Significance
It is widely believed that the superior colliculus (SC) is unable to use input from short-wavelength–sensitive cones (S-cones) in the retina. If this idea were true, the role of the SC in nearly any visual-oculomotor behavior could be tested by using an S-cone stimulus to eliminate involvement of the SC. Contrary to this assumption, we show that express saccades, an SC-dependent behavior, are sensitive to S-cone contrast. This finding demonstrates that the SC is able to use input from S-cones to guide behavior, and that S-cone stimuli cannot be used to infer SC contributions to behavior.
Keywords: S-cone, SC, macaque
Abstract
A key structure for directing saccadic eye movements is the superior colliculus (SC). The visual pathways that project to the SC have been reported to carry only luminance information and not color information. Short-wavelength–sensitive cones (S-cones) in the retina make little or no contribution to luminance signals, leading to the conclusion that S-cone stimuli should be invisible to SC neurons. The premise that S-cone stimuli are invisible to the SC has been used in numerous clinical and human psychophysical studies. The assumption that the SC cannot use S-cone stimuli to guide behavior has never been tested. We show here that express saccades, which depend on the SC, can be driven by S-cone input. Further, express saccade reaction times and changes in SC activity depend on the amount of S-cone contrast. These results demonstrate that the SC can use S-cone stimuli to guide behavior. We conclude that the use of S-cone stimuli is insufficient to isolate SC function in psychophysical and clinical studies of human subjects.
Decades of oculomotor research have led to an incongruous conclusion: The oculomotor system does not use color information to guide the eyes (1, 2). However, it is natural to direct one’s gaze to objects defined by color. Color vision in primates evolved because of the tremendous benefit of being able to discriminate colors and direct our actions accordingly (3, 4). The superior colliculus (SC) is a brainstem structure with a central role in the transformation of visual sensory signals into saccadic eye movements (2). Visual projections to the SC lack color opponent responses and appear to be dominated by luminance information (5–9). Luminance signals arise almost exclusively from long- and medium-wavelength–sensitive cones (L- and M-cones) in the retina, but not short-wavelength–sensitive cones (S-cones) (10). Instead, S-cones evolved to contribute to color vision (11). The takeaway is that the SC does not use color or S-cone input to guide saccades. This conclusion is surprising because both the SC and S-cones are evolutionarily ancient, and the SC has a central role in visually guided orienting behavior (2, 11).
The proposition that the SC cannot detect S-cone stimuli has been leveraged to test SC function in diverse clinical and human psychophysical studies (12, 13). The scope of these investigations has ranged from mechanisms of blindsight (14–16), interhemispheric transfer in patients without a corpus callosum (17), face processing (18), and visual development (19) to inhibition of return (20), nasotemporal asymmetry (21), and the gap effect (22). The rationale for these experiments comes from an influential study by Sumner et al. (23), who noted that previous physiological and anatomical experiments had failed to find S-cone input to the SC. The idea is to present subjects with either a luminance or S-cone stimulus on separate trials of a visual or oculomotor task. If behavior [usually saccadic reaction time (RT)] is different in response to the S-cone stimulus, the conclusion is that the phenomenon under study depends on the SC. The argument is that because the SC cannot detect the S-cone stimulus, it cannot generate a behavior that depends on the S-cone stimulus.
The major appeal of this strategy is that if it were true, it would allow researchers to “lesion” the SC selectively on a trial-by-trial basis in healthy human (or animal) subjects. Under this assumption, any observed visual or oculomotor behavioral phenomenon could theoretically be tested to assess whether it arises from brainstem mechanisms in the SC. Clinically, after cortical damage, an experimenter could test whether recovery or survival of any visual or oculomotor behavior is the result of neural plasticity in the SC, allowing it to take over control of the behavior.
Only recently have studies begun to challenge the view that the oculomotor system is confined to luminance channels (1, 24). White et al. (24) demonstrated color sensitivity in SC neurons. Their goals did not involve testing the assumption that the SC can be blocked using S-cone stimuli, and they did not examine how an SC-dependent behavior changes with color contrast. Our recent work shows that SC neurons in the macaque do respond to calibrated S-cone stimuli under conditions identical to the conditions used in human psychophysics (12). We showed that manipulating S-cone contrast modulates SC neural responses. The key piece of information missing from these previous physiological studies of color in the SC, and specifically S-cone sensitivity, is how it relates to behavior. Behavior is the fundamental output measured in human psychophysics. No study has demonstrated a relationship between S-cone–driven activity in the SC and an SC-dependent behavior. Researchers have continued to consider it a good strategy to use S-cone stimuli to isolate the SC (13, 25, 26). Observed differences in behavioral RT between S-cone and luminance stimuli seem to contradict the finding that S-cone and luminance stimuli activate SC neurons equally well (26). It has instead been argued that S-cone stimuli reach the SC via longer, slower visual pathways than luminance stimuli.
Although the SC plays a role in all saccadic behavior, there is a specific subclass of saccades, known as express saccades (27), that depends critically on the SC (28). Express saccades are saccades with extremely short RTs (as short as 70 ms), approaching minimum sensory and motor neuron conduction delays (29). Express saccades are not eliminated by lesions of the frontal eye fields or several other areas (30). In contrast, express saccades are completely and selectively abolished after SC inactivation or lesion (28). Fittingly, two hallmark neural correlates of express saccade behavior have been observed in the SC. First, after target presentation, SC neurons generate a large single burst of visual and oculomotor activity, compared with the two smaller distinct visual and oculomotor bursts generated on regular latency saccade trials (31). Second, SC neurons exhibit greater preparatory activity on express saccade trials than regular saccade trials. SC neurons begin to increase their activity before the saccade target is presented (32).
In the present study, we capitalized on the tight relationship between express saccade behavior and the SC to test whether SC neurons actually use the S-cone input they receive to direct behavior. We reasoned that if the SC is able to transform S-cone input into a behavioral output, then we should observe express saccades to psychophysically calibrated S-cone isolating targets. Further, express saccades to S-cone targets should depend on S-cone contrast, as has been shown for luminance contrast (33, 34). The two SC neural hallmarks of express saccade generation should also be present when S-cone targets are used. If the SC uses S-cone input to drive express saccades, varying S-cone contrast should modulate SC neuronal hallmarks of express saccades in parallel with behavior. We tested these hypotheses using the methods standard in human psychophysical studies, and demonstrate that the SC can use S-cone contrast to drive express saccades in the same manner, and as rapidly, as luminance contrast.
