Abstract
The semiflexible polymers filamentous actin (F-actin) and intermediate filaments (IF) both form complex networks within the cell, and together are key determinants of cellular stiffness. While the mechanics of F-actin networks together with stiff microtubules have been characterized, the interplay between F-actin and IF networks is largely unknown, necessitating the study of composite networks using mixtures of semiflexible biopolymers. We employ bulk rheology in a simplified in vitro system to uncover the fundamental mechanical interactions between networks of the 2 semiflexible polymers, F-actin and vimentin IF. Surprisingly, co-polymerization of actin and vimentin can produce composite networks either stronger or weaker than pure F-actin networks. We show that this effect occurs through steric constraints imposed by IF on F-actin during network formation and filament crosslinking, highlighting novel emergent behavior in composite semiflexible networks.
Keywords: actin, composite, intermediate filaments, model systems; networks, rheology, semiflexible polymers, vimentin
Abbreviations
- G-actin
globular (monomeric) actin
- F-actin
filamentous actin
- IF
intermediate filament
Introduction
The biopolymer filaments filamentous actin (F-actin), microtubules, and intermediate filaments (IF) form networks that are critical in determining cell stiffness.1-6 The network mechanics are determined by the properties of the biopolymers, as well as the interplay between them,7,8 rendering studies of the mechanics of ensembles of networks essential for understanding the cellular interior. An ideal system for such studies is the in vitro network, as it provides a well-controlled environment. Previous in vitro studies have reported the mechanics of either single filaments,9-12 or networks of filaments comprised of single biopolymer species.13-18 Studies on composite polymer networks have used either artificial interpenetrating polymers,19 or a mixture of stiff and semiflexible biopolymer filaments.20,21 However, the cell contains a combination of 2 different semiflexible polymers, F‑actin and IF, that together comprise a majority of the intracellular network.22 Since the final structural state of biopolymer networks is greatly affected by changes in assembly kinetics and steric constraints,23-25 the presence of an IF network is likely to alter the assembly of F‑actin networks. Thus, determining the impact of IF on F‑actin network formation will provide a fundamental basis for understanding the emergent behavior of composite semiflexible networks in cells.
Here, we study the mechanics of a composite network comprised of the 2 different semiflexible biopolymers found in mesenchymal cells, F-actin and vimentin IF.26 We generate a crosslinked F‑actin network interpenetrated with a vimentin IF network and use bulk rheology to investigate the composite network mechanics in both the linear and nonlinear regimes. We find that co‑polymerization with vimentin strengthens F‑actin networks when actin crosslinkers are abundant, as expected from the overall increase in the amount of polymer in the network. Surprisingly, F‑actin networks are weakened by co‑polymerization with vimentin when the F‑actin crosslinking density is low compared to the network mesh size. Based on changes in network elasticity, yield stress, and strain stiffening, we show that this counterintuitive result arises from steric constraints on F‑actin by vimentin IF, leading to a lower degree of F‑actin crosslinking in the final network.
Results
Co‑polymerization of vimentin with actin can either weaken or strength F‑actin networks
Under strain, all composite F‑actin‑vimentin IF networks examined in this work first exhibit a linear elastic regime, with the initial slope of the stress‑strain curve providing a measure for the elastic modulus . Strain stiffening occurs at a critical strain , evident as an increase in elasticity quantified by the nonlinear tangent modulus . The networks fail at a maximal stress , after which the stress decreases with strain, as shown in Figure 1. When actin is co‑polymerized with vimentin with a 1:100 ratio of biotinylated to plain actin, both the elasticity and yield stress of the composite networks are affected. At 18 actin, co‑polymerization with vimentin slightly lowers the yield stress and network elasticity, as shown in Figure 1A. In contrast, at 6 actin, co‑polymerization with vimentin increases both yield stress and elasticity, as shown in Figure 1B.
Figure 1.
