Significance
Polysaccharide capsules are protective surface layers that enhance virulence of many pathogenic bacteria. Salmonella enterica serovar Typhi is the causative agent of typhoid fever, and it produces the virulence capsular polysaccharide known as “Vi antigen.” This glycan is part of some current vaccines. In some Gram-negative bacteria, capsular polysaccharides are attached to a conserved glycolipid that anchors the polysaccharide to the cell surface and is required for its transport across the cell envelope. S. enterica Typhi follows a different strategy; this work identifies a reducing terminal lipid structure unique to the Vi antigen that is required for attachment of the capsular surface layer. This lipid is structurally (and potentially biosynthetically) related to the conserved lipid A component of bacterial lipopolysaccharides.
Keywords: polysaccharide capsule, Vi antigen, Salmonella, glycolipid, polysaccharide export
Abstract
Polysaccharide capsules are surface structures that are critical for the virulence of many Gram-negative pathogenic bacteria. Salmonella enterica serovar Typhi is the etiological agent of typhoid fever. It produces a capsular polysaccharide known as “Vi antigen,” which is composed of nonstoichiometrically O-acetylated α-1,4-linked N-acetylgalactosaminuronic acid residues. This glycan is a component of currently available vaccines. The genetic locus for Vi antigen production is also present in soil bacteria belonging to the genus Achromobacter. Vi antigen assembly follows a widespread general strategy with a characteristic glycan export step involving an ATP-binding cassette transporter. However, Vi antigen producers lack the enzymes that build the conserved terminal glycolipid characterizing other capsules using this method. Achromobacter species possess a Vi antigen-specific depolymerase enzyme missing in S. enterica Typhi, and we exploited this enzyme to isolate acylated Vi antigen termini. Mass spectrometry analysis revealed a reducing terminal N-acetylhexosamine residue modified with two β-hydroxyl acyl chains. This terminal structure resembles one half of lipid A, the hydrophobic portion of bacterial lipopolysaccharides. The VexE protein encoded in the Vi antigen biosynthesis locus shares similarity with LpxL, an acyltransferase from lipid A biosynthesis. In the absence of VexE, Vi antigen is produced, but its physical properties are altered, its export is impaired, and a Vi capsule structure is not assembled on the cell surface. The structure of the lipidated terminus dictates a unique assembly mechanism and has potential implications in pathogenesis and vaccine production.
Many bacteria produce high-molecular-weight cell-surface polysaccharides that form a hydrated layer known as a “capsule.” There is enormous diversity in capsular polysaccharide (CPS) structures resulting from variations in sugar residue composition, linkage(s), and the addition of nonsugar substituents (1). The capsule is often the outermost structure of a bacterial cell and therefore is critical for interactions with the environment. Depending on the organism, capsules assist bacteria in resisting desiccation, forming biofilms, colonizing host tissues, resisting bacteriophages, and reducing opsonophagocytosis and complement-mediated killing (2). In Salmonella enterica, the virulence capsular polysaccharide, known as “Vi antigen,” is produced by human-restricted serovar Typhi (hereafter S. Typhi, the etiological agent of typhoid fever) and serovar Paratyphi C, but it is absent in other serovars commonly associated with gastroenteritis. Vi antigen capsule is implicated in the evasion of the innate immune system (reviewed in ref. 3). The production of Vi antigen reduces serum complement binding/killing and promotes intracellular replication; Vi antigen-deficient mutants are 10,000-fold less virulent in a mouse model of infection (4). Purified Vi antigen is currently used in parenteral vaccines (5).
Despite the structural diversity of Gram-negative CPS, most are synthesized by one of two widely distributed mechanisms, with model systems provided by Escherichia coli K antigens (reviewed in ref. 1). The two pathways are differentiated by the mechanism and location of the polymerization reaction and by the machinery that exports the nascent glycan (or its biosynthetic intermediates) across the cytoplasmic membrane. One of these systems requires a pathway-defining ATP-binding cassette (ABC) transporter to export CPS that is fully polymerized in the cytoplasm. This mechanism is shared by extraintestinal pathogenic E. coli (i.e., group 2 CPS), Neisseria meningitidis, Haemophilus influenzae, Campylobacter jejuni, and other pathogens of humans and livestock. In these bacteria, the CPS glycans are attached to a (lyso)phoshatidylglycerol moiety via an oligosaccharide of five to nine β-linked 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) residues (6). The Kdo-containing glycolipid is synthesized by conserved β-Kdo transferases (known as “KpsS” and “KpsC” in E. coli) (7). The CPS glycan is built on the nonreducing end of the β-Kdo oligosaccharide linker by serotype-specific glycosyltransferases. The lipidated terminus is essential for CPS export (6) and is likely recognized by the pathway-defining ABC transporters, which are interchangeable among CPS serotypes and species (reviewed in refs. 1 and 8). CPS translocation to the cell surface is believed to involve an envelope-spanning complex composed of the ABC transporter and members of the polysaccharide co-polymerase (PCP) and outer membrane polysaccharide export (OPX) protein families (1, 8).
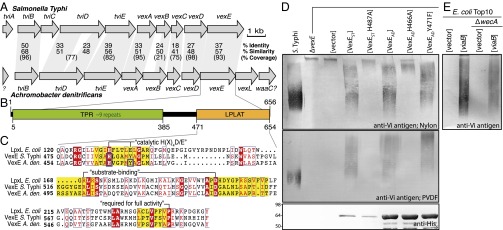
Vi antigen is a linear polymer of GalNAcA residues nonstoichiometrically O-acetylated at C-3 (9). The Vi antigen biosynthesis (viaB) operon encodes enzymes implicated in Vi antigen biosynthesis (TviA–E) as well as a characteristic ABC transporter (VexBC) and OPX (VexA) and PCP (VexD) homologs (Fig. 1A) (10, 11). Loci similar to S. Typhi viaB are found in the opportunistic pathogens Citrobacter freundii (12), Bordetella petrii (GenBank accession no. AM902716.1), and Achromobacter species (Fig. 1A), although the glycan product has not been investigated in either Bordetella or Achromobacter. The chromosomes of the Vi antigen-producing bacteria lack homologs of kpsS or kpsC that are found in all other currently known Gram-negative bacteria with ABC transporter-dependent CPS assembly pathways (8). Here we identify the structure of a glycolipid terminus unique to the Vi antigen and propose a biosynthetic origin that takes advantage of the conserved lipid A machinery in Gram-negative bacteria.
Fig. 1.
S. Typhi wild-type and ΔvexE mutant Vi antigens possess altered physical properties. (A) Shared organization and gene content in viaB loci. The viaB loci from Achromobacter sp. include an additional gene (vexL) encoding a Vi antigen lyase enzyme. The A. denitrificans sequence is deposited at GenBank (accession no. KT997721), but the same locus is found in other sequenced genomes of Achromobacter sp. [A. xylosoxidans (GenBank accession no. CP012046.1), A. arsenitoxydans (GenBank accession no. NZ_AGUF01000055.1), A. spanius (GenBank accession no. NZ_LGVG01000001.1), and A. piechaudii (GenBank accession no. ADMS01000020.1)]. (B) VexE contains a predicted N-terminal region of tetratricopeptide repeats (TPR), which form α-helical superstructures implicated in protein–protein interactions (25), and a C-terminal lysophospholipid acyltransferase (LPLAT) domain. (C) Multiple sequence alignment of the LPLAT domain of VexE from S. Typhi, A. denitrificans, and E. coli LpxL. Motifs characteristic of LPLAT are highlighted in yellow, and the putative role of particular residues is noted (17). Residues that were mutated are boxed in blue. (D) In immunoblots, Vi antigen produced by S. Typhi bound PVDF and positively charged nylon membranes, whereas Vi antigen from the ΔvexE mutant bound only nylon. The panels show immunoblots of proteinase K-digested whole-cell lysates probed with anti-Vi antigen antibody. PVDF binding was restored when the ΔvexE mutant was complemented with either S. Typhi vexE or A. denitrificans vexE. The corresponding putative catalytic mutants of VexE from either S. Typhi (H487A) or A. denitrificans (H466A) failed to restore PVDF binding. A Y471F mutation in the A. denitrificans enzyme (at position 6 of HX4(D/E) motif in VexE) had no discernible effect on its activity. VexE expression was monitored by Western blotting of hexahistidine-tagged VexE constructs from identical cell cultures. (E) Vi antigen is produced in E. coli Top10 and its ΔwecA mutant.
