibitors bafilomycin A1 (10 nM) or 3-methyladenine (3-MA; 10 mM; Sigma Aldrich) during insulin stimulation for one hour following spermidine treatment. Spermidine activates autophagy via a general increase in the expression of autophagy-related genes, which was confirmed by RT-qPCR, as described below.
Assessment of LC3-bound Puncta
In addition to measuring LC-3 protein levels in freshly isolated endothelial cells, we also assessed autophagy status by counting the number of LC3-bound puncta, which reflects autophagosome formation. For HAEC’s, we counted the number of cells with more than twenty LC3-bound puncta per cell. Cells were stained with antibody against LC3B (1:100; Abcam, Cambridge, MA) as described above. An investigator blinded to subject status or cell culture condition counted puncta in twenty cells calculated the mean. To assess reproducibility, puncta count was performed twice in a blinded fashion (n=10). The coefficient of variation was 2.4%.
Endothelial Cell Gene Expression
For gene expression studies, HAECs were trypsinized and the cell pellets were prepared per manufacturer’s instructions for gene expression using the Fastlane Cell cDNA kit (Qiagen, Germantown, MD) and stored at −80°C until processed further. RNA synthesis was completed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and quantitative real-time PCR reactions were performed using TaqMan gene expression assays (Applied Biosystems). Data were normalized to GAPDH and compared using the ΔΔct method. Gene expression results are expressed as fold change relative to the control group as indicated in the figure legend.
Statistical Analysis
Statistical analyses were carried out using SPSS version 19.0 (IBM Corp, Armonk, NY). Data are expressed as mean±standard deviation unless otherwise noted. Variables were evaluated for normality by the Shapiro-Wilk test. Variables with normal distribution were compared using unpaired t-test, paired t-tests, or chi-square tests for two-group comparisons or repeated-measures ANOVA for multi-treatment comparisons. Variables that were not normally distributed were compared with the Mann-Whitney U test to compare diabetics and controls or the Wilcoxon Signed Rank test for paired samples. Spearman correlation coefficients were used to correlate clinical characteristics, measures of vascular function, and expression of autophagy proteins. A two-tailed P<0.05 was considered to be statistically significant.
RESULTS
Study Subjects and Vascular Function
We enrolled 45 subjects with diabetes mellitus and 41 controls. As shown in Table 1, the two groups were similar in age and gender. There was a modest preponderance of black subjects in the diabetic group (P=0.047). As expected, the diabetic subjects were taking medications to lower blood glucose and had metabolic abnormalities, including higher fasting glucose, hemoglobin A1C, triglycerides, and body mass index. Diabetics had lower total and LDL cholesterol levels, likely reflecting the greater use of cholesterol-lowering medications.
Table 1.
Clinical Characteristics and Vascular Function
| Control (n=41) | Diabetic (n=45) | |
|---|---|---|
| Clinical characteristics | ||
| Age (yrs) | 52±8 | 54±11 |
| Female sex, n (%) | 24 (59%) | 27 (60%) |
| Black race, n (%) | 15 (37%) | 26 (58%)* |
| Body mass index, kg/m2 | 28.9±5.2 | 34.3±7.4* |
| Total cholesterol, mg/dL | 196±37 | 180±39* |
| LDL cholesterol, mg/dL | 124±31 | 104±36* |
| HDL cholesterol, mg/dL | 49±14 | 43±11* |
| Triglycerides, mg/dL | 117±60 | 174±120* |
| Fasting glucose, g/dL | 91±11 | 174±85* |
| Hemoglobin A1c, % | 5.4±0.4 | 8.2±2.0* |
| Systolic blood pressure, mmHg | 126±18 | 133±14 |
| Diastolic blood pressure, mmHg | 77±10 | 78±10 |
| Lipid lowering therapy, n (%) | 6 (15%) | 21 (47%)* |
| Metformin therapy, n (%) | 0 | 27 (60%)* |
| Sulfonylureas, n (%) | 0 | 10 (22%)* |
| Thiazolidinediones, n (%) | 0 | 1 (2%) |
| Insulin therapy, n (%) | 0 | 18 (40%)* |
| Antiplatelet therapy, n (%) | 4 (10%) | 16 (36%)* |
| ACE inhibitor or ARB therapy, n (%) | 5 (12%) | 22 (49%)* |
| Vascular function | ||
| Baseline brachial diameter, mm | 4.13±0.76 | 4.41±0.75 |
| Brachial artery flow-mediated dilation, % | 9.7±4.6 | 5.8±3.2* |
| Nitroglycerin-mediated dilation, % | 15.6±7.7 | 10.7±5.9* |
| Reactive hyperemia, ml/min | 903±298 | 892±318 |
P<0.05 by unpaired t test or chi square test.
Data are mean ± SD.
LDL=low density lipoprotein, HDL=high density lipoprotein, ACE=angiotensin-converting enzyme, ARB=angiotensin receptor blocker.
In regard to vascular function, brachial artery flow-mediated dilation was lower in the diabetics (P<0.001), suggesting conduit artery endothelial dysfunction. Nitroglycerin-mediated dilation also was lower (P=0.047) suggesting a concurrent defect in the vasodilator function of vascular smooth muscle.
