abstract
The early/recycling endosomes of an eukaryotic cell perform diverse cellular functions. In addition, the endosomal system generates multiple organelles, including certain cell type-specific organelles called lysosome-related organelles (LROs). The biosynthesis of these organelles possibly occurs through a sequential maturation process in which the cargo-containing endosomal vesicular/tubular structures are fused with the maturing organelle. The molecular machinery that regulates the cargo delivery or the membrane fusion during LRO biogenesis is poorly understood. Here, we describe the known key molecules, such as SNAREs, that regulate both the biogenesis and secretion of multiple LROs. Moreover, we also describe other regulatory molecules, such as Rab GTPases and their effectors that modulate the SNARE activity for cargo delivery to one such LRO, the melanosome. Overall, this review will increase our current understanding of LRO biogenesis and function.
Keywords: AP-3, BLOC-1, HPS, LRO, melanosome, SNARE
ABBREVIATIONS
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- STX
syntaxin
- VAMP
vesicle-associated membrane protein 7
- BLOC
biogenesis of lysosome-related organelles complex
- AP
adaptor protein
- Rab
Rab GTPase
- LRO
lysosome-related organelle
- TGN
trans-Golgi network
- M6PR
mannose-6-phosphate receptor
Introduction
The endosomal system, especially the post-Golgi compartments, performs multiple functions and has a diverse membrane composition and variable luminal pH. Early endosomes of this secretory system continuously undergo fusion and fission and mature into late stage organelles, such as recycling and late endosomes and lysosomes.1,2 In certain cell types, the endosomal system is partly diverted to generate specialized organelles called lysosome-related organelles (LROs).3,4 The molecular machinery that regulates endosomal fusion with maturing LROs has been determined, at least partly, for some LROs.5 However, the mechanisms of assembly and disassembly of molecular components that facilitate membrane fusion are poorly understood. Moreover, the membrane fusion process is highly regulated, and any alterations to such pathways cause defects in cargo transport, signaling, metabolism, organelle maturation and other cellular functions.6 SNAREs (Soluble NSF (N-ethylmaleimide-sensitive factor) Attachment Protein REceptor) are the critical molecular mediators of all membrane fusion events. These proteins regulate the cargo transport, secretion, autophagy and organelle biogenesis in all cells.7 Approximately 38 SNARE proteins have been described so far, and their expression varies slightly by cell type. SNAREs localize to specific organelle membranes and mediate membrane fusion into or out of the organelle.8 Additionally, SNAREs indirectly determine the specificity of cargo delivery to the target membrane. Furthermore, membrane fusion relies on several other molecules, such as Rab GTPases (Rabs), tethering factors, etc., and these molecules will bring opposing membranes closer to each other and facilitate interactions between the SNAREs.9 On the other hand, chemical cues, such as calcium level, play an important role during fusion.10 To date, approximately 11 SNAREs have been shown to localize to endocytic organelles,8 but their roles in mediating cargo delivery to an LRO or LRO secretion remain largely unknown. After fusion, SNAREs should be recycled back to their native organelle for another round of membrane fusion, but information regarding SNARE recycling is limited. In this review, we summarize our current understanding of SNARE function and discuss the regulation of SNAREs by cytosolic factors during the biogenesis or secretion of endosomal derived LROs, such as melanosomes, dense granules, lamellar bodies and secretory lysosomes. We also discuss possible methods of post-fusion SNARE recycling.
Cargo and Their Transport Routes to Maturing LROS
Approximately 13 LROs have been described in different cell types, and these organelles were predicted to be synthesized either through the endocytic system (early, recycling or late endosomes) or from Golgi in their respective cell types.4 These organelles either coexist with bonafide lysosomes, for example, melanosomes (in melanocytes or retinal pigment epithelial cells), lamellar bodies (in alveolar type II lung epithelial cells), dense granules (in platelets), and Weibel-Palade bodies (in endothelial cells), or they may act as dual function organelles such as lytic granules/secretory lysosomes (in cytotoxic T lymphocytes or natural killer cells).5 The biogenesis processes or cargo delivery pathways to most of these organelles are still unclear. Moreover, defects in the formation or functioning of LROs result in several genetic disorders such as Chediak–Higashi syndrome (CHS), Griscelli syndrome (GS) or Hermansky–Pudlak syndrome (HPS).11 In the past decade, melanosomes in melanocytes became the mainstream for understanding LRO biogenesis. This is because the cargo transport pathways to this organelle are partly known compared to other LROs (Fig. 1).5,12 However, the key endocytic SNAREs that regulate the cargo delivery to this organelle during its maturation remain unknown. Melanosome biogenesis is initiated by the sorting of PMEL, an in vivo amyloidogenic protein, from the endosomal domain (Stage I) into intraluminal vesicles where it is processed into fibrillar structures (Stage II). Then, the primary melanin-synthesising enzymes tyrosinase (TYR) and tyrosinase-related protein-1 (TYRP1), along with other proteins such as oculocutaneous albinism-2 (OCA2) and a copper transporter (ATP7A), are transported from different recycling endosomal domains to the melanosome membranes through tubular-vesicular endosomal structures (Fig. 1). These enzymes initiate melanin synthesis on the PMEL fibrils (Stage III) and the organelle eventually matures into fully pigmented (Stage IV) melanosomes (Fig. 1). The current studies suggest that cargo transport to melanosomes follows 2 distinct routes, the first pathway involves BLOC-1, an eight-subunit cytosolic HPS complex that regulates the transport of TYRP1, ATP7A and other cargo to melanosomes (referred to here as BLOC-1-dependent cargo transport), and the second pathway is dependent on AP-3, a tetra-subunit HPS complex that controls the trafficking of TYR to melanosomes (referred to here as AP-3-dependent cargo transport) (Fig. 1).5,12,13 Nevertheless, it is not clear whether these 2 distinct pathways participate in the biogenesis of other LROs.
