Abstract
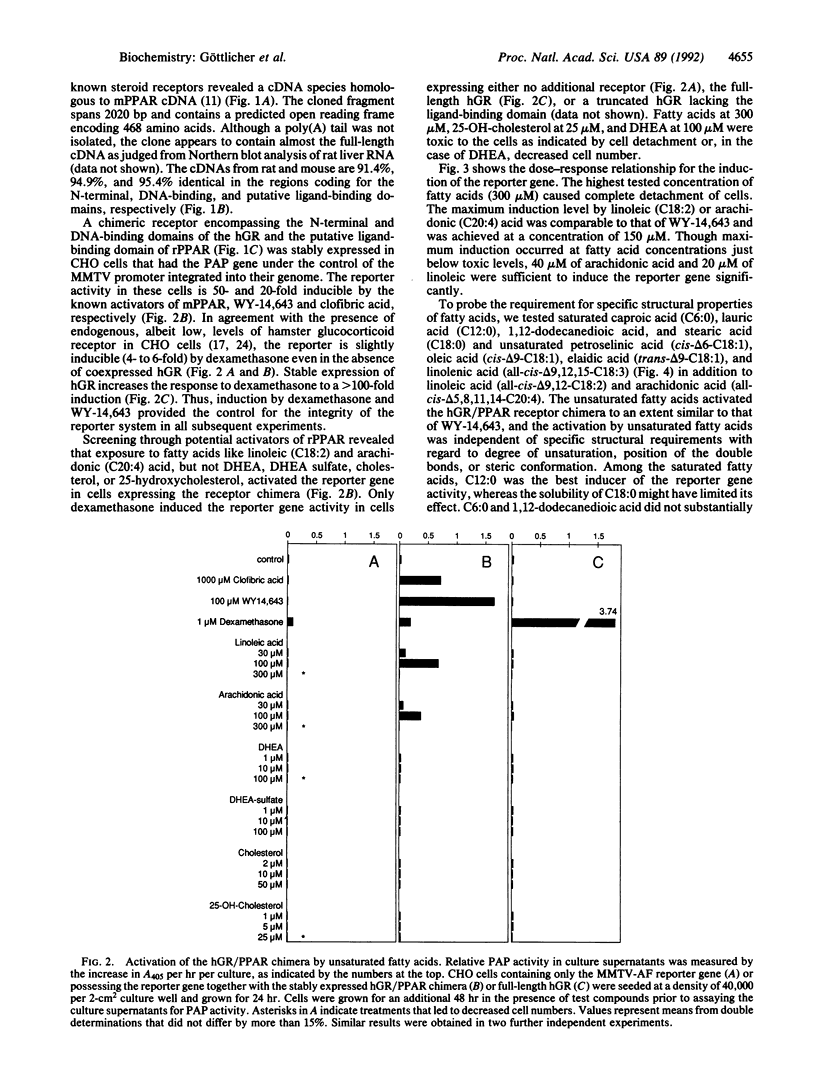
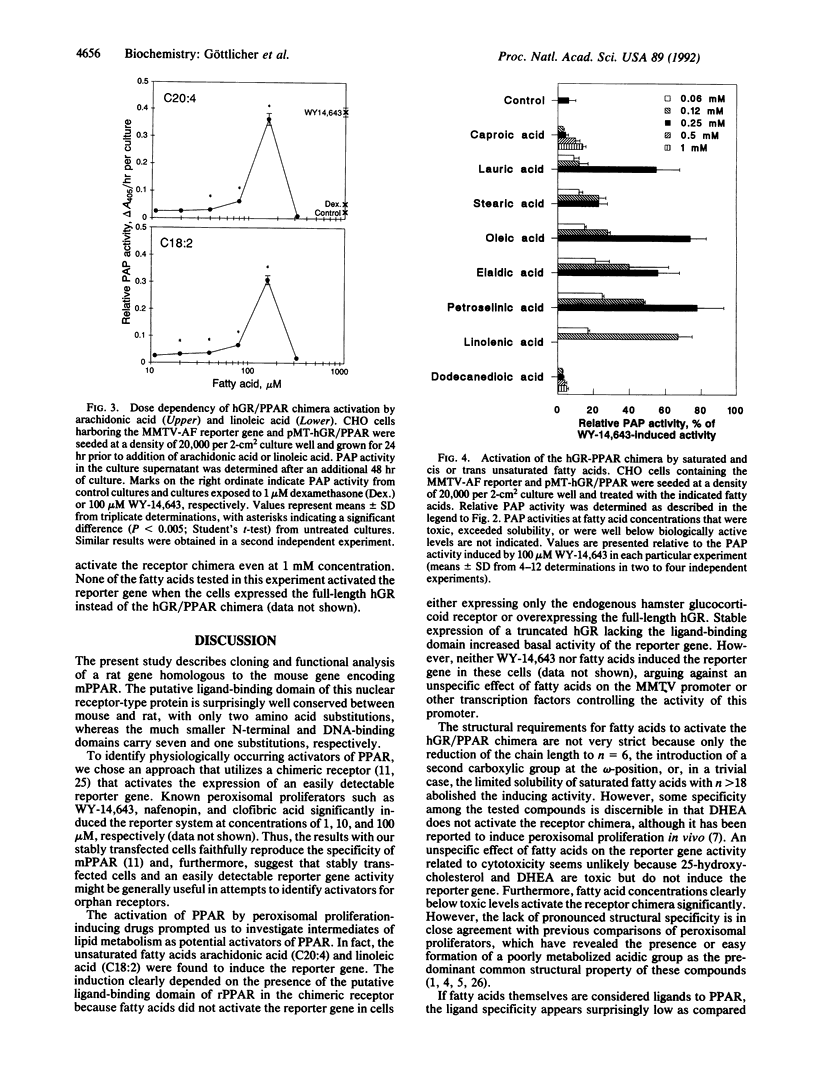
Peroxisome proliferators such as clofibric acid, nafenopin, and WY-14,643 have been shown to activate PPAR (peroxisome proliferator-activated receptor), a member of the steroid nuclear receptor superfamily. We have cloned the cDNA from the rat that is homologous to that from the mouse [Issemann, I. & Green, S. (1990) Nature (London) 347, 645-650], which encodes a 97% similar protein with a particularly well-conserved putative ligand-binding domain. To search for physiologically occurring activators, we established a transcriptional transactivation assay by stably expressing in CHO cells a chimera of rat PPAR and the human glucocorticoid receptor that activates expression of the placental alkaline phosphatase reporter gene under the control of the mouse mammary tumor virus promoter. Testing of compounds related to lipid metabolism or peroxisomal proliferation revealed that 150 microM concentrations of arachidonic or linoleic acid but not of dehydroepiandrosterone, cholesterol, or 25-hydroxy-cholesterol, activate the receptor chimera. In addition, saturated fatty acids induce the reporter gene. Shortening the chain length to n = 6 or introduction of an omega-terminal carboxylic group abolished the activation potential of the fatty acid. In conclusion, the present results indicate that fatty acids can regulate gene expression mediated by a member of the steroid nuclear receptor superfamily.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsland A., Aarsaether N., Bremer J., Berge R. K. Alkylthioacetic acids (3-thia fatty acids) as non-beta-oxidizable fatty acid analogues: a new group of hypolipidemic drugs. III. Dissociation of cholesterol- and triglyceride-lowering effects and the induction of peroxisomal beta-oxidation. J Lipid Res. 1989 Nov;30(11):1711–1718. [PubMed] [Google Scholar]
- Alksnis M., Barkhem T., Strömstedt P. E., Ahola H., Kutoh E., Gustafsson J. A., Poellinger L., Nilsson S. High level expression of functional full length and truncated glucocorticoid receptor in Chinese hamster ovary cells. Demonstration of ligand-induced down-regulation of expressed receptor mRNA and protein. J Biol Chem. 1991 Jun 5;266(16):10078–10085. [PubMed] [Google Scholar]