Expression of Autophagy Proteins in Endothelial Cells from Study Subjects
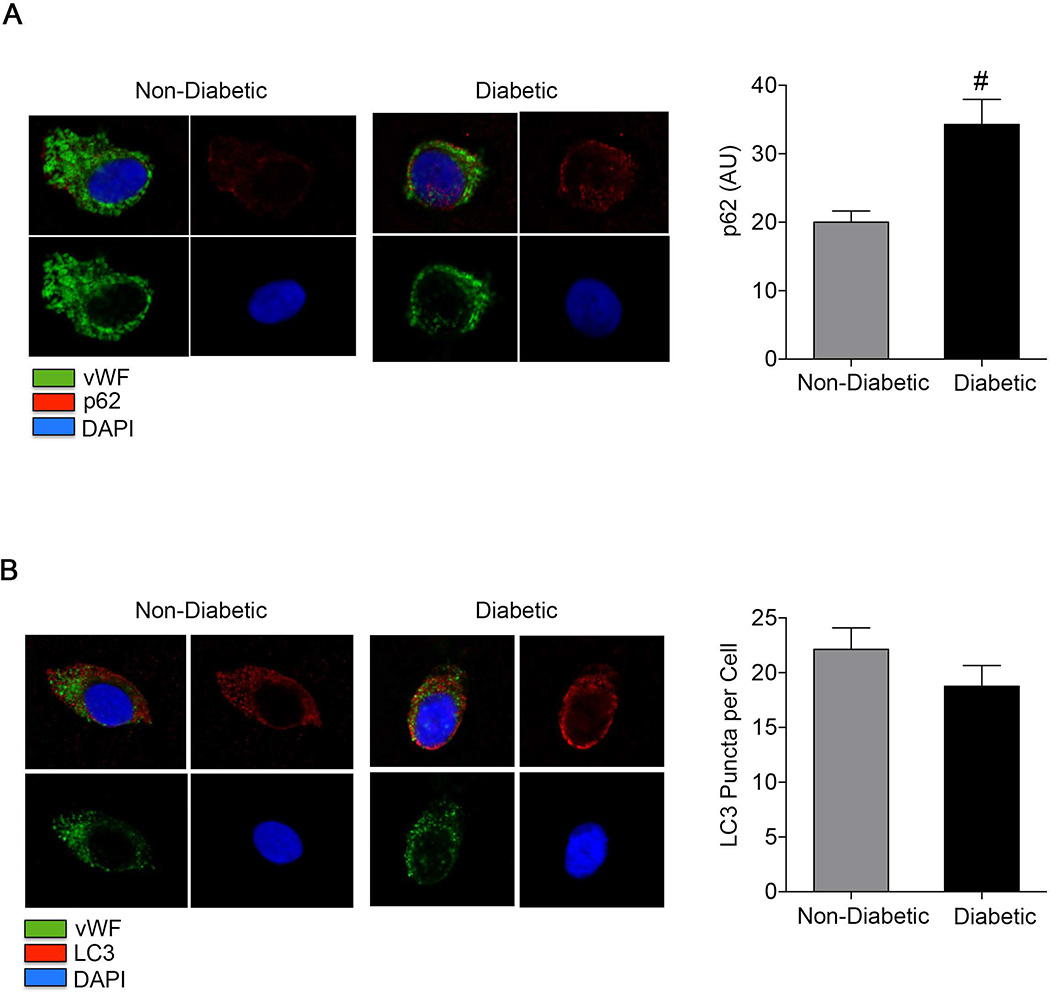
As shown in Figure 2A, p62 levels were higher in endothelial cells isolated from subjects with diabetes compared to controls (34.2±3.6 vs. 20.0±1.6, P=0.001), suggesting an overall impairment of autophagy.
Figure 2.
p62 and LC3 in freshly isolated human endothelial cells. A: Venous endothelial cells were isolated from healthy subjects and diabetic subjects as outlined in the methods. Endothelial cells from diabetic subjects (n=11) and non-diabetic controls (n=16) were stained for p62. Representative confocal images show higher levels of p62 (red channel) in a diabetic compared to a non-diabetic participant. Also shown are vWF (green channel), DAPI (blue channel) and merged images (upper left). Pooled data shows that diabetic subjects have higher levels of p62 staining compared to non-diabetic subjects (#P<0.01). B: Representative confocal images show LC3 puncta in freshly isolated endothelial cells from a non-diabetic and a diabetic. Also shown are vWF (green channel), DAPI (blue channel) and merged images (upper left). There were no differences in the number of LC3 puncta per cell in freshly isolated endothelial cells from diabetics (n=9) and non-diabetics (n=13).
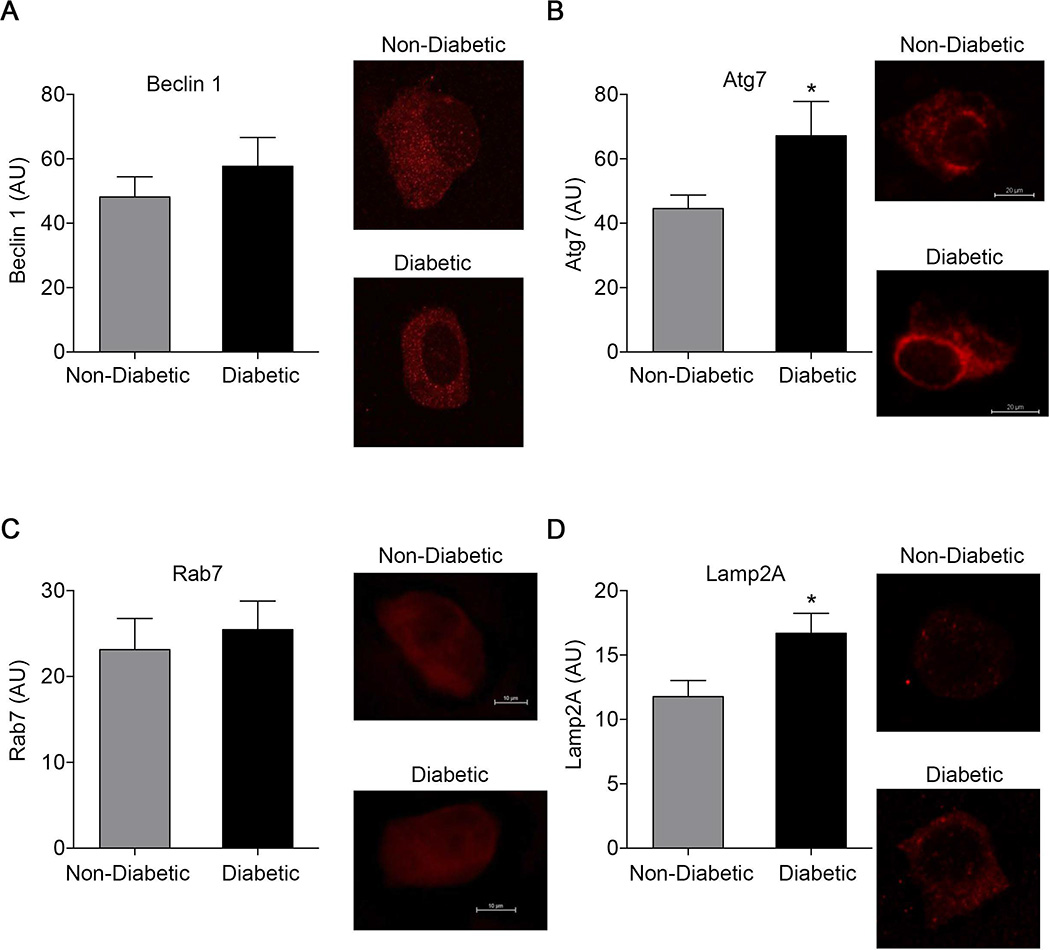
As shown in Figure 2B, the number of LC3-bound puncta (19±2 vs. 22±2 per cell, P=0.28) and total LC3 levels (35.2±6.0 vs. 38.1±4.2 AU, P=0.76) did not differ between the diabetics and the non-diabetics, respectively, suggesting that impaired autophagosome formation does not explain the impairment of autophagic flux. As shown in Figure 3A, beclin 1 levels did not differ between diabetics and non-diabetics (57.7±8.9 vs. 48.1±6.3 AU, P=0.52), further arguing against a defect in the initiation stage of autophagy, which would be expected to be associated with lower beclin 1 expression.10 In contrast, Atg7 levels were higher in endothelial cells from diabetic subjects compared to non-diabetic subjects (67.2±10.7 vs. 44.6±4.2 AU, P=0.03) (Figure 3B).
