Abstract
Aging induces structural and functional changes in the heart that are associated with increased risk of cardiovascular disease and impaired functional capacity in the elderly. Exercise is a diagnostic and therapeutic tool, with the potential to provide insights into clinical diagnosis and prognosis, as well as the molecular mechanisms by which aging influences cardiac physiology and function. In this review, we first provide an overview of how aging impacts the cardiac response to exercise and the implications this has for functional capacity in older adults. We then review the underlying molecular mechanisms by which cardiac aging contributes to exercise intolerance, and conversely how exercise training can potentially modulate aging phenotypes in the heart. Finally, we highlight the potential use of these exercise models to complement models of disease in efforts to uncover new therapeutic targets to prevent or treat heart disease in the aging population.
Keywords: exercise, aging, cardiovascular disease
Introduction
Improvements in public health over the past century have led to dramatic increases in life expectancy. By 2030, adults over the age of 65 will account for nearly 20% of the general population in the US1. Moreover, the oldest demographic groups, consisting of individuals 85 years or older, now represent the fasting growing segment in the US, and are estimated to increase by more than 230% by 20501. Thus understanding the factors limiting health and quality of life in the elderly will be increasingly important over the coming years. Among these factors, cardiovascular disease represents the leading cause of mortality in the elderly, accounting for nearly 40% of all deaths1, 2. Heart failure (HF), in particular, is reaching epidemic proportions with approximately 88% of HF deaths and greater than 75% of all HF hospitalizations in the US now occurring in adults 65 years or older3.
While advanced age is considered a major independent risk factor for HF, the mechanisms by which aging predisposes older adults to HF are not completely understood. The cumulative impact of repeated insults and injuries (i.e. myocardial infarctions, hypertension) to the heart throughout its lifetime is undoubtedly an important contributor to maladaptive myocardial remodeling and the development of HF in the elderly. However, there are also factors intrinsic to cardiac aging, occurring at a cellular and molecular level, which may impair the overall function of the heart as it approaches senescence4–6.
While it can be difficult to completely separate extrinsic from intrinsic factors in cardiac aging given the interactions between aging, disease, and environment, genome-wide transcriptome analyses of whole hearts and isolated cardiomyocytes from healthy young and old mice have provided some insights into the molecular mechanisms of aging in the heart. Specific transcriptional alterations in pathways related to stress response, mitochondrial function, fatty acid metabolism, contractility, hypertrophy, inflammation, and extracellular matrix production have been identified as key molecular phenotypes of cardiac aging, which interestingly parallel many of the transcriptional changes that occur in the failing heart7, 8. Although the aged heart is generally capable of meeting the basal energy requirements of the body, its performance under physiologic and/or pathologic stress can be significantly impaired, which can lead to exercise intolerance and dyspnea, the primary symptoms of HF. Thus, defining the mechanisms by which aging affects the heart’s ability to respond to stressful stimuli becomes integral to understanding the role of aging in HF pathophysiology.
Exercise, as an inducible form of physiologic stress, represents a powerful tool in cardiac aging research for this very reason. Exercise physiology has provided a wealth of knowledge into how age-related changes in cardiac structure and function translate to decreased exercise capacity9, 10, a strong determinant of HF prognosis, quality of life, and mortality in the elderly11, 12. Furthermore, exercise-based studies in aged animal models are now beginning to identify the molecular mechanisms of cardiac aging that contribute to exercise intolerance, and intriguingly suggest that exercise, as a therapeutic intervention, can potentially mitigate or reverse some aspects of the aging process in the heart.
Both aging and exercise are complex systemic processes that influence nearly every facet of the cardiovascular system. Here we primarily focus on the role of exercise in the aged heart, with an emphasis on cardiomyocyte biology. We first provide an overview of how aging impacts the cardiac response to acute exercise and the implications this has on declining functional capacity in the elderly. We then review the literature on exercise in aged animals, highlighting the major molecular mechanisms by which cardiomyocyte aging is thought to contribute to exercise intolerance and how exercise training potentially modulates these properties in the aged heart. Finally, given the parallels in exercise and cardiac phenotypes observed in advanced age and HF, we discuss the potential implications of these findings in the context of the growing HF epidemic in the aging population.
Effects of aging on the cardiac response to exercise
Exercise exerts a physiologic stress on the body, requiring a coordinated response by the cardiovascular, pulmonary, and nervous systems to increase blood flow and oxygen supply to the working skeletal muscle. At rest, muscle receives approximately 20% of the total blood flow, but during exercise, this can increase to over 80%13. Thus, impairments in any of these systems can lead to significant decreases in peak cardiac output (CO) and overall exercise capacity.
The heart’s contributions to augmenting CO in response to the increased metabolic demands of exercise have been well characterized, and essentially depend on dynamic regulation of two physiological parameters, heart rate (HR) and stroke volume (SV). In healthy young adults, exercise-induced adrenergic stimulation rapidly increases both HR and SV, with the latter being primarily enhanced by increased myocardial contractility and decreased peripheral vascular resistance. SV increases proportionally with exercise intensity until about 40–50% of maximal capacity, after which it tends to plateau and additional augmentation of CO is driven by a further increase in HR14.
While older adults are still capable of augmenting their CO in response to exercise, the relative increase is typically diminished compared to their younger counterparts. Reduced maximal HR, also known as chronotropic incompetence, is a major contributor to the diminished cardiac response to exercise in older adults. Normal aging results in a progressive decline in maximal HR by approximately 0.7 beats/min/year15. Although the mechanisms for chronotropic incompetence are not completely understood, degenerative changes in the conduction system along with impaired autonomic regulation likely play central roles16. Importantly, age-related decrease in peak HR strongly correlates with diminished exercise capacity, and is an independent predictor of adverse cardiovascular events and mortality17, 18.
The impact of aging on SV augmentation with exercise is not as clear with varying degrees of SV reserve reported in different studies19–22. In general, aged hearts are still capable of increasing SV in response to exercise, albeit at levels insufficient to offset the reduction in maximum HR. Interestingly, the mechanism by which the heart augments SV with exercise changes with age. While enhanced myocardial contractility is the primary means of increasing SV in young hearts, exercise increases SV in aged hearts mainly through increased end-diastolic volumes with minimal changes in contractility10.
Overall, normal aging significantly diminishes both the chronotropic and inotropic responses of the heart to exercise (Table 1). Clinically, this phenomenon is referred to as impaired cardiac reserve, which is the inability of the heart to adequately augment CO to meet the increased demands of physiologic stress, whether induced by exercise or pharmacologically (i.e. dobutamine). In conjunction with age-associated alterations in peripheral mechanisms of oxygen extraction and utilization in skeletal muscle19, 21, 23, 24, inadequate oxygen delivery from impaired cardiac reserve is a major contributor to decreased functional capacity in the elderly, especially those with HF19, 25,26. Maximum oxygen consumption (VO2max), which is the maximal rate the body can consume oxygen during incremental exercise, is an established metric of exercise capacity. With normal aging, VO2max declines by approximately 10% per decade in healthy ambulatory individuals22, but this decline notably accelerates at ages above 70 years and in HF27, suggesting that mechanisms that lead to impaired cardiac reserve in aging may be particularly relevant to the increased HF risk seen with advanced age.
Table 1.
Summary of age-associated changes in cardiovascular performance at peak exercise. Effects of aging are derived from comparison of aerobic exercise testing of healthy young adults (20–30 years) and healthy older adults (60–80 years). CV = cardiovascular. NC = no change. Data summarized from references 19–22.
| CV Parameter at Peak Exercise | Effects of Aging |
|---|---|
| Cardiac output | ↓ /NC |
| Heart rate | ↓ |
| LV stroke volume | ↑/↓ /NC |
| LV end-diastolic volume | ↑ |
| LV contractility | ↓ |
| Early diastolic filling rate | ↓ |
| VO2 max | ↓ |
| (A–V) O2 difference | ↓ |
Rodent models of cardiac aging and exercise intolerance
While human studies have provided valuable insights into how aging influences cardiovascular physiology and functional capacity, limited access to tissue has been a major obstacle to elucidating the molecular mechanisms of aging that impair cardiac reserve. In this regard, rodent models have been particularly useful because of their relatively short lifespans, genetic manipulability, and similar cardiac aging phenotypes to humans5. Based on survival data, mice and rats, around 24 months of age, are typically used to model older humans28, although even this pre-specified age cutoff must be carefully considered given the wide variation in lifespan across strains29. In general, rodent hearts at this age exhibit similar structural and functional phenotypes to older human hearts, including impaired contractile reserves, diastolic dysfunction, hypertrophy, fibrosis, and vascular stiffening30.
Importantly, despite having increased basal metabolic requirements and higher resting HR, rodents demonstrate comparable exercise physiology to humans, which can be reliably assessed when careful attention is paid to exercise testing conditions31. Continuous invasive hemodynamic monitoring in adult (3–4 month) mice has shown that they augment CO by approximately 2-fold (9.6±0.6ml/min at rest to 18.9±0.9ml/min at peak exercise) in response to acute exercise32. The increased CO is primarily derived from a marked increase in HR (489±18bpm at rest to 798±9bpm at peak exercise) and modest SV augmentation. Moreover, similar to humans, as rodents age, exercise capacity progressively declines. VO2max decreases by approximately 28% in healthy 24-month-old C57BL/6J mice, compared with 12-month-old mice33. A similar pattern is seen in Fischer 344 × Brown Norway F1 (F344/BNF1) rats, which display 10% and 33% decreases in VO2max at 24 and 35 months, respectively, compared with 12-month-old rats34.
