Abstract
The prognosis of small-cell lung cancer (SCLC) is poor despite reports suggesting modest improvement in survival. To date, chemotherapy remains the cornerstone treatment for SCLC patients, and many studies have focused on identifying the molecular characteristics of SCLC, which serve as the basis for precision treatments that improve the prognosis of SCLC. For instance, the therapeutic effect of temozolomide, recommended for patients with relapsed SCLC, is linked to 1p/19q codeletion in anaplastic oligodendroglial tumors. A subpopulation of SCLC patients may derive benefit from tyrosine kinase inhibitors targeting RET. In order to identify 1p/19q codeletion and RET rearrangement in SCLC patients, 32 SCLC resected specimens were retrospectively collected between 2008 and 2014 from the Zhejiang Cancer Hospital in People’s Republic of China. Fluorescence in situ hybridization was used to detect 1p/19q codeletion and RET rearrangement in the specimens. A 1p single deletion was detected in eight specimens, 19q single deletion was detected in three specimens, and only three specimens had a 1p/19q codeletion. None of the specimens had a RET rearrangement. The three patients whose specimens had a 1p/19q codeletion were alive after 58, 50, and 30 months of follow-up care. There was a trend toward prolonged overall survival for the patients with codeletion compared to no codeletion, 1p single deletion, 19q single deletion, and without 1p and 19q deletion (P=0.113, 0.168, 0.116, and 0.122, respectively). Our data showed that RET rearrangement may be not an ideal molecular target for SCLC therapies in People’s Republic of China. Instead, 1p/19q codeletion is a promising marker for a good prognosis and treatment with temozolomide in SCLC.
Keywords: small-cell lung cancer, 1p/19q codeletion, RET rearrangement, FISH
Introduction
Lung cancer, including small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), is the most common malignancy and the leading cause of cancer-related mortality worldwide.1 SCLC accounts for 15% of all lung cancers.1,2 Despite a trend toward modest improvement in survival, the prognosis for SCLC remains poor. The median survival time of limited- and extensive-disease SCLC is 15–20 and 8–10 months, respectively.3–5 Chemotherapy is the cornerstone treatment for SCLC. Although targeted drugs have less toxicity than chemotherapy, no targeted drugs have been approved for SCLC patients to date.6 As a precise molecular diagnosis is the basis of precision treatment, it is important to know the molecular characteristics of SCLC.
While SCLC is sensitive to chemotherapy (70%–80% response rate), it recurs easily.7,8 The National Comprehensive Cancer Network guidelines recommend temozolomide as a second-line treatment for SCLC and the drug has been used to treat relapsed SCLC, particularly with brain metastases.9,10 The 1p/19q codeletion is an important predictive and prognostic factor in anaplastic and recurrent oligodendrogliomas treated with temozolomide and was significantly correlated with response rate (P=0.04), time-to-progression (P=0.003), and overall survival (P=0.0001).11 However, it is unknown whether 1p/19q codeletion exists in SCLC and whether it can be used as a predictive and prognostic factor for SCLC.
RET rearrangements are found in 1%–2% of NSCLC patients and are drivers of tumor growth in vitro and in vivo. Cabozantinib is a multi-tyrosine kinase inhibitor that acts against RET. Cabozantinib is effective in patients with RET-rearranged lung adenocarcinoma and produces sustained responses.12 Drilon et al13 reported three patients treated with cabozantinib. Confirmed partial responses were observed in two patients, including one harboring a novel TRIM33–RET fusion, and stable disease for at least 8 months was noted in a third patient with a KIF5B–RET. A study by Dabir et al14 suggests that tyrosine kinase inhibitors targeting RET may be a beneficial treatment for a subpopulation of SCLC patients. Coupled with the presence of RET fusion proteins in NSCLC, the study indicates an emerging role for RET in SCLC. Therefore, RET rearrangements in SCLC need to be studied further.
In this paper, 1p/19q codeletion and RET rearrangements were detected in resected SCLC specimens by the use of fluorescence in situ hybridization (FISH) to serve as the basis for precision treatment against SCLC patients.