- Berge R. K., Aarsland A., Kryvi H., Bremer J., Aarsaether N. Alkylthioacetic acid (3-thia fatty acids)--a new group of non-beta-oxidizable, peroxisome-inducing fatty acid analogues. I. A study on the structural requirements for proliferation of peroxisomes and mitochondria in rat liver. Biochim Biophys Acta. 1989 Aug 22;1004(3):345–356. doi: 10.1016/0005-2760(89)90083-0. [DOI] [PubMed] [Google Scholar]
- Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988 Jun 15;66(1):1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Mitchell A. M. Peroxisome proliferation due to di(2-ethylhexyl) phthalate (DEHP): species differences and possible mechanisms. Environ Health Perspect. 1986 Dec;70:211–219. doi: 10.1289/ehp.8670211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatmark T., Christiansen E. N., Kryvi H. Evidence for a negative modulating effect of erucic acid on the peroxisomal beta-oxidation enzyme system and biogenesis in rat liver. Biochim Biophys Acta. 1983 Oct 11;753(3):460–466. doi: 10.1016/0005-2760(83)90071-1. [DOI] [PubMed] [Google Scholar]
- Frenkel R. A., Slaughter C. A., Orth K., Moomaw C. R., Hicks S. H., Snyder J. M., Bennett M., Prough R. A., Putnam R. S., Milewich L. Peroxisome proliferation and induction of peroxisomal enzymes in mouse and rat liver by dehydroepiandrosterone feeding. J Steroid Biochem. 1990 Feb;35(2):333–342. doi: 10.1016/0022-4731(90)90293-2. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Israel D. I., Kaufman R. J. Highly inducible expression from vectors containing multiple GRE's in CHO cells overexpressing the glucocorticoid receptor. Nucleic Acids Res. 1989 Jun 26;17(12):4589–4604. doi: 10.1093/nar/17.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Lock E. A., Mitchell A. M., Elcombe C. R. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annu Rev Pharmacol Toxicol. 1989;29:145–163. doi: 10.1146/annurev.pa.29.040189.001045. [DOI] [PubMed] [Google Scholar]