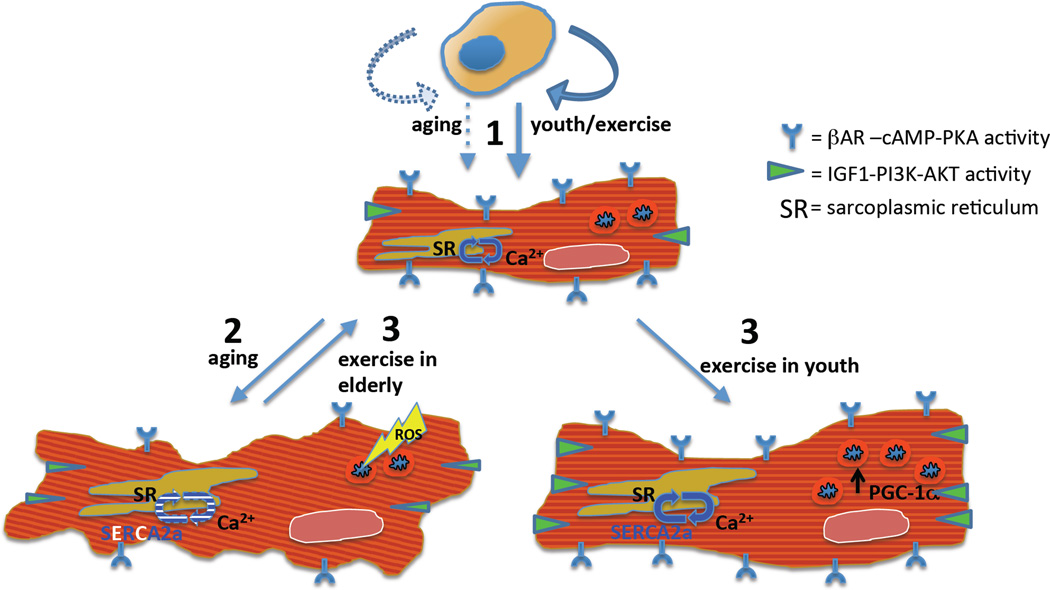
Even in rodents defining intrinsic factors of aging that influence cardiac reserve and exercise capacity is difficult. Based on the central role of the autonomic nervous system on cardiac exercise response, a substantial amount of research has focused on autonomic dysregulation in the aged heart, as described in more detail below. Recent studies by Wisloff and colleagues have used a breeding selection strategy in rats based on exercise capacity (referred to as the aerobic hypothesis)35. From 1996–2011, selective breeding of a genetically heterogeneous N:NIH rat stock (28 generations, n=11,606 rats) eventually generated two distinct lines that differed in maximal running capacity by approximately 7-fold. Comparative analyses of hearts and isolated cardiomyocytes from aged rats with low and high intrinsic running capacities subsequently identified mitochondrial dysfunction36, abnormal calcium (Ca2+) handling37, increased hypertrophy,38 and microvascular dysfunction as key molecular phenotypes in the heart associated with exercise intolerance in aging (Figure 1).
Figure 1.
Multiple mechanisms have been proposed for the impaired cardiomyocyte function observed in aging, and how exercise partially reverses their effects. (1) Diminished cardiac performance in the pathological hypertrophy of aging is linked to decreased IGF1-PI3K-AKT and bAR-cAMP-PKA signaling, decreased SERCA expression and activity and inefficient calcium handling, and mitochondrial dysfunction secondary to excessive ROS. (2) Exercise confers physiological hypertrophy and cardio-protection in the form of enhanced beta-adrenergic and IGF1 signaling, SERCA activity and calcium handling, and mitochondrial dynamics, the latter mediated largely through PGC-1a. (3) These benefits of exercise mitigate the effects of aging (Illustration Credit: Ben Smith).
We will now explore in more detail how these features of cardiomyocyte aging impair the aged heart’s response to acute exercise, and how exercise interventions potentially modulate these aging phenotypes. While adaptive changes in the vasculature are important in both aging and exercise physiology, a complete discussion of this topic is beyond the scope of this review, and we refer the interested reader to the following references as an introduction to this topic4, 39.
Exercise and autonomic regulation of the aged heart
The heart’s response to acute exercise is largely regulated by the autonomic nervous system. During exercise, increased sympathetic tone augments both HR and contractility, while concomitant parasympathetic withdrawal further enhances the chronotropic response. As the heart ages, however, its responsiveness to autonomic stimuli significantly diminishes. Evidence in humans and animals suggests that these age-associated changes in cardiac autonomic regulation play important roles in declining cardiac reserve and exercise capacity seen with aging40.
Age-associated autonomic dysregulation and impaired cardiac reserve
Sympathetic dysregulation in the aged heart is primarily derived through a process known as β-adrenergic receptor (β-AR) desensitization. With normal aging, circulating norepinephrine levels increase by 10–15% per decade41. In the heart, local norepinephrine levels also increase with age due to diminished reuptake and increased tissue spillover42. Greater β-AR occupancy by catecholamines triggers a compensatory mechanism in aged cardiomyocytes that results in desensitization of the post-synaptic machinery, and ultimately blunted intracellular Ca2+ transients and impaired inotropic and chronotropic responses to adrenergic stimulation43, 44.
The mechanisms underlying β-AR desensitization in the aged heart are complex, with alterations occurring at multiple levels along the β-AR/G-protein/adenylyl cyclase (AC) pathway. Reduced β-AR density has been reported in older human45 and rat46 hearts, implying that at least part of this process is modulated at the receptor level. Additionally, numerous alterations in downstream G-proteins and AC catalytic units have been identified in the aging myocardium. Evidence from senescent rats and guinea pigs has suggested that cardiac Gi protein levels and pertussin-toxin (PTX)-mediated Gi ribosylation increase with age47, 48. However, other studies in humans and rats have demonstrated that Gi levels are unchanged in aged cardiomyocytes, and furthermore, their reduced contractile response to adrenergic stimulation cannot be rescued by inhibiting Gi with PTX45, 46. Rather, these studies argue that age-related β-AR desensitization is primarily mediated through diminished β-AR density, reduced Gs, and impairments in AC activity.
While the mechanisms responsible for the age-dependent decline in cardiac β-AR responsiveness are not completely understood, it is clear that this process results in impaired cAMP production and protein kinase A (PKA) activity, which are necessary for augmenting intracellular Ca2+ transients and enhancing cardiac contractility during exercise49, 50. Impaired cAMP/PKA signaling may in part be due to persistent activation of Ca2+/calmodulin kinase II (CaMKII), another downstream effector of β-AR signaling. Interestingly, while constitutive β-AR stimulation leads to down-regulation of PKA signaling, CaMKII activity remains high. Persistent CaMKII activity can desensitize cardiomyocytes to PKA signaling51, 52, and moreover, has been linked to apoptosis and pathologic hypertrophy in failing cardiomyocytes53, 54.
Alterations in parasympathetic control of the aged heart have not been as extensively studied. In rats, the data has been conflicting with age-associated changes in the density and function of cardiac muscarinic M2 receptors reported to be unchanged, decreased, or increased55. In humans, the density of cardiac M2 receptors appears to decline with age.56 Moreover, aged human hearts demonstrate impaired chronotropic responses to acute parasympathetic withdrawal, suggesting that impaired muscarinic receptor activity may contribute to the blunted HR response to exercise in the elderly57, 58.
Given that exercise primarily mediates its effects on the heart through dynamic regulation of the autonomic system, it seems likely that these age-associated changes in β-adrenergic and muscarinic receptor pathways play important roles in the impaired cardiac response to exercise in older adults. Notably, down-regulation of β-AR density and activity is seen in failing hearts from younger adults, who exhibit similar declines in cardiac reserve and exercise capacity51. Likewise, acute β-AR blockade in healthy young adults recapitulates the aging cardiac response to exercise with blunted maximal HR, decreased myocardial contractility, and increased end-diastolic volumes59. Collectively, these data support an important functional role for altered sympathetic and parasympathetic signaling in cardiac phenotypes associated with aging.
Effects of exercise training on β-AR desensitization in the aged heart
There is modest evidence in older humans and rats indicating that exercise training can reverse, or “resensitize”, the aged heart to adrenergic stimuli and improve cardiac reserve60. Nine months of aerobic exercise in previously sedentary, older men (~65 years) increased exercise capacity by 28%, in addition to improving contractility and early diastolic filling rates at peak exercise61. Importantly, these exercise-induced changes were completely abrogated by acute β1-receptor blockade, suggesting that the observed effects of training on the aged heart were likely mediated through direct modulation of β-AR signaling.
Similar findings have been demonstrated in aged rats. While 12 weeks of moderate intensity treadmill running in 28-month-old Sprague-Dawley rats did not change β-AR density, it significantly decreased downstream Gi activity and enhanced isoprenaline-stimulated AC activity47. A follow-up study, in which 24-month-old Wistar-Kyoto rats were run at 70–80% VO2max for 12 weeks, demonstrated that higher intensity training increased β-AR density and AC activity in aged hearts, resulting in enhanced responsiveness to adrenergic stimulation and restoration of inotropic, lusitropic, and chronotropic properties62.
While numerous differences in experimental conditions are present between these two studies (Table 2), it is intriguing to hypothesize that exercise “dose” or subject age may have influenced the varying effects of training on β-AR density in the aged hearts. Indeed, data from humans and rodents has suggested that a threshold “dose” of exercise may be necessary to generate significant changes in the heart63–65. In adult rats, direct comparison of moderate (65–70% VO2max) and high (85–90% VO2max) intensity treadmill running demonstrated that higher intensity training not only improved exercise capacity to a greater extent, but it also correlated with a dose-dependent increase in cardiomyocyte hypertrophy, contractility/relaxation, and Ca2+ handling65. Furthermore, age also appears to play a role exercise-induced modulation of β-AR signaling. In young animals, aerobic training decreases cardiac Gi activity, but generally has little to no effect on β-adrenergic/muscarinic receptor densities or downstream AC activity47. In fact, direct comparison of high intensity (75% VO2max) treadmill running in young (3 month) versus old (23 month) F344 rats showed that adrenergic-stimulated AC activity was actually decreased in young rats, while up-regulated in older rats66.
Table 2.
