Abstract
Introduction
Depression is considered an independent risk factor for hypertension, particularly for people with recurrent episodes or a long history of depression. Another risk factor for cardiovascular disease is the Apolipoprotein E e4 allele (ApoE e4). The aim of this study was to examine how ApoE e4 was related to blood pressure (BP) in patients with depression and a control group.
Methods
A total of 78 patients, 49 with depression and 29 without, all recruited from the same hospital, underwent ApoE e genotyping (24 had at least one ApoE e4 allele) and examination of BP.
Results
In the depression group, but not in the control group, both systolic and diastolic BP were significantly higher in patients with ApoE e4 than in those without. The effect of ApoE e4 on BP differed significantly between the two groups.
Conclusion
Our findings showed that the effect of ApoE e4 on BP differed between the patients with depression and the control group. In patients with depression, ApoE e4 was associated with an increase in BP. We suggest that patients with depression and ApoE e4-positive status are particularly prone to develop BP elevation.
Keywords: depression, blood pressure, ApoE e4, ApoE, genotyping
Introduction
The literature suggests that blood pressure (BP) alterations are associated with psychopathology, especially depression.1–4 Nonetheless, the reported relationship between depression and BP alteration is inconsistent. Depression has been considered an independent risk factor for hypertension, particularly for people with recurrent episodes or a long-term history of depression.5 Yet most studies report that depression is related to lower rather than higher BP.4,6 Two longitudinal studies have shown that high levels of anxiety and depression at baseline, with an increase in these symptoms over time, were associated with lower systolic BP in an 11-year follow-up study.7,8
Depression has been associated with a high rate of cardiovascular diseases.9–11 In an 8-year follow-up study, an increased risk of stroke or transient ischemic attacks was observed in depressed patients younger than 65 years, but not among those over 65.12 In a meta-analysis, the most prominent risk factor for cardiovascular diseases was found to be depression.13 It is possible, therefore, that depression may influence factors related to cerebrovascular disorders, such as hypertension.
The human Apolipoprotein E (ApoE) has three allelic variants, e2, e3, and e4, encoded on chromosome 19. A person could be homozygotic, with two e4 alleles (one from each parent); or heterozygotic, with one e4 allele in combination with either an e2 or an e3 allele. The heterozygotic e4 variant, found in approximately 18% of the Norwegian population,14 has been associated with atherosclerosis, Alzheimer’s disease, general impaired cognitive function, and reduced hippocam-pal volume.15–19
Little is known about the association between depression and ApoE e4, but some studies have reported the e4 allele to be a risk factor.19–21 All the research we have been able to find has examined this association in older people, often in patients with cognitive deficits or dementia.22
The research question in this study was: “Is the ApoE e4 allele in persons with depression associated with systolic and diastolic BP?” At the same time, we wanted to examine a control group of patients without known depression or diagnosed disease who had presented with diffuse neurological symptoms such as tiredness or vague symptoms like their body not functioning as it should.
Materials and methods
Patients and controls
A group of 49 depressed patients were recruited from inpatient wards and outpatient clinics of the mental health unit at Innlandet Hospital Trust, Norway. They were all over 18 years of age and had been diagnosed with depression (International Statistical Classification of Diseases and Related Health Problems, tenth revision [ICD-10]; F32–34 spectre). The control group comprised 29 patients over 18 years of age, referred to the Department of Neurology at the same hospital for diffuse symptoms for which no disease could be diagnosed. They were included after exclusion of organic disorders. None of the depressed patients or the controls showed any signs of neurological disease.
Examinations and assessments
A medical history was recorded, a routine clinical examination was performed, and hematological and biochemical screening tests were carried out in all participants. The cerebrospinal fluid of all participants was examined. The first author confirmed the clinical diagnoses of depression after careful patient examinations using the ICD-10 criteria for research, which revealed mild-to-severe symptoms of depression (F32–34). A neurologist conducted a detailed examination of the patients in the Department of Neurology, and only those with no clinical or laboratory indication of a neurological diagnosis or depression were included. No cerebral neuropathology was found in any depressed patients or patients with neurological symptoms, based upon clinical examination, cerebrospinal fluid examination, or computed tomography or magnetic resonance imaging (the computed tomography or magnetic resonance imaging for the control group and in the depression group if there were any indication or suspicion of cerebral disorder).