Figure 1.
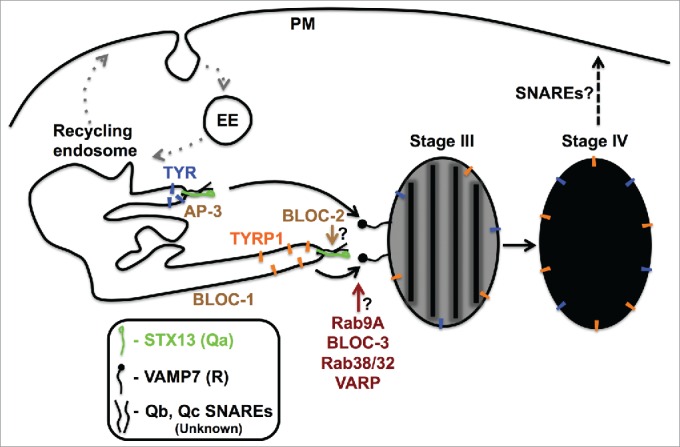
Melanosome biogenesis is a typical model for studying LRO biogenesis. The biogenesis of melanosomes begins in early endosomes (Stage I) where the amyloidogenic fibrillar protein PMEL is internalized and forms fibrils (Stage II, not shown). Further, the melanin-synthesising proteins TYRP1 (BLOC-1-dependent cargo, orange symbols) and TYR (AP-3-dependent cargo, blue symbols) were delivered from tubular-vesicular structures of recycling endosomes to maturing Stage II (not shown) or Stage III melanosomes. Upon cargo transport and melanin synthesis, Stage III melanosomes mature into Stage IV melanosomes. The cargo transport to maturing melanosomes is possibly mediated by the pairing of cytosolic SNARE domains of STX13 (Qa SNARE, green) and 2 other unknown SNAREs (Qb, Qc; black) on recycling endosomes with the SNARE domain of VAMP7 (R-SNARE, black) on Stage III or Stage II (not shown) melanosomes. Moreover, the current studies suggest that BLOC-2, Rab9A, BLOC-3, Rab38/32 and VARP molecules modulate the STX13-mediated cargo delivery to melanosomes, possibly functioning during membrane fusion. In contrast, BLOC-1 and AP-3 possibly function at early steps of cargo transport or sorting, respectively. Curved arrows represent the SNARE-mediated fusion of endosomal transport carriers with melanosomes. The dotted black arrow indicates the possible route of melanosome secretion, and the dotted gray arrows represent the different routes of the endocytic pathway. The solid black lines in the Stage III melanosome represent the PMEL fibrils. The question marks indicate the roles of those molecules that need to be validated in future studies. EE, early endosome and PM, plasma membrane.