Figure 3.
Beclin 1, Atg7, Lamp2A, and Rab7 in freshly isolated human endothelial cells. A: No differences in beclin 1 levels were observed in freshly isolated endothelial cells from non-diabetic (n=10) and diabetic subjects (n=10) assessed by immunofluorescence. B: Atg7 levels were higher in endothelial cells from diabetic subjects (n=8) compared to non-diabetics (n=11) *P<0.05. C: No differences were observed in Rab7 levels (n=11 non-diabetics and n=8 diabetics). D: Lamp2A levels were higher in endothelial cells from diabetic subjects (n=15) compared to non-diabetics (n=12) *P<0.05.
In addition, expression of the lysosomal protein Lamp2a, which plays a role in autophagosome-lysosome fusion, was higher in the endothelial cells from diabetic subjects compared to non-diabetics (16.7±1.6 vs. 11.8±1.2 AU, P=0.03, Figure 3D). Expression of Rab7a did not differ between groups (25.5±3.3 vs. 23.1±3.6 AU, P=0.65, Figure 3C). Together these findings suggest that there may be a compensatory alteration in factors regulating autophagy in the diabetic endothelium.
Effects of High Glucose Concentration on the Expression of Autophagy Proteins
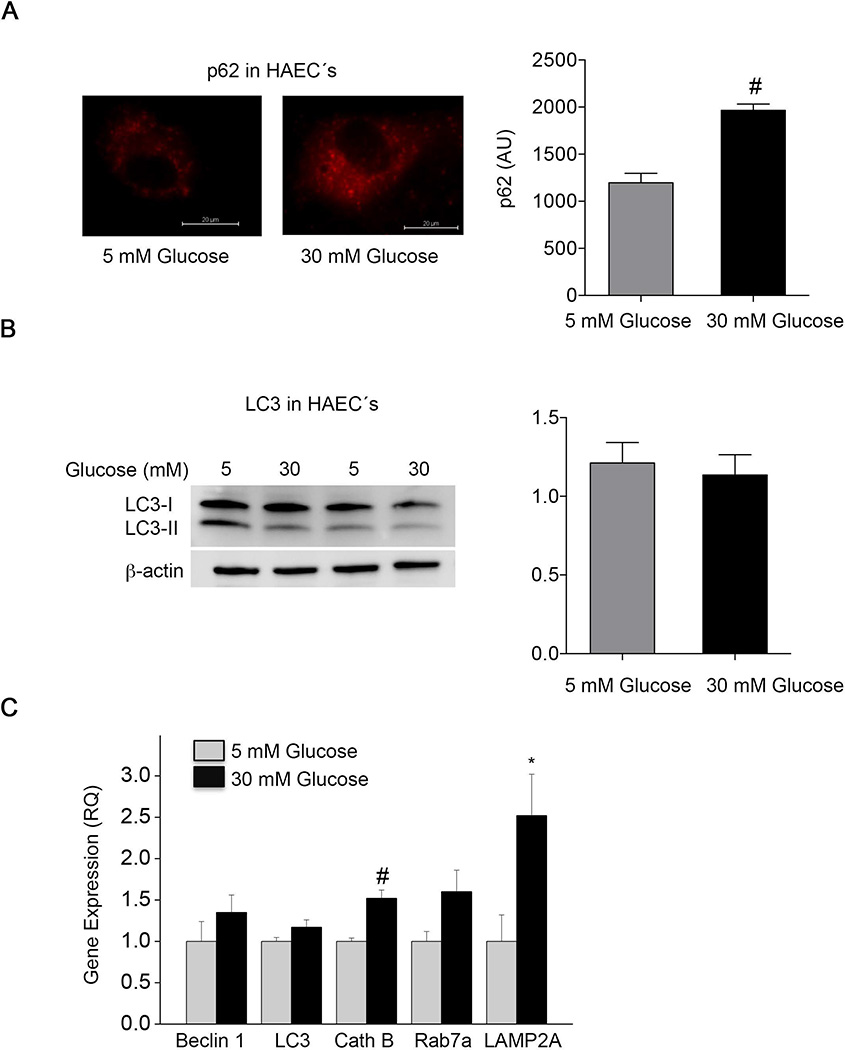
The pattern of autophagy protein expression in HAEC’s exposed to 30 mM glucose mimicked the pattern observed in endothelial cells freshly isolated from subjects with type 2 diabetes mellitus. As shown in Figure 4A, in HAEC’s exposed to 30 mM glucose, p62 expression was higher (P<0.001) whereas the ratio of LC3II (activated form of LC3) to LC3I (inactive form of LC3) (P=0.85) was similar (Figure 4B). The number of LC3 puncta (P=0.89) and total LC3 expression (P=0.88) were also similar in cells exposed to high and physiological glucose levels (Supplemental Figure 1). As shown in Figure 4C, gene expression of LAMP2A (P=0.03) and cathepsin B (P=0.001) message was higher and there was a trend for higher Rab7a (P=0.06) in cells exposed to 30 mM glucose. In contrast, expression of beclin 1 and LC3 did not differ between normal and high glucose. In addition, lysosome number (P=0.99) and acidification (P=0.67) were similar in HAEC’s exposed to high and physiological glucose levels (Supplemental Figure 2A and Figure 2B, respectively). As in the cells from diabetic subjects, these data are consistent with a possible alteration of the terminal phase or overall level of autophagy in HAEC’s exposed to high glucose.
Figure 4.