Results
Vi Antigen from a ΔvexE Mutant Has Altered Physical Properties.
Despite the absence of kpsS and kpsC from the chromosomes of viaB-positive bacteria, we speculated that a glycolipid terminus of some form may be a unifying feature for all ABC transporter-dependent CPS biosynthesis pathways. Other bacterial surface glycoconjugates exported by ABC transporters frequently use undecaprenyl diphosphate carrier lipids. To test the possibility that these lipids participated in Vi antigen production, the viaB locus was introduced into E. coli CWG1214 ΔwecA, which is unable to make undecaprenyl diphospho-N-acetylglucosamine in the obligatory first step in biosynthesis of E. coli LPS O antigens and enterobacterial common antigen (13). E. coli also lacks wbaP, whose gene product produces undecaprenyl diphospho-galactose in the corresponding initiation step for most Salmonella O antigens (13). E. coli CWG1214, transformed with the viaB locus, displayed robust Vi antigen production, evident in immunoblots (Fig. 1E), ruling out the logical candidates for undecaprenyl-active enzymes in Vi antigen assembly. All the viaB loci encode a predicted VexE protein containing a potential C-terminal lysophospholipid acyltransferase (LPLAT) motif (Fig. 1B). This motif is also found in E. coli LpxL (Fig. 1C), an acyl carrier protein (ACP)-dependent secondary acyltransferase involved in the biosynthesis of LPS lipid A, a conserved glycolipid essential for viability of almost all Gram-negative bacteria (reviewed in refs. 13 and 14) (see Fig. 4). LpxL and VexE share only 18% identity overall (e-value: 1.08 × 10−3), but the conserved LPLAT domain (cd07984) shares higher similarity (e-value: 1.67 × 10−8). The putative LPLAT motif in VexE led to the hypothesis that this protein is an acyltransferase that creates a different type of lipid terminus on Vi antigen chains.
Fig. 4.
Proposed model for the biosynthesis of the Vi antigen glycolipid terminus. The diacyl-HexNAc residue at the Vi antigen terminus originates from the secondary acylation of the UDP-activated acyl-GlcNAc product produced by LpxA in the conserved lipid A biosynthesis Raetz pathway (14).
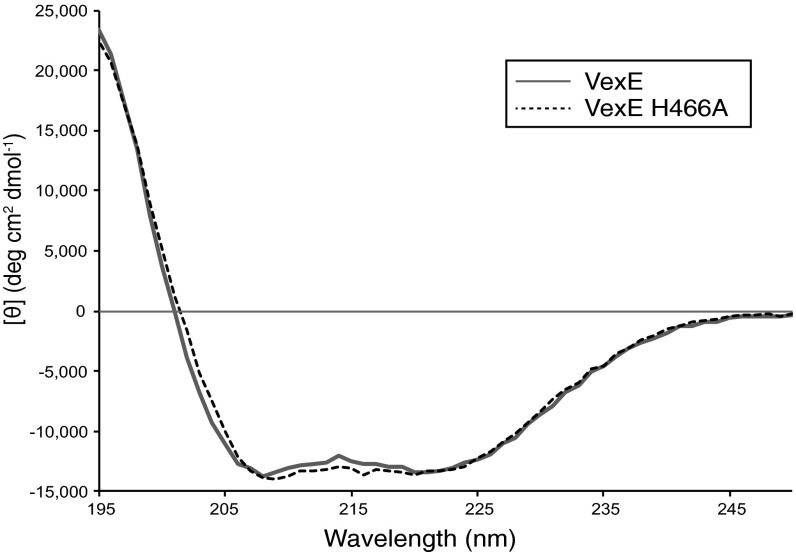
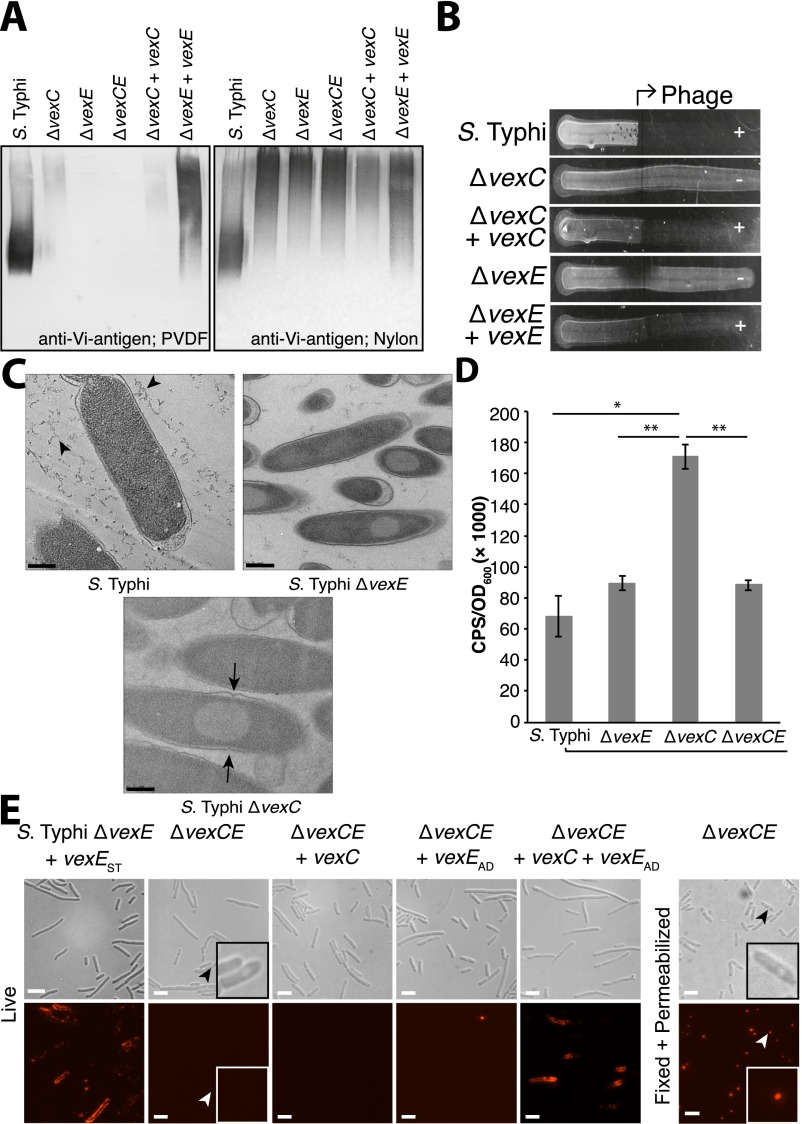
Previous analyses of Vi antigen phenotypes in viaB gene mutants were performed using recombinant E. coli transformed with plasmid-encoded viaB, but the possibility of a lipid terminus and the precise role of VexE has not been examined (10, 11). To avoid complications arising from multicopy gene expression and the unnatural host background, we examined the role of VexE using chromosomal mutations in S. Typhi. In Western immunoblots, Vi antigen in cell lysates of the parent strain bound to both hydrophobic PVDF and positively charged nylon membranes (Fig. 1D). In contrast, Vi antigen in lysates from the ΔvexE mutant bound only to nylon, indicating a change in the physical properties of Vi antigen produced by the mutant. Wild-type binding properties were restored in the mutant by the expression of VexE homologs from S. Typhi or Achromobacter denitrificans (Fig. 1D). LPLAT enzymes possess a conserved HX4D/E motif, which contains the essential catalytic His/Asp pair (Fig. 1C) (15). The corresponding H→A mutant in E. coli LpxL results in a >1,000-fold reduction in lauroyltransferase activity (16). The putative catalytic His residue was mutated in the VexE homologs from S. Typhi and A. denitrificans, and these proteins were expressed in S. Typhi ΔvexE. Vi antigen from these transformants did not bind to PVDF despite robust expression of the enzymes (Fig. 1D). Proper folding of the mutant VexE was confirmed by comparing circular dichroism spectra of purified wild-type and mutant proteins (Fig. S1). The catalytic activity of VexE therefore is linked to alterations in the physical properties of Vi antigens, resulting in differential binding to membranes with varying chemistries.
