ABSTRACT
The ankyrins are a family of well-characterized metazoan adaptor proteins that play a key role in linking various membrane-spanning proteins to the underlying spectrin-actin cytoskeleton; a mechanistic understanding of their role in tissue architecture and mechanics, however, remains elusive. Here we comment on a recent study demonstrating a key role for ankyrin-B in maintaining the hexagonal shape and radial alignment of ocular lens fiber cells by regulating the membrane organization of periaxin, dystrophins/dystroglycan, NrCAM and spectrin-actin network of proteins, and revealing that ankyrin-B deficiency impairs fiber cell shape and mechanical properties of the ocular lens. These observations indicate that ankyrin-B plays an important role in maintaining tissue cytoarchitecture, cell shape and biomechanical properties via engaging in key protein: protein interactions required for membrane anchoring and organization of the spectrin-actin skeleton, scaffolding proteins and cell adhesive proteins.
KEYWORDS: ankyrin-B, biomechanics, cytoarchitecture, cell shape, lens fibers, membrane organization
INTRODUCTION
Tissue and organ morphogenesis, architecture and function rely on cell adhesive interactions, cytoarchitectural stability, and membrane organization, which collectively enable cells to withstand the mechanical stresses encountered during growth, development and functional specialization.1-4 Various membrane scaffolding and cell adhesive proteins are thought to play a key role in linking membrane-spanning proteins to the underlying spectrin-actin cytoskeleton and the actin cytoskeleton to the extracellular matrix required for maintaining tissue cytoarchitecture and mechanical stability.1,5 The ankyrin family of proteins (ankyrin-R, ankyrin-B and ankyrin-G) play a crucial role in membrane organization and stability by tethering the spectrin-actin skeleton to membrane spanning-proteins.1,6 Although the ankyrins have been demonstrated to maintain membrane integrity and organization in various tissues and cell types including neuronal tissue, skeletal muscle, cardiomyocytes, epithelial cells and photoreceptors,2 the mechanistic bases by which these proteins influence tissue architecture, cell shape and mechanical properties is not well understood. We recently explored this question using the ocular lens from ankyrin-B (AnkB) deficient mice as a model system. The transparent and avascular ocular lens is a marvel possessing an unparalleled degree of symmetry in tissue cytoarchitecture, given the requirement for rapid deformability and resilience this tissue fulfills during visual accommodation.7 The bulk of the ocular lens is constituted by differentiated long and thin fiber cells which progressively attain a perfect hexagonal shape as they mature and become terminally differentiated (Fig. 1). Unlike many organs, the lens grows throughout life and does not shed cells. Owing to this unique characteristic, as new lens fiber cells are formed, they overlay on top of older fibers in a concentric manner, and are closely packed, compressed and arranged in perfect radial rows in the cortical region (Fig. 1).7 Lens shape, architecture and deformability depend on fiber cell shape, radial alignment, polarity and biomechanical properties, which are supported by cytoskeletal networks, cell-extracellular matrix and cell-cell adhesive interactions, and membrane protein organization.8-12 However, at a mechanistic level, it is still not completely clear how lens fibers achieve and maintain their exquisite symmetry, radial alignment and hexagonal shape. Likewise, the interrelationship between lens fiber cell shape and biomechanical properties, and the question of how the lens maintains deformability and resilience characteristics during the process of visual accommodation, are yet to be answered.13 Given the expected role of membrane scaffolding proteins in regulating cell shape, cell adhesive interactions, organization and tensile properties of the spectrin-actin membrane skeleton, our recent study took advantage of the AnkB haploinsufficient (AnkB−/+) mouse 14 to evaluate changes in lens fiber cell cytoarchitecture and stiffness, and explore the role of AnkB in fiber cell shape and mechanics .15 Importantly, our study demonstrates that deficiency of AnkB disrupts fiber cell hexagonal shape and alignment, and decreases lens stiffness, indicating a compromise in lens biomechanical properties. AnkB was found to regulate the membrane anchoring of periaxin and dystrophin (Dp71), stability of the dystrophin-glycoprotein complex (DGC), spectrin-actin membrane skeleton, and the cell adhesive protein NrCAM and maintain lens fiber cell hexagonal shape and alignment.15 These data suggest the existence of a close structural and functional relationship between the integrity of fiber cell shape and lens mechanical properties, and that AnkB plays a crucial role in integrating and regulating these characteristics.