Summary of studies in aging rodent models evaluating the effects of aerobic exercise training on cardiac aging phenotypes. Grade is 0% unless specified. Training frequency is 5 days per week unless specified. RSP= Ramped speed protocol. CP= Constant protocol. RDP= Ramped duration protocol. CM = cardiomyocyte. HW = heart weight. LV = left ventricle. BW = body weight. TL = tibial length. BP = blood pressure. Mito = mitochrondrial. (↑) = Increase. (↓) = Decrease. (NC) = No change.
| Cardiac Parameter |
Aging Animal Model | Exercise Training | Effects of Exercise Training (compared to sedentary control) |
Ref | ||||
|---|---|---|---|---|---|---|---|---|
| Species | Strain | Age (mo) |
Type | Protocol | Duration (wks) |
|||
|
Autonomic regulation |
Rat | Sprague- Dawley |
28 | Treadmill running, RSP |
30min/day, 20m/min | 12 | β-AR density (NC), M-R density (NC), Gi activity (↓), AC activity (↑) |
Bohm et al., 1993 (47) |
| Rat | Wistar- Kyoto |
24 | Treadmill running, CP |
45min/day, 17m/min, 15% (70–85% VO2max) |
12 | β-AR density (↑), AC activity (↑) | Leosco et al., 2007 (62) |
|
| Rat | F344 | 23 | Treadmill running, RDP |
75% VO2max | 9 | Gs activity (NC), AC activity (↑) | Scarpace et al., 1994 (66) |
|
| Ca2+ handling | Rat | F344 | 23–24 | Treadmill running, RDP |
60 min/day, 16 m/min, 5% |
8–10 | SERCA2a (↑), contractility (↑) | Tate et al., 1996 (80) Tate et al., 1990(81) |
| Rat | F344/ BNF1 |
24 | Treadmill running, CP |
45min/day 17m/min, 15% (70–85% VO2max) |
12 | Early diastolic filling (↑) | Brenner et al., 2001 (82) |
|
| Rat | F344BN | 29 | Treadmill running, RSP |
60min/day, 5 → 10m/min, 10% |
20–28 | SERCA2a (NC), RyR (NC), Ca2+ cycling (NC) |
Thomas et al., 2011 (84) |
|
| Rat | Wistar | 21 | Swimming CP |
90min/day, 35–37°C | 8 | SERCA2a (↑), contractility (↑) | Iemitsu et al., 2004 (83) |
|
| Mouse | C57BL/6 | 24 | Treadmill running, CP |
15min/day, 15m/min | 10 (3d/wk) |
SERCA2a (NC), NCX (NC) | Walton et al., 2015 (85) |
|
| Mouse | C57BL/6 | 12 | Treadmill running, CP |
15min/day, 15m/min | 52 (3d/wk) |
SERCA2a (↓), NCX (↓) | Walton et al., 2015 (85) |
|
| Hypertrophy | Rat | F344/ BNF1 |
24 | Treadmill running, CP |
45min/day, 17m/min, 15% (70- 85% VO2max) |
12 | HW/TL (NC) | Brenner et al., 2001 (82) |
| Rat | F344 | 25 | Treadmill running, RSP, RDP |
20 → 60min/day, 4 →15m/min (70–75% VO2max) |
12 | LV (NC), BW (↓), LV/BW (NC), BP (NC) |
Choi et al., 2009 (127) |
|
| Rat | F344/ BNF1 |
29 | Treadmill running, RSP |
60min/day (in 6×10min reps) 5 →10m/min, 10% |
20–28 | HW (↓), BW (↓), HW/BW (↑) | Wright et al., 2014 (126) |
|
| Rat | F344BN | 29 | Treadmill running, RSP |
60min/day, 5 → 10m/min, 10% |
20–28 | HW/BW (↓) | Thomas et al., 2011 (84) |
|
| Rat | F344 | 24 | Treadmill running, CP |
60min/day, 15m/min, 15% (70–75% VO2max) |
12 | CM size (↓), apoptosis (↓) | Kwak et al., 2006 (130) |
|
| Rat | Wistar- Kyoto |
21 | Treadmill running, CP |
60min/day (50–60% VO2max) |
13 | CM size (NC), LV/TL (NC), BP (NC) |
Rossoni et al., 2011 (131) |
|
| Rat | Wistar | 18 | Treadmill running, CP |
60 min/day, 30m/min |
6 | HW(↑), BW (↓), HW/BW (↑), | Wang et al., 2014 (128) |
|
| Rat | Sprague- Dawley |
28 | Treadmill running, RSP |
30min/day, 20m/min | 12 | HW/BW (NC) | Bohm et al., 1993 (47) |
|
| Rat | Wistar | 21 | Swimming CP |
90min/day, 35–37°C | 8 | LV (−), BW (↓), LV/BW (↑) | Iemitsu et al., 2004 (83) |
|
| Rat | Sprague- Dawley |
18 | Swimming RDP |
20→60min/day, 25±2°C |
12 | LV (↓), LV/TL (↓) | Liao et al., 2015 (99) |
|
| Rat | Sprague- Dawley |
18 | Swimming RDP |
20→60min/day, 25±2°C |
12 | LV (↓), LV/TL (↓), apoptosis (↓) | Lai et al., 2014 (134) |
|
| Rat | Wistar | 21 | Swimming CP |
90min/day, 35–37°C | 8 | CM size (NC), LV (NC), BP (NC) | Iemitsu et al., 2006 (132) |
|
| Mouse | C57BL/6 | 24 | Treadmill running, CP |
15min/day, 15m/min | 10 (3d/wk) |
CM size (↑), HW/BW (↑), HW/TL(↑) | Walton et al., 2015 (85) |
|
| Mouse | C57BL/6 | 12 | Treadmill running, CP |
15min/day, 15m/min | 52 (3d/wk) |
CM size (↑), HW/BW (↑), HW/TL(↑) | Walton et al., 2015 (85) |
|
| Mouse | PolG mutator |
3 | Treadmill running, CP |
45min/day, 15m/min | 20 (3d/wk) |
HW (↓), Wall thickness (↓) | Safdar et al., 2011 (172) |
|
| Mouse | C57BL/6 | 6–18 | Swimming CP |
90min session x2/day, 30–32°C |
4 | LV (↑), LV/BW (↑) | Derumeaux et al., 2008 (129) |
|
| Fibrosis | Rat | F344/BN F1 |
29 | Treadmill running, RSP |
60min/day (in 6×10min reps), 5 →10m/min, 10% |
20–28 | Fibrosis (↓), Collagen cross linking (↓) |
Wright et al., 2014 (126) |
| Rat | F344 | 25 | Treadmill running, RSP, RDP |
20 → 60min/day, 4 →15m/min (70–75% VO2max) |
12 | Fibrosis (NC), collagen cross- linking (↓), passive stiffness (↓) |
Choi et al., 2009 (127) |
|
| Rat | Sprague- Dawley |
18 | Swimming RDP |
20 →60min/day, 25±2°C, |
12 | Fibrosis (↓) | Liao et al., 2015 (99) |
|
| Mouse | C57BL/6 | 24 | Treadmill running, CP |
15min/day, 15m/min | 10 (3d/wk) |
Fibrosis (NC) | Walton et al., 2015 (85) |
|
| Mouse | C57BL/6 | 12 | Treadmill running, CP |
15min/day, 15m/min | 52 (3d/wk) |
Fibrosis (↓) | Walton et al., 2015 (85) |
|
| Mouse | C57BL/6 | 6–18 | Swimming CP |
90min session x2/day, 30–32°C |
4 | Fibrosis (↓), contractility (↑), diastolic function (NC) |
Derumeaux et al., 2008 (129) |
|
|
Mitochondrial function |
Rat | Wistar | 18 | Treadmill running, CP |
60 min/day, 30m/min, |
6 | Cardiac mito respiration (↑), ROS (↓) |
Wang et al., 2014 (128) |
| Rat | Sprague- Dawley |
5 wks |
Treadmill running, RSP |
30min/day, 4.2m/min→ 12m/min at 1m/min/30sec; |
36 | Cardiac PGC1α (↑), SIRT1 (↑), mito biogenesis (NC), |
Bayod et al., 2012 (181) |
|
| Mouse | PolG mutator |
3 | Treadmill running, CP |
45min/day, 15m/min | 20 (3d/wk) |
Cardiac PGC1α (NC), cardiac mtDNA (↑) |
Safdar et al., 2011 (172) |
|
Exercise and Ca2+ regulation in the aged heart
Calcium handling is regulated by β-adrenergic signaling in cardiomyocytes and plays a central role in modulating cellular contraction and relaxation through excitation-contraction (EC) coupling. Numerous age-related changes in key components of cardiomyocyte Ca2+ handling, however, impair both the systolic and diastolic properties of the aged heart.
Age-associated impairments in Ca2+ handling
In order to augment myocardial contractility, relaxation, and overall cardiac performance during acute exercise, EC coupling must be quickly modified within individual cardiomyocytes to increase the rate of rise and decay of intracellular Ca2+ transients. In young cardiomyocytes, peak contractions and Ca2+ transients increase and decay more rapidly at higher stimulation frequencies67, 68. While aged cardiomyocytes display similar peak contractions at slow stimulation rates, they produce much smaller increases in peak Ca2+ transients and cell shortening at more rapid pacing rates68. Additionally, rates of Ca2+ decay are significantly prolonged in aged cardiomyocytes compared with younger cells. At an organ level, these findings translate to preserved systolic function under resting conditions, but prolonged myocardial relaxation (a hallmark of age-related diastolic dysfunction) and impairments in the ability to augment contractility at the faster HR elicited by exercise.
Impairments in intracellular Ca2+ handling in aged cardiomyocytes are largely derived from age-associated changes in the proteins involved in EC coupling. Decreased levels of sarcoplasmic reticulum Ca2+–ATPase (SERCA2a) are thought to be a primary mechanism for the prolonged Ca2+ transients in the aged myocardium69–72. Cardiac SERCA2a gene transfer in senescent rats restores diastolic function back to youthful levels73. Additionally, aged-associated alterations in SERCA2a regulatory proteins, including phospholamban (PLB)74, PKA49 , and CaMKII75 have also been documented in the aged heart, with the direction of these changes expected to decrease SERCA2a activity and prolong Ca2+ transients. Evidence of age-related changes in other proteins involved in cardiomyocyte Ca2+ regulation, including the Na+/Ca2+ exchanger (NCX), ryanodine receptors (RyR), and calsequestrin have not been as consistent or would not necessarily be expected to significantly alter Ca2+ transients76.
Effects of exercise training on Ca2+ handling in the aged heart
Whether exercise training can improve intracellular Ca2+ cycling and performance of the aged heart is not entirely clear. In healthy young rodents, aerobic exercise training leads to faster rise and decay rates of Ca2+ transients in cardiomyocytes, and subsequent improvements in systolic and diastolic function65, 77. The mechanisms for these exercise-induced alterations in Ca2+ cycling in young hearts are potentially mediated through more effective coupling of L-type Ca2+ channels and RyR receptors, increased SERCA2a and NCX expression, enhanced SERCA2a function via transient CaMKII activation or PLB inhibition, and/or improved myofilament Ca2+ sensitivity77–79.
Aerobic training studies in aged rodents suggest that these benefits are not limited to young animals, and appear to be primarily driven by enhanced SERCA2a expression. Eight to ten weeks of treadmill running increases SERCA2a levels in the hearts of 24-month-old F344 rats80. Furthermore, isolated cardiomyocytes from these rats display improved Ca2+ cycling and more rapid contractility and relaxation times that are associated with increased SERCA2a expression81. Twelve weeks of high intensity (70–85% VO2max) treadmill running in young (6 month) and old (24 month) F344/BNF1 rats also largely reverses impairments in early diastolic filling rates in the older cohort. This effect is not seen in younger animals, suggesting that exercise has specific modulatory effects on age-related impairments in active myocardial relaxation, presumably through improved Ca2+ cycling82. Swimming old (21 month) Wistar rats also induces similar increases in cardiac SERCA2a expression83. However, other studies have demonstrated that SERCA2a and other related Ca2+ channels (i.e. RyR, NCX) are not increased in aged rodents by aerobic training84, 85. Notably these latter studies were done at significantly lower exercise intensities (Table 2), emphasizing the importance of evaluating exercise protocols in interpreting results of training.