Fig. S1.
Mutation of the putative catalytic histidine residue of VexE does not affect folding of the protein. The CD spectra of purified VexE and VexEH466A from A. denitrificans are identical.
To determine whether the altered binding properties reflected differences in the repeat-unit structure of the parental and mutant Vi antigens, such as alterations in O-acetylation, CPS was purified, and its structure was examined by NMR. In the initial preparations we were unable to obtain wild-type Vi antigen free from LPS contamination, and substantial amounts of wild-type Vi antigen sedimented with LPS micelles in centrifugation. Deletion of vexE abrogated this property (Fig. S2B), adding weight to the contention that VexE influenced the physical properties of Vi antigen. To obtain Vi antigen free of LPS, we created ΔwaaG mutants generating truncated LPS molecules (resulting from the loss of most of the core oligosaccharide and O antigen; reviewed in ref. 15) that could be separated from Vi antigen by gel filtration chromatography (Fig. S2 A–C). 13C NMR spectra of Vi antigens from ΔwaaG and ΔwaaG ΔvexE double mutants were identical (Fig. S2 D and E and Table S1) and were comparable to those previously published (17). The altered properties of the Vi antigen produced by the vexE mutant therefore were not caused by changes in the polysaccharide backbone structure but could be explained by alterations in a putative acylated terminus.
Fig. S2.
Purification of LPS-free Vi antigen from S. Typhi ΔwaaG. Cells with an intact viaB locus produce Vi antigen that is contaminated by LPS, so a waaG mutant was constructed to generate truncated LPS molecules that could be separated from Vi antigen by gel filtration chromatography. WaaG is a glycosyltransferase that adds the first glucose residue to the outer core of LPS (35), so ΔwaaG mutants lack the attachment site for the O antigen. (A, Upper) Phenotypes of ΔwaaG mutants. The samples are proteinase K-digested whole-cell lysates immunoblotted with anti-Vi antigen antibody. (Lower) The corresponding gels were stained with silver to visualize LPS. The Vi antigen production of ΔwaaG mutants was not affected. The ΔvexE mutants produced Vi antigen with increased average sizes, and this effect is independent of the waaG mutation. The lower amounts of O antigen-substituted LPS in complemented waaG mutants as compared with the parent strain reflects the efficiency of complementation. (B) The association between LPS and extracted Vi antigen is eliminated in the ΔvexE mutant. The panels show material extracted by phenol-water and collected by centrifugation at 100,000 × g (P) and the polysaccharide remaining in the 100,000 × g supernatant (S). (Upper) Vi antigen was detected by Western immunoblotting. (Lower) LPS was detected by silver-staining the corresponding SDS/PAGE gel. (C) Separation of Vi antigen and LPS from S. Typhi by gel filtration chromatography in the presence of detergent [0.25% (wt/vol) sodium deoxycholate]. Elution was monitored by SDS/PAGE followed by Vi antigen immunoblots (Upper) and silver staining for LPS contamination (Lower). The purified Vi antigen was free of lipid A based on silver staining, and no lipid A contamination was evident in MS analysis of the lipid-enriched fraction obtained from purified Vi antigen (Fig. 2 and Fig. S4). (D) 13C NMR spectra of purified Vi antigens from S. Typhi ΔwaaG and the ΔwaaG ΔvexE double mutant. (E) 13C NMR spectra of the same samples after chemical de–O-acetylation by base treatment. As expected, chemical removal of O-acetyl groups resulted in the loss of the O-acetyl signals at 20.06 ppm (Me) and 173.90 ppm (CO). The chemical shifts agreed with those predicted by CASPER (36) for a GalNAcA5 oligosaccharide (Table S1).
Table S1.
Chemical shifts observed in 13C spectra of purified de–O-acetylated Vi antigen
| Vi-CPS sample | δC, ppm | C1 | C2 | C3 | C4 | C5 | C6 | NAc (Me) | NAc (CO2) |
| Predicted | 98.95 | 51.35 | 68.44 | 78.89 | 72.59 | 175.87 | 22.91 | 175.43 | |
| E. coli [viaB] | Observed | 99.65 | 50.71 | 67.88 | 79.05 | 72.49 | 176.21 | 23.63 | 174.95 |
| E. coli [viaBΔvexE] | Observed | 99.39 | 50.56 | 68.01 | 78.78 | 72.61 | 176.06 | 23.55 | 175.57 |
| S. Typhi | Observed | 99.71 | 50.69 | 67.53 | 78.98 | 72.11 | 176.16 | 23.55 | 174.06 |
| S. Typhi ΔvexE | Observed | 99.68 | 50.72 | 67.80 | 79.04 | 72.54 | 176.23 | 23.61 | 174.73 |
Vi Antigen Has a Unique Glycolipid at Its Reducing Terminus.
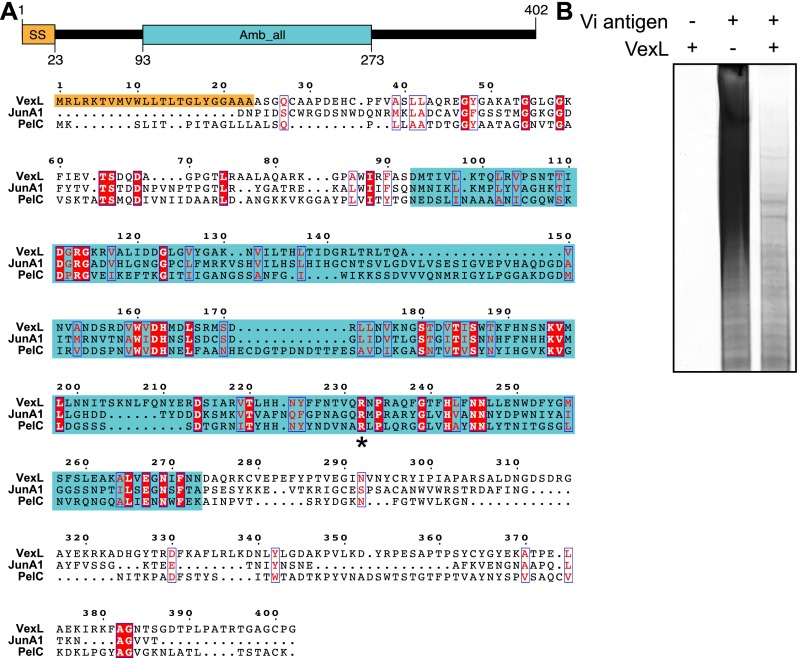
Structural investigation of the termini of high-molecular-weight polysaccharides requires a method that reduces the degree of polymerization while preserving linkages between terminal modification(s) and the remaining glycan. Previously, we exploited endoglycanase enzymes from capsule-specific bacteriophages to identify the terminal glycolipid structure from the CPS of E. coli K1 and K5 and meningococcal serotype b (6). The viaB locus of Achromobacter sp. and B. petrii contain an additional ORF located downstream of vexE in the otherwise similar locus (Fig. 1A). The predicted gene product shared sequence similarity with known pectate lyase enzymes (Fig. S3A); the corresponding gene is renamed “vexL.” Pectate lyases degrade acidic polymers, such as pectin, from plant tissues (18), and the α-1,4-linked polygalacturonic acid backbone structure of pectin superficially resembles Vi antigen. Purified A. denitrificans VexL enzyme depolymerized Vi antigen (Fig. S3B). Hydrophobic products released in these reactions were collected by solid-phase extraction and were analyzed by LC-MS.
Fig. S3.
