Abstract
Background
Characterizing relationships between pain relief and function can inform patient management decisions. This analysis explored graphically the relationship between pain relief and functional improvement in patients with neuropathic pain associated with spinal cord injury in two clinical trials of pregabalin.
Methods
This was a post hoc analysis of two randomized, double-blind, clinical trials in patients who were treated with pregabalin (n=181) or placebo (n=172) for neuropathic pain associated with spinal cord injury. The bivariate relationship between percent pain relief and absolute change in the functional outcomes with placebo and pregabalin was evaluated graphically using scatter plots, and loess curves illustrated the extent of the relationship between pain and function. Linear trend analysis evaluated the statistical significance of these relationships using Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)-based thresholds of pain reduction (<15%, 15% <30%, 30% to <50%, and ≥50%). Outcome measures included modified Brief Pain Inventory pain interference with function in one of the studies and the Medical Outcomes Study Sleep Scale (an 11-point Numeric Rating Scale) and the Hospital Anxiety and Depression Scale (HADS) for the pooled studies.
Results
Data ellipses showed a shift with pregabalin relative to placebo toward greater improvement with increasing pain relief for all outcome measures except HADS. Loess curves suggested a relationship between increased pain relief and improved function except for HADS, with the clearest relationship observed for sleep. Linear trend analysis showed significant relationships between pain and Medical Outcomes Study Sleep Scale (P<0.0001) and between pain and function on the modified Brief Pain Inventory Interference Index and most individual items (P<0.05).
Conclusion
Greater functional improvements were generally achieved at higher levels of clinically significant pain reduction. Pregabalin resulted in shifts from placebo toward greater functional improvement with greater pain relief.
Keywords: spinal cord injury, neuropathic pain, function, sleep, pregabalin
Introduction
Recent estimates suggest that as many as 273,000 individuals in the US have spinal cord injury (SCI), with ~12,000 new cases of SCI occurring annually.1 Among these individuals, ~40% also have chronic central neuropathic pain (NeP), a complication of SCI resulting from a lesion of the somatosensory pathways within the central nervous system. The presence of pain in patients with SCI further compromises function, increases disability, reduces quality of life, and has been reported to be a contributory factor to unemployment and depression.2–5 In particular, SCI-associated NeP has been shown to be associated with a substantial socioeconomic burden, with higher health care resource utilization and costs and lower health status and productivity observed at increasing levels of pain.6
Almost all patients with SCI have the potential to engage in functional activities; thus, improvement in function is a key goal of patients post injury and a major focus of SCI rehabilitation, which takes an integrated approach to the management of these patients. However, in addition to the reductions in function and quality of life noted earlier, the presence of pain negatively affects inpatient rehabilitation therapy: fewer days in rehabilitation and less rehabilitation treatment time were reported among patients with the highest pain severity levels relative to those with no pain and lower pain levels.7
When pain is present, a core element of SCI management is pain reduction to a level that the patient considers acceptable, as complete relief is rarely possible. A variety of pharmacologic interventions are available for SCI pain, and those especially used for NeP include antidepressants, antiepileptic drugs, opioids, and various intrathecal medications.8–10 While several medications have been studied in NeP associated with SCI, most of these studies utilized a small sample (≤40 subjects) and a short-treatment duration (≤4weeks).11
Currently, only pregabalin has received US Food and Drug Administration approval in the US for treatment of NeP associated with SCI.12 Approval in SCI-related NeP was based on two clinical trials in which pregabalin resulted in significantly greater pain reductions relative to placebo (P<0.05), with significantly greater proportions of patients achieving clinically relevant reductions in pain (P<0.05).13,14 In both of these trials and a pooled analysis,15 the results of secondary efficacy end points also suggested improvements in functional outcomes, and a recent meta-analysis of gabapentinoids further supported its efficacy for pain and secondary outcomes in patients with NeP associated with SCI.16
With regard to function, moderate-to-strong correlations have been observed between pain intensity and interference with daily activities using various versions of the Brief Pain Inventory (BPI); higher levels of pain intensity consistently resulted in greater interference.3,4 Given these observed associations, it may be expected that relief of NeP resulting from treatment would result in functional improvements. However, such an association between levels of pain reduction and functional improvement in patients with SCI has not been evaluated.
