Abstract
Objective
To evaluate the treatment effect of collagenase Clostridium histolyticum (CCH) in a rat model of urethral fibrosis.
Materials and Methods
Thirty male Sprague-Dawley rats (300-350 g) were divided into 5 groups. The rat urethra was injected with normal saline in the sham group and, in the other 4 groups, the rat urethra was injected with 10 μg of transforming growth factor beta 1 to create fibrosis of the urethra. Two weeks following transforming growth factor beta 1 injection, the rats were injected with varying doses of CCH or vehicles, depending on their group. The rats were then euthanized at 4 weeks after CCH or vehicle injection. Urethral tissue was harvested for histologic and molecular analyses. Type I and III collagen levels were evaluated by Western blot analysis.
Results
There was urethral fibrosis and to significant increase in collagen type I and III expressions in the urethral fibrosis group compared with the sham group (P <.05). Urethral injection of CCH appeared to be safe and significantly reduce urethral fibrosis as well as collagen type I and III expressions in the high-dose CCH treatment groups when compared with the treatment control group (P <.01).
Conclusion
This study demonstrated a beneficial effect of CCH injections in a rat model of urethral fibrosis. These findings suggest a potential role for CCH as a therapeutic option in urethral stricture patients and warrant further investigation.
The term “urethral stricture” refers to a scarring process of the urethral mucosa and the surrounding spongy tissue of the corpus spongio-sum.1 The incidence of urethral stricture disease has been estimated to be as high as 0.6% in a population of older veterans, with an estimated 5000 inpatient visits yearly and 1.5 million office visits recorded between 1992 and 2000.2 An increase in type I and III collagens leads to spongiofibrosis, scar contraction, and narrowing of the urethral lumen.3,4 This prevents normal urination and is associated with potential lower urinary tract complications including acute urinary retention, renal failure, urethral carcinoma, Fournier's gangrene, and bladder failure due to long-standing lower urinary tract obstruction.2 There are currently a number of treatment options for urethral stricture disease, which include urethral dilatation, direct visual internal urethrotomy (DVIU), and open urethroplasty. Patient symptoms and quality of life significantly improve after successful treatment.5,6 Unfortunately, successful outcomes are not always achieved. Stricture-free rates from DVIU vary from 26.9% to 68% following the first attempt, but can diminish with repeated endoscopic attempts.7-9 Furthermore, the overall recurrence rate for all reconstructive procedures is as high as 15.6% depending on the location, length, and type of stricture.10 Patients with recurrent urethral strictures can be very challenging to reconstructive urologists.
Collagenase Clostridium histolyticum (CCH) (Xiaflex; Auxilium, Chesterbrook, PA) has been Food and Drug Administration (FDA)-approved for the nonsurgical treatment of Peyronie's disease and Dupuytren's contracture. Peyronie's disease is a fibrotic wound-healing disorder of the tunica albuginea of the corpus cavernosum. There are recognized links between urethral stricture formation and Peyronie's disease. Though affecting different locations, both are recognized as fibrotic proliferative conditions. Any trauma to the urethral mucosa can lead to urethral stricture disease, and, similarly, trauma to the tunica albugenia is a widely accepted hypothesis for the development of Peyronie's disease. Furthermore, both conditions are characterized by the formation of fibrous tissue with the same types of collagen.3,4,12,13 CCH can degrade all types of collagen as demonstrated in porcine dermal connective tissue models.14 As such, because of the similarities in the molecular type and/or expression of collagen, as well as the common pathophysiology, we postulated that CCH may have therapeutic benefit for the treatment of urethral stricture disease or can be used as an adjunctive treatment for the management of recurrent urethral stricture disease.
Materials and Methods
General Protocol in Rats
All experiments were performed according to the American Guidelines for the Ethical Care of Animals and were approved by our Institutional Animal Care and Use Center Committee. A total of 30 adult male Sprague-Dawley rats (300-350 g) were purchased from Harlan Laboratories (Indianapolis, IN) and housed in a regulated environment with a 12-hour light and dark cycle in an approved experimental laboratory. The animals had free access to food and water. Animals were randomized into 5 equal groups: (1) sham (saline injection to urethra); (2) control urethral fibrosis; (3) vehicle (treatment control group); (4) low-dose CCH (0.05 mg CCH); (5) high-dose CCH (0.1 mg CCH). Rats in groups (1) and (2) were sacrificed at 2 weeks following injection of saline or transforming growth factor beta 1 (TGF-β1; Aviscera Bioscience, Santa Clara, CA). Rats in groups (3)-(5) were anesthetized again 2 weeks following TGF-β1 injection for vehicle or CCH injection. Rats in group (3) were injected with calcium chloride solution, which was used as diluent of CCH and sacrificed at 4 weeks following vehicle injection. Rats in groups (4) and (5) were injected with different doses of CCH at 2 weeks following TGF-β1 injection and sacrificed at 4 weeks following CCH injection. Urethral tissues for all groups were harvested and stored at −80°C for further analysis. Histological assessment of urethral tissues was performed. Type I and III collagen levels were evaluated by Western blot analysis.