Autophagy markers in human aortic endothelial cells (HAEC’s) under diabetic conditions. A: p62 was increased in HAEC’s exposed to 30 mM glucose compared to cells exposed to 5 mM glucose (#P<0.01). B: LC3II/LC3I was similar between HAEC grown under 5 mM and 30 mM glucose. C: Quantitative RT-PCR of cultured cells showed that HAEC grown under high glucose expressed higher levels of LAMP2A (*P<0.05) and cathepsin B (cath B, #P<0.01) compared to HAEC grown under 5 mM glucose. There were no differences in expression of beclin 1 or LC3 (data expressed as relative quantification, RQ). Data are shown as mean±SEM for 5 experiments.
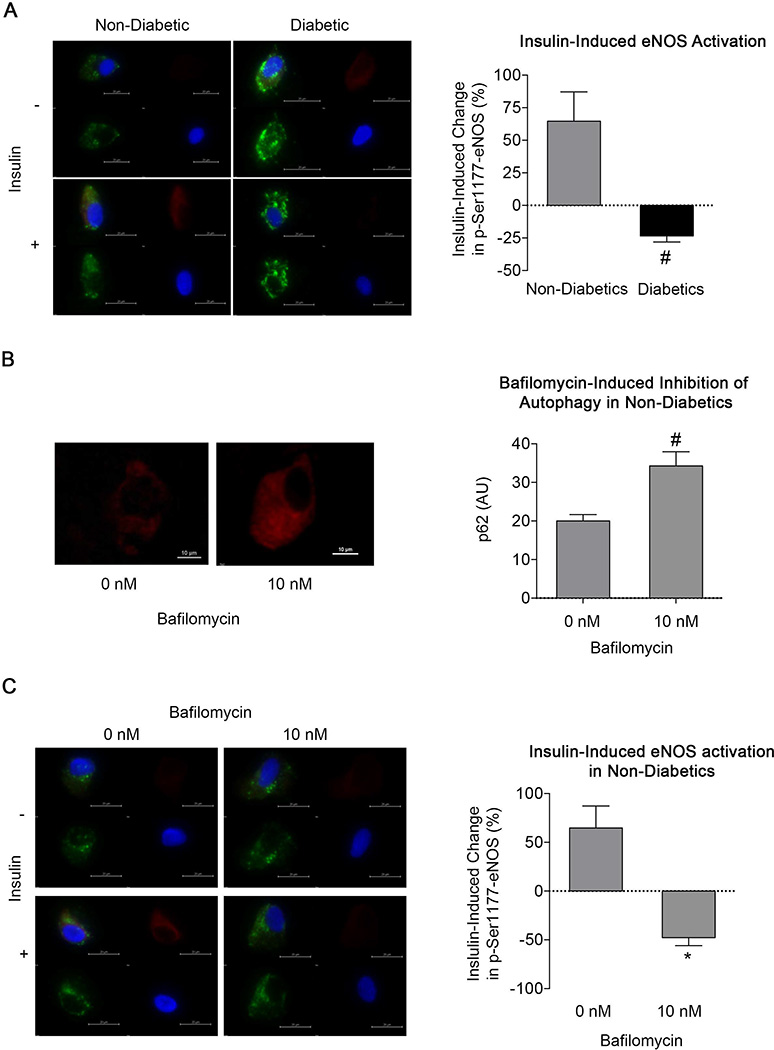
Inhibiting Autophagy Induces Endothelial Dysfunction
As shown in Figure 5A, insulin-induced eNOS activation was profoundly impaired in endothelial cells isolated from diabetic subjects compared to non-diabetics (−21.7±5.1% vs. 64±22%, respectively, P=0.003). This decrease in eNOS activation was likely not due to differences in total eNOS levels as we have previously reported similar levels of eNOS in endothelial cells from non-diabetics and diabetics.18 Bafilomycin inhibits the terminal phases of autophagy by inhibiting lysosomal acidification and autophagosome-lysosome fusion, thus preventing degradation of autophagolysosome contents.22, 23 In cultured endothelial cells, LysoSensor levels were reduced with bafilomycin treatment under glucose conditions confirming previous studies suggesting that bafilomycin inhibits lysosome acidification (5mM, P=0.002) (30mM glucose, P=0.04) (Supplemental Figure 2B).
Figure 5.
Inhibition of autophagy in endothelial cells from non-diabetics impairs endothelial function. A: Insulin-induced activation of eNOS by phosphorylation at Ser1177 (red channel) was measured in endothelial cells from non-diabetics and diabetics. Also shown are vWF (green channel), DAPI (blue channel) and merged images (upper left).Cells from diabetic subjects displayed impaired activation (#P<0.01). B: Treatment of endothelial cells isolated from non-diabetic subjects (n = 6) with 10 nM bafilomycin for 60 minutes increased p62 levels, indicating impaired autophagy (#P≤0.01). C: Bafilomycin impaired insulin-induced eNOS activation (red channel) in endothelial cells from non-diabetics (*P<0.05). Also shown are vWF (green channel), DAPI (blue channel) and merged images (upper left).
Incubation of cells from non-diabetic subjects with bafilomycin for 60 minutes (Figure 5B), increased p62 (20.7±7.9 to 29.9±4.2 AU, P=0.01), consistent with an inhibition of autophagy. Bafilomycin treatment inhibited insulin-induced eNOS activation (64.7±22% to −47.8±8.1%, P=0.04), producing a phenotype similar to cells from diabetic subjects (Figure 5C versus 5A). As shown in Supplemental Figure 3, bafilomycin did not change the basal level of eNOS phosphorylation (15.9±3.2 vs. 13.7±0.8 AU, P=0.89).