Genotyping
The whole-blood samples were collected by a bioengineer using EDTA glass vacutainers and sent to the Department of Medical Biochemistry, Oslo University Hospital, Rikshospitalet, Norway, for ApoE e analysis. Genotyping was performed by real-time polymerase chain reaction with allele-specific fluorescence energy transfer probes and melting curve analysis on the LightCycler system (Roche Diagnostics, Basel, Switzerland). DNA was extracted from 300 µL of whole blood using MagNA Pure LC DNA Isolation Kit – Large Volume on the MagNA Pure LC (Roche Diagnostics), eluted and diluted to 1 mL, of which 5 µL was used in each assay. Genotyping of the ApoE e2, e3, and e4 was performed using the LightCycler ApoE Mutation Detection Kit (Roche Diagnostics). The assay was performed as specified by the supplier, except for scaling down the total assay volume from 20 µL to 10 µL. The laboratory participates in an external quality assurance program (Equalis) that includes ApoE genotyping.
The Beck Depression Inventory-II (BDI-II)23 was used as an indicator of depression severity.
A digital Microlife BP 3 AGI apparatus (Microlife AG Swiss Corporation, Widnau, Switzerland) was used to record BP, following the standard procedure delineated in the manual. (See user manual home page: http://www.microlife.com/products/hypertension/automatic/bp-3ag1) According to ICD-10, a BP of 120/80 mmHg (systolic/diastolic) or lower is normal BP. A pressure 140/90 mmHg or higher is regarded as high BP. Systolic pressure between 120 mmHg and 139 mmHg, or diastolic between 80 mmHg and 89 mmHg is regarded as prehypertension.
Ethics
The study was reviewed and approved by the Regional Committees for Medical and Health Research Ethics, South East A, Reference no 2009/2196a, and all participants provided written informed consent form before they were included in the study.
Statistics
Independent samples t-test or chi-square analyses were performed in the two groups to test the presence/absence of ApoE e4, BP, age, sex, and antihypertensive medication. Univariate analysis of variance with and without the interaction between groups (control vs depression) and presence or absence of ApoE e4 was used to study predictors of BP. Age, sex, and antihypertensive medication were included as covariates. SPSS Version 22 (IBM Corporation, Armonk, NY, USA) was used for the analyses. Finally, the same analyses were performed without the participants who were on antihypertensive drugs.
Results
Table 1 lists characteristics of patients in the two groups. Only the score on BDI-II differed between the two groups. Many of the patients in both groups said they had more fatigue than before: 24 out of 29 in the control group and 45 out of 49 in the depression group. The typical symptom in the control group was fatigue. Apart from that, seven struggled with headache, six had some muscle pain or numbness or tendencies for tremor in arms or legs, three had some form of dizziness, one had a tendency to ataxia, and one patient had reported optic neuritis. Patients could have one or more of these symptoms.
Table 1.
Characteristics of the two groups: mean (SD), number of participants (%), and significance testing
| Participant characteristics | Depression, n=49 | Neurological symptoms (controls), n=29 | Statistics (P-value) |
|---|---|---|---|
| Females (%) | 26 (53.1%) | 20 (69.0%) | NS (P=0.17) |
| Age, years | 45.3 (14.6) | 43.45 (13.51) | NS (P=0.58) |
| ApoE e4 present | 17 (34.7%) | 13 (44.8%) | NS (P=0.37) |
| Systolic BP, mmHg | 128.35 (20.0) | 129.96 (22.0) | NS (P=0.73) |
| Diastolic BP, mmHg | 78.96 (12.79) | 81.76 (13.78) | NS (P=0.37) |
| Use of antihypertensive drugs | 9 (18.4%) | 5 (17.2%) | NS (P=0.90) |
| BDI-II | 30.1 (12.4) | 7.14 (6.87) | P<0.000001 |
Notes: Sex, ApoE e4, and use of antihypertensive drugs, analyzed by Chi-squared analysis. For the other variables, Student’s t-tests were used.