Anesthesia and Surgical Procedure
Each rat was anesthetized with 100/10 mg/kg ketamine (NWI Veterinary Supply, Boise, ID)/xylazine (Akorn, Decatur, IL) intraperitoneally. The rats were placed in the supine position, and the penile skin was retracted to expose the penis. A lubricated urinary catheter (polyethylene tube, 0.61 mm in diameter [equal to 1.8 French]) was gently inserted to facilitate urethral exposure and prevent urethral injury. Using a 5 mm penoscrotal incision, the rat urethra was meticulously dissected and exposed under a magnification lens. Using a 30-gauge needle, 0.05 mL of normal saline (sham group) was injected into spongiosal tissue at the 3 and 9 o'clock positions of the urethra. TGF-β1(10 μg) was injected by the same technique for the other 3 groups. Nonabsorbable 5-0 suture was placed into the corpus cavernosum at the same level of the urethral injection site to serve as a landmark for future site identification. The catheter was removed and the penile skin was approximated with 4-0 interrupted absorbable sutures.
Two weeks following the normal saline or TGF-β1 injections, the rats in groups (1) and (2) were euthanized by injection of 3 mL of 2.6% potassium chloride. The urethral tissues were harvested and stored. The rats in groups (3)-(5) were anesthetized with ketamine and/or xylazine and the penoscrotal incision was recreated. Varying doses of CCH (0.05 or 0.1 mg) or vehicle were injected into spongiosal tissue at the same site of the first injection. The penile skin was approximated with 4-0 interrupted sutures. Four weeks following CCH or vehicle injection, the rats were euthanized. The urethral tissues were harvested and stored for further analysis. The same investigator performed all injections and sacrifice procedures.
Histological Evaluation
Cross-sections of urethral tissue samples were fixed in 10% buffered neutral formalin and processed by routine histological methods. Multiple 4-6 μm thick sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome (MT). The degree of fibrosis (minimal, mild, moderate, and severe) was evaluated as previously described.15 The same pathologist, who was blinded to the study design, reviewed all microscopic sections. Histological examination and microphotographs were performed using a digital camera (Leica EC3, Leica Microsystems, Heerbrugg, Switzerland) coupled to an optical microscope (Leica Model DM 2500; Leica Microsystems CMS, Weltzar, Germany).
Western Blot Assay for Collagen Expression
Urethral tissue protein samples were prepared by homogenizing in radioimmunoprecipitation assay (RIPA) lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Protein contents were assessed using the Bradford assay (Bio-Rad, Hercules, CA). Western blot analyses were conducted as previously described.16 Briefly, samples containing 20 mg of protein were fractionated onto sodium dodecyl sulphate-polyarylamide gel electrophoregis (SDS-PAGE) on 4%-20% gradient gels, and the separated proteins were transferred to polyvinylidene diflouride membranes. After blocking with 5% nonfat milk in PBS + 0.05% Tween 20, the membranes were probed with antibodies to collagen type I, collagen type III (all purchased from Abcam, Cambridge, MA), and glyceraldehyde 3-phosphate dehydrogenase (Cell Signaling Technology, Inc., Beverly, MA). Immune complexes were detected with the appropriate secondary antibodies and chemiluminescence reagents (Pierce, Rockford, IL).
Statistical Analysis
Statistical analysis was performed with Prism 5.0 (GraphPad Software, San Diego, CA). All data were expressed as means (SD) and the differences between multiple groups were compared by one-way analysis of variance, followed by the Tukey multiple comparisons test (P <.05 was considered statistically significant).