Since characterizing the relationships between pain and function can inform decisions regarding appropriate patient management, the goal of the current study was to explore the relationships between improvement in pain and changes in function among patients treated with pregabalin or placebo in the two clinical trials. We hypothesized that the definition of a responder as a patient who shows improvements regardless of treatment may need to be challenged and that exploration of responders by treatment could potentially provide insight toward resolving this assumption, ie, whether relationships between pain and functional response may differ by treatment. A broad definition of function was applied in order to encompass body functions, activity, and participation as described in the International Classification of Functioning, Disability, and Health.17
Methods
Data were derived from Siddall et al13 and Cardenas et al14, two clinical trials of pregabalin in patients with SCI-associated NeP, for which details of methodology and key results of the effects of pregabalin on pain relief have been previously published. Both studies, which received approval from the appropriate Institutional Review Boards or Independent Ethics Committees, employed a randomized, placebo-controlled, double-blind design that evaluated flexible dosing of pregabalin 150–600 mg/d compared with placebo taken twice daily for 12 weeks. Patients in both studies were ≥18 years of age, with SCI and central NeP of at least moderate severity; 57.7% and 42.3% of patients in Siddall et al13 were paraplegic and tetraplegic, respectively, and the majority of patients in Cardenas et al14 were of either American Spinal Injury Association classification A (48.4%) or D (28.8%). In Siddall et al,13 pregabalin was initiated at 150 mg/d for the first week and then increased to 300 mg/d with the potential for further increase to 600 mg/d after the second week; doses could be reduced from 300 mg/d to 150 mg/d at the end of the second week and from 600 mg/d to 300 mg/d at the end of the third week if not tolerated. Cardenas et al14 initiated pregabalin at 150 mg/d for 7 days, with increases based on tolerability to 300 mg/d on day 8, 450 mg/d on day 15, and 600 mg/d on day 22, followed by a 12-week maintenance period of optimized dose with allowance of a single-level dose reduction if needed.
Cardenas et al14 included a ten-item BPI for patients with disabilities, which has been modified and validated for use in SCI,4 although these items have not been validated for their individual use. The BPI quantifies pain interference with daily function using an 11-point scale based on the question “How, during the past week, has pain interfered with your?” with responses for ten health status domains (General Activity, Enjoyment of Life, Mobility, Mood, Sleep, Normal Work, Self-Care, Relations, Recreational Activities, and Social Activities) and a range of responses from 0 (“Does not interfere”) to 10 (“Completely interferes”). The BPI is recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) as a clinically relevant assessment of functioning.18 IMMPACT is an initiative designed to provide consensus and recommendations relating to clinical trials for the treatment of pain, and especially, for use of pain and pain-related outcome measures.
The modified version of the BPI (MBPI)19 includes three additional interference items (Self-Care, Recreational Activities, and Social Activities) to broaden the range of activities, and by using Mobility (ie, getting around) instead of Walking Ability, since patients with SCI may not be able to walk. In addition to evaluating each of the MBPI items as part of this exploratory analysis, two subscales of the original BPI, Affective Interference (Relations, Mood, Sleep, Enjoyment of Life) and Activity Interference (Walking, Work, General Activities), were also evaluated.20
Efficacy end points in both studies that are included in the current analysis, evaluated as change from baseline using a last observation carried forward (LOCF) approach, are patient-reported pain evaluated in daily diaries using an 11-point Numeric Rating Scale (NRS; 0= no pain to 10= worst pain), the Medical Outcomes Study Sleep Scale (MOS-SS),21 a daily diary sleep interference NRS scale, and the Hospital Anxiety and Depression Scale (HADS).22 The MOS-SS is a patient-reported outcome that includes 12 items on subjective perception of sleep initiation, maintenance, quality, daytime somnolence, and sleep breathing disorders over a 4-week recall period; nine items are used to generate the Sleep Problems Index, with a range of 0–100 and higher scores indicating a greater impact on sleep. The daily diary sleep interference NRS rated the extent to which pain interfered with sleep over the prior 24 hours on an 11-point scale from 0= pain does not interfere to 10= pain completely interferes. The HADS is a patient-reported measure of anxiety and depression that has two subscales, one for depression (HADS-D) and one for anxiety (HADS-A), each consisting of seven items and having a score range of 0–21, with higher scores indicating greater severity.
Exploratory graphic analyses were performed on the bivariate relationship between percent change at end point (LOCF) from baseline in average pain and change at end point (LOCF) from baseline in each of the functional outcomes, including the MBPI, MOS-SS, and HADS Anxiety and Depression. These plots examined the possible shifts of the bivariate relationship between pain and function by treatment by plotting the data ellipses showing 50% and 95% confidence regions of the bivariate relationships23; these ellipses provide a graphic representation of the spread and strength of the relationship. Smoothed loess curves24 were also plotted for each pair to explore the extent of linearity or nonlinearity; loess curves represent a flexible, nonparametric method to describe associations between two variables that is robust to outliers and makes no assumptions about the distribution of the data.