Unlike melanosomes, the biogenesis of secretory lysosomes in cytotoxic T lymphocytes starts with an intermediate multivesicular structure having many inner vesicles. Furthermore, the organelle develops small dense core granules (composed of perforin, granzymes and proteoglycans) that then mature into more electron dense granules by accumulating the newly synthesized lytic granule proteins. Additionally, these intermediate structures can also fuse with mature secretory lysosomes.14 It has been shown that the transport of granzymes to secretory lysosomes occurs predominantly through the mannose-6-phosphate receptor (M6PR) from the trans-Golgi network (TGN), while an M6PR-independent route is used approximately 30% of the time. However, the method of transport of perforin to secretory lysosomes is unknown.15 Interestingly, the other cargoes CD63, CTLA-4 (CTL antigen-4) and GMP-17 (granule membrane protein of 17 kDa) were first sorted to the lysosomes and then to the secretory lysosomes. Studies have also shown that FasL (Fas ligand), a type II transmembrane protein, is directly delivered to the secretory lysosomes from the TGN. Nonetheless, the other membrane proteins that share the FasL sorting mechanism have yet to be identified.14 In contrast, dense granules (DGs) in platelets initiate their biogenesis from late endosomes/multivesicular bodies 16 and acquire different cargo through the endosomal network.17,18 The limiting membrane of DGs contains CD63 (LAMP-3) and LAMP-2 but not LAMP-1. It has been reported that the transport of LAMP-2 and VAMT2 (a putative serotonin transporter, SLC18) from early endosomes to DGs is regulated by AP-3, which sorts these cargo through its classical tyrosine- and dileucine-based sorting motifs, respectively.17 In addition, Rab32 and Rab38 have also been shown to play roles in trafficking of VMAT2 as well as MRP4 (putative adenine nucleotide transporter, ABCC4) to maturing DGs. Consistently, Rab38/32 have been shown to localize to endosome-associated tubules or transport vesicles that are pinched off and routed toward the maturing DGs.17 Interestingly, SLC35D3 (a putative sugar nucleotide transporter) has been shown to be transported to DGs or to intermediates in DG biogenesis by BLOC-1 and the AP-3 complex.18 However, the trafficking of the CD63 and ABCA3 (a lipid transporter) proteins from late endosomes to DGs is unknown. Though the lamellar bodies (LBs) in lung type II epithelial cells are one of the critical LROs, studies related to their biogenesis are very limited. These organelles are thought to form from multivesicular bodies and to acquire surfactant proteins (SPs), such as SP-A, SP-B, SP-C and phospholipids during LB maturation,19 but the mechanism is largely unknown. Although ABCA3 (phosphotidylcholine transporter) 20 and NPC1 21 were identified on mature LBs, their trafficking routes to LBs remain elusive. Overall, these studies clearly identified multiple cargoes and their transport pathways to different LROs. Nevertheless, the SNARE proteins that regulate membrane fusion during cargo delivery to maturing LROs are largely unknown.
SNAREs and their Known Regulators
Earlier studies classified the SNAREs into v (vesicle)-SNARE and t (target)-SNARE, which are associated with the transport or carrier vesicles and acceptor or target compartments, respectively. It has been observed that the donor or acceptor compartments contain more than one type of SNARE protein. To reduce the complexity, SNAREs were classified into Q-SNARE (Qa, Qb or Qc) and R-SNARE based on the crystal structure of the SNARE's complex. Bioinformatic analysis showed that syntaxin (STX) family SNAREs including SNAP23, 25 and 29 belong to the Q-SNARE family, whereas synaptobrevin and VAMP proteins belong to the R-SNARE family.8,22 In general, all SNAREs possess a conserved 60–70 amino acid SNARE motif with a C-terminal transmembrane domain, with the exception of a few SNAREs such as SNAP23, SNAP25, SNAP29, YKT6, STX11 8 and STX1C.23 During membrane fusion, cytosolic domains of Qa, Qb and Qc SNAREs on one membrane pair with R- SNARE on the opposing membrane and form trans-SNARE or SNAREpin complex. It is unknown how the SNAREs recognize each other before membrane fusion. Post fusion, the 4 SNAREs (cis-SNARE complex) on the target membrane will be disassembled by NSF and SNAP proteins, and later the SNAREs will be recycled back to the original membrane either in another active SNARE complex or in the form of inactive SNAREs.24 However, the mechanism of SNARE recycling has not been well studied.
Several Rab GTPases have been shown to recruit their effectors, such as tethering proteins, which are predicted to function in bringing the 2 membranes together and allowing the SNAREs to interact prior to membrane fusion.9,25 Moreover, SM (Sec1/Munc18-like) family proteins play regulatory roles in SNARE fusion events. These proteins bind to the N-terminus α-helical structure of STXs and form a closed SNARE conformation, which is thought to inhibit the non-specific SNARE interactions. It has also been suggested that SM proteins release the pre-associated SNARE (Qabc) complex for interaction with R-SNARE and then bind to the trans-SNARE complex in a calcium-dependent manner. This process possibly helps to stabilize the SNAREpin complex during the completion of membrane fusion. Synaptotagmins are another important type of regulators that bind to the C-terminus of SNAP proteins and sense the influx of calcium during calcium-triggered membrane fusion.10,24,26 Thus, every membrane fusion step possibly requires Rab and SM proteins in addition to SNAREs for accurate cargo delivery.