Results
The effect of CCH on the rat model was investigated by H&E stain, MT stain, and Western blot analysis at low dose and high dose compared with the treatment control (vehicle) injection. No technical problems were encountered during the study period. One rat in the high-dose CCH group developed a wound hematoma after CCH injection. This rat, however, tolerated the hematoma well until the end point of the study without any urethral or voiding complications. No urethral fistulae or episodes of urinary retention occurred during the post-injection period in any of the rats.
Comparative microscopic evaluation of representative H&E and MT-stained urethral cross-sections revealed a normal urethral structure without fibrosis in the sham group. In contrast, there was moderate fibrosis with densely packed collagenous stroma involving submucosal tissue in the fibrosis group. Mild submucosal fibrosis was detected in the vehicle group and in the low-dose CCH group. There was, however, minimal submucosal fibrosis in the high-dose CCH group (Fig. 1).
Figure 1.
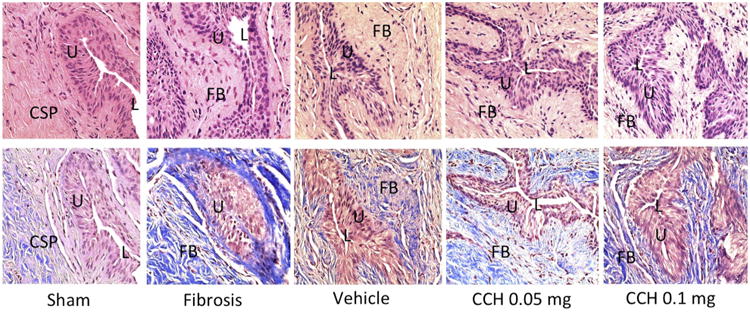
Histology of rat urethral tissue sections (top-H&E stain, bottom-MT stain-Bottom, ×400) demonstrating normal urethral lumen surrounded by normal distribution of collagen bundles and smooth muscle cells without fibrosis in the sham group, moderate fibrosis with densely packed collagenous stroma involving submucosal tissue in the urethral fibrosis group, mild submucosal fibrosis in the vehicle group, mild submucosal fibrosis in the low-dose CCH group, and minimal fibrosis in the high-dose CCH group. CSP, Corpus spongiosum; FB, fibrosis; L, Lumen; U, urothelium.
Western blot analysis in Figure 2 demonstrated significantly increased expression of type I and III collagens in the fibrosis group when compared with the sham group. Densitometry revealed collagen band intensities representing sham, which were significantly different from the fibrosis group, and both CCH treatment and vehicle groups (P <.05). There was a significant decrease in collagen type I and III expressions in both low-dose and high-dose CCH groups compared with the urethral fibrosis group (P <.05). This decrease in the expression of collagen in the CCH groups was manifested in a dose-dependent manner (P <.05). Also, the reduction of collagen type I and III expressions was more remarkable in the high-dose CCH group when compared with the vehicle group (P <.01). Finally, the collagen expression from the Western blot analysis corroborated the histologic findings.
Figure 2.
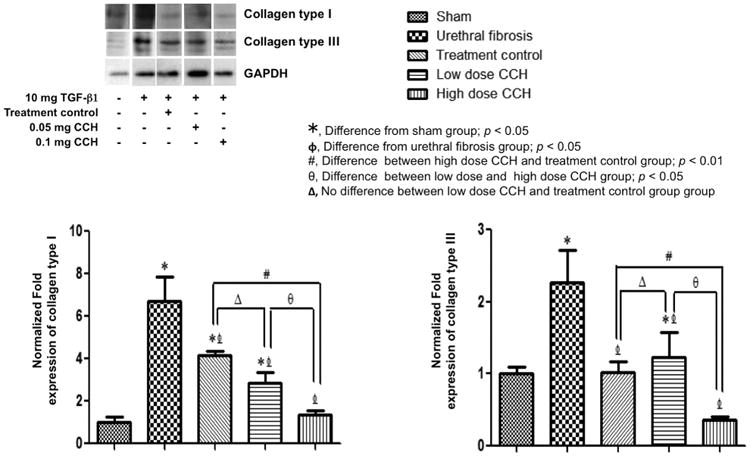
Representative Western blot demonstrating significant decreased collagen type I and type III in high-dose CCH groups compared with the treatment control group. Densitometry demonstrating relative density values of collagen proteins expressed as protein expression relevant to GAPDH control. Results are expressed as means ± standard errors and denoted as P <.05 and P <.01 when compared with sham, urethral fibrosis, and treatment control group, respectively. CCH, collagenase Clostridium histolyticum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Comment
The present study supports a role for CCH injection as a possible treatment option for urethral stricture disease. In the study, rats were treated with CCH in a TGF-β1 model of urethral fibrosis. TGF-β1 is a profibrotic factor that has been documented to play an important role in the pathophysiology of Peyronie's disease and other fibrotic diseases such as pulmonary fibrosis, systemic sclerosis, kidney fibrosis, etc.11,17 In our previous experiments, we were able to show that a TGF-β1 injection into the urethral wall produced a urethral fibrosis-like condition in the rat.15 These results had demonstrated collagen deposition in the urethral wall with a predominance of type I and type III collagens. Similar findings have been observed in cases of human urethral stricture disease.15 Our results also demonstrated that rats treated with high-dose CCH after TGF-β1 injection displayed a significantly decreased amount of fibrosis and collagen expression when compared with the treatment control group. Although we have shown that the effect of TGF-β1 in inducing urethral fibrosis may itself decrease with time, this decrease is not as significant as that seen in the high-dose CCH-treated rats.