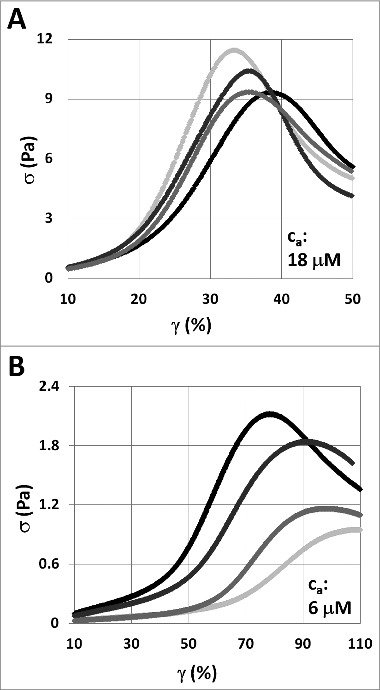
Stress‑strain curves of composite F‑actin‑vimentin IF networks with a ratio of 1:100 biotinylated actin to plain actin. Samples exhibit an initial elasticity before undergoing strain stiffening at a critical strain , evident as an increase in the tangent modulus (the slope of the stress‑strain curve). The peak of the curve indicates the yield stress , the maximal stress the network can withstand before failing. (A) At actin concentrations of 18 , the maximal stress is decreased as the vimentin concentration is increased from 0 (light gray) through 0.3 and 1.5 to 3 (black). (B) In contrast, the addition of vimentin, from 0 through 0.3 and 1.5 to 3 , increases the yield stress of the composite network at actin concentrations of 6 .
To quantify the differences between F‑actin networks and composite F‑actin‑vimentin IF networks, we calculate the absolute change in the linear elastic modulus, , and the fractional changes in the maximal tangent modulus and yield stress , each of which is extracted from the slope and the peak of the stress‑strain curves shown in Figure 1. At 6 actin, co‑polymerization with vimentin results in an increase of the network yield stress . However, this increase becomes gradually less pronounced with increasing actin concentration, and at 18 actin a decrease in is observed, as shown in Figure 2A. At low concentrations of actin, co‑polymerization with vimentin increases the linear elastic modulus , as shown in Figure 2B. This increase corresponds closely to the modulus of the single‑species vimentin IF network (black curve, Fig. 2B), suggesting that vimentin IF adds linearly to the elasticity of the F‑actin network at low actin concentrations. However, with higher actin concentrations, co‑polymerization with vimentin leads to a smaller increase or even a slight decrease in . The nonlinear elasticity shows a similar trend, as co‑polymerization with vimentin increases the maximal tangent modulus at lower actin concentrations, but decreases it at higher actin concentrations, as shown in Figure 2C.
Figure 2.
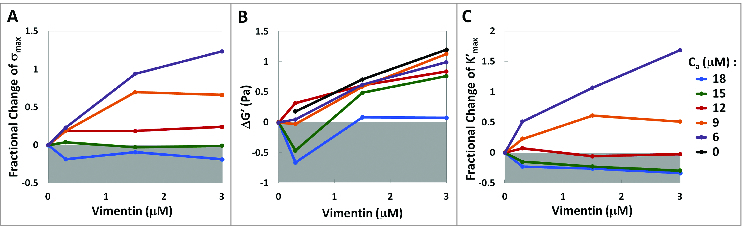
Co‑polymerization with vimentin strengthens F-actin networks at low actin concentrations, but weakens networks at high actin concentrations. (A) Fractional change in yield stress as a function of actin and vimentin concentrations. Increasing the actin concentration gradually reduces the increase in yield stress and transitions to a reduction in yield stress at sufficiently high actin concentrations. (B) Absolute change in linear elastic modulus of F-actin-vimentin IF composite networks compared to vimentin‑free networks. At low actin concentrations, vimentin IF adds roughly linearly to . At higher actin concentrations, vimentin IF contributes less to the elasticity, and can even result in a decrease of composite network elasticity. (C) Fractional change in the maximal nonlinear elasticity exhibited by the composite networks under strain. At low actin concentrations, vimentin IF adds to the nonlinear elasticity of the networks, while the nonlinear elasticity is reduced with vimentin IF at high actin concentrations. All values are measured relative to vimentin‑free F‑actin networks at a given actin concentration.