A. denitrificans viaB contains a pectate lyase homolog that depolymerizes Vi antigen. (A) A. denitrificans VexL contains an N-terminal signal sequence and an ambrosia allergen domain that is homologous to pectate lyases. Multiple sequence alignment of A. denitrificans VexL, Juniperus ashei pollen allergen (JunA1) [GenBank accession no. AAD03608.1; Protein Data Bank (PDB) ID code 1PXZ], and a bacterial pectate lyase from Erwinia chrysanthemi (PelC) (GenBank accession no. P11073.1; PDB ID code 2PEC). The putative catalytic arginine of PelC is marked with an asterisk. (B) The purified VexL lyase protein (encoded by plasmid pWQ791) depolymerizes purified Vi antigen, as shown by a decrease in its apparent molecular-weight profile, in Tris⋅HCl-boric acid-EDTA-PAGE (stained with Alcian blue and silver).
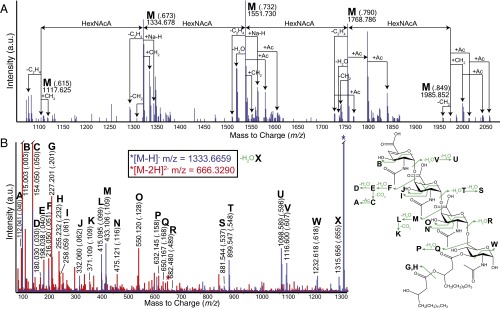
LC-MS of these molecules revealed a series of species that differed by 217.059 m/z, representing increments of one GalNAcA residue (Fig. 2A and Table S2). Oligosaccharides modified by one or more O-acetyl groups (δ m/z = 42.011) were also present. Lyase enzymes cleave polysaccharides through an eliminative mechanism and create a characteristic (anhydro) 4-deoxy-α-d-galact-4-enuronosyl residue at the nonreducing end of the oligosaccharide (18). This modification was evident in MS/MS fragmentation products (Fig. 2B). The Vi antigen oligosaccharides were linked to a reducing terminal N-acetylhexosamine (HexNAc) residue modified with either two β-hydroxymyristate chains or one β-hydroxymyristate and one β-hydroxypalmitate chain. Fragmentation of the [M-H]− ion at 1333.666 m/z produced products (including cross-ring cleavages) consistent with a structure comprising three GalNAcA residues linked to a single reducing terminal HexNAc possessing β-hydroxymyristate and β-hydroxypalmitate modifications (Fig. 2B). As confirmation, the isotopic distribution of the [M-H]− ion at 1333.666 m/z agreed with that predicted for the glycolipid (Fig. S4A), and fragmentation of the [M-2H]2- ion at m/z = 774.858 revealed the same structure extended with an additional GalNAcA residue (Fig. S4B). No ions corresponding to this glycolipid were identified when the procedure was repeated for Vi antigen purified from a ΔvexE mutant of S. Typhi.
Fig. 2.
Glycolipid terminus of the Vi antigen as determined by MS. (A) Charge deconvoluted LC-electrospray ionization (ESI)-QTOF-MS spectrum in negative mode for Vi antigen termini purified from S. Typhi. All ions correspond to a di-β-hydroxyacylated HexNAc residue linked to two or more variably O-acetylated HexNAcA residues. (B) LC-ESI-QTOF-MS/MS data for the singly charged (blue) and doubly charged (red) ions corresponding to a GalNAcA3 oligosaccharide attached to a reducing terminal diacyl-HexNAc. Overlapping signals are colored purple. Fragmentations are illustrated in green.
Table S2.
Compounds observed in charge-deconvoluted LC-QTOF-MS of S. Typhi Vi antigen glycolipid termini
| GalNAcA residues | Ac | Compounds | Species | Calculated mass, u | Observed mass, u | Difference | Error, ppm |
| 2 | 0 | anhydroHexNAcA-HexNAcA-HexNAc-(C14OH/C14OH) | [M] | 1,089.583 | 1,089.587 | 0.004 | 3.8 |
| 2 | 0 | anhydroHexNAcA-HexNAcA-HexNAc-(C14OH/C16OH) | [M] | 1,117.615 | 1,117.625 | 0.010 | 9.7 |
| 3 | 0 | anhydroHexNAcA-(HexNAcA)2-HexNAc-(C14OH/C14OH) | [M] | 1,306.642 | 1,306.643 | 0.001 | 0.5 |
| [M-H+Na] | 1,328.623 | 1,328.623 | 0.000 | −0.5 | |||
| [M-2H+2Na] | 1,350.605 | 1,350.606 | 0.001 | 0.1 | |||
| 3 | 0 | anhydroHexNAcA-(HexNAcA)2-HexNAc-(C14OH/C15OH) | [M] | 1,320.658 | 1,320.658 | 0.000 | 0.0 |
| [M-H+Na] | 1,342.639 | 1,342.639 | 0.000 | −0.4 | |||
| 3 | 0 | anhydroHexNAcA-(HexNAcA)2-HexNAc-(C14OH/C16OH) | [M] | 1,334.673 | 1,334.678 | 0.005 | 3.6 |
| [M-H+Na] | 1,356.655 | 1,356.659 | 0.004 | 2.6 | |||
| 4 | 0 | anhydroHexNAcA-(HexNAcA)3-HexNAc-(C14OH/C14OH) | [M] | 1,523.701 | 1,523.703 | 0.002 | 1.4 |
| [M-H+Na] | 1,545.682 | 1,545.683 | 0.001 | 0.6 | |||
| [M-H20] | 1,505.692 | 1,505.692 | 0.000 | 0.0 | |||
| 4 | 0 | anhydroHexNAcA-(HexNAcA)3-HexNAc-(C14OH/C16OH) | [M] | 1,551.732 | 1,551.730 | −0.002 | −1.2 |
| [M-H+Na] | 1,573.714 | 1,573.715 | 0.001 | 0.9 | |||
| 4 | 1 | anhydroHexNAcA-(HexNAcA)3-Ac-HexNAc-(C14OH/C14OH) | [M] | 1,565.713 | 1,565.712 | −0.001 | −0.6 |
| [M-H+Na] | 1,587.693 | 1,587.692 | −0.001 | −0.9 | |||
| 4 | 1 | anhydroHexNAcA-(HexNAcA)3-Ac-HexNAc-(C14OH/C16OH) | [M] | 1,593.744 | 1,593.743 | −0.001 | −0.9 |
| [M-H+Na] | 1,615.726 | 1,615.718 | −0.008 | −5.4 | |||
| 5 | 0 | anhydroHexNAcA-(HexNAcA)4-HexNAc-(C14OH/C14OH) | [M] | 1,740.759 | 1,740.761 | 0.002 | 1.2 |
| [M-H+Na] | 1,762.741 | 1,762.742 | 0.001 | 0.7 | |||
| 5 | 0 | anhydroHexNAcA-(HexNAcA)4-HexNAc-(C14OH/C16OH) | [M] | 1,768.790 | 1,768.786 | −0.004 | −2.7 |
| [M-H20] | 1,750.780 | 1,750.776 | −0.004 | −2.0 | |||
| 5 | 1 | anhydroHexNAcA-(HexNAcA)4-Ac-HexNAc-(C14OH/C14OH) | [M] | 1,782.770 | 1,782.772 | 0.002 | 1.1 |
| [M-H+Na] | 1,804.752 | 1,804.755 | 0.003 | 1.9 | |||
| 5 | 1 | anhydroHexNAcA-(HexNAcA)4-Ac-HexNAc-(C14OH/C16OH) | [M] | 1,810.801 | 1,810.796 | −0.005 | −2.5 |
| [M-H+Na] | 1,832.783 | 1,832.778 | −0.005 | −2.7 | |||
| 5 | 2 | anhydroHexNAcA-(HexNAcA)4-Ac2-HexNAc-(C14OH/C14OH) | [M] | 1,824.780 | 1,824.782 | 0.002 | 0.9 |
| [M-H+Na] | 1,846.762 | 1,846.763 | 0.001 | 0.4 | |||
| 5 | 2 | anhydroHexNAcA-(HexNAcA)4-Ac2-HexNAc-(C14OH/C16OH) | [M] | 1,852.812 | 1,852.807 | −0.005 | −2.3 |
| [M-H+Na] | 1,874.794 | 1,874.786 | −0.008 | −3.8 | |||
| 6 | 0 | anhydroHexNAcA-(HexNAcA)5-HexNAc-(C14OH/C15OH) | [M] | 1,971.833 | 1,971.829 | −0.004 | −2.3 |
| 6 | 0 | anhydroHexNAcA-(HexNAcA)5-HexNAc-(C14OH/C16OH) | [M] | 1,985.849 | 1,985.853 | 0.004 | 1.8 |
| 6 | 1 | anhydroHexNAcA-(HexNAcA)5-Ac-HexNAc-(C14OH/C14OH) | [M] | 1,999.828 | 1,999.830 | 0.002 | 1.0 |
| 6 | 1 | anhydroHexNAcA-(HexNAcA)5-Ac-HexNAc-(C14OH/C15OH) | [M] | 2,013.844 | 2,013.838 | −0.006 | −2.8 |
| 6 | 1 | anhydroHexNAcA-(HexNAcA)5-Ac-HexNAc-(C14OH/C16OH) | [M] | 2,027.860 | 2,027.852 | −0.007 | −3.6 |
| 6 | 2 | anhydroHexNAcA-(HexNAcA)5-Ac2-HexNAc-(C14OH/C14OH) | [M] | 2,041.839 | 2,041.840 | 0.008 | 0.5 |
| 6 | 2 | anhydroHexNAcA-(HexNAcA)5-Ac2-HexNAc-(C14OH/C15OH) | [M] | 2,055.855 | 2,055.862 | 0.007 | 3.7 |
| 6 | 2 | anhydroHexNAcA-(HexNAcA)5-Ac2-HexNAc-(C14OH/C16OH) | [M] | 2,069.870 | 2,069.868 | −0.002 | −0.9 |
| 6 | 3 | anhydroHexNAcA-(HexNAcA)5-Ac3-HexNAc-(C14OH/C16OH) | [M] | 2,111.881 | 2,111.883 | 0.002 | 1.2 |
u, atomic mass unit.