FIGURE 1.
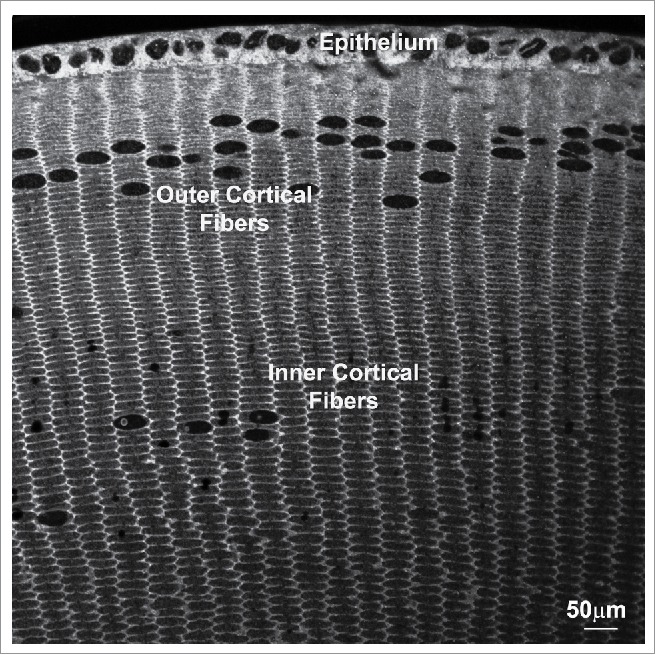
Lens fiber cell hexagonal geometry and radial alignment. Lens epithelial cells at the equator exit from the cell cycle and elongate and differentiate into long fibers. During differentiation, fiber cells progressively attain a hexagonal shape, and mature fibers in the inner cortical region of the lens lose all organelles and maintain a perfect hexagonal geometry with 2 long arms and 4 short arms with a close alignment with the neighboring fiber cells. Image is from the equatorial section of the adult mouse lens stained for β-spectrin. Scale bar: 50 μm.
Ankyrin-B and maintenance of the hexagonal geometry of lens fibers: Role of AnkB in regulating membrane organization of scaffolding and cell adhesive proteins and the spectrin-actin skeleton
As post mitotic lens fibers mature and become terminally differentiated, they attain a perfect hexagonal shape and undergo radial alignment (Fig. 1). Although numerous structural and membrane anchored proteins such as N-cadherin and NrCAM, the lens specific beaded filament proteins, and AnkB, ezrin, radixin and moesin (ERM), tropomyosin, tropomodulin 1, catenins, band 4.1 and adducin, together with the actin-spectrin membrane skeleton, are all considered to play important roles in maintaining the hexagonal shape of lens fiber cells,8,10,16 it is still unclear how the contributions from these various inputs is integrated/coordinated to influence lens structure and function. While AnkB is the most abundantly expressed ankyrin isoform in lens tissue; its role in the adult lens has not been determined.17 Toward this, our recent study which focused on determining whether AnkB is required for maintaining lens architecture and fiber cell phenotype revealed progressive and age-dependent disruptions in fiber cell shape in AnkB haploinsufficient (AnkB−/+) mice.15 Unlike in postnatal day 21 animals, lenses from 5 month-old AnkB−/+ mice exhibiting a nearly 60% reduction in AnkB levels revealed disruptions in lens cortical fiber cell architecture, fiber cell radial alignment and hexagonal shape, uncovering the importance of AnkB in maintenance of cell shape and adhesion in fiber cells.