Exercise and age-related cardiac hypertrophy
Cardiac hypertrophy, a composite of cardiomyocyte growth and increased extracellular matrix deposition, is a hallmark feature of cardiac aging86, 87, and is associated with diastolic dysfunction, HF, and mortality in the elderly88, 89. While age-related vascular remodeling undoubtedly influences cardiomyocyte growth in the aged heart, both human and animal studies indicate that mechanisms independent of changing hemodynamics also contribute86, 90. Cardiomyocyte hypertrophy in the aged heart may in part be a compensatory reaction to a cumulative loss of myocytes with normal aging91, 92. Declining regenerative potential in the aged heart93 appears insufficient to counterbalance this loss. While age-related hypertrophy minimizes myocardial wall stress and can help maintain overall cardiac function, at a cellular level, it can also be viewed as a marker of increased stress and altered homeostasis, and is generally felt to be a pathologic process associated with increased apoptosis, impaired Ca2+ regulation, and defective macroautophagy94–96.
Mechanisms of age-related cardiac hypertrophy
Many of the molecular mechanisms underlying cardiomyocyte hypertrophy in the aged heart appear similar to the intracellular signaling pathways that drive pathologic growth in hypertension and HF97. Chronically activated neurohormonal systems, including the adrenergic, endothelin, and renin-angiotensin-aldosterone systems, along with increased workload and biomechanical strain on the remaining cardiomyocytes stimulate numerous growth pathways, including the mitogen-activated protein kinases (MAPK), histone deacetylases (HDAC), calcineurin/nuclear factor of activated T cells (NFAT), and insulin-like growth factor-I (IGF-I)-phosphatidylinositol 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathways. For a detailed discussion of these growth pathways in the heart, we refer the interested reader to an excellent review by Heineke and Molkentin98.
While many of these signaling pathways, including p38, c-jun N-terminal kinase, extracellular signal-regulated kinase-1/2 (ERK-1/2), calcineurin/NFAT, and PI3K/Akt/mTOR, are up-regulated in aging rodent hearts99, 100, whether they are directly regulated by aging and what the relative contribution of each of these pathways in age-related cardiac hypertrophy is not clearly defined. Cardiac specific gain- and loss-of-function studies have provided some supportive evidence for a direct role of neurohormonal pathways in age-related cardiomyocyte hypertrophy. Genetic ablation of cardiomyocyte endothelin-A receptors attenuates age-associated cardiomyocyte hypertrophy101, while cardiomyocyte-specific β-AR1102 or angiotensin II97 overexpression induces significant myocyte growth in aging hearts. Similarly, cardiac specific suppression of PI3K103, as well as systemic mTOR inhibition with rapamycin104, reverses hypertrophy and lipofuscin accumulation in aged murine hearts. The role of HDACs, particularly NAD-dependent sirtuins, have recently emerged as important regulators of age-related cardiac hypertrophy and longevity, and will be discussed in detail later in this review.
Most recently, heterochronic parabiosis studies in mice have suggested that there may in fact be age-specific mechanisms of cardiomyocyte hypertrophy. Using a novel aptamer-based proteomics screen in this mouse model, Loffredo and colleagues found that systemic levels of growth differentiation factor-11 (GDF11), a secreted member of the TGF-β superfamily, decline with normal aging105. Interestingly, restoration of GDF11 levels in 24-month-old C57BL/6 mice reversed age-related cardiomyocyte hypertrophy and improved SERCA2a expression in the heart. Recent work by Smith and colleagues, however, found that GDF11 therapy did not alter cardiomyocyte hypertrophy in old C57BL/6 mice and moreover did not affect cardiac function106. The reason for these differing results is currently unclear as similar GDF11 interventions and aged murine strains were used in both studies.
Interestingly, GDF11 shares many structural and functional properties with myostatin, another TGF-β superfamily member (also known as GDF8). Aging studies in germline myostatin knockout mice have suggested that while systemic myostatin inhibition induces modest cardiac hypertrophy in senescent mice, it also decreases myocardial fibrosis and improves systolic function107. Indeed, both hearts and isolated cardiomyocytes from aged germline knockouts demonstrate improved β-adrenergic responsiveness, Ca2+ handling, and enhanced contractility to sympathetic stimulation107–109. Taken together, these data suggest that myostatin inhibition may induce physiologic, as opposed to pathologic hypertrophy, in the aged heart. As further evidence, chronic pressure overload through transverse aortic constriction (TAC) does not alter the hypertrophic response in cardiac specific myostatin knockout mice110 and likewise, GDF11 therapy has no effect on TAC-induced pathologic hypertrophy105. Recent work by Egerman and colleagues have highlighted the difficulty in distinguishing between myostatin and GDF11 in some of the currently available assays111, emphasizing the need for further study to understand the potentially interrelated roles of these closely related peptides in cardiac aging.
Mechanisms of exercise-induced cardiac hypertrophy
Similar to aging and HF, exercise can induce a dramatic increase in cardiac mass that is predominantly mediated by cardiomyocyte growth112, 113. However, unlike age-related cardiac hypertrophy exercise elicits a more “physiologic” growth that is felt to be cardioprotective. Not only are the outcomes of these two kinds of cardiac growth different, but the underlying molecular mechanisms are also largely distinct114.
Exercise-induced hypertrophy is mediated largely through increased IGF-1 signaling in the heart115. Cardiac-specific IGF-1 receptor knockout mice do not develop cardiac hypertrophy in response to exercise, suggesting that initial IGF-1 signaling is necessary for exercise-induced cardiac growth116. Stimulated IGF-1 receptors subsequently activate PI3K, a family of heterodimeric kinases that regulate membrane lipid phosphoinositides. Cardiac-specific expression of a dominant negative PI3K 110alpha isoform also inhibits exercise-induced cardiac growth117, 118. Similarly, germline deletion of the PI3K–effector, Akt1, abolishes exercise-induced cardiac hypertrophy119. Conversely, forced over-expression of Akt in the heart protects cardiomyocytes from hypoxic injury and apoptosis120, 121, supporting the notion that Akt could contribute to exercise-induced cardioprotection. Taken together, these studies collectively establish the IGF-1/PI3K/Akt signaling pathway as a central mediator of the cardiac exercise response.
Genome-wide transcriptome analyses comparing exercised hearts to hearts subjected to TAC, also demonstrated distinct sets of transcriptional regulators regulated in physiological and pathological hypertrophy122. Moreover, this screen identified a transcriptional pathway downstream of C/EBPβ, a member of the bHLH family of DNA-binding transcription factors, as downregulated with exercise. Reduction of C/EBPβ in vitro and in vivo was sufficient to recapitulate many of exercise-related phenotypes including a similar gene expression profile, cardiomyocyte hypertrophy, and protection against HF. Notably this pathway is connected to the cardioprotective effects of Akt signaling. Forced over-expression of C/EBPβ in cardiomyocytes blocks Akt1-induced expression of genes characteristic of physiologic hypertrophy, and conversely, Akt1 over-expression downregulates C/EBPβ expression.
In addition, work from our lab and others have shown that microRNAs (miRNAs) and exercise protocols play important roles in the cardiac growth response to exercise123. Exercise protocols vary widely, and the growth responses of the heart to different experimental designs are not identical (Table 2)124. In comparing the differential expression of miRNAs in the hearts of mice that were exercised with forced swimming versus voluntary wheel running, hearts of swum mice had 55 differentially expressed miRNAs compared to sedentary controls, while hearts from wheel run mice had 124 such miRNAs125. Sixteen miRNAs were concordantly regulated in both exercise models, with miRNA-222 proving to be a particularly potent regulator of cardiomyocyte growth and proliferation in vitro. Subsequent in vivo studies showed that miRNA-222 was required for exercise-induced hypertrophy, and its forced expression protected against adverse remodeling after ischemic injury. These results demonstrate that integrating different exercise regimens can be a particularly robust approach to identifying critical biological networks, but also underscore the differential responses elicited by distinct protocols and thus the challenges in comparing the data from one regimen in isolation.
Effects of exercise training on age-related cardiac hypertrophy
The concept of distinct forms of cardiac hypertrophy is particularly relevant in the aging heart. As opposed to young animals, in which aerobic training generally induces some degree of hypertrophy in the heart124, training studies in senescent animals have shown extensive variability in the cardiac growth response to exercise126–133 with a substantial number of studies indicating that it can paradoxically reverse aged-related hypertrophy (Table 2).
A small subset of these studies have evaluated the effects of exercise training on cardiomyocyte growth in the aged heart. Kwak and colleagues trained young (3 month) and old (24 month) F344 rats on a high intensity (75% VO2max) running protocol for 12 weeks130. While training induced cardiomyocyte hypertrophy in the young rats, it resulted in regression of cardiomyocyte size (69% decrease in cross-sectional area) in the aged cohort. Alternatively, low-moderate intensity treadmill running or swimming did not affect cardiomyocyte size in 21-month-old Wistar-Kyoto or spontaneously hypertensive rats, despite reductions in blood pressure in the latter group131, 132. Moreover, 10 weeks of low-intensity treadmill running was sufficient to induce cardiomyocyte hypertrophy in aged (24–26 month) C57BL/6 mice 85.
Differences in training protocols and animal models make it inherently difficult to directly compare studies (Table 2). Additionally, only a few studies adequately address the blood pressuring lowering effects of exercise, which are particularly relevant in assessing cardiac growth in the context of aging. However, collectively what these data again seem to indicate is that training intensity and age may be critical determinants in exercise-mediated modulation of cardiac aging phenotypes, specifically with repression of age-related cardiac hypertrophy generally occurring in older animals subjected to higher intensity protocols.
Given the discrepancies among studies, it is not surprising that the molecular basis for the potentially disparate effects of exercise-mediated growth in young versus old hearts is not entirely clear. It is postulated that exercise’s cytoprotective effects may improve survival in senescent cardiomyocytes, thus decreasing the stimulus for reactive pathologic hypertrophy. Indeed, hearts from exercise-trained aged rats demonstrate reductions in numerous apoptotic indices that are elevated in the aging myocardium130, 133, 134. However, whether these exercise-induced changes translate to less cell death and diminish the trigger for pathologic growth in the aged heart is not proven. Moreover, recent work has shown that pro-apoptotic caspase pathways can directly induce pathologic growth in adult cardiomyocytes135, suggesting an alternative mechanism by which exercise-induced inhibition of apoptotic pathways may actually directly suppress pathologic growth in the aged heart.