Fig. S4.
Confirmatory MS data. (A) Observed (blue) and expected (red) isotopic distributions for the 1333.663 m/z ion, corresponding to three GalNAcA residues attached to the reducing terminal diacyl-HexNAc. (B) Fragmentation of the Vi antigen terminal fragment with mass = 1,551.730 u (atomic mass unit). LC-ESI-QTOF-MS/MS spectrum for the doubly-charged ion ([M-2H]2-, m/z = 774.858), corresponding to four GalNAcA residues linked to a reducing terminal diacyl-HexNAc. The observed m/z values are indicated, and the expected m/z values from the fragmentation pattern are shown in parentheses.
VexE Is Required for Efficient Export and Cell-Surface Retention of Vi Antigen.
The role of the glycolipid terminus in Vi antigen export and surface assembly of the Vi capsule was investigated in S. Typhi by its susceptibility to degradation by VexL. VexL degraded almost all detectable Vi antigen in the wild type, indicating minimal amounts of intracellular (untransported) glycan. In contrast, Vi antigen profiles from the ΔvexC (lacking the ABC transporter ATPase) or ΔvexE mutant were unaffected by the presence of the enzyme (Fig. 3A). Vi antigen stability in whole cells was caused solely by inaccessibility to the lyase, as permeabilization of the mutant cells facilitated complete digestion of the glycan. Furthermore, a Vi antigen-specific bacteriophage infected the wild type but formed no plaques on the ΔvexC or ΔvexE mutants, confirming that no phage receptor is available on the mutant cell surfaces (Fig. S5B). Complementation of the mutations with the respective genes restored phage sensitivity. Wildtype S. Typhi possessed a Vi antigen capsule on the cell surface that was detectable by immunofluorescence microscopy (Fig. 3B), and complementation of the ΔvexC and ΔvexE mutants with the respective genes restored the Vi antigen capsule. Both the ΔvexC and ΔvexE mutants possessed inclusion bodies (Fig. 3B, Insets), which were labeled with Vi antigen-specific antibodies in immunofluorescence microscopy of permeabilized cells. Electron microscopy revealed that the inclusions were cytosolic in both the ΔvexC and ΔvexE mutants (Fig. S5C). To examine possible deleterious effects of these inclusions on cell physiology, the Cpx envelope stress response was assessed in S. Typhi and mutant derivatives (Fig. S5D). Surprisingly, the Cpx response was up-regulated significantly only in the ΔvexC mutant, in which no export occurs, and this increase was eliminated in a ΔvexC ΔvexE mutant, indicating that activation of a stress response by the inclusions was dependent on acylation.
Fig. 3.
VexE is required for efficient export and surface retention of Vi antigen. (A) The Vi antigen depolymerase was unable to access Vi antigen within intact cells of the ΔvexE mutant. Whole or lysed cells of S. Typhi and mutants were incubated with purified VexL, collected, digested with proteinase K, and probed for Vi antigen. VexL was able to degrade the Vi antigen in wild-type S. Typhi but not in S. Typhi ΔvexC, providing positive and negative controls for export, respectively. (B) Immunofluorescence microscopy of cells probed with anti-Vi antigen antibodies illustrated that the ΔvexE mutant possessed no Vi antigen on its surface but accumulated intracellular Vi antigen in inclusion bodies, which became accessible to antibody in permeabilized cells. (Scale bars, 10 µm.) Insets are enlarged to show a representative cell. (C) S. Typhi ΔvexE was able to export Vi antigen in a transporter-dependent manner. Growth medium from early exponential-phase cultures was collected and probed for Vi antigen and (cytosolic) RNA polymerase by Western immunoblotting.
Fig. S5.
Additional phenotypic characterization of the ΔvexE mutant S. Typhi. (A) Wild-type Vi antigen bound to both hydrophobic PVDF and negatively charged nylon membranes, whereas ΔvexE mutant Vi antigen bound only to nylon. The figures are Western blots of separated proteinase K-digested whole-cell lysates, probed with anti-Vi antigen antibody. (B) S. Typhi ΔvexE is not sensitive to Vi phage II (HER#39; Félix d’Hérelle Reference Center for Bacterial Viruses, Université Laval, Québec, Canada), indicating the absence of the Vi antigen bacteriophage receptor on the cell surface. Six microliters of overnight cultures were dropped on a Petri dish in which one half of the plate had been inoculated with 7.5 × 106 pfu bacteriophage (arrow). Cells are unable to grow on the phage-coated plate if they express surface-associated Vi antigen. (C) ΔvexE and ΔvexC mutants of S. Typhi accumulate cytosolic Vi antigen. Electron micrographs of thin sections of freeze-substituted S. Typhi and mutant derivatives. The hydrated Vi antigen capsule in the wild-type strain collapses during preparation (black arrowheads). The absence of export in the ΔvexC mutant and of proper acylation in the ΔvexE mutant results in electron-transparent inclusions that accumulate in the cytoplasm. Some inclusion bodies appear to interfere with cell division, because invaginations of the outer membrane are visible in the ΔvexC mutant surrounding an inclusion body (black arrows). (Scale bars, 400 nm.) (D) The Cpx cell envelope stress response is up-regulated in ΔvexC mutant but not in ΔvexE mutant S. Typhi. S. Typhi and mutant derivatives were transformed with pNLP15, which contains the spy promoter upstream of the luxCDABE cassette. Luciferase activity was monitored as luminescence counts per second and was normalized to the optical density of the cell culture. Data represent the mean ± SE of three independent experiments. Statistical differences were determined using a Students t test; significant differences are indicated: *P < 0.01; **P < 0.001. (E) Immunofluorescence microscopy of cells probed with anti-Vi antigen antibodies illustrated that the ΔvexCE mutant possessed no Vi antigen on its surface. However, the mutant accumulated intracellular Vi antigen in inclusion bodies, which became accessible to antibody in permeabilized cells. Complementation with plasmids containing vexC and vexE restored the surface expression of Vi antigen. (Scale bars, 10 µm.) Insets are enlarged to show a representative cell.