Interestingly, the fiber cell morphological changes recorded in AnkB deficient lenses were found to phenocopy the changes we had previously observed in periaxin deficient lenses.9,15 Periaxin, a PDZ domain-containing protein, which is expressed in the ocular lens in addition to myelinating Schwann cells, exists in a large complex with desmoyokin, ezrin, spectrin, periplakin, AnkB, NrCAM and plectin.9,18 The absence of periaxin was found to destabilize the lens fiber cell membrane, and to disrupt the membrane spectrin-actin skeleton, lens fiber cell hexagonal shape and alignment in the mouse lens.9 Based on these striking parallels between the AnkB deficient and periaxin null lenses, we postulated an AnkB requirement in the membrane association of periaxin. Interestingly, immunofluorescence analysis revealed a complete disruption of the membrane organization of periaxin in the lens fibers of both P21 and 5 month-old AnkB−/+ mice. These changes in AnkB−/+ mouse lenses were found to be associated with accumulation of periaxin in the soluble lens fraction with a concomitant decrease from the membrane fraction, indicating a requirement for AnkB in the membrane anchoring of periaxin.15 In contrast, the membrane organization of AnkB was found to be unaltered in P21 periaxin null lenses. These observations inferred that a 50% decrease in AnkB protein levels in the lens is associated with a significant dissociation of periaxin from the plasma membrane,15 indicating that AnkB is required for lens membrane targeting of periaxin.
In addition to periaxin, we also observed destabilization of the membrane organization of dystrophin-glycoprotein complex (DGC) in AnkB deficient mouse lenses. DGC is a multimeric transmembrane assembly which serves to link the extracellular matrix and actin cytoskeleton and plays a crucial role in providing mechanical support in various tissues.5,19 Dystrophin deficiency is known to be associated with development of cataract.20 AnkB is recognized to interact with the DGC complex through dystrophin-Dp71 and to coordinate adhesive interactions at the costameres and neuromuscular junctions.21,22 Similarly, periaxin interacts with dystrophin-related protein 2 (Drp-2) to maintain myelin sheath organization.23,24 These known interactions among DGC, AnkB and periaxin in non-lens tissues prompted us to explore whether these proteins interacted functionally in the context of lens fiber cell cytoarchitecture. While β-dystroglycan localizes dis-cretely to the plasma membrane in a large cluster at the center of the long arm of hexagonal fiber cells in normal lenses, the membrane organization of β-dystroglycan in the lens fibers of both AnkB−/+ and periaxin null mice was found to be disrupted, informing the importance of AnkB and periaxin in membrane organization and stability of the DGC in lens fibers.15 Furthermore, in both AnkB−/+ and periaxin null mouse lenses, Dp71 protein levels were decreased significantly in the membrane-enriched fraction with simultaneous accumulation in the soluble fraction. Collectively, the changes observed in both DGC and Dp71 in AnkB−/+ and periaxin null mouse lenses uncovered the importance of AnkB and periaxin, possibly acting both independently and synergistically, to regulate the stability and membrane organization of the DGC in lens fibers, and collectively influencing fiber cell shape.
In addition to the aforementioned changes, confocal imaging of lens inner cortical fibers derived from the 5 month-old AnkB−/+ mice in our study revealed gross disruption in the membrane organization of β-spectrin and NrCAM, and decreased levels of β-spectrin and NrCAM in the membrane-enriched fraction of AnkB−/+ lenses. These results demonstrated the importance of AnkB in stabilization of the membrane skeleton and NrCAM-based cell adhesive properties in lens fibers. Additionally, high resolution confocal microscopy-based colocalization analysis of AnkB with periaxin, NrCAM and β-spectrin in fiber cells confirmed that AnkB colocalizes with periaxin and NrCAM (based on Pearson co-localization coefficient) at the vertices and long/short arms of fiber cells, exhibiting a relatively much stronger and widespread colocalization with NrCAM. In contrast, β-spectrin colocalizes with AnkB predominantly at fiber cell vertices. Taken together, these observations provided a significant insight into the membrane organization of AnkB in lens fiber cells, suggesting that AnkB likely exists as a multiprotein complex in association with periaxin, β-spectrin and NrCAM and localizes intensely to the vertices (Fig. 2), and supports the requirement for such a multiprotein complex in maintaining the hexagonal shape of fiber cells.