To further explore the relationship between pain improvement and function for Interference Index and all individual items on the MBPI, MOS-SS sleep, and both HADS sub-scales, the pregabalin results were analyzed using linear trend analysis according to thresholds of improvement in pain from baseline at end point. These thresholds were improvements of <5%, 15% to <30%, 30% to <50%, and ≥50%, based on IMMPACT recommendations for clinically significant pain reduction25; a 15% pain reduction is considered minimally important, ≥30% is clinically significant, and a reduction ≥50% is of substantial clinical significance. At each pain response categories, the least squares mean for each continuous outcome variable was estimated using an analysis of covariance with terms for treatment and study (or center if one study was used) and adjusted for baseline value of the outcome variable. Significance of the changes across pain improvement categories was considered at an α level of <0.05.
Results
Demographic and disease characteristics of the patients in each of the trials are shown in Table 1. While age, sex, and clinical characteristics were similar between the two studies, nearly all the patients in Siddall et al13 were Caucasian (97.1%), whereas in Cardenas et al14 just over one-third were Whites (38.8%) and approximately half were Asians (50.3%). For all measures, baseline values were generally similar among the groups of patients achieving pain reduction thresholds (Table 2).
Table 1.
Demographic and clinical characteristics of the patients in the two clinical trials
| Variable | Siddall et al13
|
Cardenas et al14
|
||
|---|---|---|---|---|
| Placebo (n=67) | Pregabalin (n=70) | Placebo (n=108)a | Pregabalin (n=111) | |
| Male sex, n (%) | 54 (80.6) | 60 (85.7) | 92 (85.2) | 84 (75.7) |
| Age, years, mean (SD) | 49.8 (14.2) | 50.3 (14.3) | 45.6 (13.8) | 46.1 (12.7) |
| Race, n (%) | ||||
| Caucasian | 66 (98.5) | 67 (95.7) | 43 (39.8) | 42 (37.8) |
| Black | 0 | 0 | 8 (7.4) | 6 (5.4) |
| Asian | 1 (1.5) | 2 (2.9) | 53 (49.1) | 57 (51.4) |
| Other | 0 | 1 (1.4) | 4 (3.7) | 6 (5.4) |
| Central pain | ||||
| Duration, years, mean (SD) | 10.4 (9.8) | 9.9 (7.7) | 8.1 (8.7) | 8.2 (8.3) |
| Persistent in last 3 months, n (%) | 59 (88.1) | 62 (88.6) | 92 (85.2) | 92 (82.9) |
| Pain score, mean (SD) | 6.7 (1.4) | 6.5 (1.3)b | 6.5 (1.4) | 6.5 (1.5) |
Notes:
One patient was randomized to placebo but received pregabalin.
n=69.
Abbreviation: SD, standard deviation.
Table 2.
Baseline values of outcome measures for patients achieving pain reduction thresholds
| Measure | Mean (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <15%
|
15% to <30%
|
30% to <50%
|
≥50%
|
|||||
| Pregabalin | Placebo | Pregabalin | Placebo | Pregabalin | Placebo | Pregabalin | Placebo | |
| Pooled studies13,14 | n=60 | n=103 | n=37 | n=25 | n=31 | n=23 | n=46 | n=21 |