The underlying signaling mechanisms by which exercise potentially improves survival of aged cardiomyocytes may be related to the cardioprotective effects of the IGF1/PI3K/Akt pathway. Cardiac-specific over-expression of IGF1136, PI3K137, and Akt1121 have all been shown to improve cardiomyocyte survival in adult mouse hearts exposed to either TAC or ischemic injury. Importantly, multiple studies have also demonstrated that similar to young animals, aerobic exercise increases Akt phosphorylation in senescent rodent hearts99, 132, 138, albeit to a lesser extent132, 134. Whether lower levels of Akt activity in exercised aged hearts are sufficient to enhance cell survival and suppress pathologic growth pathways, but insufficient to promote physiologic growth may be a plausible explanation.
Ultimately, the variability in cardiac growth responses to exercise between young and old animals likely stems from differences in the substrate of a young versus senescent heart, with apoptotic and pathologic hypertrophy pathways constitutively activated in the latter. Indeed, when young (3 month) and old (18 month) rats are subjected to similar 12-week swimming protocols, while apoptotic markers, MAPK, and calcineurin/NFAT expression decrease in old hearts, they remained unchanged or increased in young hearts, despite increased Akt activity in both groups134,99. Interestingly, germline Akt1 knockout mice show an exaggerated growth response to TAC, suggesting that Akt signaling may be capable of directly suppressing pathologic growth pathways in the aged heart119. Although the mechanisms by which this occurs in cardiomyocytes are unknown, in other cell types, Akt has been shown to inhibit numerous MAPK pathways (p38, ERK) implicated in pathologic cardiac hypertrophy139–141.
In addition to Akt signaling, it is important to note that exercise also modulates other growth pathways that may be particularly relevant to the aging heart. Acute treadmill running stimulates neuregulin production in skeletal muscle142, which has demonstrated anti-apoptotic effects on cardiomyocytes through the ErbB family of tyrosine kinases and potentially downstream PI3K/AKT143. Exercise also decreases both skeletal muscle and cardiac myostatin levels in humans and rodents with pathologic hypertrophy144, 145. While the precise role of myostatin and its close homologue GDF11 in age-related cardiac hypertrophy awaits clarification, it may be that exercise-induced inhibition of this pathway induces a similar pattern of Akt activity that could potentially inhibit pathologic growth pathways in the aged heart. Indeed, in vitro studies have demonstrated that myostatin inhibition drives cardiomyocyte growth through Akt activation146. Furthermore, while physiologic versus pathologic growth pathways are largely distinct, there is some overlap. For example, while calcineurin/NFAT signaling is primarily a regulator of pathologic growth of the heart, there is evidence indicating that it also mediates cardioprotective effects and may be necessary in certain physiological growth settings147, 148. Ultimately, how exercise dynamically regulates the various signaling pathways involved in age-related cardiomyocyte hypertrophy is still largely unknown and remains a fertile area for future research.
Exercise and mitochondrial dysfunction in the aged heart
The heart requires an enormous amount of energy, primarily derived from fatty acid oxidation and subsequent ATP production within mitochondria. The ability to fulfill this energy requirement, especially under stress, is impaired in the aged heart due to mitochondrial dysfunction. The mitochondrial theory of aging is a decades-old idea149, with the underlying premise that oxidative stress increases with age and causes a gradual accumulation of mitochondrial damage and electron transport chain dysfunction150–153. Increased levels of free radicals in the aged heart lead to impaired mitochondria, which in turn produce more reactive oxygen species (ROS) resulting in a downward spiral in cardiac performance. Early studies in Drosophila overexpressing ROS scavenging enzymes and in mice with enhanced resistance to oxidative stress demonstrate increased lifespan, as do models of caloric restriction154–156. However, more recent studies suggest the relationship between ROS and mitochondrial DNA damage leading to aged phenotypes is not so straightforward, and that widespread ROS elevation in somatic tissues may not be the root cause of aging. In fact, certain levels of ROS may be instrumental for maintaining tissue homeostasis and regenerative potential. In the context of this ongoing debate, we will discuss how the interplay between ROS and mitochondrial function impacts cardiac performance during aging, and the mechanisms by which exercise may play a beneficial role in restoring cardiac energetics.
Mitochondrial dysfunction in aging
Senescence heralds an indisputable decline in mitochondrial function. Mitochondrial DNA (mtDNA) lacks protective histones and is in close proximity to high levels of ROS, and thus is particularly susceptible to oxidation157. DNA mutation rate is 10 to 20-fold higher in mitochondria than in nuclei. The role of mtDNA damage in aging is dramatically revealed in Mutator mice. These animals harbor defective proofreading by the mitochondrial DNA polymerase gamma (PolG) and thus carry significant mtDNA deletions and five-fold more point mutations158, 159. While this degree of mtDNA damage exceeds that observed in normal aging, no genetic model can represent all the progressive changes that mitochondria undergo. Nevertheless, these mice display global attributes of premature aging, and cardiac senescence in the form of hypertrophy, increased fibrosis, and impaired systolic and diastolic function by eight months of age. Tissues of PolG Mutator mice show decreased levels of mitochondrial biogenesis, diminished respiratory capacity, and increased apoptosis159, 160. Interestingly, while Mutator mice do not show increased levels of ROS159, 161, the expression of a mitochondrial specific catalase partially reverses their cardiac findings162. This supports the idea that ROS reduction ameliorates the accumulation of mtDNA mutations and that oxidative stress specifically in mitochondria is a major factor leading to the progerian phenotype.
Given that Mutator mice do not reveal dramatically altered levels of oxygen free radicals or oxidative damage, attention has turned toward possible mechanisms of premature aging that rely less on global increases in ROS, but on subtle alterations in subpopulations of cells. Cellular dysfunction or demise may result when a certain threshold of mutational burden is crossed, or if DNA damage of critical subunits of mitochondrial metabolism results in ineffective respiration, resulting in a heterogeneous response within the myocardium153, 163. Mitochondrial decline creating a cellular mosaic in aged human hearts was first exemplified by the arbitrary distribution of cardiomyocytes with undetectable cytochrome c oxidase activity164, and similarly observed in mice lacking mitochondrial transcription factor A (Tfam) in heart and skeletal muscle165. The latter develop dilated cardiomyopathy and lethal conduction blocks. A mosaic of mitochondrial dysfunction in hearts is also observed in mice with a dominant-negative, cardiac-specific mitochondrial helicase, which accelerates the accumulation of mtDNA deletions166. Aging mice carrying this mutant gene develop diffuse respiratory deficiency that ultimately manifests as arrhythmias, possibly secondary to aberrant Ca2+ handling. A heterogeneous response to mtDNA mutation that ultimately contributes to the progeroid phenotype may also derive from stem cell reservoirs that are particularly vulnerable to ROS elevation. Tissue-specific depots of somatic stem cells are crucial for repair and regeneration167. PolG Mutator mice show impaired neural and hematopoietic progenitor cell self-renewal as early as embryogenesis, which can be rescued by administering the antioxidant N-acetylcysteine (NAC) to pregnant females168.
Benefits of exercise training on mitochondrial preservation
Substantial evidence supports a role for exercise in mitochondrial preservation169, 170. Four weeks of voluntary treadmill running in 7–9 week old mice increases the mitochondrial number and volume in their left ventricles171. Exercising Mutator mice on a treadmill for five months attenuates their cardiac hypertrophy and fibrosis, in addition to protecting against apoptosis and the decrease in complexes of the mitochondrial respiratory chain in the heart172. As with catalase overexpression, the cardioprotective benefits of exercise in PolG mice likely involve ROS detoxifying mechanisms. Indeed, exercise in a variety of tissues, including the heart, has been shown to increase antioxidant capacity by augmenting ROS scavenging enzymes such as catalase, superoxide dismutase, and glutathione peroxidase173–178.
An integral relationship exists between exercise and transcriptional regulators that limit ROS levels. These factors include the family of PPAR-gamma coactivators, nuclear factor-erythroid-derived 2-like 2 (NFE2L2), and the sirtuin family (SIRTs, silent information regulators). PGC-1α and β, regulators of mitochondrial biogenesis and respiratory capacity, coactivate nuclear respiratory factor 1 and 2 (NRF1 and 2) and estrogen-related receptors in the induction of genes important for oxidative phosphorylation and other mitochondrial processes179, 180. Chief among these is Tfam, which controls mitochondrial gene transcription as well as replication. Long-term and short-term endurance exercise increases PGC-1α expression in cardiac and skeletal muscle181–184. Exercise, at least in part through β-adrenergic signaling, augments PGC-1α activity and nuclear translocation, resulting in greater mitochondrial biogenesis183, 185. In contrast, PGC-1 shows reduced muscle expression in aging, coincident with decreased mitochondrial function186, 187. The lower mtDNA content, impaired complex IV activity, and decreased ejection fraction of PolG hearts are largely corrected by the forced expression of PGC-1α188. The cardio-protective effects of PGC-1 likely are due, at least in part, to its ROS-lowering effects, as PGC-1α induces GPx1 and SOD2 in models of neurodegeneration 189. It is unclear whether the benefit comes solely from increased levels of PGC-1α in the heart, or from a systemic contribution from concurrent PGC-1α overexpression in skeletal muscle. Aged mice carrying this MCK-PGC-1α transgene have improved whole body metabolism in the form of greater insulin sensitivity and reduced sarcopenia and chronic inflammation190.
Exercise enhances antioxidant defenses and restores redox homeostasis in the aging myocardium via NFE2L2 as well. NFE2L2 trans-activates genes of the antioxidant response191, and is coactivated by PGC-1α during oxidative stress192, 193. The loss of redox capacity seen in aging is similarly observed in hearts lacking NFE2L2 194, 195. While aging hearts exhibit reduced NFE2L2-dependent antioxidant mechanisms, both acute exercise and several weeks of moderate exercise training in aged mice increase NFE2L2 activity and induction of its target pathways to near normal levels seen in young counterparts194, 196.