Materials and methods
Patient characteristics
A total of 32 SCLC patient specimens were retrospectively collected from the Zhejiang Cancer Hospital in People’s Republic of China between 2008 and 2014. All specimens were obtained from resected SCLC tumors from conventional SCLC patients. The pathological diagnosis of SCLC was based on the standard criteria, defined by the World Health Organization. Specimens were obtained from six female and 26 male patients, ranging in age from 38 to 77 years. The median patient age was 58 years. Of the 32 patients, five were nonsmokers, two light smokers, five moderate smokers, and 20 heavy smokers. The median pack-years was 30. The breakdown of the stages of SCLC (as defined by the seventh edition of the TNM classification for lung cancer) was as follows: nine patients with stage IA, five patients with IB, four patients with IIA, three patients with IIB, ten patients with IIIA, and one patient with IIIB. The patient specimens were retrospectively collected, and some patients have died, so there was no written informed patient consent. This study was approved by the ethics committee of Zhejiang Cancer Hospital.
Detection of 1p/19q codeletion and RET rearrangements
1p/19q and RET FISH were performed on formalin-fixed paraffin-embedded tumor tissues using ZytoLight SPEC RET Dual Color Break Apart Probe, ZytoLight SPEC 1p36/1q25 Dual Color Probe, and 19q13/19p13 Dual Color Probe (Zyto-Vision; GeneDiagnostic Inc., Hangzhou, People’s Republic of China) according to the manufacturers’ instructions.
The SPEC 1p36/1q25 Dual Color Probe is a mixture of an orange fluorochrome direct labeled 1p36 probe specific for the smallest region of consistent deletion at 1p36.31 and a green fluorochrome direct labeled 1q25 probe specific for 1q25.3. The SPEC 19q13/19p13 Dual Color Probe is a mixture of an orange fluorochrome direct labeled 19q13 probe specific for the region of common deletion at 19q13.32–q13.33 and a green fluorochrome direct labeled 19p13 probe specific for 19p13.3.
The SPEC RET Dual Color Break Apart Probe is a mixture of two direct labeled probes hybridizing to the 10q11.21 band. The orange fluorochrome direct labeled probe hybridizes proximal to the RET gene; the green fluorochrome direct labeled probe hybridizes distal to that gene.
A quantity of 4–5 μm thick formalin-fixed paraffin-embedded sections were deparaffinized, treated with warmed heat Pretreatment Solution Citric at 98°C, and digested in pepsin solution. The probe (10 μL) was added to each slide. Target DNA and probes were co-denatured at 75°C for 10 minutes and incubated at 37°C overnight in a humidified hybridization chamber. Three post-hybridization washes were performed in 1× Wash Buffer A at 37°C for 5 minutes each. Finally, the slides were air dried and counterstained with DAPI/antifade solution. Signals for each locus-specific FISH probe were assessed under an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan).
FISH manual analysis was performed by two independent researchers. One hundred non-overlapping cells were assessed for green “G” and orange “O” signals for each hybridization. 100 non-overlapping nuclei were counted per hybridization. Deletions of 1p and 19q were defined as 20% of tumor nuclei containing the ratio<0.87 as recommended by guidelines.15 Cells that were positive for RET rearrangement cells with a break apart pattern, with one fusion signal and two separated G and O signals (1F1G1O), were considered positive for RET rearrangement. Only signals that were more than one signal diameter apart from each other were counted as breaks. A case was considered positive for RET rearrangement if >15% of the cells showed split signals.16
The negative and positive controls with known 1p/19q status are applying the standard protocol procedure.
Follow-up
The follow-up deadline was January 15, 2016. Fourteen patients were alive, 16 patients died, and two patients were lost to follow-up. The survival time was calculated from the date of pathological diagnosis.
Statistical analyses
Where appropriate, the statistical analyses between dichotomous variables were determined by the Pearson’s chi-squared or Fisher’s exact tests in groups with different deletion status. The log-rank test was used to estimate and compare survival. All statistical analyses were carried out on an intention-to-treat basis using the SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA).
Results
We detected 1p single deletion in eight specimens and 19q single deletion in three specimens. Only three specimens had a 1p/19q codeletion and no patients had RET rearrangements (Figures 1 and S1). The rate of 1p/19q codeletion in our specimens was 9.4%. There was no difference between the clinical characteristics (including age, sex, smoking history, stage, and cycles of chemotherapy) for the patients with codeletion compared to no codeletion, 1p single deletion, 19q single deletion, and without 1p and 19q deletion (Table 1). There was a trend toward prolonged overall survival for the patients with codeletion compared to no codeletion, 1p single deletion, 19q single deletion, and without 1p and 19q deletion (P=0.113, 0.168, 0.116, and 0.122, respectively) (Figures 2–5). We discuss each of the cases in detail below.