| HADS-A | 6.8 (4.1) | 7.4 (4.2) | 6.7 (4.1) | 8.0 (4.5) | 6.3 (4.3) | 8.4 (4.5) | 6.8 (4.0) | 6.9 (3.8) |
| HADS-D | 5.6 (4.1) | 6.5 (3.9) | 6.0 (4.0) | 6.5 (4.7) | 5.3 (3.8) | 6.8 (3.0) | 4.9 (3.5) | 5.8 (4.0) |
| MOS-SS | 4.5 (2.7) | 5.0 (2.5) | 4.9 (2.5) | 5.8 (1.9) | 4.4 (2.5) | 5.1 (2.0) | 4.8 (2.4) | 4.7 (2.5) |
| Cardenas et al14 | n=35 | n=55 | n=22 | n=17 | n=17 | n=17 | n=31 | n=16 |
| Brief pain inventory | ||||||||
| Pain interference index | 5.1 (2.2) | 4.8 (2.4) | 5.3 (2.1) | 5.1 (2.3) | 4.1 (2.0) | 5.0 (1.8) | 4.0 (2.2) | 4.4 (2.2) |
| Mood | 5.9 (2.1) | 4. 9 (2.6) | 4.9 (2.7) | 5.5 (2.6) | 4.8 (2.3) | 5.4 (2.3) | 4.0 (2.2) | 4.6 (2.8) |
| General activity | 5.9 (2.5) | 5.3 (2.8) | 5.8 (1.5) | 5.6 (2.3) | 5.3 (2.1) | 5.5 (1.8) | 4.5 (1.9) | 5.4 (2.5) |
| Mobility | 5.2 (3.0) | 5.0 (3.3) | 5.6 (2.7) | 4.9 (2.8) | 4.8 (3.0) | 5.7 (2.6) | 4.1 (3.3) | 4.6 (3.1) |
| Normal work | 5.2 (3.1) | 5.5 (3.1) | 5.5 (2.6) | 4.5 (3.3) | 5.1 (2.8) | 5.9 (2.4) | 4.1 (2.9) | 4.4 (3.0) |
| Relations | 4.1 (3.0) | 3.9 (2.9) | 3.9 (3.2) | 3.9 (3.2) | 2.5(1.9) | 3.8 (2.6) | 3.0 (2.8) | 3.4 (2.3) |
| Sleep | 5.4 (3.0) | 5.2 (2.7) | 5.8 (2.2) | 6.1 (2.6) | 4.5 (3.1) | 5.2 (2.5) | 5.0 (2.6) | 5.1 (2.8) |
| Enjoyment of life | 5.3 (2.7) | 5.2 (3.0) | 5.4 (2.8) | 6.0 (2.7) | 3.9 (2.4) | 5.1 (2.2) | 4.4 (3.0) | 4.7 (3.2) |
| Self-care | 4.6 (3.4) | 4.1 (3.2) | 5.1 (3.2) | 4.1 (3.5) | 3.6 (2.9) | 4.2 (2.9) | 3.3 (2.8) | 3.3 (3.2) |
| Recreational activities | 4.8 (3.2) | 4.9 (2.9) | 5.6 (2.9) | 5.2 (2.8) | 4.1 (2.8) | 4.8 (2.7) | 4.1 (2.8) | 4.3 (3.2) |
| Social activities | 4.8 (3.3) | 4.3 (2.9) | 5.3 (2.8) | 5.5 (3.4) | 2.8 (2.4) | 4.9 (2.5) | 3.5 (3.2) | 4.3 (3.3) |
Abbreviations: HADS-A, Hospital Anxiety and Depression Scale – Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale – Depression subscale; MOS-SS, Medical Outcomes Study Sleep Scale; SD, standard deviation.
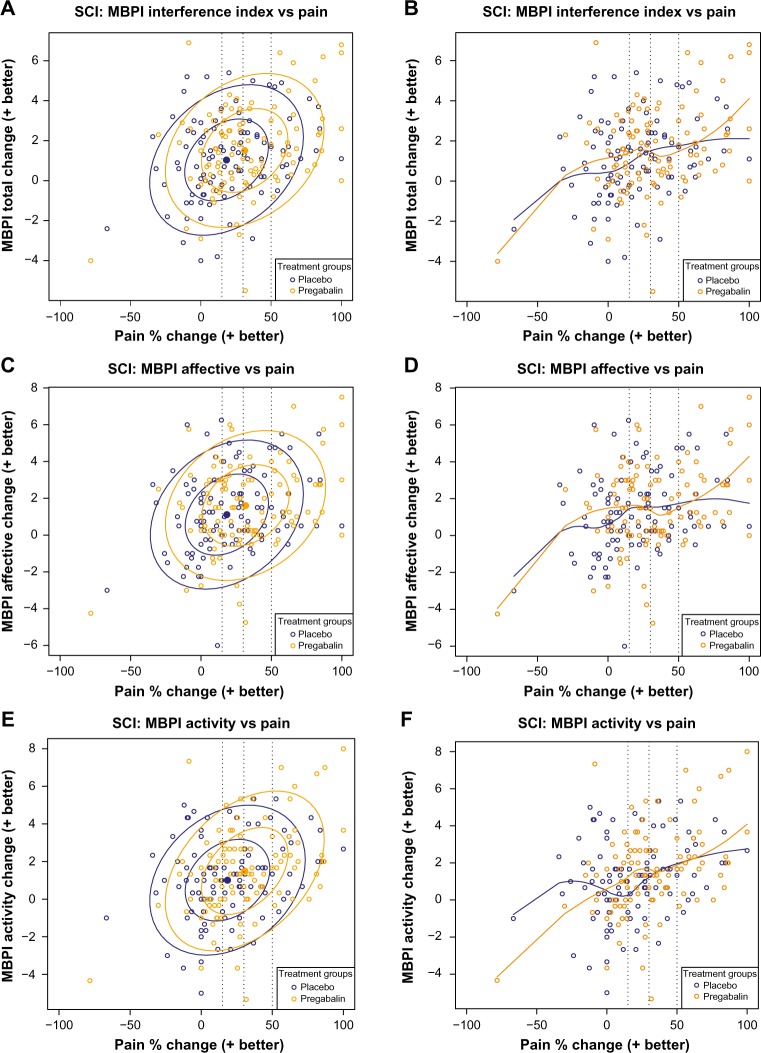
In Cardenas et al,14 data ellipses of percent change in pain versus change in the ten-item MBPI Pain Interference Index (Figure 1A) showed that for pregabalin relative to placebo, there was a shift toward greater functional improvement with greater pain relief. Both the 50% and 95% data ellipses shifted toward the upper right quadrant of the plot with pregabalin, indicating greater improvements in both pain and function relative to placebo. The loess curve (Figure 1B) confirmed the trend toward greater functional improvement with increasing pain relief, with a greater slope observed for pregabalin compared with placebo at pain improvement ≥30%. Similar trends were observed on the Affective Interference (Figure 1C and D) and Activity Interference (Figure 1E and F) subscales of the seven-item BPI, with the loess curve of the Activity Interference subscale (Figure 1F) suggesting a more linear relationship between pain and function with pregabalin not only relative to placebo but also relative to the Affective subscale.