SIRTs are NAD+-dependent deacetylases that regulate cellular health and longevity. As sensors of nutrient flux and redox states, they help to maintain metabolic homeostasis. Two members in particular, SIRT1 and SIRT3, play important roles both in the aged myocardium and in antioxidant pathways. SIRT1 activates hypertrophic pathways via activation of AKT, and its forced high expression produces cardiac dysfunction197. In contrast, more moderate levels of SIRT1 transgenic expression reduce age-related hypertrophy, fibrosis, and dysfunction, as well as damage from oxidative stress from paraquat198, 199. Mice lacking SIRT3 have the hallmarks of premature aging, and show greater hypertrophy and fibrosis in response to the pressure overload of transverse aortic banding, while SIRT3 overexpression confers resistance to hypertrophy driven by angiotensin-II200, 201. Like SIRT1, SIRT3 protects against oxidative stress, in large part through FOXO3a-dependent mechanisms that induce superoxide dismutase and catalase202. Notably, these two sirtuins are upregulated during exercise in heart and/or skeletal muscle, and are positive modulators of PGC-1α activity 134, 203–208. Caloric restriction, likely in concert with SIRTs, helps preserve energy handling in the aging heart and reduce cardiomyocyte apoptosis8. Like exercise, it induces PGC-1α in the heart and leads to preserved mitochondrial function during aging 209.
Even in the setting of a heterogeneous response to mtDNA damage within the myocardium during aging, it is likely that exercise nevertheless mitigates damage to discrete subsets of cells that are more susceptible to the effects of ROS. In skeletal muscle at least, moderate intensity endurance exercise in rats protects against the age-associated loss of satellite cells210. Interestingly, despite the large body of evidence supporting a causal relationship between ROS and mitochondrial dysfunction in cardiac senescence, nonspecific reduction of ROS has led to surprising results. In clinical trials, antioxidant dietary supplements are not associated with reduced mortality, but rather, in the case of beta carotene, vitamin A, and vitamin E, increased mortality211. Some studies paint a more complex picture, suggesting that an exercise-induced increase in ROS signals to and activates endogenous mechanisms of antioxidant defense. In both human and rat skeletal muscle, oral administration of the antioxidant vitamin C reduces mitochondrial biogenesis induced by exercise, and lowers the expression of PGC-1α, NRF1, Tfam, and cytochrome c212. The combination of vitamins C and E likewise blunt exercise-mediated increases in PGC-1α, PGC-1β, and ROS scavengers in skeletal muscle in healthy human subjects213. These studies highlight the delicate balance between the harmful effects of excessive ROS that accelerates senescence and the requirement for some basal level of ROS that maintains critical signaling pathways and cellular homeostasis. In the aging heart, this concept of “mitohormesis” surely plays a crucial role in conveying exercise’s benefits.
Can exercise reverse cardiac aging in humans?
As highlighted throughout this review, exercise training in aged animal models has raised the exciting possibility that exercise can reverse cardiac aging phenotypes associated with HF. Whether similar effects can be derived from exercise in older humans, however, has yet to be defined.
Cross-sectional studies comparing sedentary and athletic older adults, have suggested that lifelong physical activity is associated with less age-related changes in the heart63, 214–216. However, inherent limitations in cross-sectional analyses include potential selection bias of “fitter” individuals and those adhering to healthier lifestyles leading to unrecognized confounding or even “reverse causality” in which individuals with better cardiac function are more likely to be lifelong exercisers. Thus, it is impossible to conclude from such studies whether lifelong exercise is causally related to these changes. Furthermore, whether exercise can actually reverse established age-related myocardial changes, and if so, whether these changes are directly causal in improving exercise capacity or cardiovascular outcomes in the elderly, remain unknown.
A number of small prospective studies have attempted to address such questions by looking at the effects of exercise training on cardiac structure and function in previously sedentary older adults, with our without HF. While some studies have suggested that training improves resting cardiac parameters associated with HF in the elderly, including diastolic dysfunction217, systolic reserve capacity61, and chronotropic incompetence19, there are an equal number of studies that have shown that training, while similarly improving exercise capacity, does not significantly alter any of these cardiac aging phenotypes24, 218–220. Rather, these latter studies argue that exercise-mediated improvements in functional capacity in older adults are primarily derived from peripheral mechanisms of oxygen extraction in the skeletal muscle.
The reasons for these discrepancies are not entirely clear, but similar to training studies in animals, potentially stems from differences in exercise protocols, techniques for measuring cardiac structure/function, and the varying ages of participants studied. Cardiac senescence, as with other aging processes, is a progressive phenomenon. Thus, the often-used inclusion criteria for older adults as simply greater than 65 years, can yield variable results since a 65 year-old’s heart is often quite different from an 85 year-old’s. Furthermore, with emerging data from aged animals (Table 2) indicating that a sufficient “dose” of exercise is likely necessary to alter established aging phenotypes in the heart63, 64, what the requisite or optimal dose needed for older humans remains to be determined. Moreover, whether the intensity of exercise utilized in animal studies can be realistically achieved by frail older adults with cardiovascular disease may not be feasible. Ultimately, well-controlled, dose-response studies are needed to begin to answer some of these questions. However, what the growing body of exercise literature in aged animals provides is unique insights into how exercise can modulate the aging process in the heart, and thus, a framework for potentially identifying novel targets for treating age-related heart diseases.
Conclusion
With the rapidly changing distribution of age now occurring at this stage of human evolution, it is becoming increasingly important that we develop a deeper understanding of how cardiac aging impacts the health of our aging population. As highlighted in this review, exercise testing has already provided valuable insights into how cardiac physiology changes with age, and with further refinements will inevitably be a powerful tool for generating and translating discoveries from preclinical animal models. While it still remains to be defined how much exercise training impacts cardiac aging phenotypes in humans, emerging data from aging rodent models has suggested the exciting possibility that exercise can effectively modulate some of the aging process in the heart, and with that provides the promise of identifying novel targets for developing age-specific, tailored therapies for the older patient.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the NIH (AR[R01HL110733, R01HL122987], Roh[T32HL073734], and Rhee[T32GM007592]). Dr. Roh was also supported by a LaDue Fellowship Award from Harvard Medical School. AR is a principal faculty member of the Harvard Stem Cell Institute.
Non-standard Abbreviations and Acronyms
- HF
Heart failure
- CO
Cardiac output
- HR
Heart rate
- SV
Stroke volume
- VO2max
Maximum oxygen consumption
- Ca2+
Calcium
- β-AR
β-adrenergic receptor
- AC
Adenylyl cyclase
- PTX
Pertussin toxin
- PKA
Protein kinase A
- CaMKII
Ca2+/Calmodulin kinase 2
- EC
Excitation-contraction
- SERCA2a
Sarcoplasmic reticulum Ca2+–ATPase
- PLB
Phospholamban
- NCX
Na+/Ca2+ exchanger
- RyR
Ryanodine receptor
- MAPK
Mitogen activated protein kinase
- HDAC
Histone deacetylases
- NFAT
Nuclear factor of activated T cells
- IGF-1
Insulin like growth factor-1
- mTOR
Mammalian target of rapamycin
- GDF11
Growth differentiation factor 11
- TAC
Transverse aortic constriction
- ROS
Reactive oxygen species
- mtDNA
Mitochondrial DNA
- PolG
Polymerase gamma
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1α
- NFE2L2
Nuclear factor-erythroid-derived 2-like 2
- SIRT
Silent information regulator
- NRF
Nuclear respiratory factor
- Tfam
Mitochondrial transcription factor A
Footnotes
DISCLOSURES
None.
References
- 1.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clinics in geriatric medicine. 2009;25:563–577. doi: 10.1016/j.cger.2009.07.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. Journal of the American College of Cardiology. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 5.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circulation research. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circulation research. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodyak N, Kang PM, Hiromura M, Sulijoadikusumo I, Horikoshi N, Khrapko K, Usheva A. Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res. 2002;30:3788–3794. doi: 10.1093/nar/gkf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. Journal of the American College of Cardiology. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 10.Kappagoda T, Amsterdam EA. Exercise and heart failure in the elderly. Heart failure reviews. 2012;17:635–662. doi: 10.1007/s10741-011-9297-4. [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. The New England journal of medicine. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 12.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Jama. 1996;276:205–210. [PubMed] [Google Scholar]
- 13.Laughlin MH. Cardiovascular response to exercise. The American journal of physiology. 1999;277:S244–S259. doi: 10.1152/advances.1999.277.6.S244. [DOI] [PubMed] [Google Scholar]
- 14.Vella CA, Robergs RA. A review of the stroke volume response to upright exercise in healthy subjects. British journal of sports medicine. 2005;39:190–195. doi: 10.1136/bjsm.2004.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: the st. James women take heart project. Circulation. 2010;122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 16.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. The American journal of cardiology. 1986;57:1374–1379. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 18.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. Jama. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 20.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 21.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- 22.Fleg JL, O’Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. Journal of applied physiology. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 23.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. Journal of applied physiology. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 24.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. Journal of the American College of Cardiology. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. Journal of applied physiology. 1997;82:1411–1415. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- 27.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 28.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 29.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 31.Platt C, Houstis N, Rosenzweig A. Using exercise to measure and modify cardiac function. Cell metabolism. 2015;21:227–236. doi: 10.1016/j.cmet.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lujan HL, DiCarlo SE. Cardiac output, at rest and during exercise, before and during myocardial ischemia, reperfusion, and infarction in conscious mice. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R286–R295. doi: 10.1152/ajpregu.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Experimental gerontology. 1996;31:387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 34.Olfert IM, Balouch J, Mathieu-Costello O. Oxygen consumption during maximal exercise in Fischer 344 × Brown Norway F1 hybrid rats. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59:801–808. doi: 10.1093/gerona/59.8.b801. [DOI] [PubMed] [Google Scholar]
- 35.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends in cardiovascular medicine. 2012;22:29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics. 2008;33:100–109. doi: 10.1152/physiolgenomics.00269.2007. [DOI] [PubMed] [Google Scholar]
- 37.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation research. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie RH, Leo CH, Qin C, Stephenson EJ, Bowden MA, Buxton KD, Lessard SJ, Rivas DA, Koch LG, Britton SL, Hawley JA, Woodman OL. Low intrinsic exercise capacity in rats predisposes to age-dependent cardiac remodeling independent of macrovascular function. American journal of physiology Heart and circulatory physiology. 2013;304:H729–H739. doi: 10.1152/ajpheart.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaye DM, Esler MD. Autonomic control of the aging heart. Neuromolecular Med. 2008;10:179–186. doi: 10.1007/s12017-008-8034-1. [DOI] [PubMed] [Google Scholar]
- 41.Esler M, Skews H, Leonard P, Jackman G, Bobik A, Korner P. Age-dependence of noradrenaline kinetics in normal subjects. Clin Sci (Lond) 1981;60:217–219. doi: 10.1042/cs0600217. [DOI] [PubMed] [Google Scholar]
- 42.Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. The American journal of physiology. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 43.Davies CH, Ferrara N, Harding SE. Beta-adrenoceptor function changes with age of subject in myocytes from non-failing human ventricle. Cardiovascular research. 1996;31:152–156. [PubMed] [Google Scholar]