Abbreviations: ApoE e4, Apolipoprotein E e4 allele; BP, blood pressure; BDI-II, Beck Depression Inventory-II; SD, standard deviation; NS, not significant.
In the control group, there was one participant with 2/3 allele combination, 15 with 3/3, 12 with 3/4, and one with 4/4. In the depression group, three had 2/3, 29 had 3/3, 15 had 3/4, and two had 4/4 allele combination.
Table 2 lists systolic and diastolic BP (mmHg), age, sex, and antihypertensive medication in patients with and without ApoE e4, with separate analysis for the depressed and the control group. As listed in Table 2, the BP analyses were also performed excluding the subjects on antihypertensive drugs, showing no significant differences in results.
Table 2.
The control and depression groups divided according to if the ApoE e4 allele was present or not
| Groups | ApoE e4 present | ApoE e4 absent | Statistics (P-value) |
|---|---|---|---|
| Depression | n=17 | n=32 | |
| All patients | |||
| Systolic BP, mmHg | 142.4 (19.7) | 120.9 (15.1) | 0.0001 |
| Diastolic BP, mmHg | 87.3 (11.1) | 75.5 (11.4) | 0.0005 |
| Age, years | 43.2 (14.9) | 49.2 (13.5) | 0.18 |
| Sex (females/males) | 5/12 | 21/11 | 0.02 |
| Antihypertensive drugs | n=7 | n=6 | 0.17 |
| Patients not using antihypertensive drugs | n=10 | n=26 | |
| Systolic BP, mmHg | 141.4 (21.0) | 120.8 (13.6) | 0.001 |
| Diastolic BP, mmHg | 85.8 (11.5) | 74.0 (10.4) | 0.005 |
| Neurological symptoms (controls) | n=13 | n=16 | |
| All patients | |||
| Systolic BP, mmHg | 123.7 (10.4) | 135.1 (24.7) | 0.13 |
| Diastolic BP, mmHg | 79.5 (10.2) | 83.6 (16.2) | 0.43 |
| Age, years | 42.0 (9.4) | 45.2 (15.7) | 0.47 |
| Sex (females/males) | 10/3 | 12/6 | 0.70 |
| Antihypertensive drugs | n=1 | n=4 | 0.40 |
| Patients not using antihypertensive drugs | n=12 | n=12 | |
| Systolic BP, mmHg | 123.8 (10.9) | 124.8 (11.0) | 0.81 |
| Diastolic BP, mmHg | 79.5 (10.7) | 76.8 (9.3) | 0.52 |
Notes: Values are given as mean (SD) for systolic and diastolic BP and age. Student’s t-test and Chi-square analysis were performed.
Abbreviations: ApoE e4, Apolipoprotein E e4 allele; BP, blood pressure; SD, standard deviation.
Both systolic and diastolic BP were significantly higher among the depressed ApoE e4 allele group as compared to the depressed non-ApoE e4 group. The nondepressed group demonstrated no such differences.
Univariate analyses of variance were then performed, with systolic and diastolic BP as the dependent variables; with group (control vs depression) and ApoE e4 (yes or no) as independent variables; and age, sex, and antihypertensive medications as covariates. The results are listed in Table 3.
Table 3.