CCH is FDA-approved as a nonsurgical treatment of 2 fibrotic diseases: Dupuytren's contracture and Peyronie's disease. CCH consists of 2 collagenases that cleave collagen strands at different sites. AUX I (a class I C. histolyticum collagenase) cleaves the terminal ends of collagen, and AUX II (a class II C. histolyticum collagenase) cleaves internal sections of collagen.18 In a study of Dupuytren's contracture, CCH hydrolyzed collagen, weakening the contracted cord and improving elasticity and the range of motion.19,20 In Peyronie's disease studies, CCH imparted both a 34% reduction in penile curvature, and a significant improvement in the Peyronie's disease symptom bother score.21
Both urethral stricture disease and Peyronie's disease are fibrotic conditions with increased expression of the same types of scar-forming collagen.3,4,12,13 To our knowledge, this is the first study to investigate the role of CCH in an animal model of urethral fibrosis.
Treatment outcomes for urethral stricture disease vary according to treatment options. Stricture-free rates after DVIU can vary from 26.9% to 68% following the first attempt but can diminish with repeated endoscopic attempts.7-9 “In an attempt to decrease recurrence rates following stricture management, intraurethral captopril gel and local injection of steroids and mitomycin C have been investigated. Captopril is an angiotensin-converting enzyme-inhibitor with known antifibrotic properties secondary to its inhibition of the TGF-β1 pathway.22 Shirazi et al reported significant decreases in recurrence rates after DVIU in patients who received 0.1% and 0.5% captopril gel intraurethrally compared to the placebo group.23 No further studies, however, have been reported to confirm this finding. Steroids have the ability to decrease tissue fibrosis by decreasing collagen and glycosaminoglycans synthesis, increasing collagenase production, and reducing levels of collagenase inhibitors.24 They can be applied locally by injection or lubrication. Unfortunately, a recent meta-analysis on the outcomes of local steroid injection or lubrication after DVIU did not demonstrate any significant improvements in recurrence rates after intervention. It did, however, significantly delay the time to recurrence in urethral stricture patients undergoing DVIU.25”
Adjunct mitomycin C injection significantly reduced the recurrent rates after internal urethrotomy in anterior urethral stricture patients.26 Mitomycin C, however, is considered a chemotherapeutic agent, which raises the concern of local toxicity to adjacent tissues.27 Redshaw et al have reported rectourethral fistula and necrosis of bladder neck following intralesional injection of mitomycin C during transurethral injection of the bladder neck contracture.28 As such, we believe that CCH may be a safer alternative adjunctive treatment option for the endoscopic management of urethral stricture disease. Urethroplasty is accepted to be a gold standard for definitive treatment of urethral stricture disease.29 We do not suggest that CCH injection will be a replacement for urethroplasty, but this treatment may be used as a combination treatment to improve treatment outcomes or may be suitable in postsurgical patients with localized disease. Further animal and clinical studies are, however, needed to confirm our current findings.