These results are consistent with the intracellular accumulation of Vi antigen in the ΔvexC and ΔvexE mutants, suggesting that both mutations resulted in export defects. However, published mutant phenotypes indicated extensive Vi antigen export in a vexE mutant of an E. coli recombinant containing viaB (11). Because lyase treatment and immunofluorescence microscopy of whole cells cannot account for Vi antigen released into the growth medium, we examined the cell-free supernatants from early exponential-phase cultures of S. Typhi and its mutant derivatives for Vi antigen release (Fig. 3C). Wild-type cells released some Vi antigen into the medium, as expected with any encapsulated bacterium and as is consistent with published observations (10, 11). The ΔvexC mutant released only a trace of Vi antigen; release could be explained by small amounts of lysis during growth and is consistent with the release of cytosolic RNA polymerase in the same cultures (Fig. 3C). Export and release of Vi antigen was restored when the ΔvexC mutation was complemented with vexC. In contrast, ΔvexE cells released large quantities of Vi antigen. This material was eliminated in a ΔvexCE double mutant (Fig. 3C and Fig. S5E), indicating an active process involving the ABC transporter rather than elevated leakage resulting from the vexE defect.
Discussion
Vi antigen has a lipid terminus that differs from those of any other known CPS assembled in an ABC transporter-dependent pathway. It is composed of a reducing terminal HexNAc residue modified with two β-hydroxy fatty acids and resembles one half of the structure of lipid A (Fig. 4). This structure, together with the similarity shared by VexE and LpxL, a secondary acyl-ACP–dependent acyltransferase from lipid A biosynthesis, is consistent with the proposal that VexE is an acyltransferase that transfers a β-hydroxymyristate or β-hydroxypalmitate chain to the terminus of Vi antigen. The action of VexE would be comparable to that of the secondary acyltransferases in lipid A biosynthesis, although LpxL and LpxM transfer nonhydroxylated fatty acids (Fig. 4) (16). VexE is the only acyltransferase encoded by the viaB locus. There is no precedent for such enzymes being able to transfer both acyl chains, and doing so would require radically different acceptor specificities in a single catalytic site. A logical origin of this terminal moiety involves secondary acylation of the UDP-activated β-hydroxymyristoyl-GlcNAc product of LpxA in lipid A biosynthesis (Fig. 4) (19). Such a reaction could divert this intermediate for use in Vi antigen biosynthesis and, to our knowledge, would represent the first off-pathway use of an Lpx-pathway intermediate. We investigated the possibility that VexE interfered with normal lipid A biosynthesis in E. coli. Expression of VexE slowed the growth of E. coli slightly, but this effect was likely caused by protein overexpression and was independent of VexE catalytic activity (Fig. S6A). In addition, VexE was unable to modify lipid A (Fig. S6B). The inability of VexE activity to influence lipid A biosynthesis is perhaps not surprising, given the regulation of the essential Raetz pathway process. The LpxA reaction equilibrium favors the reverse reaction, and the first committed step of lipid A biosynthesis (LpxC) is tightly regulated (15, 16, 20), so pathway flow is regulated according to lipid A requirement. We pursued the possibility that ΔvexE Vi antigen possesses a single acyl chain (the product of LpxA) at its reducing terminus. However, we were unable to detect either diacyl- or monoacyl-HexNAc in either extracellular or intracellular (accumulated) Vi antigen from the ΔvexE mutant. This negative result could reflect an absolute requirement for diacylated UDP-GlcNAc, offering an additional means of separation from the lipid A pathway. However, we cannot rule out technical issues in which monoacylated Vi antigen termini lack sufficient hydrophobicity for separation protocols (consistent with altered PVDF binding).
Fig. S6.
Overexpression of VexE does not affect cell growth or lipid A structure in E. coli. (A) Viable cell count growth curves of E. coli Top10 harboring plasmids encoding VexE and VexEH466A. Overexpression of VexE, induced by the addition of l-arabinose [final concentration 0.02% (wt/vol)] at 2.5 h (arrow), slightly hindered growth of E. coli, independently of catalytic activity, because a similar profile was obtained with VexEH466A. Data represent the mean ± SE of three independent experiments. Light micrographs depict cells after 8.5 h growth. (Scale bars, 2 μm.) (B) ESI-MS of lipid A species isolated from E. coli BKT09, which possesses mutations in lpxL, lpxM, lpxP, and pagP and therefore produces only tetraacylated lipid A. Overexpression of the secondary acyltransferases LpxL and LpxM resulted in the expected addition of laurate and palmitate modifications, respectively, generating pentaacylated lipid A. Overexpression of VexE did not alter lipid A structure.
The apparent ability to synthesize Vi antigen in the absence the acylated terminus could reflect the mutations creating conditions that facilitate polymer synthesis on nonphysiological acceptors, as is the case in the E. coli and N. meningitidis kpsS and kpsC mutants (6). However, this assumption requires that the diacyl-HexNAc actually serves as an acceptor, but the identity and mechanism of the Vi antigen polymerase is unknown. Vi antigen potentially could be synthesized by growth at the reducing terminus in a process similar to class I hyaluronan synthases. These enzymes use UDP-GlcNAc or UDP-glucuronic acid (GlcA) as acceptors and the nascent [3)-GlcNAc-β-(1→4)-GlcA-β-(1→]n-UDP chain as the donor during chain extension (21). It is unknown how the terminal UDP moiety is removed in the final glycan product. In such a scenario, the addition of diacyl-HexNAc could represent the last step in Vi antigen biosynthesis before export, explaining the ability to synthesize Vi antigen in the absence of VexE. Biochemical characterization of the Vi antigen polymerase(s) is required to resolve this question.
In the E. coli group 2 CPS assembly, export is dependent on the presence of the glycolipid terminus (6). In contrast, defective acylation of the Vi antigen in the ΔvexE mutant does not prevent export, but accumulation of intracellular Vi antigen (which is not seen in the wild type) also occurs. Accumulation of Vi antigen could reflect altered recognition by the export machinery. For example, the LPS ABC transporter MsbA from E. coli is highly selective for completed (hexaacylated) LPS molecules. Export of tetraacylated precursors occurs only at low levels (reviewed in ref. 13). Alternatively, the export defect in the ΔvexE mutant could reflect alteration of essential interactions that couple synthesis to export in a multiprotein complex. However, altered interactions are unlikely because the phenotype resulting from the VexE catalytic-site mutation (which should preserve protein–protein interactions) is indistinguishable from the vexE deletion (Fig. 3C). Interestingly, intracellular Vi antigen in transport-defective mutants showed an increase in the average chain length (Fig. 3C and Fig. S5A), whereas overexpression of VexE caused a reduction. Lowering chain length requires VexE catalytic activity rather than a simple structural requirement for the protein, because the size reduction was not evident in ΔvexE cells expressing VexEH466A. Altered chain lengths can be explained by an elongation phase differing from the normal assembly process occurring with molecules with a complete glycolipid terminus. There is precedent for the modulation of glycan chain length by competition between export and extension in other bacterial systems with ABC transporters (22, 23).
The use of a conserved intermediate from the lipid A–biosynthesis pathway to create the lipid terminus potentially facilitates Vi antigen production in diverse Gram-negative bacteria by horizontal transfer of the viaB locus with a limited gene complement. This diversity is evident in the possession of the locus by Achromobacter, Bordetella, and Citrobacter sp. and expression in E. coli, but why some Vi antigen producers possess the additional VexL component is unknown. It is also unknown whether the terminal lipid itself is important in the interaction of Vi antigen with the host immune system. In the context of Vi antigen-based vaccines, a production strain lacking vexE may offer advantages because it exports Vi antigen with altered micellar properties and a reduced association with LPS.
Methods
Strains and Plasmids.
The bacterial strains used in this study are listed in Table S3. The background for the generation of viaB mutants was S. Typhi H251.1 (aroC); clean mutations were generated by recombineering using the λ-red system (see SI Methods for details). Strains and transformants were grown at 37 °C in lysogeny broth (LB) medium supplemented with 100 µg/mL 2,3-dihydroxybenzoic acid and antibiotics where appropriate. Complementation of mutations was performed using l-arabinose–inducible pBAD-based vectors described in Table S3.