SNARES Regulate Cargo Delivery to Maturing LROs
As we discussed above, the type of cargo to be transported to different LROs is varied and specific to the organelle function.5 We predict that the machinery that regulates the delivery of different cargo to their respective LROs might be conserved, at least for some LROs. For example, BLOC-1 and -3 complexes are predicted to function in the biogenesis of multiple LROs such as melanosomes, dense granules and lamellar bodies.3,11 However, it is not clear whether the SNARE proteins are also conserved for the transport of cargo to these organelles. Moreover, the SNARE proteins involved in these transport pathways are uncharacterized. Nonetheless, recent studies have illustrated the role of several SNAREs in the transport to melanosomes. An initial study reported that B16 melanoma cells upregulate the expression of multiple SNAREs, such as STX7 (Qa), STX13 (Qa), VAMP7 (R) and VAMP8 (R) with an increase in melanogenesis,27 indicating a possible link between SNAREs and melanosome biogenesis. A more recent study demonstrated that STX13 (Qa) and VAMP7 (R) localize to the recycling endosomes and melanosomes, respectively, and regulate the transport of TYRP1 and TYR to melanosomes. Consistently, the depletion of either SNARE in melanocytes blocks melanosome maturation due to mistargeting of both TYRP1 and TYR to the lysosomes for degradation. Additional studies using live cell imaging and steady state localization have shown that VAMP7 mislocalizes to lysosomes in STX13-knockdown cells and the length of STX13-positive tubular structures is affected in VAMP7-knockdown cells, indicating that STX13 (Qa) and VAMP7 (R) are essential for melanosome biogenesis.28 Nevertheless, the cognate SNAREs Qb and Qc or Qbc of STX13 need to be identified in the future studies.
Are the SNAREs solely responsible for the specificity to the targeted membrane fusion/LRO maturation? Several in vitro membrane fusion assays demonstrated that SNAREs could mediate membrane fusion. However, studies in cellular systems have shown that the process of membrane fusion requires multiple factors such as Rab GTPases and tethering factors in addition to SM proteins. Likewise, several Rabs and their effectors, such as Rab38, Rab32 and VARP (effector of Rab38), have been shown to regulate cargo delivery to melanosomes.29,30 In addition, these Rab proteins have been shown to be activated by the GEF (guanine exchange factor) BLOC-3 complex (composed of HPS4 and HPS1 subunits), which is known to cause HPS disease. Additionally, the HPS4 subunit of BLOC-3 physically interacts with a late endosomal/lysosomal localized Rab9A.31,32 How these molecules regulate SNAREs during cargo delivery to melanosomes is an open question. Moreover, none of the tethering factors have been implicated in the biogenesis of melanosomes. To answer these questions, recent studies have attempted to understand the regulation between Rabs and SNAREs during cargo transport using 2 methods: (1) analysis of the tubular-vesicular structures of recycling endosomes by the live imaging of SNARE STX13 and (2) overexpression studies utilizing the dominant active mutant of STX13.
Analysis of tubular-vesicular structures of recycling endosomes in mouse melanocytes: STX13 has been characterized as a marker for recycling endosomes in non-melanocytes.33 However, in melanocytes, STX13 has been shown to regulate cargo transport to melanosomes, and STX13 cycles between recycling endosomes and melanosomes.28 Moreover, STX13-positive tubular structures in melanocytes originate from Rab5-positive early endosomes and colocalize with Rab11, which resembles the characteristics of recycling endosomes.34 In addition, immunoelectron microscopic (IEM) studies have shown that STX13-postive tubular structures possess melanosomal cargo 28,34 and can make contact with the maturing melanosome,35 suggesting that STX13-postive tubular structures mediate cargo delivery to melanosomes. Consistent with the IEM studies, live imaging studies of GFP-STX13 in wild type melanocytes showed that STX13 localized to long tubular structures that exist for a minute and make frequent contact with melanosomes.34 Moreover, a shorter length of STX13-positive tubular structures in melanocytes was indicative of less efficient cargo delivery or membrane fusion with melanosomes. In addition, it was assumed that the cargo present in these shorter tubular structures entered into other pathways from the recycling endosomes or, by default, these were targeted to the lysosomes. Interestingly, recent studies have shown the presence of shorter STX13-positive tubular structures having reduced contacts with melanosomes in BLOC-2 (composed of HPS3, 5 and 6 subunits)-deficient HPS cell lines. Moreover, the cargo (such as TYRP1) in BLOC-2-deficient melanocytes was accumulated in the Golgi, suggesting that BLOC-2 possibly functions either in stabilizing the longer STX13-positive tubular structures or is required for