In Peyronie's disease, CCH is contraindicated in patients who have Peyronie's plaques that involve the penile urethra due to the potential risk of damage to the urethra. The aforementioned study by Rydevik et al showed that CCH had no significant detrimental effects on nerve or vascular tissue in animal models.30,31 As a matter of fact, injection of CCH directly into the induced fibrosis rat urethra caused no severe adverse events specific to the urethra. Only one rat developed penile hematoma, which is a self-limited adverse event in the Peyronie's disease patients treated with CCH. Hence, we may further assume that CCH should not be contra-indicated in patients who have ventral Peyronie's plaques that involve the penile urethra. Further clinical studies are needed to further confirm this hypothesis.
This study was limited by the use of an animal model. The improvement in the abnormal collagen content in the rat urethral specimens might not directly translate to improvements in symptoms or clinical outcomes in the urethral stricture patients. Even though the effect of TGF-β1 in inducing fibrosis might decrease with time, in a previous TGF-β1 study, significant fibrosis lasted as long as 45 days after TGF-β1 injection.32 Furthermore, more studies are warranted to demonstrate any benefit with CCH in conditions associated with dense or severe spongiofibrosis. Finally, even though our results demonstrated no severe adverse events to the rat urethra, one must acknowledge potential risks associated with any injection into the urethra such as fistulae, necrosis, increased fibrosis, and damage to the adjacent corpus cavernosum. Further studies are certainly needed to confirm the safety profile of this drug injected into the urethra. The present study, however, provides a proof of concept and suggests a promising role for CCH in the nonsurgical management of this condition.
Conclusion
CCH injection decreases collagen expression and urethral fibrosis in a rat model of urethral fibrosis. Furthermore, injection of CCH into an induced fibrotic urethra did not incur any serious adverse complications to the urethra. These findings suggest a potential role for CCH as a therapeutic option for urethral stricture patients and warrants further investigation.
Acknowledgments
Dr Hellstrom has received lecture honoraria and research grants from, has been a consultant to, and has been on advisory boards for Abbvie, Allergan, AMS, Antares, Apricus, Astellas, Auxilium (grant 552666), Coloplast, Endo, Lilly, NERI, NIH (Board member), Pfizer, Promescent, Repros, and Theralogix (Board member).
Funding Support: This study was supported in part by funding from Auxilium Pharmaceuticals, Inc, Malvern, PA, Department of Urology Andrology Fund, Tulane University School of Medicine and Tulane National Primate Research Center (TNPRC) base grant (grant number: P51OD011104). Premsant Sangkum is a research fellow of Andrology, Department of Urology, Tulane University School of Medicine and received an international mobility grant from Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand.
Footnotes
Financial Disclosure: The remaining authors declare that they have no relevant financial interests.
References
- 1.Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol. 2009;182:983–987. doi: 10.1016/j.juro.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol. 2007;177:1667–1674. doi: 10.1016/j.juro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcanti AG, Costa WS, Baskin LS, et al. A morphometric analysis of bulbar urethral strictures. BJU Int. 2007;100:397–402. doi: 10.1111/j.1464-410X.2007.06904.x. [DOI] [PubMed] [Google Scholar]
- 4.Da-Silva EA, Sampaio FJ, Dornas MC, et al. Extracellular matrix changes in urethral stricture disease. J Urol. 2002;168:805–807. [PubMed] [Google Scholar]
- 5.Barbagli G, De Angelis M, Romano G, Lazzeri M. Clinical outcome and quality of life assessment in patients treated with perineal urethrostomy for anterior urethral stricture disease. J Urol. 2009;182:548–557. doi: 10.1016/j.juro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Veeratterapillay R, Pickard RS. Long-term effect of urethral dilatation and internal urethrotomy for urethral strictures. Curr Opin Urol. 2012;22:467–473. doi: 10.1097/MOU.0b013e32835621a2. [DOI] [PubMed] [Google Scholar]
- 7.Albers P, Fichtner J, Bruhl P, Muller SC. Long-term results of internal urethrotomy. J Urol. 1996;156:1611–1614. [PubMed] [Google Scholar]