Figure 1.
Scatter plots of the relationship between pain reduction and pain interference with function assessed by the ten-item MBPI Total Interference score (A and B) and the Affective (C and D) and Activity (E and F) subscales derived from the seven-item BPI.
Notes: Data are for patients in Cardenas et al.14 Panels on the left show bivariate data ellipses at 50% and 95% confidence regions for the observed spread and strength of the relationship between pain and function. Panels on the right are loess curves exploring the extent of the relationship. Broken vertical lines indicate clinically relevant pain improvement thresholds of 15%, 30%, and 50%.
Abbreviations: MBPI, modified Brief Pain Inventory; SCI, spinal cord injury.
Consistent with the MBPI interference index and the two subscales, data ellipses of all individual MBPI items showed a shift toward greater functional improvement with greater pain relief for pregabalin relative to placebo (Figure S1). The loess curves for the individual MBPI scales (Figure S1) showed that the clearest relationship between pain and function with pregabalin was for Sleep, with an almost linear relationship across the range of percent change in pain. Domains related to mental components (Mood, Normal Relations, and Social Activities) appeared to have a more complex relationship relative to domains related to physical activity, although the overall trends still suggested greater function with increased pain relief.
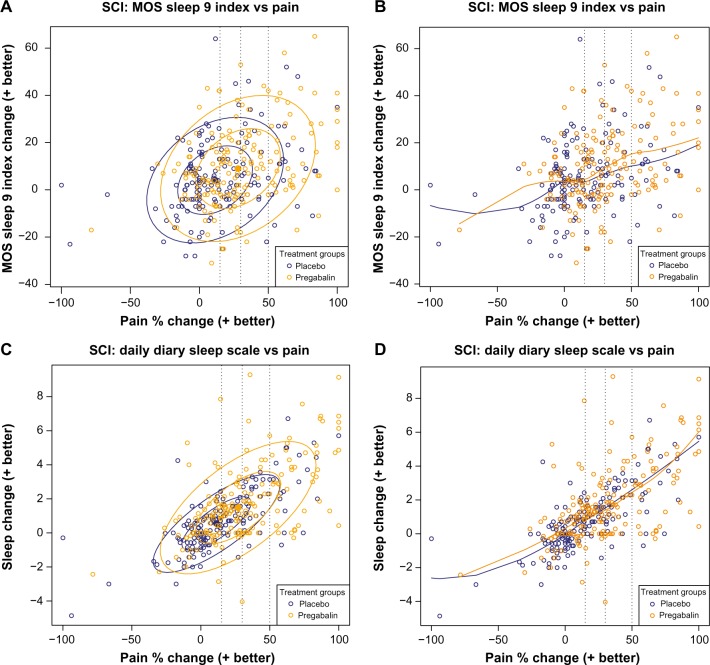
For the combined results of both studies, the data ellipses of the MOS-SS showed that pregabalin resulted in a shift from placebo toward greater improvements in sleep at higher levels of pain relief (Figure 2A). The loess curve (Figure 2B) showed that the relationship between pain and MOS-SS appeared to be almost linear for both pregabalin and placebo, but the magnitude of the improved sleep was greater with pregabalin relative to placebo for pain relief ≥30%. While the data ellipses of the patient diary sleep NRS (Figure 2C) showed a trend similar to the MOS-SS, the loess curves of placebo and pregabalin almost overlap, with both demonstrating an almost linear relationship between pain relief and improved sleep (Figure 2D).
Figure 2.
Scatter plots of the relationship between pain reduction and sleep assessed using the MOS-SS (A and B) and the patient daily diary using an 11-point sleep interference Numeric Rating Scale (C and D).
Notes: Data are for patients from the two studies.13,14 Panels on the left show data ellipses at 50% and 95% confidence regions for the observed spread and strength of the relationship between pain and function. Panels on the right are loess curves exploring the extent of the relationship. Broken vertical lines indicate clinically relevant pain improvement thresholds of 15%, 30%, and 50%.
Abbreviations: MOS-SS, Medical Outcomes Study Sleep Scale; SCI, spinal cord injury.