directing these tubular structures toward the melanosomes.34 The latter hypothesis is consistent with the observed phenotypes in BLOC-2-deficient melanocytes, in which the melanosomal proteins (TYRP1 but not TYR) were misrouted to the plasma membrane, endosomes and Golgi, and a cohort to lysosomes. Currently, it is unclear how BLOC-2 regulates the targeting of recycling endosomal tubules to the melanosomes.34 Additionally, shorter GFP-STX13 tubular structures similar to BLOC-2-deficient cells were also observed in BLOC-3 (composed of HPS1 and 4 subunits)-deficient HPS cell lines and in melanocytes depleted for Rab9A, Rab38, Rab32 or VARP molecules.13 However, in contrast to BLOC-2-defient melanocytes, the cargo in these cells was mislocalized to lysosomes. These results indicate that Rab9A, BLOC-3, Rab38/Rab32 and VARP possibly regulate SNAREs (see below) during membrane fusion between recycling endosomes and melanosomes (Fig. 1).13 Consistent with this hypothesis, Rab9A, Rab38, Rab32, VARP and BLOC-3 have been shown to partly localize to both of the organelles.13,29,30,32 Additionally, VARP has been shown to interact with the SNARE VAMP7 and regulate its SNARE activity.36
Overexpression of a constitutively active mutant of STX13 in mouse melanocytes:Recently, it has been shown that the deletion of the N-terminus (1–129 amino acids, STX13Δ129) or the regulatory domain alone (STX13Δ14-129) in STX13 increases both the SNARE activity and the cargo transport to melanosomes 28 in wild type melanocytes, indicating that these mutants function as constitutively active SNAREs inside the cell. Moreover, a recent study tested whether the Rab9A, Rab38 or Rab32 proteins could regulate the activity of these active STX13 mutants in mouse melanocytes. In this study, the authors overexpressed the constitutively active STX13 mutants in Rab-depleted melanocytes with the assumption that these SNARE mutants would rescue the hypopigmentation defects of the cells. Surprisingly, neither of the STX13 mutants rescued the pigmentation defects observed in Rab-knockdown melanocytes, suggesting that Rab9A, Rab38, and Rab32 are required for STX13 activity, most likely for the transport of cargo to melanosomes.13 Consistently, the N-terminal deleted STX13 mutants have been shown to localize to the melanosomes in wild type cells, whereas these mutants were absent from hypopigmented melanosomes in Rab9A-, Rab38- or Rab32-knockdown cells. Overall, these studies clearly suggest that Rabs and their effectors, VARP and possibly BLOC-2 (shown to interact with Rab38), regulate the biogenesis of LROs, such as melanosomes. It would be of great interest to determine the roles of these Rabs in the maturation of other LROs.
SNARE Recycling is Possibly Required for LRO Biogenesis
SNAREs acts as fusogens in any membrane fusion event. Therefore, the membrane localization of these proteins is stringently regulated both at the steady-state level and during membrane fusion. It has been hypothesized that post fusion, the cis-SNARE complex on the target membrane or an LRO would be disassembled and would allow the SNAREs to recycle back to their donor membrane or vesicle/tubular endosomal membrane (in the case of melanosome biogenesis). However, the perturbation of the recycling of SNAREs may possibly inhibit LRO maturation. Moreover, the mechanisms regulating SNARE recycling are not very well understood. Studies have shown that clathrin coat components, such as adaptor proteins (APs) interact with cargo, as well as with SNAREs (mostly with the N-terminal unstructured region), by recognizing canonical dileucine- or tyrosine-based motifs. Likewise, AP-1 regulates the recycling of VAMP4 (R) from endosomes to trans-Golgi through a dileucine motif.37 In addition, adaptor-related proteins, like GGA, epsins and AP-180/CALM have also been shown to interact with SNAREs. For example, epsinR, a monomeric adaptor required for the distribution of Vti1b (Qb) in HeLa cells and AP-180/CALM regulate the retrieval of synaptic proteins by interacting with synaptobrevin in neurons.38,39 Interestingly, AP-3 has been implicated in recycling the SNARE STX13 (Qa) from melanosomes to endosomes in melanocytes 40 and VAMP7 (R) in a cis-SNARE complex from early/recycling endosomes to late endosomes.41 Consistent with this hypothesis, the absence of expression of the AP-3 subunit, such as delta or beta3A, results in mislocalization of STX13 to melanosomes in melanocytes 28,40 and mislocalization of VAPM7 (in a cis-SNARE complex) to the Golgi in non-melanocytes. Furthermore, recycling of VAMP7 by AP-3 has been shown to be dependent on its N-terminal longin domain.42 Similarly, a recent study showed that AP-3 indirectly regulates the recycling of STX13 through a non-canonical binding motif other than the classical dileucine or tyrosine-based motifs in the protein.28 Thus, SNARE recycling is an important aspect of SNARE function and more of the underlying mechanisms need to be explored in the future.