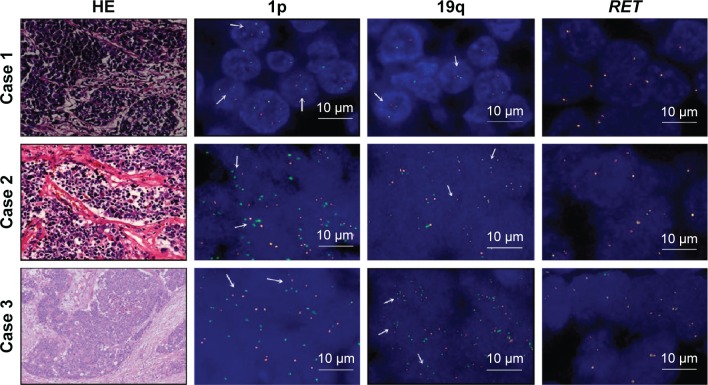
Figure 1.
The results of 1p/19q codeletion and RET rearrangements.
Notes: Only three patients had a 1p/19q codeletion and no patients had RET rearrangements. The quantification results of ploidy (O:G): 1p was 26% and 19q was 25% in case 1; 1p was 58% and 19q was 24% in case 2; 1p was 40% and 19q was 48% in case 3; 1p was 52% and 19q was 66% in positive control; and 1p was 12% and 19q was 6% in negative control. The arrows mean there is a ratio of O:G<0.87 in the cells. Magnification is 200×.
Abbreviations: G, green; O, orange; HE, hematoxylin-eosin.
Table 1.
Clinical features of small-cell lung cancer patients
| Codeletion | Without codeletion | 1p single deletion | 19q single deletion | Without 1p and 19q deletion | |
|---|---|---|---|---|---|
| Number of patients | 3 | 29 | 8 | 3 | 18 |
| Age (median) | 58 | 58 | 61 | 62 | 56 |
| Sex (female/male) | 0/3 | 6/23 | 1/7 | 0/3 | 5/13 |
| Smoking history (median pack-years) | 20 | 30 | 35 | 30 | 26.5 |
| Stage | |||||
| IA | 0 | 14 | 5 | 0 | 9 |
| IB | 1 | 4 | 0 | 3 | 1 |
| IIA | 1 | 6 | 1 | 0 | 5 |
| IIB | 1 | 2 | 2 | 0 | 0 |
| IIIA | 0 | 2 | 0 | 0 | 2 |
| IIIB | 0 | 1 | 0 | 0 | 1 |
| Cycles of chemotherapy | |||||
| ≥4 | 2 | 16 | 5 | 0 | 11 |
| <4 | 1 | 13 | 3 | 3 | 7 |
Figure 2.
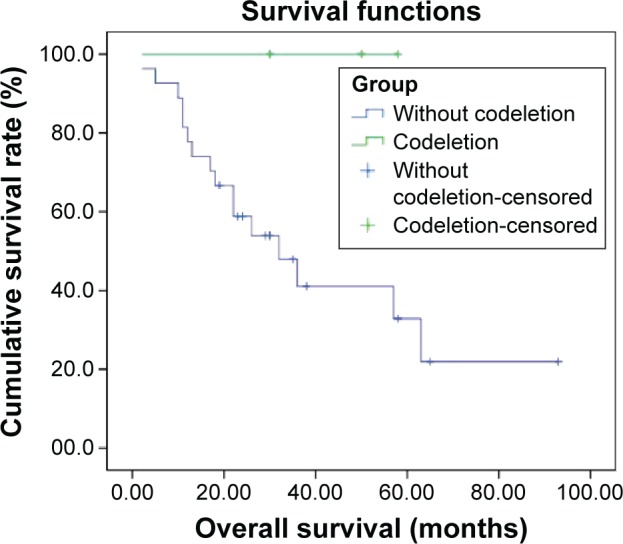
Overall survival of patients with 1p/19q codeletion (n=3) compared to patients without 1p/19q codeletion (n=29) (P=0.113).
Figure 3.