Vimentin strengthening or weakening of F‑actin networks is dependent on F‑actin crosslinker density
We conduct rheology on 15 F‑actin networks co‑polymerized with vimentin while varying the density of biotinylated actin monomers, thereby controlling the average distance between sites of F‑actin crosslinking. We quantify the elasticity and yield stress of the networks, as well as the critical strain at which the networks undergo strain stiffening. When sites of F‑actin crosslinking are closely spaced, corresponding to a high density of biotinylated actin monomers in the F‑actin filaments, co‑polymerization with 3 vimentin results in an increase in both linear modulus and yield stress, and a lower critical strain. Conversely, when biotinylated actin monomers are sparse and crosslinking sites farther apart, co‑polymerization with 3 vimentin decreases the yield stress and linear modulus, and delays the onset of strain stiffening, as shown in Figure 3.
Figure 3.
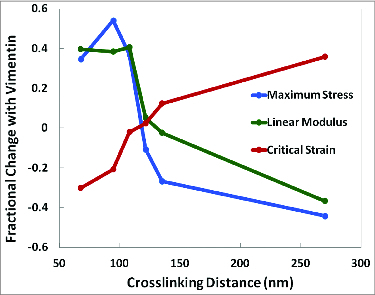
Vimentin strengthening or weakening of F-actin networks is dependent on the density of F‑actin crosslinkers. In a 15 F‑actin network, at high crosslinking densities (small average distance between crosslinkers), co‑polymerization with 3 vimentin increases the maximal composite network stress and linear elastic modulus, while lowering the critical strain of composite network strain stiffening. The opposite trend is seen at lower crosslinking densities (larger average distance between crosslinkers), as 3 vimentin delays the onset of strain stiffening, but decreases the linear elastic modulus and maximal composite network stress. All fractional changes are measured relative to a vimentin‑free 15 F-actin network.
Discussion
Our data show that co‑polymerization of vimentin and actin can result in composite networks that are either stronger or weaker than pure F‑actin networks, depending on the concentration of actin and the density of F‑actin crosslinkers. These composite networks consist of interpenetrating F-actin and vimentin IF networks of comparable mesh sizes, as shown in Figure 4. We estimate the mesh size of the F‑actin network at these concentrations to range from 0.26 to 0.45 , based on the dependence on monomer concentration and the protein volume fraction.27 As there are 370 actin monomers per of F-actin,28 a 1:100 biotinylation ratio results in an average spacing between biotinylated sites of , roughly comparable to the F‑actin network mesh size. Vimentin forms an IF network with a mesh size ranging from 0.80 to 2.5 , assuming a similar protein mass density of actin and vimentin.29
Figure 4.
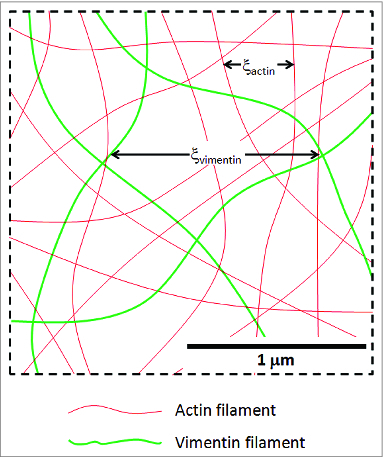
Illustration of the approximate F-actin‑vimentin IF network geometry for an actin concentration of 15 and a vimentin concentration of 3 . Actin filaments are bound to each other through biotin‑neutravidin crosslinks, while vimentin IF are crosslinked by magnesium. The result is an interpenetrating network of the 2 species of biopolymers. At a 1:100 actin biotinylation ratio, the average distance between actin crosslinking sites is comparable to the actin network mesh size.