Results
We asked whether the primate SC uses S-cone input to guide behavior. We measured behavioral responses from two monkeys and neuronal responses from 138 intermediate layer SC neurons (112 from monkey CA, 26 from monkey FS). Behavioral and neural responses were measured while monkeys performed a memory-guided gap task (Fig. 1), a task that frequently elicits express saccades. Removing the fixation cross at a fixed time before target presentation both frees fixation-related inhibition and creates a temporal cue, which allows visual stimulation to drive quick behavioral responses, including express saccades (32, 35–37). Three different saccade targets were used: a luminance target, a high-contrast S-cone target, and a low-contrast S-cone target. S-cone targets were psychophysically calibrated at six spatial locations in each monkey (38). Calibration is critical because a true S-cone isolating stimulus varies across individuals and retinal locations. Using a luminance target in addition allows us to compare results using S-cone isolating targets with a large body of evidence linking the SC and express saccades. All targets were only presented at retinal locations where S-cone stimuli had been calibrated in each animal. SC neurons with receptive fields at calibrated target locations were sought for recording. The targets were presented on a flickering background of luminance noise to mask target luminance artifacts and replicate methods previously used in human subjects (23).
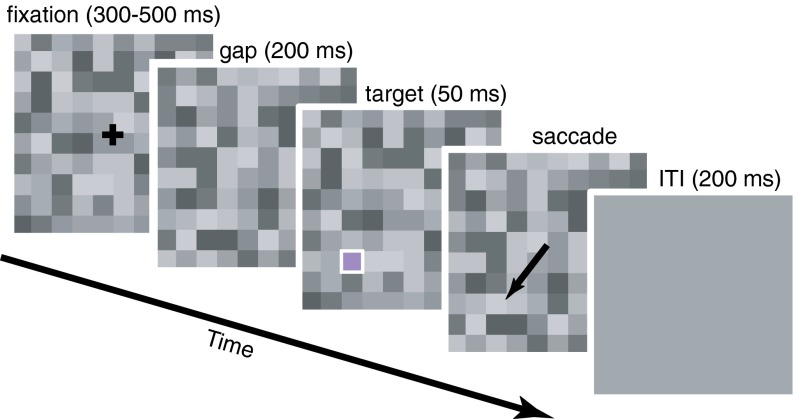
Fig. 1.
Memory-guided gap saccade task with luminance noise. After the initial fixation period, the fixation cross disappeared. After fixation offset, a fixed gap period began, followed by target presentation (outlined in white for clarity). The target was either in the receptive field of the recorded SC neuron or in the mirrored location in the opposite hemifield. Monkeys then made a saccade to the remembered target location. Target color and location were selected randomly interleaved on each trial. Locations were 45° above, 45° below, or on the horizontal meridian. Targets were 4° eccentricity in monkey FS and 6° eccentricity in monkey CA. ITI, intertrial interval.
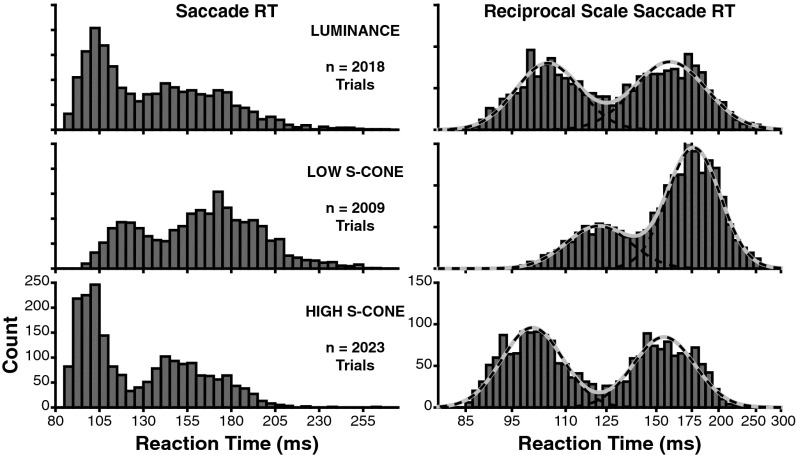
We computed saccadic RT during the gap task to determine whether express saccades were present to S-cone–defined targets (Fig. 2). The early mode of each RT distribution represents express saccades (27). Both animals exhibited express saccades to all three target types with qualitatively similar modes (Fig. S1). Data were therefore pooled in further analyses. The low contrast of our targets yielded express saccades with relatively long latencies compared with previous work using high-contrast white targets on black backgrounds (33). We used the luminance target trials as a baseline for express saccade generation (Fig. 2, top row). The novel finding is that express saccades are induced by S-cone targets (Fig. 2, bottom two rows). Importantly, express saccades depend critically on the S-cone contrast of the target. To measure this dependency, we plotted RTs on a reciprocal scale (Fig. 2, Right), which makes RTs follow a Gaussian distribution (39). We then fit a sum of two Gaussian distributions to quantify the latency and probability of express and regular RTs to each target type. Both the latency and probability of express saccades are modulated by the amount of S-cone contrast. Compared with luminance target trials, high S-cone contrast reduced the average latency (luminance = 104.5 ms, high S-cone = 100.2 ms; P < 0.05) and increased the proportion of express saccades (luminance = 46.4% of trials, high S-cone = 52.8% of trials; P < 0.05). When S-cone contrast was reduced, the latency of express saccades increased (121.8 ms) and their probability decreased (29.9% of trials) compared with both the high-contrast S-cone (latency and proportion both P < 0.05) and luminance (latency and proportion both P < 0.05) target trials.
Fig. 2.