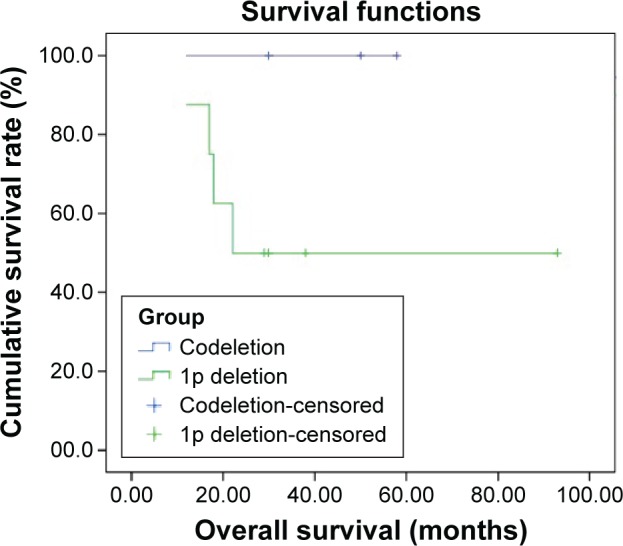
Overall survival of patients with 1p/19q codeletion (n=3) compared to patients with 1p single deletion (n=8) (P=0.168).
Figure 4.
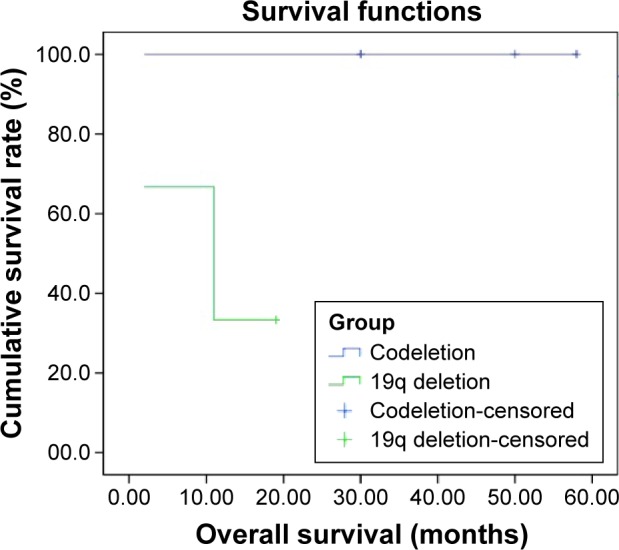
Overall survival of patients with 1p/19q codeletion (n=3) compared to patients with 19q single deletion (n=3) (P=0.116).
Figure 5.
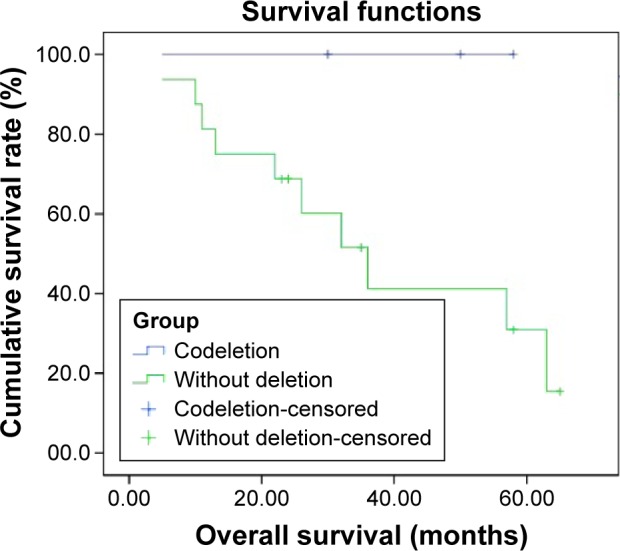
Overall survival of patients with 1p/19q codeletion (n=3) compared to patients without 1p and 19q deletion (n=18) (P=0.122).
Case 1
The first patient was a 46-year-old male heavy smoker whose chief complaint was pain in his left shoulder and back for 3 months. A chest computerized tomography (CT) revealed a mass in his left upper lung lobe with mediastinal lymph node metastasis. The patient received two cycles of chemotherapy with gemzar and cisplatin followed by radical resection of the left upper lung cancer. His pathological diagnosis was SCLC and immunohistochemical stains revealed that his specimen was positive for synaptophysin and carcinoembryonic antigen and negative for chromogranin A and neuron-specific enolase. The size of the resected tumor was 5×4× 3.5 cm, and his pathological TNM stage was T2aN2M0 (IIIA). After surgery, he received two cycles of chemotherapy (gemzar and cisplatin) and thoracic radiotherapy (planning target volume 56Gy/28F). The patient was followed up for 58 months and is still alive with no recurrence.