- 8.Tunc M, Tefekli A, Kadioglu A, et al. A prospective, randomized protocol to examine the efficacy of postinternal urethrotomy dilations for recurrent bulbomembranous urethral strictures. Urology. 2002;60:239–244. doi: 10.1016/s0090-4295(02)01737-5. [DOI] [PubMed] [Google Scholar]
- 9.Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol. 1996;156:73–75. [PubMed] [Google Scholar]
- 10.Meeks JJ, Erickson BA, Granieri MA, Gonzalez CM. Stricture recurrence after urethroplasty: a systematic review. J Urol. 2009;182:1266–1270. doi: 10.1016/j.juro.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 11.El-Sakka AI, Hassoba HM, Pillarisetty RJ, et al. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997;158:1391–1394. [PubMed] [Google Scholar]
- 12.Somers KD, Sismour EN, Wright GL, Jr, et al. Isolation and characterization of collagen in Peyronie's disease. J Urol. 1989;141:629–631. doi: 10.1016/s0022-5347(17)40920-7. [DOI] [PubMed] [Google Scholar]
- 13.Luangkhot R, Rutchik S, Agarwal V, et al. Collagen alterations in the corpus cavernosum of men with sexual dysfunction. J Urol. 1992;148:467–471. doi: 10.1016/s0022-5347(17)36630-2. [DOI] [PubMed] [Google Scholar]
- 14.Friedman K, Pollack SV, Manning T, Pinnell SR. Degradation of porcine dermal connective tissue by collagenase and hyaluronidase. Br J Dermatol. 1986;115:403–408. doi: 10.1111/j.1365-2133.1986.tb06234.x. [DOI] [PubMed] [Google Scholar]
- 15.Sangkum P, Gokce A, Tan RB, et al. TGF-beta1 induced urethral fibrosis in a rat model. J Urol. 2015;194:820–827. doi: 10.1016/j.juro.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Naura AS, Errami Y, et al. Cordycepin blocks lung injury-associated inflammation and promotes BRCA1-deficient breast cancer cell killing by effectively inhibiting PARP. Mol Med. 2011;17:893–900. doi: 10.2119/molmed.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 18.French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681–687. doi: 10.1021/bi00377a004. [DOI] [PubMed] [Google Scholar]
- 19.Starkweather KD, Lattuga S, Hurst LC, et al. Collagenase in the treatment of Dupuytren's disease: an in vitro study. J Hand Surg Am. 1996;21:490–495. doi: 10.1016/S0363-5023(96)80368-6. [DOI] [PubMed] [Google Scholar]
- 20.Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 21.Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel JL, Jr, Sundell CL, Wang S, et al. Role of angiotensin II in the expression and regulation of transforming growth factor-beta in obstructive nephropathy. Kidney Int. 1995;48:1233–1246. doi: 10.1038/ki.1995.407. [DOI] [PubMed] [Google Scholar]
- 23.Shirazi M, Khezri A, Samani SM, et al. Effect of intraurethral captopril gel on the recurrence of urethral stricture after direct vision internal urethrotomy: Phase II clinical trial. Int J Urol. 2007;14:203–208. doi: 10.1111/j.1442-2042.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Qi E, Zhang Y, et al. Efficacy and safety of local steroids for urethra strictures: a systematic review and meta-analysis. J Endourol. 2014;28:962–968. doi: 10.1089/end.2014.0090. [DOI] [PubMed] [Google Scholar]
- 26.Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol. 2007;51:1089–1092. doi: 10.1016/j.eururo.2006.11.038. discussion 1092. [DOI] [PubMed] [Google Scholar]
- 27.Mundy AR. Adjuncts to visual internal urethrotomy to reduce the recurrence rate of anterior urethral strictures. Eur Urol. 2007;51:1467–1468. doi: 10.1016/j.eururo.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 28.Redshaw JD, Broghammer JA, Smith TG, 3rd, et al. Intralesional injection of mitomycin C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS Study Group. J Urol. 2015;193:587–592. doi: 10.1016/j.juro.2014.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampson LA, McAninch JW, Breyer BN. Male urethral strictures and their management. Nat Rev Urol. 2014;11:43–50. doi: 10.1038/nrurol.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rydevik B, Brown MD, Ehira T, Nordborg C. Effects of collagenase on nerve tissue. An experimental study on acute and long-term effects in rabbits. Spine. 1985;10:562–566. doi: 10.1097/00007632-198507000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Rydevik B, Ehira T, Linder L, et al. Microvascular response to locally injected collagenase. An experimental investigation in hamsters and rabbits. Scand J Plast Reconstr Surg Hand Surg. 1989;23:17–21. doi: 10.3109/02844318909067503. [DOI] [PubMed] [Google Scholar]
- 32.Gokce A, Abd Elmageed ZY, Lasker GF, et al. Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie's disease. Andrology. 2014;2:244–251. doi: 10.1111/j.2047-2927.2013.00181.x. [DOI] [PubMed] [Google Scholar]