Saccadic RT distributions during the gap task to the three target types. Distributions include data pooled across both monkeys, and both spatial locations (in receptive field and mirrored), for each target type. (Left) Raw RT distributions in 5-ms bins. (Right) Same RT data on a reciprocal time scale, which creates a normal distribution. Data are shown in 50 bins evenly spaced on a reciprocal scale. Solid gray curves show the best fit of a Gaussian mixture model. Dashed black lines show the fit of each individual component of the Gaussian mixture. Gaussian fits were rescaled to overlay the raw histograms for visualization purposes.
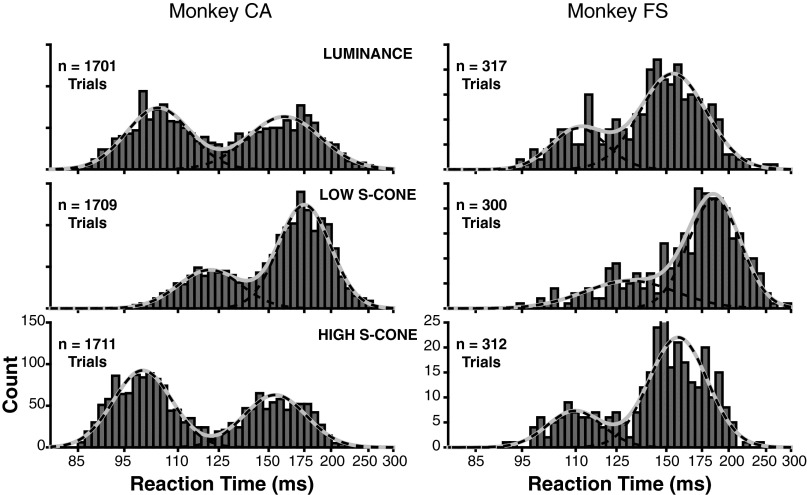
Fig. S1.
Individual animal saccadic RT distributions during the gap task to the three target types. (Left) Data from monkey CA. (Right) Data from monkey FS. Conventions are the same as in Fig. 2, Right. The MLE for both animals shows that the mean express saccade RT to the low-contrast S-cone target was significantly later than to the luminance and high-contrast S-cone targets. No significant differences between the luminance and high-contrast S-cone targets are detectable within the individual animals, but are present in data pooled across both animals (Fig. 2).
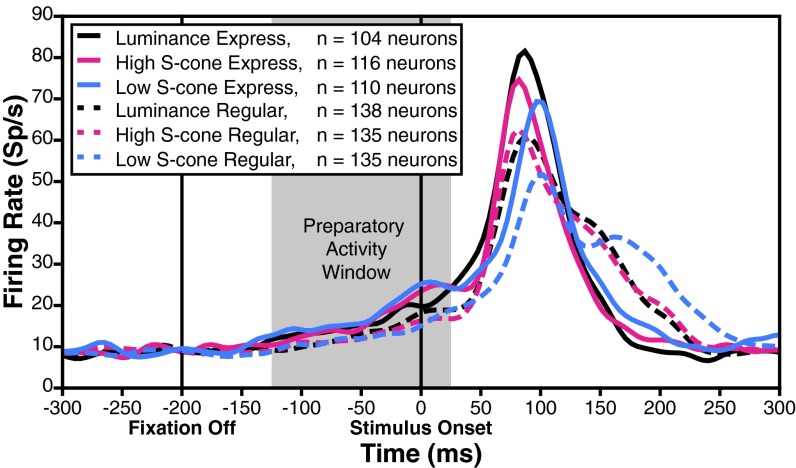
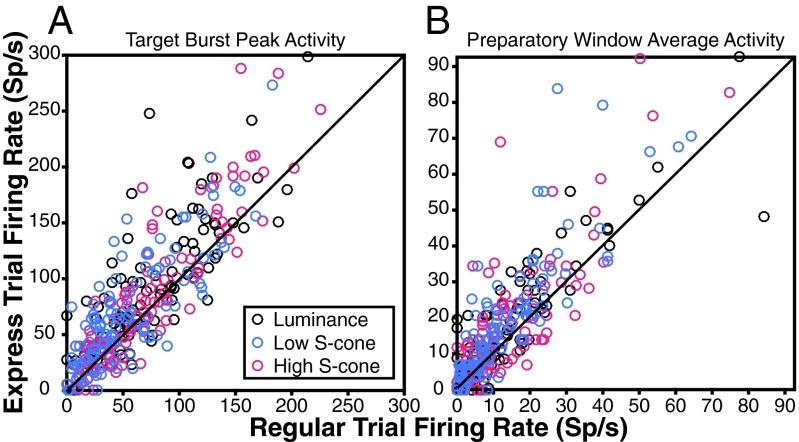
The first neural hallmark of express saccades is a larger initial burst of activity on express saccade trials compared with regular saccade trials. Trials were classified as either regular or express saccade latency using the distributions fit in Fig. 2, Right. Classification was done separately for each of the three target types, creating six separate categories of trials. During regular saccade trials (Fig. 3, dashed curves), average neuronal activity to the three target types showed an initial visual response, followed by a second burst indicative of a saccadic command. During express saccade trials (Fig. 3, solid curves) neural activity manifested as a larger unified burst. The express trial peak burst activity exceeds the peak burst activity on regular trials as expected for the luminance target [Fig. 3, black curves and Fig. 4A, black circles; Monte Carlo, P = 0.0002; Holm–Bonferroni (HB)-corrected, P < 0.05]. The S-cone target responses show the same neural hallmark. Express saccades to both the high-contrast S-cone and the low-contrast S-cone targets showed a greater peak than regular saccade trials (high S-cone: Fig. 3, magenta curves and Fig. 4A, magenta circles; Monte Carlo, P = 0.0232; low S-cone: Fig. 3, blue curves and Fig. 4A, blue circles; Monte Carlo, P < 0.0001; both HB-corrected, P < 0.05). This effect was found throughout the neuron population for all target types (Fig. 4A, most data points lie above the unity line). A majority of neurons tested had greater peak bursts on express saccade trials to all three target types (luminance: 70 of 104; binomial test, P = 2.671e−4; high S-cone: 70 of 116; binomial test, P = 0.0161; low S-cone: 71 of 110; binomial test, P = 0.0015; all HB-corrected, P < 0.05).