Table S3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or property | Reference and/or source |
| E. coli | ||
| Top10 | F−, mcrA, Δ(mrr-hsdRMS-mcrBC), ϕ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, rpsL(Strr), endA1 | Invitrogen |
| CWG1241 | Top10 ΔwecA::cat; Cmr | This study |
| BKT09 | Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ−, rph-1, Δ(rhaD-rhaB)568, hsdR514, ΔpagP, ΔlpxP, ΔlpxM, ΔlpxL::Kanr | (37) R. Bishop, McMaster University, Canada |
| S. enterica | ||
| H251.1 | S. enterica serovar Typhi Ty2 trp, cys, ΔaroC1019 | (38) K. E. Sanderson, Salmonella Genetic Stock Centre, University of Calgary, Canada. |
| CWG1235 | S. Typhi H251.1 ΔvexC | This study |
| CWG1236 | S. Typhi H251.1 ΔvexE | This study |
| CWG1237 | S. Typhi H251.1 ΔvexC ΔvexE | This study |
| CWG1238 | S. Typhi H251.1 ΔwaaG::kan; Kmr | This study |
| CWG1239 | CWG1236 ΔwaaG::kan; Kmr | This study |
| A. denitrificans | ||
| CWG1240 | A. denitrificans; Smr, Ampr, Kanr | G. D. Wright, McMaster University, Canada |
| Plasmids | ||
| pACYC184 | Cloning vector containing tetracycline and chloramphenicol resistance cassettes; Tetr, Cmr | (39) |
| pBAD24 | Plasmid vector with l-arabinose–inducible promoter; Apr | (40) |
| pKD3 | Source of frt-flanked chloramphenicol-resistance cassette; Cmr | (26) |
| pKD4 | Source of frt-flanked kanamycin-resistance cassette; Kmr | (26) |
| pSIM6 | λ-red recombinase helper plasmid; Apr | (27) |
| pCP20 | Source of Flp recombinase, temperature-sensitive replicon; Apr | (26) |
| pGVXN158 | pLAFR1 derivative encoding S. Typhi BRD948 viaB locus; Tetr | (11) |
| pNLP15 | spy (Spheroplast protein Y) reporter; spy promoter fused to promoterless luxCDABE operon; Kmr | (33) |
| pWQ284 | pBAD24 containing a chloramphenicol-resistance cassette; Cmr | (41) |
| pWQ782 | pACYC184 derivative containing an oligo cassette containing Asc1 and Spe1 restriction sites; Tetr | This study |
| pWQ783 | pWQ782 containing the Salmonella Typhi viaB operon; Tetr | This study |
| pWQ784 | Derived from pWQ783; viaB containing a deletion of vexE; Tetr | This study |
| pWQ785 | Derived from pWQ783; viaB containing a deletion of vexC; Tetr | This study |
| pWQ786 | pBAD24 derivative encoding Salmonella Typhi VexE-His6; Apr | This study |
| pWQ787 | pBAD24 derivative encoding A. denitrificans VexE-His6; Apr | This study |
| pWQ788 | pWQ786 containing a H487A mutation; Apr | This study |
| pWQ789 | pWQ787 containing a H466A mutation; Apr | This study |
| pWQ790 | pWQ787 containing a Y471F mutation; Apr | This study |
| pWQ791 | pBAD24 derivative encoding A. denitrificans VexL-His6; Apr | This study |
| pWQ792 | pBAD24 derivative encoding VexC-His10; Apr | This study |
| pWQ793 | pWQ284 derivative encoding VexC-His10; Cmr | This study |
| pWQ794 | pWQ284 derivative encoding A. denitrificans VexE-His6; Cmr | This study |
| pWQ795 | pWQ284 derivative encoding S. Typhi LpxL-His6; Cmr | This study |
| pWQ796 | pWQ284 derivative encoding S. Typhi LpxM; Cmr | This study |
| pWQ903 | pBAD24 derivative encoding WaaG from E. coli F470 | (42) |
Primers.
Oligonucleotide primers used to amplify genes from S. Typhi, A. denitrificans, and E. coli genomic DNA were obtained from Sigma-Aldrich and are described in Table S4.
Table S4.
Sequences of oligonucleotide primers used to generate recombinant plasmids, site-directed mutants, and genomic deletions
| Plasmid | Primer | Sequence (5′→3′) | Features |
| pWQ782 | SL024 | gatcggcgcgccGGTACCactagtAGCATTCATCAGGCGGGCAAG | Forward primer for amplification of pACYC184 introducing Asc1 and Spe1 restriction sites |
| SL025 | gatcggcgcgccCATCCGGAATTCCGTATGGCAATG | Reverse primer for amplification of pACYC184 introducing Asc1 and Spe1 restriction sites | |
| pWQ783 | SL035 | cattggcgcgccTCATTTCCGAAGCAGTCAC | Forward primer for amplification of viaB; Asc1 restriction site |
| MM17 | acatactagtCTTAGGCTGGGGTGTCTTTGC | Forward primer for the amplification of viaB; Asc1 restriction site. | |
| pWQ784 | SL026 | gatcactagtTTATTCACGCATCCGCCTGATG | Reverse primer for the amplification of tviABCDEvexABCD; Spe1 restriction site. |
| pWQ785 | MNL05 | AGGAGAGACGCATTCtaaTTAGTAAATATACGGATAGAGTGTGG | Forward mutagenesis primer for vexC deletion in pSDL033 |
| MNL06 | CTCTATCCGTATATTTACTAAttaGAATGCGTCTCTCCTGAAGC | Forward mutagenesis primer for vexC deletion in pSDL033 | |
| pWQ786 | AZG1 | gcgcgcgaattcaccATGAGCTTGCACTCTACATTT | Forward primer for the amplification of vexE-His6; EcoRI restriction site (S. Typhi) |
| AZG2-2 | gcgcgctctagatcagtggtggtggtggtggtgACTATCCCTACGTATAAT | Reverse primer for the amplification of vexE-His6; XbaI restriction site (S. Typhi) | |
| pWQ787 | vexeAdHis-for | tactgaattcaccATGGTTGATACGGTCATTGAAAGC | Forward primer for the amplification of vexE-His6; EcoRI restriction site (A. denitrificans) |
| vexeAdHis-rev | tacttctagattaatggtgatggtgatggtgCGCGGACGGATCCGCCGCCG | Reverse primer for the amplification of vexE-His6; EcoRI restriction site (A. denitrificans) | |
| pWQ788 | SL030 | GCATTATTGTATCGGCTgcgCTGGGCGCAATGTATGCC | Forward mutagenesis primer for H487A in VexE (S. Typhi) |
| SL031 | GGCATACATTGCGCCCAGcgcAGCCGATACAATAATGC | Reverse mutagenesis primer for H487A in VexE (S. Typhi) | |
| pWQ789 | SL032 | GGTCGCCACCGCCgccGTCGGGCCGATG | Forward mutagenesis primer for H466A in VexE (A. denitrificans) |
| SL033 | CATCGGCCCGACggcGGCGGTGGCGACC | Reverse mutagenesis primer for H466A in VexE (A. denitrificans) | |
| pWQ790 | SL063 | CACGTCGGGCCGATGtttGCGGGCCTGATGGCG | Forward mutagenesis primer for Y471F in VexE (A. denitrificans) |
| SL064 | CGCCATCAGGCCCGCaaaCATCGGCCCGACGTG | Reverse mutagenesis primer for Y471F in VexE (A. denitrificans) | |
| pWQ792 | SL083 | cagggaattcaTGTTCGGTTTATTAGGTTG | Forward primer for the amplification of vexC-His10; EcoRI restriction site (S. Typhi) |
| SL084 | gatcaagcttttaatggtggtgatggtggtgatggtggtgatgTATATCAAAGGAATAATCTTCAG | Reverse primer for the amplification of vexC-His10; HindIII restriction site (S. Typhi) | |
| pWQ791 | SL079 | cattgaattcATGCGACTTCGAAAGACAGTGATGG | Forward primer for amplification of vexL-His6; EcoRI restriction site (A. denitrificans) |
| SL081 | cattaagcttttagtggtggtggtggtggtgGCCCGGGCAACCCGCGCC | Reverse primer for amplification of vexL-His6; HindIII restriction site (A. denitrificans) | |
| pWQ795 | SL102 | cagggaattcATGACGAAGTTGCCTAAGTTC | Forward primer for amplification of LpxL-His6; EcoRI restriction site (S. Typhi) |
| SL103 | gatcaagcttttaatggtggtgatggtggtgATAGCGCGACGGTACGCCTTC | Reverse primer for amplification of LpxL-His6; HindIII restriction site (S. Typhi) | |
| pWQ796 | SL119 | caccggtacctATGGAAACCAAAAAAAATAATAGTG | Forward primer for amplification of LpxM; KpnI restriction site (S. Typhi) |
| SL120 | gatcaagcttTTATTTGATGGGATAAAGATC | Reverse primer for amplification of LpxM; HindIII restriction site (S. Typhi) | |