Despite the qualitative similarities between F‑actin-vimentin IF networks and vimentin‑free F‑actin networks seen in Figure 1, vimentin IF affects both the linear and nonlinear behavior of the composite networks. To highlight the effect of vimentin IF on the F‑actin networks, we quantify these changes as the difference in elasticity and yield stress in networks with and without vimentin IF. At low actin concentrations, the composite network is stronger and stiffer with the addition of vimentin IF, consistent with the overall increase in polymer concentration. These composite networks exhibit a relative increase in yield stress , an increase in the linear elasticity , and an increase in the maximal nonlinear elasticity , as shown in Figure 2. This increase in linear elasticity is equal to the sum of the elasticity of the individual networks. Surprisingly, at higher actin concentrations, vimentin IF results in a reduction of and , and a linear elasticity that is lower than the sum of the parts. F‑actin networks co-polymerized with vimentin thus exhibit 2 mechanical regimes: at low actin concentrations, vimentin IF strengthens the composite network and increases its elasticity, while at high actin concentrations, the additional polymer results in an unexpectedly weaker composite network with a lower elasticity and yield stress.
Strikingly, the crossover between the strengthening and weakening regimes observed in the composite network rheology occurs when the estimated F‑actin network mesh size is comparable to the distance between F‑actin crosslinking sites. To understand the transition between these 2 regimes, we consider the degree of crosslinking in the system, since the rheological properties of a crosslinked network are highly dependent on both polymer concentration and crosslinker density.27 In order to become crosslinked, actin filaments undergo thermal fluctuations to find a crosslinking partner. When crosslinks between actin filaments are formed, the amplitudes of filament fluctuations are reduced, impeding the formation of additional crosslinks. The degree of crosslinking in the final network is thus dependent on the density of crosslinkers, as well as the volume each filament can thermally explore, resulting in some unutilized crosslinking sites in this final state. In our networks, we estimate F‑actin crosslinking sites to be nearly fully utilized when the volume a biotinylation site can explore through thermal fluctuations contains at most one other biotinylated site. A freely fluctuating F‑actin segment of length undergoes transverse motion with a root‑mean‑square amplitude of , where denotes the filament persistence length.30 In a network, however, the surrounding filaments limit these fluctuations. Thus, the magnitude of these thermal transverse fluctuations in our networks are limited by the F‑actin network mesh size , resulting in near‑full utilization of crosslinking sites when . Remarkably, the predicted mesh size at which crosslinkers are fully utilized coincides with the observed transition between the strengthening and weakening regimes, suggesting that co‑polymerization with vimentin IF weakens F‑actin networks when actin crosslinking sites are sparse. Since vimentin IF causes a reduction in yield stress and network elasticity when , we hypothesize that the additional steric constraint imposed by vimentin IF results in a loss of F‑actin crosslinking in this case.
If vimentin IF indeed weakens F‑actin networks through a disruption of actin crosslinking when crosslinkers are sparse, we expect to recover the network strengthening regime by increasing the F‑actin crosslinking density. We therefore conduct rheology experiments at a fixed actin concentration of 15 while varying the actin biotinylation ratio. As before, both and are reduced with the addition of vimentin IF when crosslinking sites are far apart, corresponding to a low ratio of biotinylated to plain actin. In contrast, by increasing the number of crosslinking sites, we are able to recover a relative increase in both and , as depicted in Figure 3, suggesting that vimentin IF disrupts F‑actin crosslinking when crosslinkers are sparse. This is further verified by investigating the onset of strain stiffening , which in single‑species semiflexible polymer networks is expected to scale as , and therefore provides a measure of changes in crosslinking in the network. 15, 31 When F‑actin crosslinking sites are abundant, we observe a shift toward earlier strain stiffening, as shown in Figure 3, suggesting that the effective crosslinking of the composite network is higher than for the plain F-actin network. We attribute this to the interpenetration of the 2 networks, effectively increasing the sites of interaction between filaments in the composite network. In contrast, the strain stiffening onset is delayed by the co‑polymerization with vimentin IF when the average distance between biotinylated sites in F‑actin is large, pointing to a loss of F‑actin crosslinking in the network. Notably, the transition between these 2 regimes coincides with the observed crossover in and . As predicted, all 3 rheological changes are attributable to the same underlying reduction in F‑actin crosslinking, confirming our hypothesis that the presence of vimentin IF can reduce the volume explored by actin filaments during network formation, as indicated by the shaded regions in Figure 5. When F‑actin crosslinkers are abundant, the reduction in actin filament fluctuations due to the presence of vimentin IF will not reduce the overall crosslinking, as alternate crosslinking sites are available, as shown schematically in Figure 5A and B. At low crosslinking densities, this same reduction in fluctuations can lead to a loss of all neighboring crosslinking sites, as illustrated in Figure 5C and D. As a result of this reduction in actin filament fluctuations, the addition of vimentin IF can lead to either a strengthening of the composite network through the additional polymer present, or a weakening through the disruption of physical F‑actin crosslinking sites. This emergent behavior highlights the importance of considering biopolymers in composite networks when seeking to understand the material properties of complex networks such as the cytoskeleton.