Fig. 3.
Neural responses to the three target types during express and regular latency saccade trials. Curves show the SDF across all neurons aligned on target onset for trials in which the target appeared in the receptive field. The gray-shaded region indicates the preparatory activity analysis window. Sp/s, spikes per second.
Fig. 4.
Individual neuron activity differences between express and regular saccade trials to each target type. Points above the unity line indicate neurons with greater response during express saccade trials for either the initial activity burst peak (A) or average preparatory window activity (B) (n = 104 luminance, 116 high S-cone, and 110 low S-cone neurons). Sp/s, spikes per second.
We next asked whether the first hallmark depends on the amount of S-cone contrast. We observed that the low-contrast S-cone target burst was later (72.9 ms) than both the high S-cone and luminance target bursts (Fig. 3, solid blue curve lags behind solid magenta and black curves; high S-cone: 60.0 ms; bootstrap, P < 0.05; luminance: 61.0 ms; bootstrap, P < 0.05). Changes in neuronal burst timing are in direct correspondence with the differences observed in express saccade RTs as S-cone contrast decreased (Fig. 2). Neural latency increased by 12.9 ms, and behavioral RT increased by 21.5 ms. Effects of S-cone target intensity on peak burst were examined in the subset of neurons with express saccade data to all three target types (94 neurons). We considered peak responses to the high-contrast S-cone and luminance targets together because they were similar and combining them increases statistical power. The median peak response was significantly lower to the low-contrast S-cone target than to the other targets combined (Fig. 3, solid curves, blue peak is less than the median of black and magenta peaks, and Fig. 4A, blue circles fall lower along the vertical axis than black and magenta circles; Monte Carlo, P = 0.0005). In fact, the majority of this neural subset had lower peak activity to the low-contrast S-cone target than to both the luminance and high S-cone targets considered separately (57 of 94; binomial test, P = 2.833e−13). Express saccade bursts in most SC neurons are present in response to S-cone targets, and sensitive to changes in S-cone contrast.
The second neural hallmark of express saccade generation is stronger preparatory activity in SC neurons on express saccade trials. Preparatory activity is reflected in the slow rise of the solid curves compared with the dashed curves in the preparatory window leading up to target response (Fig. 3, gray shading, 125 ms before to 25 ms after target presentation). We confirmed the expected presence of greater express than regular trial preparatory activity to the luminance target (Fig. 3, black curves and Fig. 4B, black circles; Monte Carlo, P = 0.0030; HB-corrected, P < 0.05). We then inspected the data for preparatory activity on S-cone target trials. Preparatory activity was considerably greater on express compared with regular trials for both contrasts of S-cone target (high S-cone: Fig. 3, magenta curves and Fig. 4B, magenta circles; Monte Carlo, P = 0.0053; low S-cone: Fig. 3, blue curves and Fig. 4B, blue circles; Monte Carlo, P < 0.0001; both HB-corrected, P < 0.05). Preparatory activity was evident at the single neuron level (Fig. 4B, most data points lie above the unity line). The majority of SC neurons exhibited express saccade trial preparatory activity to all three target types (luminance: 66 of 104; binomial test, P = 0.0039; high S-cone: 74 of 116; binomial test, P = 0.0019; low S-cone: 85 of 110; binomial test, P = 3.916e−4; all HB-corrected, P < 0.05).
Perhaps more important than the presence of the preparatory activity, per se, is whether preparatory activity is greater when S-cone contrast is low. If preparatory activity brings SC neurons closer to saccadic threshold, then greater preparatory activity should be present to trigger an express saccade when visual input is weaker (32, 34, 40). On trials when preparatory activity was atypically high, the relatively weak visual burst to the low-contrast S-cone stimulus was more likely to drive an express saccade. Our data provide resounding support for this hypothesis. Preparatory activity on express saccade trials was greater for the low-contrast S-cone target than for the luminance and high S-cone targets combined (Fig. 3, solid curves, blue preparatory activity is greater than the median of black and magenta; Fig. 4B, blue circles tend to lie higher along the vertical axis than black and magenta circles; Monte Carlo, P = 0.0294). Moreover, many SC neurons in this subset exhibited greater preparatory activity on low S-cone express trials than on both the luminance and high S-cone trials considered separately (42 of 94; binomial test, P = 2.603e−5). The SC displays express saccade preparatory activity that varies according to S-cone contrast, and the effects are present in individual cells.
Discussion
S-cone targets can drive express saccades, a behavior that depends on the SC. Two hallmarks of express saccade-related neural activity in the SC are present when S-cone targets are used: (i) a single visual-motor burst and (ii) preparatory activity. Both express saccade behavior and SC neural correlates depend on the amount of S-cone contrast in the target. This contrast modulation indicates that our results do not stem from trivial target selection or attention factors that could be computed at higher cortical levels. We conclude that the SC uses S-cone input to guide visual-oculomotor behavior.
Express saccade RT to an S-cone target can be made arbitrarily shorter or longer than RT to a luminance target by changing S-cone contrast. The total cone contrast of the luminance target in our study fell between the total cone contrast of the S-cone targets. Correspondingly, express saccade RTs to the luminance target were between RTs to the S-cone targets. It has been suggested that express saccades to S-cone targets are guesses or anticipations in human subjects (22). The fact that RT changed with S-cone contrast in the present experiments rules out this possibility. The shift in RT with contrast demonstrates that express saccades to S-cone targets were stimulus-dependent. Our data indicate that SC-dependent behavior hinges upon total cone contrast in the retina, rather than a special presence or absence of S-cone input.