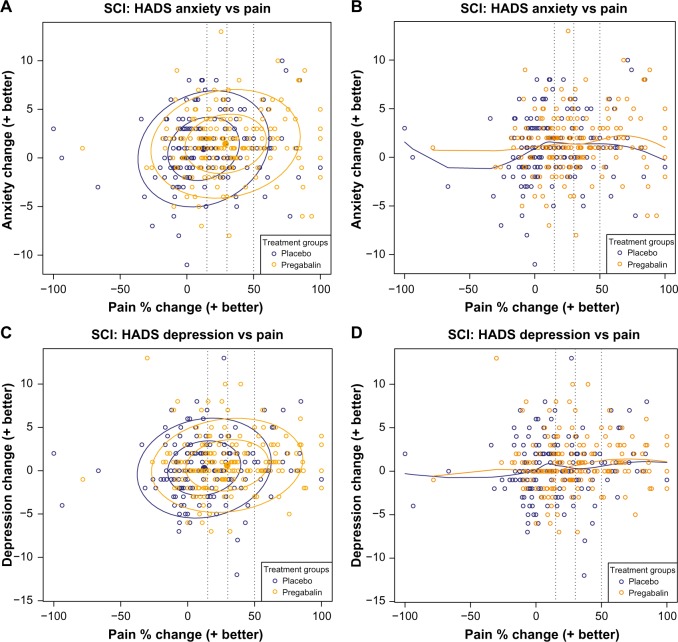
Data ellipses of the changes in HADS scores showed that relative to placebo, pregabalin results shifted toward the right, indicating an improvement in pain, but there was no shift upward in the direction of improvements in anxiety or depression (Figure 3A and C). Loess curves were flat for both placebo and pregabalin, further indicating a lack of relationship between pain relief and improvement in anxiety or depression (Figure 3B and D).
Figure 3.
Scatter plots of the relationship of pain reduction with changes in anxiety (A and B) and depression (C and D) measured using the HADS.
Notes: Data are for patients from the two studies.13,14 Panels on the left show bivariate data ellipses at 50% and 95% confidence regions for the observed spread and strength of the relationship between pain and function. Panels on the right are loess curves exploring the extent of the relationship. Broken vertical lines indicate clinically relevant pain improvement thresholds of 15%, 30%, and 50%.
Abbreviations: HADS, Hospital Anxiety and Depression Scale; SCI, spinal cord injury.
Additionally, when the relationship between pain and function was explored regarding pregabalin treatment using linear trend analysis, significance (P<0.0001) was observed for the MBPI Pain Interference Index across the pain improvement thresholds (Figure S2). The greatest reduction in pain interference, a change of −3.00, was observed at the highest threshold of pain improvement (≥50%).
When similar linear trend analyses were used to evaluate the changes in individual items of the MBPI (Figure S3), significant relationships were observed for all of the ten functional domains, except for Relationships. Consistent with the data ellipses and loess curves, the clearest relationship was between pain and interference of pain with Sleep (P<0.0001). A similar clear and significant relationship was observed between pain reduction and sleep measured on the MOS-SS (Figure S4). With each increasing level of pain reduction, there was a consistent incremental improvement in sleep.
Analysis of the changes in HADS scores showed no statistically significant relationship between pain reduction and either the anxiety or depression subscales with pregabalin treatment (data not shown).
Discussion
This study is the first to explore the relationship between pain relief and improvement in function among patients with SCI-associated NeP. The results reported here suggest that pain reduction is likely associated with improvement in several different domains of function. While similar trends in the association between pain reduction and function were observed both for pregabalin- and placebo-treated patients, suggesting robustness of the observations, the magnitude of the effects was consistently greater with pregabalin. These results also support the concept that those who benefit in terms of pain relief also benefit in other areas.26 These effects were especially noted in the data ellipses, which showed a shift with pregabalin relative to placebo toward the upper right quadrant, suggesting greater improvement in both pain and function. However, the small incremental improvements in function are all that could be reasonably expected to be demonstrated, given the overall severe multiple SCI-related contributors to impairments in function.
The effects of pain reduction were in particular manifested by the changes in the pain interference scores of the MBPI, primarily in domains related to daily physical function, including Sleep, Mobility, Normal Work, and Self-Care. Overall, the most consistent relationship between pain and function appeared to be for sleep as measured by the MOS-SS, and the aforementioned MBPI pain interference Sleep item and the patient daily NRS diary, such that the loess curves suggested a linear relationship in the improvements in sleep with increasing pain reduction. This linear relationship was further observed in the linear trend analysis, which showed incremental improvements in sleep across the pain relief thresholds. Notably, only sleep outcomes appeared to show a linear progression across pain relief thresholds in the linear trend analysis. For other functional outcomes, although statistical significance was shown in the linear trend analysis, this significance was primarily driven by the large change at ≥50% pain relief, with less distinction at the lower thresholds.