Figure 5.
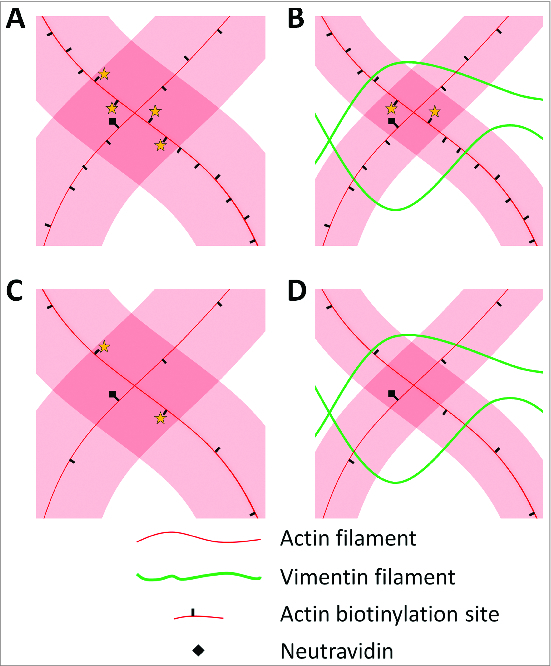
Vimentin IF restricts F-actin fluctuations, which leads to a loss of F‑actin crosslinking when F‑actin crosslinkers are scarce. Shaded areas indicate the volume explored by an actin filament through thermal fluctuations before these are reduced by crosslinking. (A) When F‑actin crosslinkers are abundant, several biotins are within reach of a given biotin‑neutravidin site, indicated by stars, and any of these can bind to crosslink the 2 actin filaments. (B) Co‑polymerization with vimentin to form IF constrains actin filament fluctuations, but biotin sites are still within reach at high biotin densities, and an F‑actin crosslink can form. (C) At low biotin densities, reachable crosslinking partners are fewer and farther apart. (D) Steric constraints by vimentin IF can now result in a loss of F‑actin crosslinking, weakening the resulting composite network.
Previous microrheological studies of a composite network of rigid and semiflexible biopolymer found rheological properties between those of the single‑species networks,20 while bulk rheological studies showed that microtubules in crosslinked F‑actin networks induced actin strain stiffening.21 Here, we observe a new phenomenon in composite semiflexible networks, in which the composite network is either strengthened or weakened by the addition of a second semiflexible polymer species, depending on the concentrations and density of crosslinkers. In cells, the exact behavior of composite cytoskeletal networks will depend on the nature of the crosslinkers that mediate their interconnections and molecular crosstalk. For example, many F‑actin crosslinkers form F‑actin bundles and are dynamically binding and unbinding to and from F-actin,32,33 cyto-linker proteins form crossbridges between IF and F‑actin,34 and molecular motors can form connections between microtubules and IF.35,36 Further studies using biological crosslinkers will help to better understand more complex biopolymer networks, but the studies presented here provide a necessary foundation for understanding the interplay between semiflexible polymers in composite networks.
Methods and Materials
Proteins
Actin is purified from rabbit skeletal muscle37 and stored as G‑actin in G‑buffer (0.2 mM CaCl2, 0.2 mM ATP, 0.2 mM DTT, 0.005% NaN3, and 2 mM Tris‑HCl, pH 8.0) at -80 ºC until the day before the experiment. Biotinylated G‑actin is purchased from Cytoskeleton (Denver, CO) as a lyophilized powder and resuspended in G‑buffer one day prior to experiments. Neutravidin is from Sigma (St. Louis, MO). Vimentin is expressed in and purified from Escherichia coli38 and stored in a dialysis buffer of 5 mM Tris-HCl, 1 mM EDTA, 0.1 mM EGTA, and 1 mM DTT, pH 8.4 at -80 ºC until the day of the experiment.