Case 2
The second patient was a 61-year-old male heavy smoker who reported a cough and sputum with blood for over 10 days. A chest CT revealed a mass (5.5×4 cm) in his left upper lung lobe. The patient underwent a left total pneumonectomy. His pathological diagnosis was SCLC, and his specimen stained positive for CD56, neuron-specific enolase, and carcinoembryonic antigen, and negative for chromogranin A and synaptophysin. His pathological TNM stage was T2bN1M0 (IIB). He received six cycles of chemotherapy (etoposide and cisplatin), thoracic radiotherapy, and prophylactic cranial irradiation. The patient was followed up for 50 months and is still alive with no recurrence.
Case 3
The third patient was a 58-year-old male moderate smoker. A mass (3.1×2.5 cm) was found in his right upper lung lobe during a physical examination. The patient received two cycles of chemotherapy with etoposide and cisplatin followed by a right upper lobectomy with systematic mediastinal lymphadenectomy. His pathological diagnosis was SCLC and his tumor stained positive for chromogranin A, synaptophysin, and CD56, and negative for TTF-1 and CK5/6. His pathological TNM stage was T2aN0M0 (IB). He received two cycles of chemotherapy with etoposide and cisplatin and prophylactic cranial irradiation (25Gy/10F) after surgery. The patient was followed up for 30 months and is still alive with no recurrence.
Discussion
Although few SCLC patients are eligible for surgery, all our study specimens were obtained from surgery because they reflect clinicopathologic features better than biopsy specimens. The codeletion of the 1p and 19q chromosomal arms is a characteristic and early genetic event in oligodendroglial tumors, which is associated with a better prognosis and enhanced response to therapy.17–19 In our study, we had three patients with a 1p/19q codeletion and all three patients had good prognosis and were alive for at least 30 months after surgery. The results show no significant difference to the overall survival for the patients with codeletion compared to no codeletion, 1p single deletion, 19q single deletion, and without 1p and 19q deletion, which may be attributed to the limited number of patients. Due to a trend toward prolonged overall survival for the patients with 1p/19q codeletion, 1p/19q codeletion may be a promising marker for a good prognosis of SCLC and needs to be validated in a larger number of patients. Several different methods for 1p/19q testing are available: polymerase chain reaction-based loss of heterozygosity analysis, multiplex ligation-dependent probe amplification, array comparative genomic hybridization, and FISH. FISH is the most commonly used method for the detection of 1p/19q codeletion in glioma specimens.19,20 FISH was used to detect 1p/19q codeletion and RET rearrangement for SCLC specimens in our study. Although 1p single deletion rate is not low, our results showed that 1p/19q codeletion rate is only 9.4%. Temozolomide has been approved for relapsed SCLC, but the relationship between 1p/19q codeletion and therapeutic value of temozolomide in SCLC is unknown. Our study provides the basis for further research into whether 1p/19q codeletion can be used as a predictive factor for treatment with temozolomide.
Using the combined analysis of massively parallel whole-genome and transcriptome sequencing for cancer and paired normal tissue, Ju et al21 reported a novel fusion gene between KIF5B and the RET proto-oncogene caused by a pericentric inversion of 10p11.22–q11.21 in a 33-year-old lung adenocarcinoma patient who was a never-smoker with no familial cancer history. This fusion gene overexpressed chimeric RET receptor tyrosine kinase, which spontaneously induced cellular transformation.21 Of 936 patients with NSCLC, the RET fusion gene was detected in 13 patients of whom nine patients had a KIF5B–RET fusion, three patients had a CCDC6–RET fusion, and one patient had a novel NCOA4–RET fusion.22 An activating M918T RET somatic mutation in a metastatic SCLC tumor specimen was identified. RET expression in SCLC cells is significantly higher than in adenocarcinoma lung cells. A subpopulation of SCLC patients may derive benefit from tyrosine kinase inhibitors targeting RET.14 Dowlati et al23 reported that RET mutation in SCLC is of a low-frequency. No RET rearrangements were found in the 32 patients in our study, which may be related to the limited number of specimens examined. New molecular and genetic targets are urgently needed to improve SCLC prognosis and RET rearrangements may not be the ideal molecular target for SCLC in People’s Republic of China. However, 1p/19q codeletion is a promising marker for a good prognosis and treatment with temozolomide in SCLC.