The observed relationship between pain relief and improved sleep was expected for several reasons. Not only is the relationship between pain and sleep disturbance reported to be bidirectional such that each contributes to the manifestation of the other,27–29 but also pain frequency and intensity are associated with worse sleep quality in patients with SCI.2,30 Furthermore, the consistently large shifts with pregabalin in the 50% and 95% data ellipses may reflect that pregabalin, in other NeP conditions, has been associated with decreased pain-related sleep interference in addition to its analgesic effect,31 and in fibromyalgia, a condition characterized by a dysfunction in central pain processing, improvements in sleep with pregabalin were observed using polysomnography.32 The effect on sleep may be especially relevant in SCI, since sleep problems are common after SCI,33 and many sedative hypnotics that may be used to treat these problems, such as benzodiazepines, are prone to tolerance and dependence.
In contrast, no statistically significant relationships were observed on either HADS-A or HADS-D using the linear trend analysis, nor did there appear to be a shift with pregabalin toward improvements in anxiety and depression; the data ellipses only shifted to the right, not upward. Because mean baseline HADS scores were in the range considered “normal”, it is likely that the lack of an observable relationship may be due to a floor effect. Alternatively, as suggested by the data ellipses, it may also be possible that effects on depression and anxiety may be at least partially independent of pain.
An important limitation of this analysis is that it was based on results from clinical trials, and thus may not be representative of clinical practice. Generalizability may also be limited since the population was predominantly male and comprised primarily of patients who were Whites or Asians. Additionally, it should be noted that the ten individual items on the version of the MBPI that was used in this study have not been validated, and therefore, no inferences can be made from this exploratory analysis. Nevertheless, the data suggest that there is likely a relationship between pain and function, such that patients who have greater improvements in pain achieve higher levels of function.
Conclusion
For patients with SCI-related NeP in two clinical trials of pregabalin, greater improvements in several functional domains associated with daily activities and quality of life were achieved at higher levels of pain reduction. While the greatest improvements were experienced by patients who obtained the highest level of pain relief regardless of treatment allocation, pregabalin resulted in a shift to more pain relief and better function relative to placebo. Further evaluation of these relationships in the clinical setting is warranted, with a view toward better understanding of how to achieve improvements in both pain and function.
Supplementary materials
Scatter plots and their associated loss curves of percent change in pain versus change in score for all items (A-T) on the ten-item modified Brief Pain Inventory (MBPI).
Notes: Ellipses show 50% and 95% confidence regions for the observed spread and strength of the relationship between pain and function. Broken vertical lines indicate clinically relevant pain improvement thresholds of 15%, 30%, and 50%.
Linear trend analysis of the relationship between pain improvement thresholds and pain interference with function assessed by the MBPI Interference Index among pregabalin-treated patients in Cardenas et al.1
Abbreviation: MBPI, modified Brief Pain Inventory.
Linear trend analysis of the relationship between pain improvement thresholds and pain interference on individual items of the MBPI (A–J) among pregabalin-treated patients in Cardenas et al.1
Abbreviation: MBPI, modified Brief Pain Inventory.
Linear trend analysis of the relationship between pain improvement thresholds and sleep assessed using the MOS-SS. Note: Data are for pregabalin-treated patients pooled from the two studies.1,2
Abbreviation: MOS-SS, Medical Outcomes Study Sleep Scale.
References
- 1.Cardenas DD, Nieshoff EC, Sanin L, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80:533–539. doi: 10.1212/WNL.0b013e318281546b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddall P, Cousins M, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury. A placebo-controlled trial. Neurology. 2006;67:1792–1800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
Footnotes
Disclosure
This study was funded by Pfizer Inc. Birol Emir, Bruce Parsons, and Alesia Sadosky are all employees of Pfizer Inc. and hold stock in Pfizer. Edward C Nieshoff collaborated with Pfizer on the project; he was not financially compensated for his participation or the development of this manuscript. Editorial support was provided by E Jay Bienen who was funded by Pfizer Inc. The authors report no other conflicts of interest in this work.
References
- 1.National Spinal Cord Injury Statistical Center [webpage on the Inter-net] Spinal Cord Injury. Facts and Figures at a Glance. 2013. [Accessed August 22, 2013]. Available from: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202013.pdf.