- 44.Xiao RP, Spurgeon HA, O’Connor F, Lakatta EG. Age-associated changes in beta-adrenergic modulation on rat cardiac excitation-contraction coupling. The Journal of clinical investigation. 1994;94:2051–2059. doi: 10.1172/JCI117559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM, et al. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation. 1994;90:1225–1238. doi: 10.1161/01.cir.90.3.1225. [DOI] [PubMed] [Google Scholar]
- 46.Xiao RP, Tomhave ED, Wang DJ, Ji X, Boluyt MO, Cheng H, Lakatta EG, Koch WJ. Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. The Journal of clinical investigation. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohm M, Dorner H, Htun P, Lensche H, Platt D, Erdmann E. Effects of exercise on myocardial adenylate cyclase and Gi alpha expression in senescence. The American journal of physiology. 1993;264:H805–H814. doi: 10.1152/ajpheart.1993.264.3.H805. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara N, Bohm M, Zolk O, O’Gara P, Harding SE. The role of Gi-proteins and beta-adrenoceptors in the age-related decline of contraction in guinea-pig ventricular myocytes. Journal of molecular and cellular cardiology. 1997;29:439–448. doi: 10.1006/jmcc.1996.0397. [DOI] [PubMed] [Google Scholar]
- 49.Jiang MT, Moffat MP, Narayanan N. Age-related alterations in the phosphorylation of sarcoplasmic reticulum and myofibrillar proteins and diminished contractile response to isoproterenol in intact rat ventricle. Circulation research. 1993;72:102–111. doi: 10.1161/01.res.72.1.102. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N, O’Gara P, Wynne DG, Brown LA, del Monte F, Poole-Wilson PA, Harding SE. Decreased contractile responses to isoproterenol in isolated cardiac myocytes from aging guinea-pigs. Journal of molecular and cellular cardiology. 1995;27:1141–1150. doi: 10.1016/0022-2828(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 51.Fowler MB, Laser JA, Hopkins GL, Minobe W, Bristow MR. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation. 1986;74:1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circulation research. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- 53.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. The Journal of clinical investigation. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Backs J, Worst BC, Lehmann LH, Patrick DM, Jebessa Z, Kreusser MM, Sun Q, Chen L, Heft C, Katus HA, Olson EN. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. The Journal of cell biology. 2011;195:403–415. doi: 10.1083/jcb.201105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 56.Brodde OE, Konschak U, Becker K, Ruter F, Poller U, Jakubetz J, Radke J, Zerkowski HR. Cardiac muscarinic receptors decrease with age. In vitro and in vivo studies. The Journal of clinical investigation. 1998;101:471–478. doi: 10.1172/JCI1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. Journal of the American College of Cardiology. 1997;29:187–193. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- 58.Stratton JR, Levy WC, Caldwell JH, Jacobson A, May J, Matsuoka D, Madden K. Effects of aging on cardiovascular responses to parasympathetic withdrawal. Journal of the American College of Cardiology. 2003;41:2077–2083. doi: 10.1016/s0735-1097(03)00418-2. [DOI] [PubMed] [Google Scholar]
- 59.Fleg JL, Schulman S, O’Connor F, Becker LC, Gerstenblith G, Clulow JF, Renlund DG, Lakatta EG. Effects of acute beta-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–2341. doi: 10.1161/01.cir.90.5.2333. [DOI] [PubMed] [Google Scholar]
- 60.Leosco D, Parisi V, Femminella GD, Formisano R, Petraglia L, Allocca E, Bonaduce D. Effects of exercise training on cardiovascular adrenergic system. Frontiers in physiology. 2013;4:348. doi: 10.3389/fphys.2013.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spina RJ, Turner MJ, Ehsani AA. Beta-adrenergic-mediated improvement in left ventricular function by exercise training in older men. The American journal of physiology. 1998;274:H397–H404. doi: 10.1152/ajpheart.1998.274.2.H397. [DOI] [PubMed] [Google Scholar]
- 62.Leosco D, Rengo G, Iaccarino G, Filippelli A, Lymperopoulos A, Zincarelli C, Fortunato F, Golino L, Marchese M, Esposito G, Rapacciuolo A, Rinaldi B, Ferrara N, Koch WJ, Rengo F. Exercise training and beta-blocker treatment ameliorate age-dependent impairment of beta-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. American journal of physiology Heart and circulatory physiology. 2007;293:H1596–H1603. doi: 10.1152/ajpheart.00308.2007. [DOI] [PubMed] [Google Scholar]
- 63.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. Journal of the American College of Cardiology. 2014;64:1257–1266. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wisloff U, Ellingsen O, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exercise and sport sciences reviews. 2009;37:139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 65.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–1672. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Scarpace PJ, Shu Y, Tumer N. Influence of exercise training on myocardial beta-adrenergic signal transduction: differential regulation with age. Journal of applied physiology. 1994;77:737–741. doi: 10.1152/jappl.1994.77.2.737. [DOI] [PubMed] [Google Scholar]
- 67.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. Journal of molecular and cellular cardiology. 2000;32:2075–2082. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- 68.Isenberg G, Borschke B, Rueckschloss U. Ca2+ transients of cardiomyocytes from senescent mice peak late and decay slowly. Cell calcium. 2003;34:271–280. doi: 10.1016/s0143-4160(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 69.Janczewski AM, Lakatta EG. Modulation of sarcoplasmic reticulum Ca(2+) cycling in systolic and diastolic heart failure associated with aging. Heart failure reviews. 2010;15:431–445. doi: 10.1007/s10741-010-9167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cain BS, Meldrum DR, Joo KS, Wang JF, Meng X, Cleveland JC, Jr, Banerjee A, Harken AH. Human SERCA2a levels correlate inversely with age in senescent human myocardium. Journal of the American College of Cardiology. 1998;32:458–467. doi: 10.1016/s0735-1097(98)00233-2. [DOI] [PubMed] [Google Scholar]
- 71.Taffet GE, Tate CA. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. The American journal of physiology. 1993;264:H1609–H1614. doi: 10.1152/ajpheart.1993.264.5.H1609. [DOI] [PubMed] [Google Scholar]
- 72.Lompre AM, Lambert F, Lakatta EG, Schwartz K. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circulation research. 1991;69:1380–1388. doi: 10.1161/01.res.69.5.1380. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 74.Lim CC, Liao R, Varma N, Apstein CS. Impaired lusitropy-frequency in the aging mouse: role of Ca(2+)-handling proteins and effects of isoproterenol. The American journal of physiology. 1999;277:H2083–H2090. doi: 10.1152/ajpheart.1999.277.5.H2083. [DOI] [PubMed] [Google Scholar]
- 75.Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. The American journal of physiology. 1998;275:H2087–H2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- 76.Fares E, Howlett SE. Effect of age on cardiac excitation-contraction coupling. Clin Exp Pharmacol Physiol. 2010;37:1–7. doi: 10.1111/j.1440-1681.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- 77.Kemi OJ, Ellingsen O, Smith GL, Wisloff U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Frontiers in bioscience : a journal and virtual library. 2008;13:356–368. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]
- 78.Kemi OJ, Ceci M, Condorelli G, Smith GL, Wisloff U. Myocardial sarcoplasmic reticulum Ca2+ ATPase function is increased by aerobic interval training. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2008;15:145–148. doi: 10.1097/HJR.0b013e3282efd4e0. [DOI] [PubMed] [Google Scholar]
- 79.Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisloff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. Journal of molecular and cellular cardiology. 2007;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tate CA, Helgason T, Hyek MF, McBride RP, Chen M, Richardson MA, Taffet GE. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. The American journal of physiology. 1996;271:H68–H72. doi: 10.1152/ajpheart.1996.271.1.H68. [DOI] [PubMed] [Google Scholar]
- 81.Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. The American journal of physiology. 1990;258:H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 82.Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104:221–226. doi: 10.1161/01.cir.104.2.221. [DOI] [PubMed] [Google Scholar]
- 83.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Matsuda M, Yamaguchi I. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. American journal of physiology Heart and circulatory physiology. 2004;286:H1696–H1705. doi: 10.1152/ajpheart.00761.2003. [DOI] [PubMed] [Google Scholar]
- 84.Thomas MM, Vigna C, Betik AC, Tupling AR, Hepple RT. Cardiac calcium pump inactivation and nitrosylation in senescent rat myocardium are not attenuated by long-term treadmill training. Experimental gerontology. 2011;46:803–810. doi: 10.1016/j.exger.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Walton RD, Jones SA, Rostron KA, Kayani AC, Close GL, McArdle A, Lancaster MK. Interactions of Short-Term and Chronic Treadmill Training With Aging of the Left Ventricle of the Heart. The journals of gerontology Series A, Biological sciences and medical sciences. 2015 doi: 10.1093/gerona/glv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–278. doi: 10.1161/01.cir.56.2.273. [DOI] [PubMed] [Google Scholar]
- 87.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Annals of internal medicine. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 88.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. European heart journal. 2008;29:741–747. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 89.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. The New England journal of medicine. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 90.Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, Angeli F, Yeghiazarians Y, Lee R. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Experimental gerontology. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation research. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 92.Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. Journal of the American College of Cardiology. 1986;8:1441–1448. doi: 10.1016/s0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- 93.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovascular research. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 95.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 96.van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovascular research. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 97.Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–432. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 98.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 99.Liao PH, Hsieh DJ, Kuo CH, Day CH, Shen CY, Lai CH, Chen RJ, Padma VV, Kuo WW, Huang CY. Moderate exercise training attenuates aging-induced cardiac inflammation, hypertrophy and fibrosis injuries of rat hearts. Oncotarget. 2015 doi: 10.18632/oncotarget.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manne ND, Kakarla SK, Arvapalli R, Rice KM, Blough ER. Molecular mechanisms of age-related cardiac hypertrophy in F344XBN rat model. J Clin Exp Cardiolog. 2014;5:1–6. [Google Scholar]
- 101.Ceylan-Isik AF, Dong M, Zhang Y, Dong F, Turdi S, Nair S, Yanagisawa M, Ren J. Cardiomyocyte-specific deletion of endothelin receptor A rescues aging-associated cardiac hypertrophy and contractile dysfunction: role of autophagy. Basic Res Cardiol. 2013;108:335. doi: 10.1007/s00395-013-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, Shioi T. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. doi: 10.1161/CIRCULATIONAHA.109.871137. [DOI] [PubMed] [Google Scholar]
- 104.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. GDF11 Does Not Rescue Aging-Related Pathological Hypertrophy. Circulation research. 2015 doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging cell. 2009;8:573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jackson MF, Luong D, Vang DD, Garikipati DK, Stanton JB, Nelson OL, Rodgers BD. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J Endocrinol. 2012;213:263–275. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 109.Rodgers BD, Interlichia JP, Garikipati DK, Mamidi R, Chandra M, Nelson OL, Murry CE, Santana LF. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. The Journal of physiology. 2009;587:4873–4886. doi: 10.1113/jphysiol.2009.172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell metabolism. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.DeMaria AN, Neumann A, Lee G, Fowler W, Mason DT. Alterations in ventricular mass and performance induced by exercise training in man evaluated by echocardiography. Circulation. 1978;57:237–244. doi: 10.1161/01.cir.57.2.237. [DOI] [PubMed] [Google Scholar]
- 113.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 114.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 115.Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, Michelucci A, Colella A, Galanti G. Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circulation research. 2001;89:977–982. doi: 10.1161/hh2301.100982. [DOI] [PubMed] [Google Scholar]