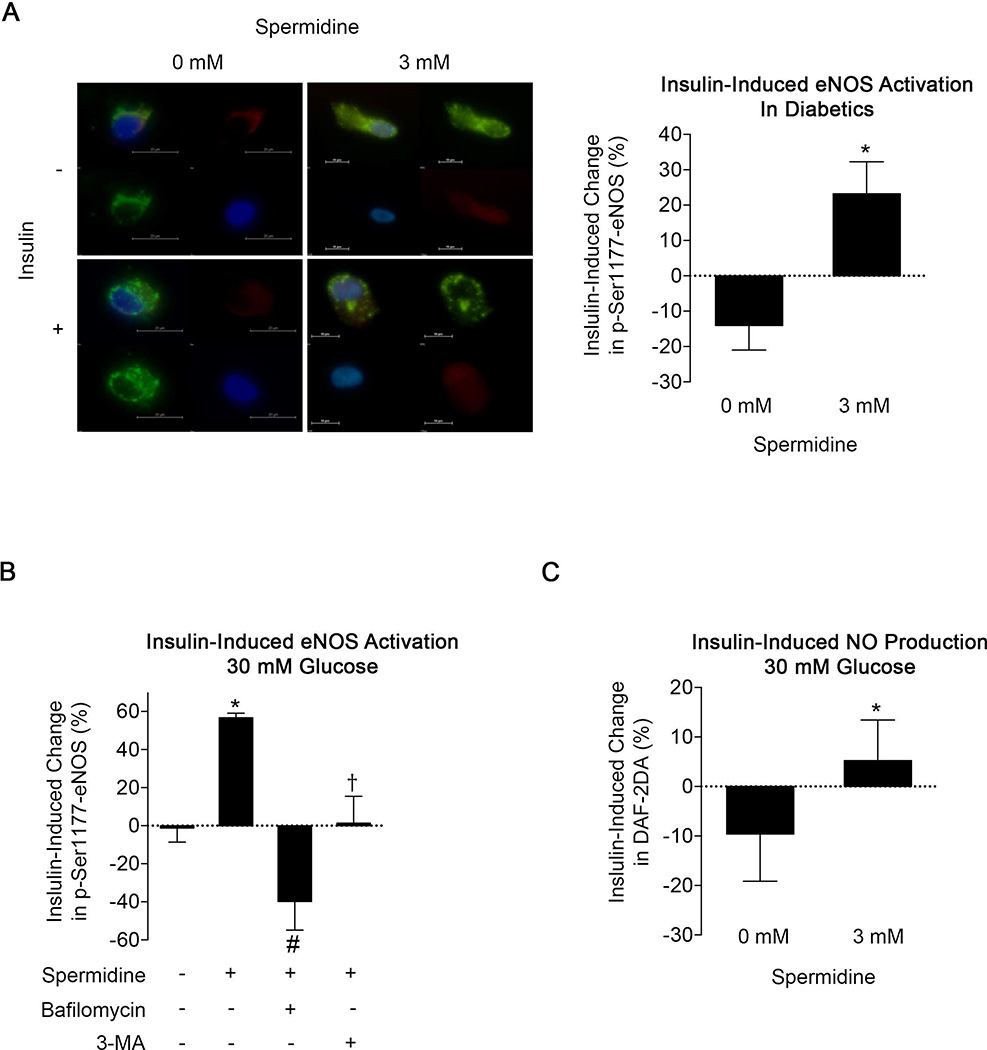
Activating Autophagy Reverses Endothelial Dysfunction in Diabetes
Spermidine is a polyamine compound that activates autophagy through deacetylation of specific residues on histone 3 leading to the upregulation of genes related to multiple stages of the autophagic process.10, 24–26 As shown in Supplemental Figure 4A, 3 mM spermidine incubation for 6 hours decreased nuclear levels of acetylated Lys9 histone 3 (P=0.004) in HAEC’s exposed to 30 mM glucose. Under these conditions, spermidine also increased expression of multiple autophagy genes, including Atg5, Atg12, beclin 1, BNIP3L, p62, and PINK (all P<0.05) with trends (P<0.10) for increased ATG4B, cathepsin B, Rab7a, and LAMP2A (Supplemental Figure 4B).
In endothelial cells from diabetic patients, treatment with spermidine improved insulin-mediated eNOS phosphorylation (P=0.01, Figure 6A), consistent with the possibility that augmenting autophagy improves eNOS activation in diabetes. Similarly, spermidine treatment reversed the impairment in insulin-mediated eNOS phosphorylation induced by high glucose exposure in HAEC’s (P=0.04, Figure 6B vs Supplemental Figure 5A and 5B). Co-treatment with autophagy inhibitors bafilomycin or 3-MA prevented the beneficial effect of spermidine on eNOS activation in HAEC’s exposed to high glucose suggesting that the effects of spermidine on eNOS signaling are mediated through autophagy (Figure 6B). Further, activating autophagy with spermidine restored insulin-induced nitric oxide production (P=0.04, Figure 6C) in HAEC’s exposed to high glucose, suggesting that enhanced eNOS activation was associated with higher nitric oxide bioavailability. Spermidine had no significant effect on eNOS phosphorylation (P=0.72) or nitric oxide production (P=0.72) in cells exposed to 5 mM glucose arguing against a non-specific effect (Supplemental Figures 6A and 6B, respectively). Collectively, these findings suggest that activation of autophagy with spermidine reverses endothelial dysfunction associated with diabetes and thus implicates impaired autophagy as a mechanism of endothelial dysfunction in this setting.
Figure 6.
Autophagy activation with spermidine restored eNOS activation and nitric oxide production. A: Treatment of freshly isolated endothelial cells from diabetic subjects (n=5) with spermidine for 24 hours improves insulin-induced eNOS activation (red channel, #P≤0.01). Also shown are vWF (green channel), DAPI (blue channel) and merged images (upper left). B: Insulin-induced activation of eNOS at Ser1177 was assessed by immunofluorescence in HAEC’s under high glucose in the presence and absence of spermidine treatment. Treatment of HAEC’s under high glucose with spermidine restored insulin-induced eNOS activation, which was blocked by autophagy inhibitors bafilomycin and 3-MA (RM-ANOVA P=0.004, *P<0.05 versus control, #P≤0.01 versus spermidine, †P≤0.01 versus spermidine). C: Spermidine improved nitric oxide production in HAEC’s exposed to high glucose (*P<0.05). Data are shown as mean±SEM for 4 experiments.
As shown in Supplemental Figure 6D, total eNOS protein expression increased following spermidine incubation in cells exposed to 30 mM glucose (P=0.03) but not 5 mM glucose (P=0.23). As shown in Supplemental Figure 6C, this change in total eNOS protein was accompanied by an increase in basal (unstimulated) phosphorylated eNOS (P=0.04). These changes would not explain our findings, since an increase in the basal level of phosphorylated eNOS or NO production would tend to decrease rather than increase the insulin-induced changes in these measures of endothelial function.