SNAREs FOR LRO SECRETION OR EXOCYTOSIS
In neurons, the biogenesis of synaptic vesicles require BLOC-1 and AP-3 complexes for the delivery of cargo (neurotransmitters).43 Even through synaptic vesicles are not typical LROs, their mechanisms of secretion resemble LRO exocytosis or the release of LRO contents into the extracellular milieu.26 Secretion of LRO contents or exocytosis of the entire LRO are essential for their function. For example, melanocytes secrete melanosomes for skin pigmentation, lytic granules release their contents at the site of infection or upon T-lymphocyte signaling and platelets release their dense granule components at the site of vascular injury. Interestingly, the processes of both exocytosis and secretion also require specific SNAREs on the organelle and on the plasma membrane, which are predicted to be different than the SNAREs involved in cargo transport or biogenesis. Studies have shown that the fusion complex of neuronal exocytosis consists of the SNAREs STX1 (Qa) and synaptobrevin (R), which are anchored in membranes by their transmembrane domains, whereas SNAP-25 (Qbc) is tethered to the plasma membrane via several cysteine-linked palmitoyl chains.44 However, only few SNAREs responsible for the secretion/exocytosis of LROs are known. Studies have shown that the exocytosis of lamellar bodies through the plasma membrane is regulated by the SNAREs STX2 (Qa) and SNAP-23 (Qbc) and their co-factor, α-SNAP, on the plasma membrane and VAMP2 (R) on the LRO.45,46 Furthermore, the exocytosis of dense granules in platelets requires the SNAREs VAMP8 (R) on the LRO and STX11 (Qa), SNAP23 (Qbc) and Munc18b on the target/plasma membrane.47 Unlike the LRO, the exocytosis of insulin granules (a non-LRO) is regulated by STX1 (Qa) and SNAP25 (Qbc) on the plasma membrane and VAMP2 (R) on the vesicles.48 Moreover, similar to other membrane fusion events, the process of LRO secretion/exocytosis appears to be dependent on the influx of calcium at the site of fusion. Additionally, synaptotagmin family proteins have been described as calcium sensors during organelle exocytosis. For example, synaptotagmin I in neuronal cells 49 and synaptotagmin 7 in alveolar type-II cells 50 have been shown to act as calcium sensors for the exocytosis of synaptic vesicles and lamellar bodies, respectively. In addition, synaptotagmins interact with C-terminus of SNAP (Qbc SNARE) upon calcium influx and induce fusion by binding to the Qa and R SNAREs present on both membranes. Dense granule exocytosis is also dependent on calcium flux but the synaptotagmin or SNAP proteins that are involved have not yet been identified. Interestingly, studies have also shown that complexin, a repressor for the fusion machinery, has been shown to be modulated by interaction with synaptogamin. In the future, it will be crucial to identify the SNAREs that are responsible for the secretion of other LROs. Moreover, it would be of interest to determine whether the SNARE machinery involved in LRO exocytosis also functions in LRO biogenesis.
Unexpectedly, AP-3 has been shown to regulate the function of secretory lysosomes, where the deficiency of AP-3 results in enlarged secretory lysosomes with defects in microtubule-dependent movement during secretion. However, the sorting of lytic proteins to these granules was not affected in AP-3-deficient cells, suggesting that AP-3 is required to deliver the secretory lysosome contents through the plasma membrane. In contrast, the size and number of lamellar bodies in alveolar type II cells of chocolate (Rab38-knockout) mice are increased along with the levels of phosphatidylcholine and surfactant protein B in the lung tissues. Moreover, the malfunction in the secretion of lamellar bodies in these mice is possibly due to the decreased in alveolar space in the lung tissue, and subsequent cellular accumulation of these organelles.51 In addition, studies have shown that LRRK2, leucine-rich repeat kinase 2, regulates the lamellar body size and exocytosis in alveolar type II cells by modulating intracellular Ca2+ signaling upon stimulation with ATP.52 Thus, these studies illustrate that multiple regulatory mechanisms exist for the secretion or exocytosis of different LROs.
Future Direction and Prospects
Several Qb and Qc SNAREs have been implicated in melanosome biogenesis, but their precise roles need to be tested. Moreover, it is unknown whether the SNAREs STX13 and VAMP7, which have reported roles in melanosome biogenesis, regulate the formation of other LROs. In addition, the recycling mechanisms and functions of SNAREs in LRO secretion remain to be determined. Likewise, studying the process of SNARE sorting along with cargo sorting during vesicle formation will provide clues to the specificity of membrane fusion.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Wellcome Trust-DBT India Alliance Senior Fellowship (500122/Z/09/Z to S.R.G.S.), CEFIPRA Project (4903-1 to S.R.G.S. and G. Raposo), DBT-RNAi task-force (BT/PR4982/AGR/36/718/2012 to S.R.G.S), IISc-DBT partnership program (to S.R.G.S) and UGC fellowship (2120930821/2009 to R.A.J.).