Test of between-subjects effects
| Dependent variables | Covariates
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group (neuro/depressed)
|
ApoE e4 (absent/present)
|
Sex (f/m)
|
Age (years)
|
Antihypertensive treatment (no/yes)
|
||||||
| B (95% CI) | P-value | B (95% CI) | P-value | B (95% CI) | P-value | B (95% CI) | P-value | B (95% CI) | P-value | |
| Systolic BP (all patients) | −3.17 (−11.6:5.3) | 0.46 | 6.1 (−2.2:14.4) | 0.15 | 5.2 (−3.2:13.6) | 0.22 | 0.5 (0.2:0.8) | 0.004 | 4.8 (−6.3:15.9) | 0.39 |
| Diastolic BP (all patients) | −3.77 (−9.5:2.0) | 0.20 | 4.67 (−1.0:10.4) | 0.11 | 3.0 (−0.2:7.8.7) | 0.30 | 0.20 (−0.03:0.43) | 0.08 | 6.4 (−1.2:14.0) | 0.10 |
| Systolic BP (patients not using antihypertensive drugs) | 3.80 (−4.1:11.7) | 0.34 | 9.68 (1.7:17.6) | 0.02 | 5.7 (−2.1:13.5) | 0.15 | 0.31 (0.01:0.60) | 0.04 | ||
| Diastolic BP (patients not using antihypertensive drugs) | 0.48 (−5.3:6.3) | 0.87 | 7.29 (1.41:13.2) | 0.02 | 2.1 (−3.7:7.9) | 0.46 | 0.11 (10.1:0.3) | 0.33 | ||
Notes: Univariate ANOVA was performed with a significance level set to 0.05. Dependent variables were systolic and diastolic BP (in mmHg); independent variables (fixed factors) were grouped (depression vs controls) and possessing at least one ApoE e4 allele (yes vs no). Covariates were age, sex, and antihypertensive medication. The analyses were redone after excluding those on antihypertensive drugs (the last two rows in the table).
Abbreviations: ApoE e4, Apolipoprotein E e4 allele; BP, blood pressure; CI, confidence interval.
A main effect was seen for age relative to systolic BP for all participants, which was still there after excluding those on antihypertensive drugs. When those on antihypertensive drugs were excluded, a main effect was also seen for ApoE e allele for both systolic and diastolic BP.
The interaction between group (depressed/not depressed) and ApoE e (e4 or not) regarding BP is listed in Table 4.
Table 4.
The interaction between group (depressed/not depressed) and ApoE e (e4 or not) regarding systolic and diastolic BP
| Systolic BP (all patients) B (95% CI); P-value | Diastolic BP (all patients) B (95% CI); P-value | Systolic BP (patients not using antihypertensive drugs) B (95% CI); P-value | Diastolic BP (patients not using antihypertensive drugs) B (95% CI); P-value | |
|---|---|---|---|---|
| Interaction group/ApoE e4 | 26.7 (10.0:43.4); P=0.002 | 12.4 (0.6:24.2); P=0.04 | 20.6 (5.3:35.9); P=0.009 | 8.8 (−3.0:20.6); P=0.1 |
Notes: The analysis was first done for all participants (first two columns). In the last two columns, those on antihypertensive drugs were excluded. Univariate analysis of variance with the interaction between groups (control or depression) and ApoE e4 (presence or absence).
Abbreviations: ApoE e4, Apolipoprotein E e4 allele; BP, blood pressure; CI, confidence interval.
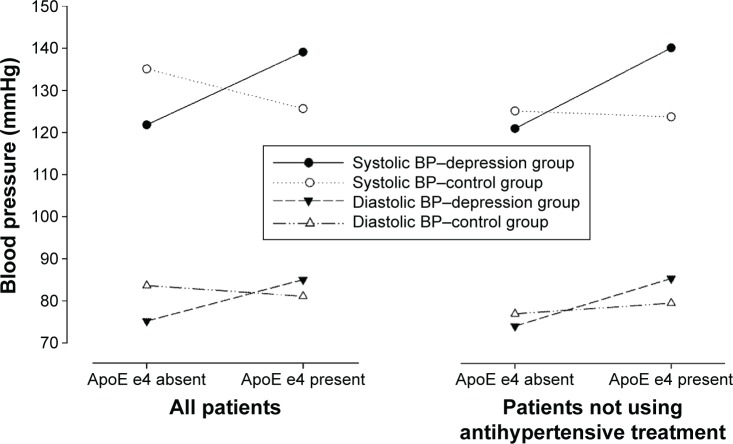
The interaction between group and ApoE e was highly significant (F=10.18, P=0.002 for systolic BP), with a moderate close to high eta2 (0.13). The estimated marginal means for the systolic and diastolic BP are shown in Figure 1.
Figure 1.
Estimated marginal means where the dependent variable was systolic and diastolic BP in all patients and in patients without antihypertensive treatment.