Supplementary material
(A) 1p negative control; (B) 19q negative control; (C) RET negative control; (D) RET positive control.
Acknowledgments
This work was supported by the Zhejiang Province Medical Science Fund Project of China (No 2015ZHA006) and the 1022 Talent Training Program of Zhejiang Cancer Hospital.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Xing P, Fan Y, et al. Current small cell lung cancer treatment in China. Thorac Cancer. 2015;6(3):233–238. doi: 10.1111/1759-7714.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol. 2013;24(8):2088–2092. doi: 10.1093/annonc/mdt140. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Yang KH, Guan QL, Mi DH, Wang J. Cisplatin plus etoposide versus other platin-based regimens for patients with extensive small-cell lung cancer: a systematic review and meta-analysis of randomised, controlled trials. Intern Med J. 2012;42(12):1297–1309. doi: 10.1111/j.1445-5994.2012.02821.x. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007;97(2):162–169. doi: 10.1038/sj.bjc.6603810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu HY, Wang XJ, Mao WM. Targeted therapies in small cell lung cancer (review) Oncol Lett. 2013;5(1):3–11. doi: 10.3892/ol.2012.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ztloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21(9):1810–1816. doi: 10.1093/annonc/mdq036. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Foster J, Gooding W, Evans T, Sulecki M, Belani CP. Phase 2 study of irinotecan and paclitaxel in patients with recurrent or refractory small cell lung cancer. Cancer. 2010;116(5):1344–1349. doi: 10.1002/cncr.24753. [DOI] [PubMed] [Google Scholar]
- 9.Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18(4):1138–1145. doi: 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 10.Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer. 2014;86(2):237–240. doi: 10.1016/j.lungcan.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol. 2006;24(29):4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 12.Drilon AE, Sima CS, Somwar R, et al. Phase II study of cabozantinib for patients with advanced RET-rearranged lung cancers. J Clin Oncol. 2015;33(suppl) abstr 8007. [Google Scholar]
- 13.Drilon A, Wang L, Hasanovic A, et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013;3(6):630–635. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabir S, Babakoohi S, Kluge A, et al. RET mutation and expression in small-cell lung cancer. J Thorac Oncol. 2014;9(9):1316–1323. doi: 10.1097/JTO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 15.Horbinski C, Nikiforova MN, Hobbs J, et al. The importance of 10q status in an outcomes-based comparison between 1p/19q fluorescence in situ hybridization and polymerase chain reaction-based microsatellite loss of heterozygosity analysis of oligodendrogliomas. J Neuropathol Exp Neurol. 2012;71(1):73–82. doi: 10.1097/NEN.0b013e318240fa65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SE, Lee B, Hong M, et al. Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma. Mod Pathol. 2015;28(4):468–479. doi: 10.1038/modpathol.2014.107. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain MC, Born D. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. J Neurooncol. 2015;125(2):249–251. doi: 10.1007/s11060-015-1906-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZY, Chan AK, Ng HK, et al. Surgically treated incidentally discovered low-grade gliomas are mostly IDH mutated and 1p19q co-deleted with favorable prognosis. Int J Clin Exp Pathol. 2014;7(12):8627–8636. [PMC free article] [PubMed] [Google Scholar]
- 19.Woehrer A, Sander P, Haberler C, et al. FISH-based detection of 1p 19q codeletion in oligodendroglial tumors: procedures and protocols for neuropathological practice – a publication under the auspices of the Research Committee of the European Confederation of Neuropathological Societies (Euro-CNS) Clin Neuropathol. 2011;30(2):47–55. doi: 10.5414/npp30047. [DOI] [PubMed] [Google Scholar]
- 20.Singh VY, Chacko G, Chacko AG, et al. Fluorescence in situ hybridization for 1p, 19q status in a cohort of glial neoplasms. Neurol India. 2014;62(1):32–36. doi: 10.4103/0028-3886.128275. [DOI] [PubMed] [Google Scholar]
- 21.Ju YS, Lee WC, Shin JY, Rajshekhar V. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22(3):436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30(35):4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 23.Dowlati A, Lipka MB, McColl K, et al. Clinical correlation of extensive-stage small-cell lung cancer genomics. Ann Oncol. 2016;27(4):642–647. doi: 10.1093/annonc/mdw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) 1p negative control; (B) 19q negative control; (C) RET negative control; (D) RET positive control.