- 2.Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001;82(11):1571–1577. doi: 10.1053/apmr.2001.26068. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43(12):704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- 4.Raichle KA, Osborne TL, Jensen MP, Cardenas D. The reliability and validity of pain interference measures in persons with spinal cord injury. J Pain. 2006;7(3):179–186. doi: 10.1016/j.jpain.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Murray RF, Asghari A, Egorov DD, et al. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord. 2007;45(6):429–436. doi: 10.1038/sj.sc.3102022. [DOI] [PubMed] [Google Scholar]
- 6.Mann R, Schaefer C, Sadosky A, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51(7):564–570. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]
- 7.Zanca JM, Dijkers MP, Hammond FM, Horn SD. Pain and its impact on inpatient rehabilitation for acute traumatic spinal cord injury: analysis of observational data collected in the SCIRehab study. Arch Phys Med Rehabil. 2013;94(4 Suppl):S137–S144. doi: 10.1016/j.apmr.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Attal N, Mazaltarine G, Perrouin-Verbe B, Albert T. Chronic neuropathic pain management in spinal cord injury patients. What is the efficacy of pharmacological treatments with a general mode of administration? (oral, transdermal, intravenous) Ann Phys Rehabil Med. 2009;52(2):124–141. doi: 10.1016/j.rehab.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009;47(5):352–359. doi: 10.1038/sc.2008.136. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snedecor SJ, Sudharshan L, Cappelleri JC, et al. Systematic review and comparison of pharmacologic therapies for neuropathic pain associated with spinal cord injury. J Pain Res. 2013;6:539–547. doi: 10.2147/JPR.S45966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfizer Inc . Lyrica® [pregabalin] Capsules Prescribing Information. New York, NY: Pfizer, Inc; 2012. [Google Scholar]
- 13.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury. A placebo-controlled trial. Neurology. 2006;67(10):1792–1800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas DD, Nieshoff EC, Suda K, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80(6):533–539. doi: 10.1212/WNL.0b013e318281546b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardenas DD, Emir B, Parsons B. Examining the time to therapeutic effect of pregabalin in spinal cord injury patients with neuropathic pain. Clin Ther. 2015;37(5):1081–1090. doi: 10.1016/j.clinthera.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, McIntyre A, Dijkers M, Loh E, Teasell RW. Gabapentinoids are effective in decreasing neuropathic pain and other secondary outcomes after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2014;95(11):2180–2186. doi: 10.1016/j.apmr.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) [webpage on the Internet] International Classification of Functioning, Disability and Health (ICF) 2001. [Accessed January 16, 2013]. Available from: http://www.who.int/classifications/icfbrowser/
- 18.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 20.Lapane KL, Quilliam BJ, Benson C, Chow W, Kim M. One, two, or three? Constructs of the Brief Pain Inventory among patients with non-cancer pain in the outpatient setting. J Pain Symptom Manage. 2014;47(2):325–333. doi: 10.1016/j.jpainsymman.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Hays R, Stewart A. Sleep measures. In: Stewart A, Ware J, editors. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. pp. 235–259. [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Friendly M, Monette G, Fox J. Elliptical insights: understanding statistical methods through elliptical geometry. Stat Sci. 2013;28(1):1–39. [Google Scholar]
- 24.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 25.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain. 2010;149(2):360–364. doi: 10.1016/j.pain.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 27.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 28.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 29.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137(1):202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norrbrink Budh C, Hultling C, Lundeberg T. Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord. 2005;43(2):85–95. doi: 10.1038/sj.sc.3101680. [DOI] [PubMed] [Google Scholar]
- 31.Roth T, van Seventer R, Murphy TK. The effect of pregabalin on pain-related sleep interference in diabetic peripheral neuropathy or postherpetic neuralgia: a review of nine clinical trials. Curr Med Res Opin. 2010;26(10):2411–2419. doi: 10.1185/03007995.2010.516142. [DOI] [PubMed] [Google Scholar]
- 32.Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res (Hoboken) 2012;64(4):597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Hirsh AT, Molton IR, Bamer AM. Sleep problems in individuals with spinal cord injury: frequency and age effects. Rehabil Psychol. 2009;54(3):323–331. doi: 10.1037/a0016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots and their associated loss curves of percent change in pain versus change in score for all items (A-T) on the ten-item modified Brief Pain Inventory (MBPI).
Notes: Ellipses show 50% and 95% confidence regions for the observed spread and strength of the relationship between pain and function. Broken vertical lines indicate clinically relevant pain improvement thresholds of 15%, 30%, and 50%.
Linear trend analysis of the relationship between pain improvement thresholds and pain interference with function assessed by the MBPI Interference Index among pregabalin-treated patients in Cardenas et al.1
Abbreviation: MBPI, modified Brief Pain Inventory.
Linear trend analysis of the relationship between pain improvement thresholds and pain interference on individual items of the MBPI (A–J) among pregabalin-treated patients in Cardenas et al.1
Abbreviation: MBPI, modified Brief Pain Inventory.
Linear trend analysis of the relationship between pain improvement thresholds and sleep assessed using the MOS-SS. Note: Data are for pregabalin-treated patients pooled from the two studies.1,2
Abbreviation: MOS-SS, Medical Outcomes Study Sleep Scale.