FIGURE 2.

Schematic drawing of a hexagonal lens fiber cell and localization of AnkB, periaxin, NrCAM and spectrin at the vertices. Lens fiber cells from the inner cortical region exhibit a perfect hexagonal shape with 2 long arms and 4 short arms. To attain the hexagonal shape, the fiber cells form tricellular junctions (vertices) with neighboring cells. At these fiber cell vertices, AnkB colocalizes with periaxin, β-spectrin and NrCAM. While AnkB has been demonstrated to interact directly with both β-spectrin and NrCAM in different cell types, it is yet to be confirmed whether such protein-protein interactions between AnkB, β-spectrin, periaxin or NrCAM occur in lens fibers (indicated with?). In the deficiency of AnkB and periaxin, fiber cells lose their hexagonal geometry and become easily collapsible, as depicted.
Ankyrin-B and lens biomechanics
In addition to optical properties, the focusing ability of the lens depends on its finely tuned tensile properties.13,25 The mechanical properties of the lens fibers have therefore evolved to be permissive to rapid shape changes that occur during accommodation. Although the amplitude of lens accommodation is controlled largely by ciliary muscle contraction and lens zonular tension, questions remain regarding how the fiber cell cytoarchitecture and membrane skeleton remodel during these events to maintain integrity and biomechanical properties of the lens.13,25 Given the effects of AnkB deficiency on lens fiber cell shape, including disruption of fiber cell hexagonal geometry and alignment, we postulated that AnkB deficiency likely impacts the mechanical properties of the ocular lens. Indeed, upon conducting a stress/strain analysis of AnkB−/+ and periaxin null mouse lenses using a microstrain analyzer, we found a significant decrease in the Young's modulus (stiffness) in both AnkB−/+ and periaxin null lenses compared to age-matched normal lenses, implying a direct role for AnkB and periaxin in maintenance of tensile properties of the lens.15 These observations further support the importance of AnkB along with other proteins such as periaxin, beaded filament proteins (phakinin and filensin), NrCAM, tropomyosin and tropomodulin in regulating the integrity and organization of the membrane skeleton (i.e. spectrin-actin network) and in maintaining lens biomechanical properties. It is also likely that the role of AnkB and periaxin in lens mechanics is closely linked to the membrane organization of the DGC complex in lens fibers, which is disrupted in the absence of AnkB and periaxin. Deficiency of phakinin and filensin, which are components of the beaded filaments distributed exclusively in lens fibers, and are anchored to the lens fiber cell plasma membrane, also impairs lens fiber cell geometry and mechanical properties.25 Additionally, the beaded filament is thought to biochemically interact with the spectrin-actin network through tropomyosin and tropomodulin in the lens, and to synergistically maintain lens fiber cell shape and biomechanical properties.13 Whether AnkB interacts with and regulates beaded filament membrane organization in lens fibers is not known and needs exploration in future studies. Although maintenance of lens mechanical strength is necessary for preventing stress-induced injury during lens ac-commodation, it is still largely unknown how transmission of mechanical force takes place within the lens through the tightly packed fiber mass. Intriguingly, lens fibers exhibit elaborate interdigitations at their lateral surfaces including the ball and socket processes and protrusions and these membrane formations are reported to be enriched with various regulatory proteins of cytoskeleton and cell adhesive interactions.26,27 Whether these fiber cell lateral membrane processes are comprised of AnkB and its associated proteins including periaxin, NrCAM and ion channel proteins, and involved in transmission of signals related to the mechanical properties of lens, needs to be investigated. However, the existence of a definitive link between the fiber cell hexagonal geometry and lens biomechanical properties is now becoming