Composite networks
Composite networks are formed using 6‑18 F‑actin with a 1:100 molar ratio of biotinylated actin to plain actin. Actin is co‑polymerized with 0.3‑3 vimentin crosslinked with magnesium.18 Networks are formed by first pre‑polymerizing plain G‑actin together with biotinylated actin monomers for 60 seconds at room temperature.39 This is done to ensure an even incorporation of the biotinylated actin monomers into the actin filaments. F‑actin crosslinking is then initiated by the addition of a 2‑fold molar excess of neutravidin together with unpolymerized vimentin in dialysis buffer, after which the network is mixed and loaded immediately onto a rheometer for mechanics measurements. The final buffer conditions of 2 mM MgCl2, 0.2 mM CaCl2, 100 mM KCl, 0.5 mM ATP, 0.2 mM DTT, and 2 mM Tris‑HCl allow for the crosslinking of F‑actin in the presence of faster‑forming vimentin IF network,40 while the use of a biotin-neutravidin F‑actin crosslinker allows for a final network structure that depends only on the network formation kinetics.
Bulk rheology
The network mechanics are characterized at 25 ºC on an Ares G2 stress controlled shear rheometer (TA Instruments, New Castle, DE) using a stainless steel parallel plate geometry with a 40-mm-diameter top plate, to which actin and vimentin attach nonspecifically.14,39 90 minutes after loading, during which the proteins polymerize and form a network, the sample is subjected to a strain ramp at a rate of 0.25 %/s, during which the stress and the strain are measured. The elasticity is quantified as the initial linear elastic modulus and the nonlinear tangent modulus (the slope of the stress‑strain curve). We also quantify the onset of strain stiffening , and the yield stress as the maximal stress the networks can withstand before failing, as previously described.39
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We thank Prof. Frederick MacKintosh for discussions and insights. This work was supported by the National Institutes of Health program project grant P01 GM096971, by the National Science Foundation (DMR‑1310266) and by the Harvard MRSEC (DMR‑0820484).
References
- 1. Nogales E. Structural insights into microtubule function. Annu Rev Biochem 2000; 69:277-302; PMID:10966460 [DOI] [PubMed] [Google Scholar]
- 2. Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science 1998; 279:514-9; PMID:9438837, http://dx.doi.org/ 10.1126/science.279.5350.514 [DOI] [PubMed] [Google Scholar]
- 3. Fuchs E, Weber K. Intermediate filaments - structure, dynamics, function, and disease. Annu Rev Biochem 1994; 63:345-82; PMID:7979242, http://dx.doi.org/ 10.1146/annurev.bi.63.070194.002021 [DOI] [PubMed] [Google Scholar]
- 4. Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell-surface and through the cytoskeleton. Science 1993; 260:1124-7; PMID:7684161, http://dx.doi.org/ 10.1126/science.7684161 [DOI] [PubMed] [Google Scholar]
- 5. Smith PG, Deng LH, Fredberg JJ, Maksym GN. Mechanical strain increases cell stiffness through cytoskeletal filament reorganization. Am J Physiol-Lung C 2003; 285:L456-L63; PMID:12704020 [DOI] [PubMed] [Google Scholar]
- 6. Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, Fredberg JJ, Goldman RD,, Weitz DA. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J 2013; 105:1562-8; PMID:24094397, http://dx.doi.org/ 10.1016/j.bpj.2013.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 2003; 116:1157-73; PMID:12615960 [DOI] [PubMed] [Google Scholar]