- Lundgren B., Meijer J., DePierre J. W. Examination of the structural requirements for proliferation of peroxisomes and mitochondria in mouse liver by hypolipidemic agents, with special emphasis on structural analogues of 2-ethylhexanoic acid. Eur J Biochem. 1987 Mar 2;163(2):423–431. doi: 10.1111/j.1432-1033.1987.tb10815.x. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Drug metabolism. Growth signal pathways. Nature. 1990 Oct 25;347(6295):709–710. doi: 10.1038/347709a0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Nestel P. J. Effects of N-3 fatty acids on lipid metabolism. Annu Rev Nutr. 1990;10:149–167. doi: 10.1146/annurev.nu.10.070190.001053. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W. Did eucaryotic steroid receptors evolve from intracrine gene regulators? Endocrinology. 1989 Sep;125(3):1119–1120. doi: 10.1210/endo-125-3-1119. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Phillipson B. E., Rothrock D. W., Connor W. E., Harris W. S., Illingworth D. R. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985 May 9;312(19):1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis. 1987 May;8(5):631–636. doi: 10.1093/carcin/8.5.631. [DOI] [PubMed] [Google Scholar]
- SCHRADE W., BOEHLE E., BIEGLER R., TEICKE R., ULLRICH B. [Gas chromatographic studies on serum fatty acids in man. Part 3. The fatty acids of cholesterol esters, phospholipids and triglycerides, and of unsaturated fatty acids in healthy subjects and in arteriosclerosis]. Klin Wochenschr. 1960 Aug 1;38:739–753. doi: 10.1007/BF01487275. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Marnett L. J. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta. 1991 Apr 24;1083(1):1–17. doi: 10.1016/0005-2760(91)90119-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wu H. Q., Masset-Brown J., Tweedie D. J., Milewich L., Frenkel R. A., Martin-Wixtrom C., Estabrook R. W., Prough R. A. Induction of microsomal NADPH-cytochrome P-450 reductase and cytochrome P-450IVA1 (P-450LA omega) by dehydroepiandrosterone in rats: a possible peroxisomal proliferator. Cancer Res. 1989 May 1;49(9):2337–2343. [PubMed] [Google Scholar]