The two SC neuronal hallmarks of express saccade generation changed in concert in an S-cone contrast-dependent manner. Previous models of saccadic RT (39, 40) and the role of intermediate-layer SC neurons in generating express saccades (31, 32, 34, 36, 41) predict a specific relationship between the initial burst and preparatory activity. If the initial visual burst is sufficient to push SC activity over saccadic threshold, an express saccade is triggered. Preparatory activity brings SC neurons closer to saccadic threshold, making it more likely that the initial burst will breach threshold and an express saccade will occur. This model offers a critical test of whether express saccades truly depend on SC responses to S-cone targets: greater preparatory activity should be present in order for the weak visual burst to the low-contrast S-cone target to push SC activity over threshold. Our results support this model prediction. Decreasing S-cone contrast decreased the visual burst, and greater preparatory activity was required to trigger an express saccade. Increasing S-cone contrast generated a larger burst that required less preparatory activity to trigger an express saccade. It should be understood here that the monkeys are not able to predict the upcoming target contrast. All target types were randomly interleaved. Rather, by chance, some trials have greater preparatory activity. On these trials, it is more likely that an express saccade will be elicited. When preparatory activity happens to be sufficiently high, an express saccade can be elicited on low-contrast S-cone target trials. The decreased proportion of express saccades made to the low-contrast S-cone target shows the behavioral impact of random variability in preparatory activity. Our findings support previous models of saccade generation and expand them to include color-specific contrast. The fact that data using S-cone targets fits well with a large body of research based on luminance targets implies that the SC makes use of S-cone contrast as it does luminance contrast.
RT differences between S-cone and luminance stimuli in previous studies can likely be interpreted as the result of using an S-cone stimulus that was too weak compared with the luminance control. A low S-cone contrast would lead to weaker SC neuronal responses and slower RTs to the S-cone stimulus. Our data show that behavioral and neural responses to the luminance and high-contrast S-cone targets were very similar, despite the fact that the high contrast S-cone target contained much greater total cone contrast. Total cone contrast of the luminance and low-contrast S-cone targets was much more similar, but their neural and behavioral results were very different. Most previous behavioral studies did not test different levels of S-cone contrast, and matching stimuli in contrast across cone types and visual mechanisms is a very difficult problem (42). Even when stimuli are equated in detectability (43), it is likely that S-cone and luminance channels function at different speeds (10, 44). To avoid the contrast-matching problem, we have shown that behavior and SC neuronal responses are sensitive to changes in S-cone contrast and can be shifted relative to a fixed luminance contrast. This sensitivity predicts that increasing S-cone contrast and/or decreasing luminance contrast could reverse results from previous psychophysical work that compared S-cone and luminance stimuli.
It has been argued that SC-dependent behavior to luminance stimuli might use the faster direct retinotectal pathway, whereas SC-dependent behavior to S-cone stimuli is slowed by rerouting through cortex (26). Although our experiments cannot distinguish whether S-cone stimuli traverse the retinotectal or corticotectal pathway, they do indicate that it is impossible to infer a specific pathway behaviorally. Express saccades are among the most rapid visual-motor behaviors, and approach the minimum required visual and motor neuron conduction delays via the corticotectal route (29). We have demonstrated that S-cone and luminance stimuli can trigger express saccades at the same latency. The parsimonious explanation is that both stimulus types use the same pathway(s). RTs of SC-dependent behavior to S-cone and luminance stimuli depend principally on the level of contrast. S-cone stimuli cannot be used to stop the SC from participating in behavior, and they cannot be used to deduce the contributions of specific visual pathways.
Materials and Methods
Two adult male rhesus monkeys (Macaca mulatta) were used in these experiments. Animals were cared for in accordance with NIH guidelines. The University of Pittsburgh Institutional Animal Care and Use Committee approved all experimental protocols. Monkeys weighed 13 kg and 8.5 kg (monkey CA and monkey FS, respectively). Surgical procedures and chamber placement have been described elsewhere (12).
Data Acquisition and Analysis.
Task timing and behavioral monitoring were continuously monitored and controlled online (Cortex software, provided by Robert Desimone, Massachusetts Institute of Technology, Cambridge, MA). Timing accuracy of our setup was verified with a photodiode to be within ±4 ms. Data were saved for offline analysis on a Plexon MAP system (Plexon, Inc.), along with spike timing. Our neuronal recording procedure, physiological identification of the SC, and cathode ray tube monitor calibration have been described previously (12). All data analyses were performed offline using custom MATLAB software (MathWorks).
Stimuli and Background.
The target and background presentation and calibration procedures have been described in full previously (12, 38). Briefly, targets were defined by either luminance or S-cone contrast with respect to an equal energy gray (EEG) background of luminance noise (23, 45). The background was a full screen array of 1° × 1° squares whose individual luminance changed at random every four monitor frames (∼47 ms at 85 Hz). This flickering background removes potential artifacts created by stimuli that are not exactly equiluminant with the background. Luminance values ranged from 18.78 to 22.55 cd/m2, spaced in increments of ∼0.5 cd/m2. The mean background luminance across all possible values was 20.73 cd/m2.
We converted targets and background to Derrington–Krauskopf-Lennie (DKL) contrast space to make the effects of contrast explicit (42, 46). The mean color and luminance of the background were used as the basis for conversion to DKL space. Following the procedure described by Brainard (42), we normalized DKL space such that a stimulus isolating a specific mechanism in DKL space with unit pooled cone contrast would correspond to a contrast of 100%. Contrasts are given in terms of each DKL visual mechanism with coordinates of the form [L + M, L − M, S − (L+M)]. The maximum decrement of the luminance noise background was (−16.2298, 0.0091, 1.0320), and the maximum increment was (15.3497, −0.0402, −0.2187). Targets were 1° × 1° squares embedded in the luminance noise background. The luminance contrast target (24.92 cd/m2) isolates the luminance mechanism (35.1922, 0.1817, 0.5641). The other two targets isolated the S-cone opponent mechanism. Their exact DKL coordinates varied by session and animal but spanned a relatively narrow range. The high-contrast S-cone target coordinates ranged from (−5.4318, 0.0157, 95.7475) to (−8.7634, −0.1684, 95.9704). The low-contrast S-cone target coordinates ranged from (−2.7450, 0.0462, 28.3539) to (−6.2916, −0.2824, 29.5714). Both S-cone targets slightly decreased DKL luminance and contained only small, inconsistent contrasts in the DKL L-M–opponent mechanism. Both deviations remained within the range covered by the background noise. S-cone targets presented on the noisy background were therefore detectable only on the basis of S-cone contrast, and are thus S-cone isolating.
Behavioral Tasks.