REFERENCES
- 1.Gautreau A, Oguievetskaia K, Ungermann C. Function and regulation of the endosomal fusion and fission machineries. Cold Spring Harbor Perspect Biol 2014; 6:a016832; PMID:24591520; http://dx.doi.org/17878918 10.1101/cshperspect.a016832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harbor Perspect Biol 2014; 6:a016857; PMID:24851870; http://dx.doi.org/17878918 10.1101/cshperspect.a016857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raposo G, Marks MS. Melanosomes–dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol 2007; 8:786-97; PMID:17878918; http://dx.doi.org/ 10.1038/nrm2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol 2007; 19:394-401; PMID:17628466; http://dx.doi.org/ 10.1016/j.ceb.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol 2013; 25:495-505; PMID:23726022; http://dx.doi.org/ 10.1016/j.ceb.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell 2003; 112:519-33; PMID:12600315; http://dx.doi.org/ 10.1016/S0092-8674(03)00112-0 [DOI] [PubMed] [Google Scholar]
- 7.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 2006; 7:631-43; PMID:16912714; http://dx.doi.org/ 10.1038/nrm2002 [DOI] [PubMed] [Google Scholar]
- 8.Hong W. SNAREs and traffic. Biochim Biophys Acta 2005; 1744:120-44; PMID:15893389; http://dx.doi.org/ 10.1016/j.bbamcr.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 9.Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol 2011; 22:18-26; PMID:20643221; http://dx.doi.org/ 10.1016/j.semcdb.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 2009; 323:474-7; PMID:19164740; http://dx.doi.org/ 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet 2008; 9:359-86; PMID:18544035; http://dx.doi.org/ 10.1146/annurev.genom.9.081307.164303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitaram A, Marks MS. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology 2012; 27:85-99; PMID:22505665; http://dx.doi.org/ 10.1152/physiol.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahanty S, Ravichandran K, Chitirala P, Prabha J, Jani RA, Setty SR. Rab9A is required for delivery of cargo from recycling endosomes to melanosomes. Pigment Cell Melanoma Res 2015; 29:43-59; PMID:26527546; http://dx.doi.org/15582491 10.1111/pcmr.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol 2005; 17:87-94; PMID:15582491; http://dx.doi.org/ 10.1016/j.smim.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 15.Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J Cell Biol 1993; 120:885-96; PMID:8432729; http://dx.doi.org/ 10.1083/jcb.120.4.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youssefian T, Cramer EM. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood 2000; 95:4004-7; PMID:10845941 [PubMed] [Google Scholar]
- 17.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood 2012; 120:4072-81; PMID:22927249; http://dx.doi.org/ 10.1182/blood-2012-04-420745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng R, Wang Y, Yao Y, Zhang Z, Harper DC, Heijnen HF, Sitaram A, Li W, Raposo G, Weiss MJ, et al.. SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood 2012; 120:404-14; PMID:22611153; http://dx.doi.org/ 10.1182/blood-2011-11-389551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 2002; 13:263-70; PMID:12243725; http://dx.doi.org/ 10.1016/S1084952102000551 [DOI] [PubMed] [Google Scholar]
- 20.Ridsdale R, Na CL, Xu Y, Greis KD, Weaver T. Comparative proteomic analysis of lung lamellar bodies and lysosome-related organelles. PLoS One 2011; 6:e16482; PMID:21298062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roszell BR, Tao JQ, Yu KJ, Huang S, Bates SR. Characterization of the Niemann-Pick C pathway in alveolar type II cells and lamellar bodies of the lung. Am J Physiol Lung Cell Mol Physiol 2012; 302:L919-32; PMID:22367786; http://dx.doi.org/ 10.1152/ajplung.00383.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell 2007; 18:3463-71; PMID:17596510; http://dx.doi.org/ 10.1091/mbc.E07-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama T, Kamiguchi H, Akagawa K. Syntaxin 1C, a soluble form of syntaxin, attenuates membrane recycling by destabilizing microtubules. J Cell Sci 2012; 125:817-30; PMID:22421360; http://dx.doi.org/ 10.1242/jcs.081943 [DOI] [PubMed] [Google Scholar]
- 24.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged? Annu Rev Cell Dev Biol 2012; 28:279-308; PMID:23057743; http://dx.doi.org/ 10.1146/annurev-cellbio-101011-155818 [DOI] [PubMed] [Google Scholar]
- 25.Hong W, Lev S. Tethering the assembly of SNARE complexes. Trends Cell Biol 2014; 24:35-43; PMID:24119662; http://dx.doi.org/ 10.1016/j.tcb.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012; 490:201-7; PMID:23060190; http://dx.doi.org/ 10.1038/nature11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J Biol Chem 2001; 276:19820-7; PMID:11278762; http://dx.doi.org/ 10.1074/jbc.M010838200 [DOI] [PubMed] [Google Scholar]