increasingly evident.9,13,15 It is likely that the hexagonal geometry of the fiber cells regulated by AnkB and its associated proteins is a critical determinant of the load bearing properties of these cells. There are many proteins within the lens which exhibit fiber cell specific distribution with little to no influence on lens development and differentiation including phakinin, filensin, tropomodulin, periaxin and NrCAM.13,17,25 The deficiency of these proteins, however, impairs lens fiber cell shape, alignment and mechanical properties, indicating that protein: protein interactions and membrane organization of the resulting complexes render them key determinants of lens architecture and mechanics.9,13,17,25 Another means by which AnkB regulates lens biomechanics could be related to its known involvement in membrane organization of the L-type calcium channels.28 Interestingly enough, in AnkB−/+ mouse lenses we have observed decreased levels of L-type calcium proteins in the lens fiber membrane fraction, together with decreased myosin light chain phosphorylation, suggesting a possible compromise in calcium regulated myosin II activity and lens mechanical properties in these lenses.15
Concluding remarks
Our recent study demonstrated that AnkB plays a crucial role in maintaining integrity of fiber cell hexagonal shape, membrane organization and biomechanical characteristics, which are key determinants of lens cytoarchitecture, optical clarity and tensile properties through different molecular mechanisms. Importantly, we obtained evidence for a close association between integrity of fiber cell hexagonal shape and maintenance of biomechanical properties of the ocular lens. Our study documents the ability of AnkB to serve as an adaptor protein that engages in key protein: protein interactions to regulate cell shape, membrane organization and mechanical properties. In future studies, it is important to determine the role of AnkB in supporting the resilience of lens cytoarchitecture following compression, and in transmission of mechanical force during lens accommodation.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were dis-closed.
ACKNOWLEDGMENTS
We thank Drs. Vann Bennett, Peter Brophy and Mark Walters for their collaboration on the lens ankyrin-B and periaxin studies.
Funding
This work is supported by the National Institute of Health grants to PVR (R01EY025096 and R01EY018590).
REFERENCES
- 1.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 2001; 81:1353-92; PMID:11427698 [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Lorenzo DN. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Topics Membr 2013; 72:1-37; PMID:24210426; http://dx.doi.org/ 10.1016/B978-0-12-417027-8.00001-5 [DOI] [PubMed] [Google Scholar]
- 3.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol 1999; 11:424-31; PMID:10449327; http://dx.doi.org/ 10.1016/S0955-0674(99)80061-1 [DOI] [PubMed] [Google Scholar]
- 4.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al.. Collective cell guidance by cooperative intercellular forces. Nat Mat 2011; 10:469-75; PMID:21602808; http://dx.doi.org/ 10.1038/nmat3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 2006; 119:199-207; PMID:16410545; http://dx.doi.org/ 10.1242/jcs.02814 [DOI] [PubMed] [Google Scholar]
- 6.Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol 2009; 1:a003012; PMID:20457566; http://dx.doi.org/ 10.1101/cshperspect.a003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci 2011; 366:1250-64; PMID:21402584; http://dx.doi.org/ 10.1098/rstb.2010.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak RB, Fischer RS, Zoltoski RK, Kuszak JR, Fowler VM. Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. J Cell Biol 2009; 186:915-28; PMID:19752024; http://dx.doi.org/ 10.1083/jcb.200905065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddala R, Skiba NP, Lalane R 3rd, Sherman DL, Brophy PJ, Rao PV. Periaxin is required for hexagonal geometry and membrane organization of mature lens fibers. Dev Biol 2011; 357:179-90; PMID:21745462; http://dx.doi.org/ 10.1016/j.ydbio.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci 2003; 116:4985-95; PMID:14625392; http://dx.doi.org/ 10.1242/jcs.00815 [DOI] [PubMed] [Google Scholar]