Receptive fields of recorded SC neurons were identified with a standard memory-guided saccade task. The memory-guided gap saccade task was used to measure behavioral and neuronal responses to the three target types. The fixation cross was 1° × 1° in size and black (<0.01 cd/m2). Animals fixated the central cross for 300–500 ms, and the fixation cross was then turned off. Fixation offset marks the beginning of the 200-ms (17-frame) gap period during which the background continued to flicker but no other stimuli were presented on the screen. The targets appeared for four frames synchronously with changes in the luminance noise background. The three target types and two possible spatial locations were randomly interleaved in each session. Animals were required to maintain fixation within a 1.5° × 1.5° square window for the duration of the fixation, gap, and target presentation. This procedure discouraged early guesses (only ∼1% of all trials were aborted during this interval across both animals) and ensured that the monkeys were presented with the target before being allowed to choose a saccade location. The monkeys were given 300 ms to initiate a saccade after the target was turned off. After leaving the fixation window, subjects were required to reach the 2° × 2° square target window within 30 ms to prevent corrective saccades. Monkeys maintained fixation within the target window for 200–400 ms to receive a liquid reward. During the intertrial interval (ITI), the computer screen was uniform EEG and the same luminance as the mean luminance of the background noise.
The memory-guided gap task parameters and brief ITI motivate the monkeys to make rapid saccades to targets but also prevent guessing and maintain accuracy. All target types, especially the low-contrast S-cone target, were subjectively difficult to detect for a human observer, and this difficulty was reflected in the monkeys’ behavior. Behavior was correct for 72–80% of the low-contrast S-cone target trials and for 85–93% of the high-contrast S-cone and luminance target trials. Errors were typically the result of failure to detect the target largely due to its low contrast. Nearly all errors were failures to initiate a saccade after target presentation or failures to reach the correct target.
Behavioral RT.
We analyzed behavior by computing saccadic RT on correct trials of the memory-guided gap saccade task. RT was measured as the time between target onset and the time at which the eye velocity first exceeded 30° per second. Trials with RTs less than 80 ms or more than 2 SDs below the mean express saccade mode (discussed below) were considered anticipations, and RTs greater than 300 ms were considered late. Both were removed from further analysis. RTs were combined across the two possible spatial locations for each target type. These procedures left a total of 2,018 luminance trials (1,701 for monkey CA, 317 for monkey FS), 2,009 low-contrast S-cone trials (1,709 for monkey CA, 300 for monkey FS), and 2,023 high-contrast S-cone trials (1,711 for monkey CA, 312 for monkey FS).
Reciprocal RT data were fit with a mixture of two Gaussians (39) using maximum likelihood estimates (MLEs). The MLEs were performed using a five-parameter probability density function (PDF) of the form:
| [1] |
where x is the reciprocal RT (1/RT), μ1 and μ2 are the mean of the first and second modes of the distribution (express and regular RTs), and σ1 and σ2 represent their SDs. The parameter P determines the proportion of the total probability that lies in each mode, and corresponds to the proportion of express saccades. The parameter P was constrained to lie on the interval [0,1], and σ1 and σ2 were constrained to be positive. To eliminate additional outliers, trials with RTs 2 SDs below the mean express saccade modality were removed (22–30 trials per target type). The data were then fit again with these outliers removed. The means and probabilities of the express and regular RTs are reported as the values of these fitted parameters. All behavioral statistical analyses reported in this paper were derived from the simultaneous 95% confidence intervals of the MLE parameters (Sidak-corrected for three comparisons: low S-cone vs. high S-cone, low S-cone vs. luminance, and high S-cone vs. luminance; i.e., 98.3% confidence intervals). Confidence intervals were obtained using a parametric bootstrap procedure performed by resampling (with a sample size equal to the original sample) the fitted distribution 1,000 times and performing a MLE of the parameters for each sample.
Neuronal Analyses.
We recorded from 138 neurons (112 from monkey CA, 26 from monkey FS) in the intermediate layers of the SC. Neuronal data were only considered for trials in which the target appeared in the receptive field of the recorded neuron. The spike trains from each neuron for each trial were sorted as belonging to either an express or regular saccade trial. Sorting for each trial was based on the fit of the Gaussian mixture PDF to the RT distributions. Only trials whose posterior probability of being in one of the two modes was at least 95% were included in the neuronal analysis. The remaining ambiguous trials were not included.
Our neural analysis has three target types and, in addition, express and regular saccades to each target type, yielding a total of six possible trial categories. We cannot control the number of express saccades generated to each target type in a given session or the number of trials excluded as ambiguous. Express saccades also varied with target type. We aimed to perform statistical tests using a repeated-measures design to reduce the impact of different firing rates across neurons and increase statistical power. Each neural analysis included as many neurons as possible, given these constraints. Many analyses were thus performed on subsets of the total recorded neural population. Neurons from both animals showed qualitatively similar neuronal response profiles and target differences during the gap task and were pooled for further analysis. It should be noted that despite the relatively smaller contribution of neurons from monkey FS, the data from that monkey contributed signal to the main results. Inclusion of monkey FS’s neurons in the analysis decreased P values of the statistical tests performed beyond what would be expected by a simple increase in sample size. The impact of data from monkey FS was assayed by resampling data from monkey CA (leaving out data from monkey FS) with n = the total number of neurons from both animals. Resampled results from monkey CA were then compared with results using the full dataset, including data from monkey FS.
To create the spike density functions (SDFs) in Fig. 3, spike trains for each neuron were placed in 5-ms bins. The average firing rate in each bin was computed and smoothed by convolving with a Gaussian kernel with σ = 10 ms. The SDFs for each individual neuron were then averaged together to create the population SDF. This procedure was done separately for each of the six categories, including all neurons with at least one trial in a given category. For express saccade trials, the totals were 104 neurons on luminance trials (91 from monkey CA, 13 from monkey FS), 116 neurons on high-contrast S-cone trials (103 from monkey CA, 13 from monkey FS), and 110 neurons on low-contrast S-cone trials (97 from monkey CA, 13 from monkey FS). For the regular saccade trials, data were included from 138 neurons on luminance trials (112 from monkey CA, 26 from monkey FS), 135 neurons on high-contrast S-cone trials (112 from monkey CA, 23 from monkey FS), and 135 neurons on low-contrast S-cone trials (112 from monkey CA, 23 from monkey FS).