Notes: ApoE e4 (yes/no) and the two groups (depression vs controls), age, sex, use of antihypertensive medication, and the interaction between group and ApoE e4 were covariates.
Abbreviations: ApoE e4, Apolipoprotein E e4 allele; BP, blood pressure.
Discussion
We found a clear interaction between depression and ApoE e4 regarding BP, especially for systolic, but also for diastolic pressure. The combination of ApoE e4 and depression resulted in a 21.5 mmHg difference in systolic BP between patients with and without the ApoE e4 in the depression group. Thus, our findings indicate that there is a tendency for higher BP in depressed patients carrying an ApoE e4 allele compared to depressed patients with no such gene. The same tendencies could be seen for diastolic as for systolic BP, although the tendency was slightly weaker.
Earlier studies regarding depression and BP have been inconclusive, showing not only that hypertension was related to depression,5 but also that depressed patients exhibited lower levels of BP than did healthy control subjects.4,6 As for our study, where no ApoE e4 is present, there is a tendency for lower levels of BP in the depressed group than among controls (Table 1 and Figure 1). The interaction between ApoE e4 and depression became weaker, especially for diastolic BP, when those on antihypertensive medication were excluded. This effect was probably due to the lower n in the last analyses. It could be that the two previous studies showing low levels of BP in depressed patients had included fewer patients with an ApoE e4 allele than were included in our study. Neither of those studies examined ApoE genotypes.
The ApoE e4 allele was initially recognized for its importance in lipoprotein metabolism and cardiovascular disease, such as atherosclerosis, and related cerebrovascular disease.24,25 Research has shown depression to be associated with systemic immune activation and inflammation, endothelial dysfunction, and increased cardiovascular morbidity and mortality. Depression and cardiovascular disease are interrelated: depression predicts cardiovascular disease onset and adverse outcome in patients with known cardiovascular disease.26 The combination of depression and ApoE e4 seems to be related to elevated BP, which may then be associated with cardiovascular disease. There are probably different interacting relationships between ApoE e4 and depression. On the background of the difficulties described in association with ApoE e4, it is likely that this allele is some type of vulnerability gene, which put the person with that gene at risk for several diseases and disorders. It has also been repeatedly seen that ApoE e4 is a risk factor for dementia. Likewise, depression may be the first symptom of or a risk factor for dementia in old age. We suggest that the driving force is ApoE e4’s influence on BP and that biology (ApoE e4) and psychology (depression) are closely interwoven in the elevation of BP.
The proportion of persons with an ApoE e4-positive status in this sample of patients with depression and neurological symptoms was higher than that found in a representative sample of the general Norwegian population based in the same catchment area:27 38% vs 18.2%. Furthermore, no significant difference existed between the two groups. The reason for the high number of individuals with the e4 allele in this sample is unclear. It could be that persons with a positive ApoE e4 status (a vulnerability gene variant) are more susceptible to multiple disorders, including depression.
Strengths and limitations
The strengths of our study are that it is a case–control design where all the patients were from the same county and hospital. Furthermore, the control group had many of the same symptoms (especially fatigue) as the depressed patients, but no affective disorder.
One limitation to this study is the sample size, requiring that conclusions must be drawn with caution.
Conclusion
The presence of ApoE e4, which has been associated with inflammation, cardiovascular risk factors, and metabolic disturbances, was associated with elevated systolic and diastolic BP in patients with depression – but not in patients with neurological symptoms. The results of this study suggest that patients with depression and ApoE e4-positive status are particularly prone to develop BP elevation.