- 28.Jani RA, Purushothaman LK, Rani S, Bergam P, Setty SR. STX13 regulates cargo delivery from recycling endosomes during melanosome biogenesis. J Cell Sci 2015; 128:3263-76; PMID:26208634; http://dx.doi.org/ 10.1242/jcs.171165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell 2009; 20:2900-8; PMID:19403694; http://dx.doi.org/ 10.1091/mbc.E08-12-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J Biol Chem 2012; 287:19550-63; PMID:22511774; http://dx.doi.org/ 10.1074/jbc.M112.351908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kloer DP, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem 2010; 285:7794-804; PMID:20048159; http://dx.doi.org/ 10.1074/jbc.M109.069088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol 2012; 22:2135-9; PMID:23084991; http://dx.doi.org/ 10.1016/j.cub.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol 1998; 143:957-71; PMID:9817754; http://dx.doi.org/ 10.1083/jcb.143.4.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Delevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, et al.. BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J Cell Biol 2015; 209:563-77; PMID:26008744; http://dx.doi.org/ 10.1083/jcb.201410026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJ, Verkleij AJ, Salamero J, Marks MS, et al.. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol 2009; 187:247-64; PMID:19841138; http://dx.doi.org/ 10.1083/jcb.200907122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schafer IB, Hesketh GG, Bright NA, Gray SR, Pryor PR, Evans PR, Luzio JP, Owen DJ. The binding of Varp to VAMP7 traps VAMP7 in a closed, fusogenically inactive conformation. Nat Struct Mol Biol 2012; 19:1300-9; PMID:23104059; http://dx.doi.org/ 10.1038/nsmb.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peden AA, Park GY, Scheller RH. The Di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J Biol Chem 2001; 276:49183-7; PMID:11598115; http://dx.doi.org/ 10.1074/jbc.M106646200 [DOI] [PubMed] [Google Scholar]
- 38.Miller SE, Collins BM, McCoy AJ, Robinson MS, Owen DJ. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature 2007; 450:570-4; PMID:18033301; http://dx.doi.org/ 10.1038/nature06353 [DOI] [PubMed] [Google Scholar]
- 39.Koo SJ, Puchkov D, Haucke V. AP180 and CALM: Dedicated endocytic adaptors for the retrieval of synaptobrevin 2 at synapses. Cell Logist 2011; 1:168-72; PMID:22279617; http://dx.doi.org/ 10.4161/cl.1.4.18897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, et al.. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell 2007; 18:768-80; PMID:17182842; http://dx.doi.org/ 10.1091/mbc.E06-12-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hesketh GG, Perez-Dorado I, Jackson LP, Wartosch L, Schafer IB, Gray SR, McCoy AJ, Zeldin OB, Garman EF, Harbour ME, et al.. VARP Is Recruited on to Endosomes by Direct Interaction with Retromer, Where Together They Function in Export to the Cell Surface. Dev Cell 2014; 29:591-606; PMID:24856514; http://dx.doi.org/ 10.1016/j.devcel.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent HM, Evans PR, Schafer IB, Gray SR, Sanderson CM, Luzio JP, Peden AA, Owen DJ. Structural basis of the intracellular sorting of the SNARE VAMP7 by the AP3 adaptor complex. Dev Cell 2012; 22:979-88; PMID:22521722; http://dx.doi.org/ 10.1016/j.devcel.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell'Angelica EC, Peden AA, Werner E, et al.. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell 2006; 17:4014-26; PMID:16760431; http://dx.doi.org/ 10.1091/mbc.E06-02-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 1998; 395:347-53; PMID:9759724; http://dx.doi.org/ 10.1038/26412 [DOI] [PubMed] [Google Scholar]
- 45.Abonyo BO, Gou D, Wang P, Narasaraju T, Wang Z, Liu L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry 2004; 43:3499-506; PMID:15035620; http://dx.doi.org/ 10.1021/bi036338y [DOI] [PubMed] [Google Scholar]
- 46.Wang P, Howard MD, Zhang H, Chintagari NR, Bell A, Jin N, Mishra A, Liu L. Characterization of VAMP-2 in the lung: implication in lung surfactant secretion. Cell Biol Int 2012; 36:785-91; PMID:22571236; http://dx.doi.org/ 10.1042/CBI20110146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marks MS. SNARing platelet granule secretion. Blood 2012; 120:2355-7; PMID:22996656; http://dx.doi.org/ 10.1182/blood-2012-07-442756 [DOI] [PubMed] [Google Scholar]
- 48.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm 2009; 80:473-506; PMID:19251047; http://dx.doi.org/ 10.1016/S0083-6729(08)00616-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saraswati S, Adolfsen B, Littleton JT. Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release. Proc Natl Acad Sci U S A 2007; 104:14122-7; PMID:17709738; http://dx.doi.org/ 10.1073/pnas.0706711104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuland K, Sharma N, Frick M. Synaptotagmin-7 links fusion-activated Ca(2)(+) entry and fusion pore dilation. J Cell Sci 2014; 127:5218-27; PMID:25344253; http://dx.doi.org/ 10.1242/jcs.153742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011; 301:L461-77; PMID:21764986; http://dx.doi.org/ 10.1152/ajplung.00056.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miklavc P, Ehinger K, Thompson KE, Hobi N, Shimshek DR, Frick M. Surfactant secretion in LRRK2 knock-out rats: changes in lamellar body morphology and rate of exocytosis. PLoS One 2014; 9:e84926; PMID:24465451; http://dx.doi.org/ 10.1371/journal.pone.0084926 [DOI] [PMC free article] [PubMed] [Google Scholar]