Neuronal response latency was determined by finding the time at half-height of the peak response for the population SDF in the window from 25 to 150 ms after target onset. Half-height was considered as half of the peak SDF rate plus the average SDF rate from 200 to 0 ms before fixation offset. Statistical analysis for neuronal latency was performed by using bootstrap resampling (1,000 samples) of neurons for each category to create Sidak-corrected (for three comparisons) simultaneous 95% confidence intervals.
Rate comparisons between express and regular saccade trials (i.e., tests to establish the presence of neural hallmarks) were performed on the subset of neurons with at least one regular and one express trial to a given target type. This subset included 104 neurons on luminance trials (91 from monkey CA, 13 from monkey FS), 116 neurons on high-contrast S-cone trials (103 from monkey CA, 13 from monkey FS), and 110 neurons on low-contrast S-cone trials (97 from monkey CA, 13 from monkey FS). Peak activity analysis was performed on the SDF, computed as above, but for each neuron individually. The peak activity was defined as the maximum rate in a 50-ms window beginning at the population neuronal response latency for each of the six trial categories. Average preparatory activity was computed on the same subsets of neurons. The mean rate from 125 ms before to 25 ms after target onset was used in this case.
We used a Monte Carlo shuffling procedure with a repeated-measures design to determine activity differences between express and regular trials. The same procedures were used for both peak activity and mean preparatory activity comparisons. The activity of each neuron during express and regular latency trials was randomly shuffled (i.e., the label of being an express or regular trial was randomly assigned within each neuron). The test statistic was computed as the difference in median neuronal activity between the randomly assigned express and regular trials. The test statistic was computed 10,000 times to create the null distribution. Reported P values are given as the number of null distribution samples that equaled or exceeded the test statistic computed on the actual data, divided by 10,000. We then used binomial tests to determine the proportion of neurons that demonstrated this effect. The proportion of neurons with strictly greater express than regular trial activity (“successes”) was compared against an expected random proportion of 0.50. For both tests, we report raw actual P values and the HB-corrected P values for three comparisons at an alpha level of 0.05.
We also tested for differences between the low-contrast S-cone target activity and the combined luminance and high S-cone activity during express saccade trials. Neurons included in these analyses were required to have at least one express saccade trial to all three of the target types (94 neurons: 84 from monkey CA, 10 from monkey FS). To test whether activity differed between the target types, we again used a matched-pairs Monte Carlo shuffling procedure. This time, the activity of each neuron to each of the three target types was randomly shuffled (i.e., the label of being a luminance, high S-cone, or low S-cone trial was randomly assigned within each neuron). The test statistic was computed as the difference between the randomly assigned low S-cone trials’ median and the median of the combined randomly assigned luminance and high S-cone trials. The null distribution and P values were computed as above. We used binomial tests to determine the proportion of neurons that demonstrated a contrast effect. Neurons with lower peak activity (higher preparatory activity) on low-contrast S-cone trials than both luminance and high S-cone trials were counted as successes. All other neurons, including cases of strictly equal activity, were considered “failures.” The proportion of successes was compared with a null proportion of 0.25. This value represents the uniform probability of low-contrast S-cone activity falling into one of four possible categories: (i) less than high S-cone and luminance, (ii) less than high S-cone and greater than luminance, (iii) greater than high S-cone and less than luminance, and (iv) greater than high S-cone and luminance.
Acknowledgments
We thank Karen McKracken for technical support and our colleagues at the University of Pittsburgh and Center for the Neural Basis of Cognition for comments and feedback. This work was supported by NIH Grant MH103204.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6591.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600095113/-/DCSupplemental.
References
- 1.White BJ, Kerzel D, Gegenfurtner KR. Visually guided movements to color targets. Exp Brain Res. 2006;175(1):110–126. doi: 10.1007/s00221-006-0532-5. [DOI] [PubMed] [Google Scholar]
- 2.Schiller PH. The neural control of visually guided eye movements. In: Richards JE, editor. Cognitive Neuroscience of Attention: A Developmental Perspective. Lawrence Erlbaum Associates; Mahwah, NJ: 1998. pp. 3–50. [Google Scholar]
- 3.Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410(6826):363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- 4.Mollon JD. “Tho’ she kneel’d in that place where they grew...” The uses and origins of primate colour vision. J Exp Biol. 1989;146(1):21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- 5.de Monasterio FM. Properties of ganglion cells with atypical receptive-field organization in retina of macaques. J Neurophysiol. 1978;41(6):1435–1449. doi: 10.1152/jn.1978.41.6.1435. [DOI] [PubMed] [Google Scholar]
- 6.de Monasterio FM. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]
- 7.Schiller PH, Malpeli JG. Properties and tectal projections of monkey retinal ganglion cells. J Neurophysiol. 1977;40(2):428–445. doi: 10.1152/jn.1977.40.2.428. [DOI] [PubMed] [Google Scholar]
- 8.Schiller PH, Malpeli JG, Schein SJ. Composition of geniculostriate input of superior colliculus of the rhesus monkey. J Neurophysiol. 1979;42(4):1124–1133. doi: 10.1152/jn.1979.42.4.1124. [DOI] [PubMed] [Google Scholar]
- 9.Finlay BL, Schiller PH, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. IV. Corticotectal cells. J Neurophysiol. 1976;39(6):1352–1361. doi: 10.1152/jn.1976.39.6.1352. [DOI] [PubMed] [Google Scholar]
- 10.Calkins DJ. Seeing with S cones. Prog Retin Eye Res. 2001;20(3):255–287. doi: 10.1016/s1350-9462(00)00026-4. [DOI] [PubMed] [Google Scholar]
- 11.Hunt DM, Peichl L. S cones: Evolution, retinal distribution, development, and spectral sensitivity. Vis Neurosci. 2014;31(2):115–138. doi: 10.1017/S0952523813000242. [DOI] [PubMed] [Google Scholar]