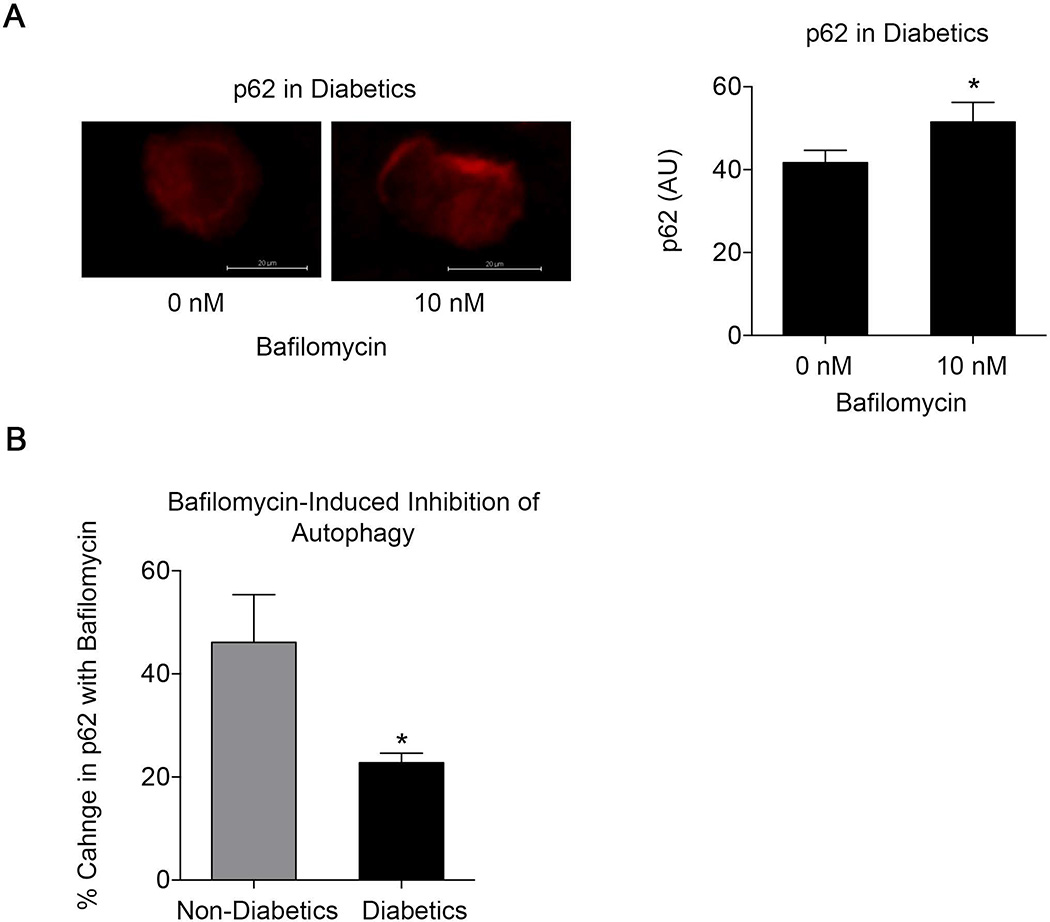
Diabetes is Associated with Inadequate Autophagic Flux
Bafilomycin blocks lysosome acidification and autophagolysosome maturation.22, 23 Since p62 is normally degraded along with the contents of the autophagosome, monitoring p62 levels following bafilomycin exposure provides insight into the state of autophagic flux. If the terminal stages of autophagy are completely blocked, bafilomycin should have no effect on p62 levels. In contrast, an increase in p62 following bafilomycin exposure under diabetic conditions would support the contention that autophagolysosome maturation and clearance are ongoing, but inadequate. As shown in Figure 7A, bafilomycin increased p62 levels in endothelial cells from diabetic subjects (P=0.01), providing support for the latter mechanism of impaired autophagic flux. However, the increase in p62 following bafilomycin treatment was lower in endothelial cells from subjects with diabetes as compared to controls (Figure 7B) consistent with lower autophagic flux in the diabetic endothelium (P=0.04).
Figure 7.
Autophagic flux is ongoing, but inadequate in endothelial cells from diabetic subjects. A: Bafilomycin increased p62 levels in endothelial cells from diabetic subjects assessed by immunofluorescence (n = 6, #P≤0.01). B: Augmentation of p62 levels with bafilomycin treatment was higher in endothelial cells non-diabetics (n=5) compared to cells from diabetics (n=8, *P<0.05).
Clinical Characteristics, Vascular Function, and Autophagy Proteins
As noted, there was a modest preponderance of black subjects in the diabetic group. After adjusting for black race, the observed differences in p62 (P<0.001), Lamp2A (P=0.04), and insulin-mediated eNOS phosphorylation (P=0.002) remained statistically significant, suggesting that race does not account for the findings.
When adjusting for the use of renin-angiotensin system inhibitors, the differences in flow-mediated dilation and autophagy (p62) remain significant between control and diabetic subjects (P<0.001, P=0.002, respectively).27
Finally, we investigated whether vascular function related to autophagy proteins in freshly isolated endothelial cells. There was a trend for an inverse correlation between p62 and reactive hyperemia (R= −0.42, P=0.096). There were no significant correlations between the other measures of vascular function and autophagy proteins.
DISCUSSION
The present study provides evidence that inadequate autophagy contributes to endothelial dysfunction in diabetes. We observed higher p62 levels in endothelial cells from diabetics, indicating impaired autophagy. The lack of a difference in LC3 puncta count in cells from diabetics and non-diabetic controls and in cells exposed to high and low glucose suggests that the initiation phase is intact, while the increased levels of Lamp2a and cathepsin B under diabetic conditions implicates a defect at the terminal phase of autophagy. The finding that bafilomycin increases p62 to a lesser extent in cells from diabetics compared to non-diabetics suggests that autophagic clearance is ongoing, but reduced. We also showed a link between autophagy and endothelial function. Inhibiting autophagy in cells from healthy controls impairs eNOS activation to an extent comparable to that observed in cells from diabetics. In endothelial cells from patients with diabetes, activation of autophagy with spermidine restores insulin-mediated eNOS activation in endothelial cells from patients with diabetes and enhanced nitric oxide production in cultured endothelial cells in high glucose conditions. Restoration of eNOS activation by spermidine in cultured endothelial cells was blocked by autophagy inhibitors, bafilomycin and 3-MA, suggesting an autophagy-dependent mechanism. Taken together, these findings implicate inadequate autophagic flux as a mechanism of endothelial dysfunction in diabetes.