- 8. Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 2010; 189:541-56; PMID:20421424, http://dx.doi.org/ 10.1083/jcb.200909113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin-filaments measured from thermal fluctuations in shape. J Cell Biol 1993; 120:923-34; PMID:8432732, http://dx.doi.org/ 10.1083/jcb.120.4.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isambert H, Venier P, Maggs AC, Fattoum A, Kassab R, Pantaloni D, Carlier MF. Flexibility of actin-filaments derived from thermal fluctuations - effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J Biol Chem 1995; 270:11437-44; PMID:7744781, http://dx.doi.org/ 10.1074/jbc.270.19.11437 [DOI] [PubMed] [Google Scholar]
- 11. Venier P, Maggs AC, Carlier MF, Pantaloni D. Analysis of microtubule rigidity using hydrodynamic flow and thermal fluctuations. J Biol Chem 1994; 269:13353-60; PMID:7909808 [PubMed] [Google Scholar]
- 12. Mucke N, Kreplak L, Kirmse R, Wedig T, Herrmann H, Aebi U, Langowski J. Assessing the flexibility of intermediate filaments by atomic force microscopy. J Mol Biol 2004; 335:1241-50; PMID:14729340, http://dx.doi.org/ 10.1016/j.jmb.2003.11.038 [DOI] [PubMed] [Google Scholar]
- 13. Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol 1991; 113:155-60; PMID:2007620, http://dx.doi.org/ 10.1083/jcb.113.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science 2004; 304:1301-5; PMID:15166374, http://dx.doi.org/ 10.1126/science.1095087 [DOI] [PubMed] [Google Scholar]
- 15. Tharmann R, Claessens MM, Bausch AR. Viscoelasticity of isotropically cross-linked actin networks. Phys Rev Lett 2007; 98:088103; PMID:17359131 [DOI] [PubMed] [Google Scholar]
- 16. Lin YC, Koenderink GH, MacKintosh FC, Weitz DA. Viscoelastic properties of microtubule networks. Macromolecules 2007; 40:7714-20; http://dx.doi.org/ 10.1021/ma070862l [DOI] [Google Scholar]
- 17. Schopferer M, Bar H, Hochstein B, Sharma S, Mucke N, Herrmann H, Willenbacher N. Desmin and Vimentin Intermediate Filament Networks: Their Viscoelastic Properties Investigated by Mechanical Rheometry. J Mol Biol 2009; 388:133-43; PMID:19281820, http://dx.doi.org/ 10.1016/j.jmb.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 18. Lin YC, Yao NY, Broedersz CP, Herrmann H, MacKintosh FC, Weitz DA. Origins of Elasticity in Intermediate Filament Networks. Phys Rev Lett 2010; 104:058101; PMID:20366795 [DOI] [PubMed] [Google Scholar]
- 19. Sperling LH, Mishra V. The current status of interpenetrating polymer networks. Polym Advan Technol 1996; 7:197-208; http://dx.doi.org/ 10.1002/(SICI)1099-1581(199604)7:4%3c197::AID-PAT514%3e3.0.CO;2-4 [DOI] [Google Scholar]
- 20. Pelletier V, Gal N, Fournier P, Kilfoil ML. Microrheology of microtubule solutions and actin-microtubule composite networks. Phys Rev Lett 2009; 102:188303; PMID:19518917 [DOI] [PubMed] [Google Scholar]
- 21. Lin YC, Koenderink GH, MacKintosh FC, Weitz DA. Control of non-linear elasticity in F-actin networks with microtubules. Soft Matter 2011; 7:902-6; http://dx.doi.org/ 10.1039/c0sm00478b [DOI] [Google Scholar]
- 22. Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity. diffusion, intracellular surface area. Int Rev Cytol 2000; 192:189-221; PMID:10553280 [DOI] [PubMed] [Google Scholar]
- 23. Falzone TT, Lenz M, Kovar DR, Gardel ML. Assembly kinetics determine the architecture of alpha-actinin crosslinked F-actin networks. Nat Commun 2012; 3:861; PMID:22643888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kayser J, Grabmayr H, Harasim M, Herrmann H, Bausch AR. Assembly kinetics determine the structure of keratin networks. Soft Matter 2012; 8:8873-9; http://dx.doi.org/ 10.1039/c2sm26032h [DOI] [Google Scholar]