- 11.Yoon KH, Blankenship T, Shibata B, Fitzgerald PG. Resisting the effects of aging: a function for the fiber cell beaded filament. Investigat Ophthalmol Visual Sci 2008; 49:1030-6; PMID:18326727; http://dx.doi.org/ 10.1167/iovs.07-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S, Landsbury A, Dahm R, Liu Y, Zhang Q, Quinlan RA. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest 2009; 119:1837-48; PMID:19587458; http://dx.doi.org/ 10.1172/JCI38277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, Sah RL, Clark JI, Fowler VM. Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PLoS One 2012; 7:e48734; PMID:23144950; http://dx.doi.org/ 10.1371/journal.pone.0048734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol 1999; 147:995-1008; PMID:10579720; http://dx.doi.org/ 10.1083/jcb.147.5.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddala R, Walters M, Brophy PJ, Bennett V, Rao PV. Ankyrin-B directs membrane tethering of Periaxin and is required for maintenance of lens fiber cell hexagonal shape and mechanics. Am J Physiol Cell Physiol 2016; 310:115-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol 2011; 349:363-77; PMID:20969840; http://dx.doi.org/ 10.1016/j.ydbio.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.More MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J Cell Biol 2001; 154:187-96; PMID:11449000; http://dx.doi.org/ 10.1083/jcb.200104038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillespie CS, Sherman DL, Blair GE, Brophy PJ. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 1994; 12:497-508; PMID:8155317; http://dx.doi.org/ 10.1016/0896-6273(94)90208-9 [DOI] [PubMed] [Google Scholar]
- 19.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 2002; 12:349-61; PMID:12076680; http://dx.doi.org/ 10.1016/S0959-437X(02)00309-X [DOI] [PubMed] [Google Scholar]
- 20.Fort PE, Darche M, Sahel JA, Rendon A, Tadayoni R. Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization. Mol Vis 2014; 20:1480-90; PMID:25489223 [PMC free article] [PubMed] [Google Scholar]
- 21.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 2008; 135:1189-200; PMID:19109891; http://dx.doi.org/ 10.1016/j.cell.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, Bennett V. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem 2011; 286:7370-78; PMID:21186323; http://dx.doi.org/ 10.1074/jbc.M110.187-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman DL, Fabrizi C, Gillespie CS, Brophy PJ. Specific disruption of a schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron 2001; 30:677-87; PMID:11430802; http://dx.doi.org/ 10.1016/S0896-6273(01)00327-0 [DOI] [PubMed] [Google Scholar]
- 24.Sherman DL, Wu LM, Grove M, Gillespie CS, Brophy PJ. Drp2 and periaxin form Cajal bands with dystroglycan but have distinct roles in Schwann cell growth. J Neurosci 2012; 32:9419-28; PMID:22764250; http://dx.doi.org/ 10.1523/JNEUROSCI.1220-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fudge DS, McCuaig JV, Van Stralen S, Hess JF, Wang H, Mathias RT, FitzGerald PG. Intermediate filaments regulate tissue size and stiffness in the murine lens. Investigat Ophthalmol Visual Sci 2011; 52:3860-67; PMID:21345981; http://dx.doi.org/ 10.1167/iovs.10-6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas SK, Brako L, Gu S, Jiang JX, Lo WK. Regional changes of AQP0-dependent square array junction and gap junction associated with cortical cataract formation in the Emory mutant mouse. Exp Eye Res 2014; 127:132-42; PMID:25088353; http://dx.doi.org/ 10.1016/j.exer.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou CJ, Lo WK. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Exp Eye Res 2003; 77:423-32; PMID:12957142; http://dx.doi.org/ 10.1016/S0014-4835(03)00171-4 [DOI] [PubMed] [Google Scholar]
- 28.Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, et al.. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 2011; 124:1212-22; PMID:21859974; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.111.023986 [DOI] [PMC free article] [PubMed] [Google Scholar]