Prior studies have shown disordered autophagy in traditional insulin target tissues in experimental models diabetes. In the liver, mouse models of insulin resistance and obesity are associated with inadequate autophagy, and inhibiting autophagy by genetic deletion of Atg7 or Atg5 induces insulin resistance.28, 29 As was recently reviewed, inadequate autophagy also contributes to ectopic fat deposition, including the development of hepatic steatosis.30 In skeletal muscle, exercise prevents the development of insulin resistance and associated metabolic abnormalities in mice consuming a high fat diet, but the benefits of exercise are blocked in a genetically modified mouse model with an inability to induce autophagy in response to stress.31 In adipose tissue, autophagy is required for adipogenesis and, interestingly, excess autophagy may contribute to excessive release of free fatty acids from lipid droplets and promote cellular toxicity.30 In pancreatic islet cells, inadequate autophagic flux and abnormalities of lysosomal function under diabetic conditions reduce insulin secretion and, thus, may contribute to the pathogenesis and progression of type 2 diabetes.8, 15, 32
In the present study, we now provide evidence linking impaired autophagy to endothelial dysfunction in diabetes mellitus. Importantly, we demonstrate the presence of inadequate autophagy in vascular cells taken from patients with clinical diabetes. These findings add to the accumulating evidence that altered autophagy affects the endothelium in other disease states. Aging is strongly associated with endothelial dysfunction, and a recent study showed elevated p62 and reduced beclin-1 in freshly isolated endothelial cells from older subjects.10 Those results suggest impairment in the initiation phase of autophagy in aging and potentially implicate a different mechanism from our findings in cells from diabetics. In that study, beclin-1 expression correlated with endothelium-dependent vasodilation in the forearm, further supporting the idea that impaired autophagy initiation relates to endothelial dysfunction in aging. In an animal model of arterial aging, impaired autophagy was associated with endothelial dysfunction, and activators of autophagy, including spermidine and trehalose, restored endothelial dependent vasodilation, decreased oxidative stress, and attenuated inflammation.10, 33 The beneficial effects of spermidine on vascular function and endothelial phenotype in this model were lost when Atg12, a key initiator of autophagy, was knocked down suggesting that the effects are autophagy-driven.10, 28 The present study goes beyond the work of LaRocca and colleagues. In addition to showing differences in basal levels of autophagy proteins in freshly isolated endothelial cells, we studied functional responses to activators and inhibitors of autophagy to link inadequate autophagy to endothelial dysfunction in diabetes mellitus. Further, we demonstrate that induction of autophagy improves eNOS signaling in endothelial cells taken from patients with diabetes. We have previously reported in two published studies that eNOS activation measured by the phosphorylation of the activation site Ser1177 in endothelial cells freshly isolated from non-diabetics and diabetic subjects correlates with flow-mediated vasodilation. Thus, improvements in eNOS activation are linked to clinically relevant measures of endothelial function. Our work in the present study supports future studies to investigate whether systemic treatment with autophagy activators improve endothelial function in patients with diabetes.
In addition to eNOS activation, recent studies suggest that autophagy plays a protective role for other aspects of endothelial function. For example, activation of autophagy maintains cell viability in endothelial cells exposed hemin34 and advanced glycation end-products35. Furthermore, the polyphenolic compound curcumin increased endothelial cell survival following hydrogen peroxide exposure in a manner that depended on activation of autophagy via inhibition of mTOR and activation of FOXO1.36 Autophagy has been shown to regulate the release of von Willibrand factor from endothelial cells37 and angiogenic activity as reflected by endothelial sprouting, proliferation, and tube formation.38, 39 Finally, autophagy is important for clearance of oxidized low-density lipoprotein from endothelial cells, and thus may be relevant to atherogenesis.40 Consistent with this possibility, p62 gene expression was higher in sections of human aorta containing fatty streaks compared to unaffected sections, although that investigation did not specifically examine the endothelium.41 Thus, there is a growing body of evidence that autophagy is important for multiple aspects of endothelial function, but there has been a paucity of studies that examined these issues in human subjects.
Combining the present findings with the results of our recent studies using the same methodology suggests a mechanism to explain how inadequate autophagy may promote endothelial dysfunction. We previously observed higher nitrotyrosine levels and mitochondrial fragmentation in endothelial cells freshly isolated from subjects with type 2 diabetes and higher mitochondrial ROS production and mitochondrial fragmentation in endothelial cells exposed to high glucose.18, 19 Abnormal insulin-induced eNOS activation under diabetic conditions was linked to increased oxidative stress and was reversed by a scavenger of reactive oxygen species and by inhibiting mitochondrial fusion, which dramatically reduces mitochondrial ROS production.18, 19 As has been previously observed in pancreatic islet cells,42 these results suggest a problem with mitochondrial quality control and impaired autophagic clearance, leading to the accumulation of dysfunctional organelles that produce excess ROS and promote endothelial dysfunction. Excess ROS production by mitochondria has been shown to not only scavenge nitric oxide but also alter kinase signaling pathways that may be relevant in eNOS activation.4, 5
The observed pattern of autophagy protein expression (higher p62, unchanged LC3 puncta, increased Lamp2a) and the responses to spermidine (improved endothelial function) all suggest that autophagosome formation is intact or even increased and that there is a defect in the terminal phase of autophagy in endothelial cells under diabetic conditions. This situation may occur when there is a state of cellular stress with a high burden of damaged organelles and macromolecules, leading to activation of autophagosome formation and ongoing, but inadequate clearance from the cell. Our findings comparing the response to bafilomycin in diabetics compared to non-diabetics suggest that autophagic flux is lower in the diabetic endothelium and may be inadequate to clear the burden of organelles and macromolecules.
Our study has a number of limitations. The diabetic subjects had concomitant risk factors that might have contributed to our findings and the relatively modest sample size reduces the power to perform multivariable analyses to distinguish the relative importance of the metabolic risk factors associated with diabetes mellitus. We observed a trend for an inverse correlation between p62 levels and reactive hyperemia that might have been significant with a larger sample size. We examined venous endothelial cells rather than arterial endothelial cells, which may be more relevant to atherosclerosis. We point out, however, that venous endothelial cells are exposed to the same systemic risk factors in diabetes, and previous studies have shown correlated findings in arterial and venous endothelial cells collected with this methodology.17, 43 Additionally, the number of endothelial cells obtained using the venous endothelial cell biopsy precludes the use of some methodology commonly utilized to study the mechanisms of autophagy impairment. As with all pharmacologic agents, spermidine likely has additional non-autophagy related cellular effects that may influence endothelial function. However, previous studies have shown that the beneficial effects of spermidine on vascular function appear to be specific to alterations in autophagy as knock down of Atg12 in endothelial cells in an aging model lose this beneficial effect.10, 42 A concentration of 30 mM (540 mg/dL) was used to induce acute diabetic-like conditions in cultured HAEC’s but acute higher glucose levels may differ in effect from the chronic elevations with greater variability observed in patients with diabetes. The range of fasting blood glucose in the diabetic subjects was 84–268 mg/dL (4.6 mM– 14.9 mM) and it is likely that post-prandial levels are considerably higher and may influence endothelial phenotype. Regulation of p62 levels involves both autophagic degradation and transcription modulation. Taken together with the findings using autophagy modulators, differential p62 expression in the diabetic endothelium appears to reflect inadequate autophagy. Finally, autophagy is a highly regulated and complex mechanism, and additional work will be required to determine the precise alterations in diabetes that influence eNOS signaling and other functions of the vascular endothelium.