- 12.Hall NJ, Colby CL. S-cone visual stimuli activate superior colliculus neurons in old world monkeys: Implications for understanding blindsight. J Cogn Neurosci. 2014;26(6):1234–1256. doi: 10.1162/jocn_a_00555. [DOI] [PubMed] [Google Scholar]
- 13.Smithson HE. S-cone psychophysics. Vis Neurosci. 2014;31(2):211–225. doi: 10.1017/S0952523814000030. [DOI] [PubMed] [Google Scholar]
- 14.Alexander I, Cowey A. Edges, colour and awareness in blindsight. Conscious Cogn. 2010;19(2):520–533. doi: 10.1016/j.concog.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Marzi CA, Mancini F, Metitieri T, Savazzi S. Blindsight following visual cortex deafferentation disappears with purple and red stimuli: A case study. Neuropsychologia. 2009;47(5):1382–1385. doi: 10.1016/j.neuropsychologia.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Leh SE, Mullen KT, Ptito A. Absence of S-cone input in human blindsight following hemispherectomy. Eur J Neurosci. 2006;24(10):2954–2960. doi: 10.1111/j.1460-9568.2006.05178.x. [DOI] [PubMed] [Google Scholar]
- 17.Savazzi S, et al. Interhemispheric transfer following callosotomy in humans: Role of the superior colliculus. Neuropsychologia. 2007;45(11):2417–2427. doi: 10.1016/j.neuropsychologia.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Higashida N, Kitazawa S. Facilitation of face recognition through the retino-tectal pathway. Neuropsychologia. 2013;51(10):2043–2049. doi: 10.1016/j.neuropsychologia.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Nakano T, Nakatani K. Cortical networks for face perception in two-month-old infants. Proc R Soc Lond B Biol Sci. 2014;281(1793):20141468. doi: 10.1098/rspb.2014.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumner P, Nachev P, Vora N, Husain M, Kennard C. Distinct cortical and collicular mechanisms of inhibition of return revealed with S cone stimuli. Curr Biol. 2004;14(24):2259–2263. doi: 10.1016/j.cub.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Bompas A, Sterling T, Rafal RD, Sumner P. Naso-temporal asymmetry for signals invisible to the retinotectal pathway. J Neurophysiol. 2008;100(1):412–421. doi: 10.1152/jn.90312.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson AJ, Carpenter RHS. The effect of stimuli that isolate S-cones on early saccades and the gap effect. Proc Biol Sci. 2008;275(1632):335–344. doi: 10.1098/rspb.2007.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumner P, Adamjee T, Mollon JD. Signals invisible to the collicular and magnocellular pathways can capture visual attention. Curr Biol. 2002;12(15):1312–1316. doi: 10.1016/s0960-9822(02)01020-5. [DOI] [PubMed] [Google Scholar]
- 24.White BJ, Boehnke SE, Marino RA, Itti L, Munoz DP. Color-related signals in the primate superior colliculus. J Neurosci. 2009;29(39):12159–12166. doi: 10.1523/JNEUROSCI.1986-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spering M, Carrasco M. Acting without seeing: eye movements reveal visual processing without awareness. Trends Neurosci. 2015;38(4):247–258. doi: 10.1016/j.tins.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizzi R, Michael GA. The role of the collicular pathway in the salience-based progression of visual attention. Behav Brain Res. 2014;270:330–338. doi: 10.1016/j.bbr.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 1983;260(1):21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- 28.Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57(4):1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- 29.Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci. 1993;16(3):553–567. [Google Scholar]
- 30.Schiller PH, Lee K. The effects of lateral geniculate nucleus, area V4, and middle temporal (MT) lesions on visually guided eye movements. Vis Neurosci. 1994;11(2):229–241. doi: 10.1017/s0952523800001590. [DOI] [PubMed] [Google Scholar]
- 31.Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol. 1996;76(2):908–926. doi: 10.1152/jn.1996.76.2.908. [DOI] [PubMed] [Google Scholar]
- 32.Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17(21):8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: Reaction times versus intensity, size, duration, and eccentricity of their targets. Exp Brain Res. 1984;55(2):223–231. doi: 10.1007/BF00237273. [DOI] [PubMed] [Google Scholar]
- 34.Marino RA, Levy R, Munoz DP. Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus. J Neurophysiol. 2015;114(2):879–892. doi: 10.1152/jn.00047.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paré M, Munoz DP. Saccadic reaction time in the monkey: Advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76(6):3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- 36.Sommer MA. Express saccades elicited during visual scan in the monkey. Vision Res. 1994;34(15):2023–2038. doi: 10.1016/0042-6989(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 37.Sommer MA. The spatial relationship between scanning saccades and express saccades. Vision Res. 1997;37(19):2745–2756. doi: 10.1016/s0042-6989(97)00078-3. [DOI] [PubMed] [Google Scholar]
- 38.Hall NJ, Colby CL. Psychophysical definition of S-cone stimuli in the macaque. J Vis. 2013;13(2):1–18. doi: 10.1167/13.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter RHS, editor. Oculomotor Procrastination. Lawrence Erlbaum Associates; Hillsdale, NJ: 1981. [Google Scholar]
- 40.Carpenter RHS, Williams MLL. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377(6544):59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- 41.Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18(17):7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brainard DH. Cone contrast and opponent modulation color spaces. In: Kaiser PK, Boynton RM, editors. Human Color Vision. 2nd Ed. Optical Society of America; Washington, DC: 1996. pp. 563–579. [Google Scholar]
- 43.Bompas A, Sumner P. Sensory sluggishness dissociates saccadic, manual, and perceptual responses: An S-cone study. J Vis. 2008;8(8):1–13. doi: 10.1167/8.8.10. [DOI] [PubMed] [Google Scholar]
- 44.Smithson HE, Mollon JD. Is the S-opponent chromatic sub-system sluggish? Vision Res. 2004;44(25):2919–2929. doi: 10.1016/j.visres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Birch J, Barbur JL, Harlow AJ. New method based on random luminance masking for measuring isochromatic zones using high resolution colour displays. Ophthalmic Physiol Opt. 1992;12(2):133–136. doi: 10.1111/j.1475-1313.1992.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984;357(1):241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]