- 25. Kayser J, Haslbeck M, Dempfle L, Krause M, Grashoff C, Buchner J, Herrmann H, Bausch AR. The small heat shock protein Hsp27 affects assembly dynamics and structure of keratin intermediate filament networks. Biophys J 2013; 105:1778-85; PMID:24138853, http://dx.doi.org/ 10.1016/j.bpj.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci 1999; 112:4615-25; PMID:10574710 [DOI] [PubMed] [Google Scholar]
- 27. Degennes PG, Pincus P, Velasco RM, Brochard F. Remarks on polyelectrolyte conformation. J Phys-Paris 1976; 37:1461-73. [Google Scholar]
- 28. Huxley HE, Brown W. Low-angle x-ray diagram of vertebrate striated muscle and Its behaviour during contraction and rigor. J Mol Biol 1967; 30:383- 434; PMID:5586931, http://dx.doi.org/ 10.1016/S0022-2836(67)80046-9 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt CF, Barmann M, Isenberg G, Sackmann E. Chain dynamics, mesh size, and diffusive transport in networks of polymerized actin - a quasielastic light-scattering and microfluorescence study. Macromolecules 1989; 22:3638-49; http://dx.doi.org/ 10.1021/ma00199a023 [DOI] [Google Scholar]
- 30. MacKintosh FCM. Elasticity and dynamics of cytoskeletal filaments and their networks. In Poon WCK, Andelman D, editors. Soft Condensed Matter Physics in Molecular and Cell Biology. New York: Taylor and Francis; 2006. p. 139-155. [Google Scholar]
- 31. Mackintosh FC, Kas J, Janmey PA. Elasticity of Semiflexible Biopolymer Networks. Phys Rev Lett 1995; 75:4425-8; PMID:10059905, http://dx.doi.org/ 10.1103/PhysRevLett.75.4425 [DOI] [PubMed] [Google Scholar]
- 32. Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol 1998; 10:102-11 [DOI] [PubMed] [Google Scholar]
- 33. Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci 2005; 118:651; PMID:15701920, http://dx.doi.org/ 10.1242/jcs.01670 [DOI] [PubMed] [Google Scholar]
- 34. Yang YM, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell 1996; 86:655-65; PMID:8752219, http://dx.doi.org/ 10.1016/S0092-8674(00)80138-5 [DOI] [PubMed] [Google Scholar]
- 35. Prahlad V, Yoon M, Moir RD, Vale RD, Goldman RD. Rapid movements of vimentin on microtubule tracks: Kinesin-dependent assembly of intermediate filament networks. J Cell Biol 1998; 143:159-70; PMID:9763428, http://dx.doi.org/ 10.1083/jcb.143.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helfand BT, Mikami A, Vallee RB, Goldman RD. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol 2002; 157:795-806; PMID:12034772, http://dx.doi.org/ 10.1083/jcb.200202027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spudich JA, Watt S. Regulation of rabbit skeletal muscle contraction .1. Biochemical studies of interaction of tropomyosin-troponin complex with actin and proteolytic fragments of myosin. J Biol Chem 1971; 246:4866; PMID:4254541 [PubMed] [Google Scholar]
- 38. Herrmann H, Hofmann I, Franke WW. Identification of a Nonapeptide Motif in the Vimentin Head Domain Involved in Intermediate Filament Assembly. J Mol Biol 1992; 223:637-50; PMID:1542111, http://dx.doi.org/ 10.1016/0022-2836(92)90980-X [DOI] [PubMed] [Google Scholar]
- 39. Jensen MH, Morris EJ, Gallant CM, Morgan KG, Weitz DA, Moore JR. Mechanism of Calponin Stabilization of Cross-Linked Actin Networks. Biophys J 2014; 106:793-800; PMID:24559982, http://dx.doi.org/ 10.1016/j.bpj.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirmse R, Portet S, Mucke N, Aebi U, Herrmann H, Langowski J. A quantitative kinetic model for the in vitro assembly of intermediate filaments from tetrameric vimentin. J Biol Chem 2007; 282:18563-72; PMID:17403663, http://dx.doi.org/ 10.1074/jbc.M701063200 [DOI] [PubMed] [Google Scholar]