Acknowledgments
We are grateful to Marit Hansen Hallberg and the staff in the Department of Medical Biochemistry, Oslo University Hospital, Rikshospitalet, Oslo, Norway, for technical support on DNA extraction and genotyping; to Gunnar Børre Thoresen and his team at Innlandet Hospital Trust for data collection; and to Christa Kristiansen for data collection. The authors also thank the study participants and the participating institutions for their cooperation, and Lillehammer University College and Innlandet Hospital Trust for funding.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Matthews KA, Katholi CR, McCreath H, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110(1):74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 2.Markovitz JH, Matthews KA, Kannel WB, Cobb JL, D’Agostino RB. Psychological predictors of hypertension in the Framingham Study. Is there tension in hypertension? JAMA. 1993;270(20):2439–2443. [PubMed] [Google Scholar]
- 3.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med. 2001;63(5):737–743. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Licht CM, de Geus EJ, Seldenrijk A, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53(4):631–638. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- 5.Meyer CM, Armenian HK, Eaton WW, Ford DE. Incident hypertension associated with depression in the Baltimore Epidemiologic Catchment area follow-up study. J Affect Disord. 2004;83(2–3):127–133. doi: 10.1016/j.jad.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Palinkas LA. Low blood pressure and depression in older men: a population based study. BMJ. 1994;308(6926):446–449. doi: 10.1136/bmj.308.6926.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildrum B, Mykletun A, Holmen J, Dahl AA. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. Br J Psychiatry. 2008;193(2):108–113. doi: 10.1192/bjp.bp.107.045013. [DOI] [PubMed] [Google Scholar]
- 8.Hildrum B, Romild U, Holmen J. Anxiety and depression lowers blood pressure: 22-year follow-up of the population based HUNT study, Norway. BMC Public Health. 2011;11:601. doi: 10.1186/1471-2458-11-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102(15):1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 10.Almeida OP, Flicker L, Norman P, et al. Association of cardiovascular risk factors and disease with depression in later life. Am J Geriatr Psychiatry. 2007;15(6):506–513. doi: 10.1097/01.JGP.0000246869.49892.77. [DOI] [PubMed] [Google Scholar]
- 11.Ford DE, Mead LA, Chang PP, Cooper-Patrick L, Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in men: the precursors study. Arch Intern Med. 1998;158(13):1422–1426. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- 12.Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38(1):16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 13.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 14.Hestad K, Kveberg B, Engedal K. Low blood pressure is a better predictor of cognitive deficits than the apolipoprotein e4 allele in the oldest old. Acta Neurol Scand. 2005;111(5):323–328. doi: 10.1111/j.1600-0404.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 15.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 16.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deary IJ, Whiteman MC, Pattie A, et al. Cognitive change and the ApoE epsilon 4 allele. Nature. 2002;418(6901):932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- 18.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of ApoE in mild cognitive impairment. Neurology. 2004;63(10):1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan KR, Tupler LA, Ritchie JC, Jr, et al. Apolipoprotein E-epsilon 4 frequency in geriatric depression. Biol Psychiatry. 1996;40(1):69–71. doi: 10.1016/0006-3223(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 20.Rigaud AS, Traykov L, Caputo L, et al. Association of the apolipo-protein E epsilon4 allele with late-onset depression. Neuroepidemiology. 2001;20(4):268–272. doi: 10.1159/000054801. [DOI] [PubMed] [Google Scholar]
- 21.Yen YC, Rebok GW, Gallo JJ, Yang MJ, Lung FW, Shih CH. ApoE4 allele is associated with late-life depression: a population-based study. Am J Geriatr Psychiatry. 2007;15(10):858–868. doi: 10.1097/JGP.0b013e3180f63373. [DOI] [PubMed] [Google Scholar]
- 22.Nose M, Kodama C, Ikejima C, et al. ApoE4 is not associated with depression when mild cognitive impairment is considered. Int J Geriatr Psychiatry. 2013;28(2):155–163. doi: 10.1002/gps.3803. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 24.McCarron MO, Delong D, Alberts MJ. ApoE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53(6):1308–1311. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 25.Mahley RW. Apolipoprotein E: cholesterol transport protein with expandinrole in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 26.Hollan I, Meroni PL, Ahearn JM, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmunity Rev. 2013;12(10):1004–1015. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Hestad K, Kveberg B, Engedal K. Low blood pressure is a better predictor of cognitive deficits than the apolipoprotein e4 allele in the oldest old. Acta Neurol Scand. 2005;111(5):323–328. doi: 10.1111/j.1600-0404.2005.00397.x. [DOI] [PubMed] [Google Scholar]