- 116.Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, Abel ED. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 119.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 120.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3’-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 121.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 122.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Wisloff U, Kemi OJ. Animal models in the study of exercise-induced cardiac hypertrophy. Physiol Res. 2010;59:633–644. doi: 10.33549/physiolres.931928. [DOI] [PubMed] [Google Scholar]
- 125.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell metabolism. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wright KJ, Thomas MM, Betik AC, Belke D, Hepple RT. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Experimental gerontology. 2014;50:9–18. doi: 10.1016/j.exger.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 127.Choi SY, Chang HJ, Choi SI, Kim KI, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Kim HS, Kim CH, Oh BH, Kim MH. Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. Journal of Korean medical science. 2009;24:32–39. doi: 10.3346/jkms.2009.24.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang W, Zhang H, Xue G, Zhang L, Zhang W, Wang L, Lu F, Li H, Bai S, Lin Y, Lou Y, Xu C, Zhao Y. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid Med Cell Longev. 2014;2014:457429. doi: 10.1155/2014/457429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derumeaux G, Ichinose F, Raher MJ, Morgan JG, Coman T, Lee C, Cuesta JM, Thibault H, Bloch KD, Picard MH, Scherrer-Crosbie M. Myocardial alterations in senescent mice and effect of exercise training: a strain rate imaging study. Circulation Cardiovascular imaging. 2008;1:227–234. doi: 10.1161/CIRCIMAGING.107.745919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:791–793. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- 131.Rossoni LV, Oliveira RA, Caffaro RR, Miana M, Sanz-Rosa D, Koike MK, Do Amaral SL, Michelini LC, Lahera V, Cachofeiro V. Cardiac benefits of exercise training in aging spontaneously hypertensive rats. Journal of hypertension. 2011;29:2349–2358. doi: 10.1097/HJH.0b013e32834d2532. [DOI] [PubMed] [Google Scholar]
- 132.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. American journal of physiology Heart and circulatory physiology. 2006;291:H1290–H1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 133.Huang CY, Yang AL, Lin YM, Wu FN, Lin JA, Chan YS, Tsai FJ, Tsai CH, Kuo CH, Lee SD. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. Journal of applied physiology. 2012;112:883–891. doi: 10.1152/japplphysiol.00605.2011. [DOI] [PubMed] [Google Scholar]
- 134.Lai CH, Ho TJ, Kuo WW, Day CH, Pai PY, Chung LC, Liao PH, Lin FH, Wu ET, Huang CY. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 2014;36:9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Putinski C, Abdul-Ghani M, Stiles R, Brunette S, Dick SA, Fernando P, Megeney LA. Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2013;110:E4079–E4087. doi: 10.1073/pnas.1315587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circulation research. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- 137.Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, Franke TF, Qian H, Bogoyevitch MA, Woodcock EA, Febbraio MA, Gregorevic P, McMullen JR. Phosphoinositide 3-kinase p110alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circulation Heart failure. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 138.Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH, Ho TJ, Kuo WW, Chang CH, Huang CY, Lin WT. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age (Dordr) 2014;36:9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 140.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 141.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 142.Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–C1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 143.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. Journal of molecular and cellular cardiology. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 144.Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, Schuler G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. European journal of heart failure. 2009;11:342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 145.Lenk K, Erbs S, Hollriegel R, Beck E, Linke A, Gielen S, Winkler SM, Sandri M, Hambrecht R, Schuler G, Adams V. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19:404–411. doi: 10.1177/1741826711402735. [DOI] [PubMed] [Google Scholar]
- 146.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circulation research. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Molkentin JD. Parsing good versus bad signaling pathways in the heart: role of calcineurin-nuclear factor of activated T-cells. Circulation research. 2013;113:16–19. doi: 10.1161/CIRCRESAHA.113.301667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chung E, Yeung F, Leinwand LA. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovascular research. 2013;100:402–410. doi: 10.1093/cvr/cvt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harman D. The biologic clock: the mitochondria? Journal of the American Geriatrics Society. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 150.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 151.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends in cardiovascular medicine. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khrapko K, Bodyak N, Thilly WG, van Orsouw NJ, Zhang X, Coller HA, Perls TT, Upton M, Vijg J, Wei JY. Cell-by-cell scanning of whole mitochondrial genomes in aged human heart reveals a significant fraction of myocytes with clonally expanded deletions. Nucleic acids research. 1999;27:2434–2441. doi: 10.1093/nar/27.11.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 155.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 156.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 159.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 160.Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PloS one. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T. Mitochondrial threshold effects. The Biochemical journal. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. The American journal of pathology. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson NG. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nature genetics. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 166.Baris OR, Ederer S, Neuhaus JF, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stockigt F, Schrickel JW, Wiesner RJ. Mosaic Deficiency in Mitochondrial Oxidative Metabolism Promotes Cardiac Arrhythmia during Aging. Cell metabolism. 2015;21:667–677. doi: 10.1016/j.cmet.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 167.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 168.Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, Suomalainen A. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell metabolism. 2012;15:100–109. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 169.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, Pallardo FV. Mitochondrial biogenesis in exercise and in ageing. Advanced drug delivery reviews. 2009;61:1369–1374. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 171.Eisele JC, Schaefer IM, Randel Nyengaard J, Post H, Liebetanz D, Bruel A, Muhlfeld C. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic research in cardiology. 2008;103:12–21. doi: 10.1007/s00395-007-0684-x. [DOI] [PubMed] [Google Scholar]
- 172.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 174.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. The Journal of experimental medicine. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Demirel HA, Powers SK, Caillaud C, Coombes JS, Naito H, Fletcher LA, Vrabas I, Jessup JV, Ji LL. Exercise training reduces myocardial lipid peroxidation following short-term ischemia-reperfusion. Medicine and science in sports and exercise. 1998;30:1211–1216. doi: 10.1097/00005768-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 176.Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. Journal of applied physiology. 1985;59:1298–1303. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- 177.Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. The American journal of physiology. 1993;265:H2094–H2098. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- 178.Somani SM, Frank S, Rybak LP. Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacology, biochemistry, and behavior. 1995;51:627–634. doi: 10.1016/0091-3057(94)00427-k. [DOI] [PubMed] [Google Scholar]
- 179.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 180.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. The Journal of clinical investigation. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bayod S, Del Valle J, Lalanza JF, Sanchez-Roige S, de Luxan-Delgado B, Coto-Montes A, Canudas AM, Camins A, Escorihuela RM, Pallas M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Experimental gerontology. 2012;47:925–935. doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 182.Kavazis AN, Smuder AJ, Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. Journal of applied physiology. 2014;117:223–230. doi: 10.1152/japplphysiol.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. The Journal of biological chemistry. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 184.Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R912–R917. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- 185.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21401–21476. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. The Journal of clinical investigation. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dillon LM, Rebelo AP, Moraes CT. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dillon LM, Williams SL, Hida A, Peacock JD, Prolla TA, Lincoln J, Moraes CT. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Human molecular genetics. 2012;21:2288–2297. doi: 10.1093/hmg/dds049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 190.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxidants & redox signaling. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 192.Cherry AD, Suliman HB, Bartz RR, Piantadosi CA. Peroxisome proliferator-activated receptor gamma co-activator 1-alpha as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. The Journal of biological chemistry. 2014;289:41–52. doi: 10.1074/jbc.M113.512483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR. p53 orchestrates the PGC-1alpha-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxidants & redox signaling. 2013;18:386–399. doi: 10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free radical biology & medicine. 2012;52:366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.He X, Ma Q. Redox regulation by nuclear factor erythroid 2-related factor 2: gatekeeping for the basal and diabetes-induced expression of thioredoxin-interacting protein. Molecular pharmacology. 2012;82:887–897. doi: 10.1124/mol.112.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KJ, Odelberg SJ, Firpo MA, Paine R, 3rd, Hoidal JR, Abel ED, Rajasekaran NS. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PloS one. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circulation research. 2014;114:368–378. doi: 10.1161/CIRCRESAHA.113.300536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circulation research. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 199.Hsu CP, Odewale I, Alcendor RR, Sadoshima J. Sirt1 protects the heart from aging and stress. Biological chemistry. 2008;389:221–231. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 200.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP. Mitochondrial SIRT3 and heart disease. Cardiovascular research. 2010;88:250–256. doi: 10.1093/cvr/cvq250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a–dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation research. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 204.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovascular research. 2011;90:276–284. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 205.Sugden MC, Caton PW, Holness MJ. PPAR control: it’s SIRTainly as easy as PGC. The Journal of endocrinology. 2010;204:93–104. doi: 10.1677/JOE-09-0359. [DOI] [PubMed] [Google Scholar]
- 206.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS letters. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochimica et biophysica acta. 2009;1790:1059–1066. doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PloS one. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 212.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. The American journal of clinical nutrition. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 213.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Forman DE, Manning WJ, Hauser R, Gervino EV, Evans WJ, Wei JY. Enhanced left ventricular diastolic filling associated with long-term endurance training. Journal of gerontology. 1992;47:M56–M58. doi: 10.1093/geronj/47.2.m56. [DOI] [PubMed] [Google Scholar]
- 215.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 216.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. The American journal of cardiology. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. Journal of the American College of Cardiology. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 218.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stratton JR, Cerqueira MD, Schwartz RS, Levy WC, Veith RC, Kahn SE, Abrass IB. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation. 1992;86:504–512. doi: 10.1161/01.cir.86.2.504. [DOI] [PubMed] [Google Scholar]
- 220.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation. 2001;104:1358–1366. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.