In conclusion, we observed inadequate autophagy in endothelial cells isolated from subjects with diabetes mellitus and cultured cells exposed to a high glucose concentration. Furthermore, we showed that intact autophagy is required for eNOS signaling in cells from healthy subjects and that generalized activation of autophagy reverses endothelial dysfunction under diabetic conditions in cells exposed to high glucose. These findings suggest that inadequate autophagy is a contributing mechanism for endothelial dysfunction in diabetes mellitus. Given the importance of endothelial dysfunction for the pathogenesis and clinical expression of atherosclerotic cardiovascular disease, these results are likely to be clinically relevant.44 Interventions directed toward restoring normal autophagic flux, particularly therapies directed toward the terminal phases of autophagy and autophagic clearance may have therapeutic potential.
Supplementary Material
Acknowledgments
Sources of Funding
The project was supported by National Heart, Lung, and Blood Institute grants HL81587 and HL11539. Dr. Fetterman is supported by the NIH-sponsored Boston University Medical Center Multidisciplinary Training Program in Cardiovascular Research (T32 HL007224). Drs. Hamburg and Vita receive support from the Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr Hamburg is supported by NIH grants HL102299 and HL109790. Dr. Gokce is supported by NIH grants HL081587 and HL115775. Dr. Farb is supported by an American Heart Association Postdoctoral Fellowship 12POST11780028.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
J.L.F. conducted experiments, wrote the manuscript. M.H. recruited participants. N.F. recruited participants and carried out patient visits. B.F. carried out patient visits and conducted several experiments. R.B.F. reviewed/edited the manuscript. E.A.L., B.D.B., and M.A.D. performed vascular testing and aided in patient visits. M.G.F., N.G., and O.S.S. reviewed/edited the manuscript. J.A.V. and N.M.H. conceived the overall experimental strategy and design and directed the studies.
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Barkoudah E, Skali H, Uno H, et al. Mortality rates in trials of subjects with type 2 diabetes. Journal of the American Heart Association. 2012;1:8–15. doi: 10.1161/JAHA.111.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabit CE, Chung WB, Hamburg NM, et al. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxidants & redox signaling. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard M, Shirihai OS, Gentil BJ, et al. Mitochondrial morphology transitions and functions: implications for retrograde signaling? American journal of physiology. Regulatory, integrative and comparative physiology. 2013;304:R393–R406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill BG, Benavides GA, Lancaster JR, Jr, et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biological chemistry. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 10.LaRocca TJ, Henson GD, Thorburn A, et al. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuervo AM, Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 2000;1:570–583. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez MG, Munafo DB, Beron W, et al. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 13.Goldman S, Zhang Y, Jin S. Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy. 2010;6:179–181. doi: 10.4161/auto.6.1.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez CD, Lee MS, Marchetti P, et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011;7:2–11. doi: 10.4161/auto.7.1.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Las G, Shirihai OS. The role of autophagy in beta-cell lipotoxicity and type 2 diabetes. Diabetes Obes Metab. 2010;12(Suppl 2):15–19. doi: 10.1111/j.1463-1326.2010.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo PC, Ashton AW, Celaj S, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol (1985) 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 18.Tabit CE, Shenouda SM, Holbrook M, et al. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton-Romero RFB, Holbrook M, Fetterman JL, Linder EA, Berk BD, Inagaki E, Gokce N, Fuster JJ, Walsh K, Vita JA, Hamburg NM. Wnt5a/JNK signaling mediates endothelial dysfunction in human diabetes. Arteriosclerosis, thrombosis, and vascular biology. 2016 doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leikert JF, Rathel TR, Muller C, et al. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001;506:131–134. doi: 10.1016/s0014-5793(01)02901-5. [DOI] [PubMed] [Google Scholar]
- 22.Kawai A, Uchiyama H, Takano S, et al. Autophagosome-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimori T, Yamamoto A, Moriyama Y, et al. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 24.Larque E, Sabater-Molina M, Zamora S. Biological significance of dietary polyamines. Nutrition. 2007;23:87–95. doi: 10.1016/j.nut.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 26.Morselli E, Marino G, Bennetzen MV, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 28.Liu HY, Han J, Cao SY, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Li P, Fu S, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Las G, Serada SB, Wikstrom JD, et al. Fatty acids suppress autophagic turnover in beta-cells. J Biol Chem. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, et al. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higdon AN, Benavides GA, Chacko BK, et al. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy, American journal of physiology. Heart and circulatory physiology. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, You SJ, Zhang YL, et al. Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep. 2011;4:459–464. doi: 10.3892/mmr.2011.460. [DOI] [PubMed] [Google Scholar]
- 36.Han J, Pan XY, Xu Y, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- 37.Torisu T, Torisu K, Lee IH, et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med. 2013;19:1281–1287. doi: 10.1038/nm.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Yu S, Zhang H, et al. Angiogenesis impairment in diabetes: role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS One. 2012;7:e46720. doi: 10.1371/journal.pone.0046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim KW, Paul P, Qiao J, et al. Autophagy mediates paracrine regulation of vascular endothelial cells. Lab Invest. 2013;93:639–645. doi: 10.1038/labinvest.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YL, Cao YJ, Zhang X, et al. The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun. 2010;394:377–382. doi: 10.1016/j.bbrc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 41.Shchelkunova TA, Albert EA, Morozov IA, et al. Contents of mRNAs encoding endosome/lysosome components in normal human aorta and in stage II of atherogenesis: a hidden regulation. Biochemistry (Mosc) 2011;76:1178–1184. doi: 10.1134/S0006297911100129. [DOI] [PubMed] [Google Scholar]
- 42.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silver AE, Christou DD, Donato AJ, et al. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.