| CWG1236 | SL089 | CCTGATAACCATCAGGCGGATGCGTGAATAAGGCTGAGTAAGGAAATATAgtgtaggctggagctgcttc | λ-red recombination primer for vexE deletion (S. Typhi) |
| SL090 | ATTTTCAGCTCTGAAGTACAAATTTTCATCTACCGCAATTAAATCGCTTAcatatgaatatcctccttag | λ-red recombination primer for vexE deletion (S. Typhi) | |
| CWG1235, CWG1237 | SL092 | GATTTGTGACACTGAAAATCACTGACTTTAGCTTCAGGAGAGACGCATTCgtgtaggctggagctgcttc | λ-red recombination primer for vexC deletion (S. Typhi) |
| SL093 | CATTTTTTAATACGTTCTGAATTTTCCACACTCTATCCGTATATTTACTAAcatatgaatatcctccttag | λ-red recombination primer for vexC deletion (S. Typhi) | |
| CWG1239, CWG1240 | SL096 | GAAAAAATGCTGCCGCATGAGGCACGCCCCATAGATTTGGACAGCCTGCTgtgtaggctggagctgcttc | λ-red recombination primer for waaG deletion (S. Typhi) |
| SL097 | CCTCAAAAGCATCTTTACCGCGCCATAGTGTGGTTAACGGCGCTTTCAGCcatatgaatatcctccttag | λ-red recombination primer for waaG deletion (S. Typhi) | |
| CWG1241 | LRWecA-f | GGTCTTCGTGGTTATACTTCTGCTAATAATTTTCTCTGAGAGCATGCATTgtgtaggctggagctgcttc | λ-red recombination primer for wecA deletion (E. coli) |
| LRWecA-r | AGCGTCTTCGGCCGGTTTCCCAGGCATTGGTTGTGTCATCACATCCTCATcatatgaatatcctccttag | λ-red recombination primer for wecA deletion (E. coli) |
Restriction sites are underlined. Nonchromosomal sequences are lowercase. Point mutations are boldface.
Purification of Vi Antigen.
Vi antigen was purified from the supernatant of a hot aqueous phenol extract of lyophilized S. Typhi ΔwaaG and mutant derivatives (24). Secreted Vi antigen was precipitated from culture supernatants using hexadecyltrimethylammonium bromide (11). The polysaccharide preparations were digested with DNase, RNase, and Proteinase K and were separated from residual LPS by gel filtration chromatography in the presence of detergent (see SI Methods for details).
Isolation of the Vi Antigen Glycolipid Terminus.
Twenty milligrams of purified Vi antigen were resuspended at 1 mg/mL in 50 mM sodium bicarbonate, 0.1 mM CaCl2 (pH 7.5). Purified VexL-His6 was added to a final concentration of 100 µg/mL; the reaction mixture was incubated at 37 °C for 5 h and then was loaded into a SepPak C18 cartridge. The column was washed with 10 mL of water, and bound hydrophobic material was eluted in 70% (vol/vol) acetonitrile. Eluted material was dried by SpeedVac and was resuspended in 100 µL 25% (vol/vol) acetonitrile in water.
MS.
LC-MS analyses of the glycolipid terminus were performed on an Agilent 1200 high-performance liquid chromatograph interfaced with an Agilent ultra-high-definition (UHD) 6530 quadrupole TOF (QTOF) mass spectrometer. A C18 column was used for chromatographic separation. Conditions for LC and MS are described in SI Methods.
Digestion of Cell Surface and Intracellular VI Antigen with VexL Lyase.
Cultures were grown until OD600 = 0.5 was reached. Cells equivalent to one OD600 unit were collected by centrifugation and were resuspended in PBS supplemented with 0.1 mM CaCl2, with and without VexL-His6 (100 µg/mL final concentration). Cell suspensions were incubated at 37 °C for 1 h and were collected by centrifugation. The cells were solubilized in SDS/PAGE buffer and were analyzed by Western immunoblotting using mouse monoclonal antibody P2B1G2/A9, which is specific for Vi antigen (20). To ensure that the undigested Vi antigen resulted only from its inaccessibility, aliquots of cells were lysed by French press, unbroken cells were removed by centrifugation, and VexL-His6 was added, incubated, and analyzed as above.
Detection of Cell-Free Vi Antigen in Culture Supernatants.
LB cultures (50 mL) were grown at 37 °C until an OD600 of 0.5 was reached. Cells then were collected by centrifugation at 5,000 × g for 15 min. The supernatant was dialyzed against water for 2 d, using a dialysis membrane with a 3,500 molecular weight cutoff (MWCO). The dialyzed supernatant was lyophilized, resuspended in 1 mL of water, and examined by Western immunoblotting.
Immunofluorescence Microscopy.
Live and fixed/permeabilized cells were probed with Vi antigen-specific monoclonal antibody (P2B1G2/A9) (20) and were labeled with rhodamine red-conjugated goat anti-mouse IgG. See SI Methods for details.
SI Methods
Generation of λ-Red Mutants.
Mutants were generated in S. Typhi aroC (H251.1) using the λ-red recombinase system (26, 27). Cells were transformed with the recombination helper plasmid pSIM6, followed by linear PCR products that were amplified from pKD3 or pKD4 using the primers described in Table S4. PCR products contained frt-flanked chloramphenicol- or kanamycin-resistance cassettes, and recombinants were selected by growth on the appropriate antibiotic. The kan cassette was removed using the Flp recombinase encoded by pCP20 (26). Double mutants were constructed sequentially after removal of the kan cassette. The kan cassette was left in the final step in double mutants. Lack of polar effects was confirmed by restoration of wild-type phenotypes in complementation with single cloned genes.
Sequencing of A. denitrificans.
A 5-mL culture of A. denitrificans was grown for 48 h in Brain-Heart Infusion (BHI) broth (Sigma-Aldrich) at 37 °C, and genomic DNA was extracted using a PureLink Genomic DNA Mini Kit (Invitrogen) per the manufacturer’s instructions. Genomic sequencing was conducted with the help of C. Cooper (University of Guelph, Guelph, ON, Canada) and B. Coombes (McMaster University, Hamilton, ON, Canada), using Illumina MiSeq technology at the Farncombe Metagenomics Facility (McMaster University, Hamilton, ON, Canada). A paired-end assembly was generated using MIRA. The genomic DNA sequence from A. denitrificans containing the viaB locus has been submitted to GenBank (accession no. KT99772).
Bioinformatic Analyses.
Putative domains were identified in vexE using the conserved domain database (28) and TPRpred (29). Multiple sequence alignments were generated using Clustal Omega (30) and are presented using ESPript (31).
SDS/PAGE and Western Immunoblotting.
Unless noted otherwise, polysaccharide samples consisted of proteinase K-digested whole-cell lysates, prepared by collecting cells from 1 OD600 unit-equivalent of culture (32). They were separated on 10% SDS/PAGE resolving gels. Protein samples were heated at 100 °C for 10 min before analysis by SDS/PAGE using 12% resolving gels. For immunoblotting, Vi antigen samples (purified polysaccharide or whole-cell lysates) were transferred to PVDF or positively charged nylon membranes; protein was transferred to nitrocellulose membranes. For Vi antigen, membranes were probed with mouse Vi antigen-specific monoclonal antibody P2B1G2/A9 (20) diluted 1:200. Alkaline phosphatase-conjugated goat anti-mouse secondary antibody diluted 1:3,000 and the substrates nitroblue terazolium and X-Gal were used for detection. For protein, membranes were probed with mouse anti-His5 monoclonal antibody (Qiagen) diluted 1:2,000 or with mouse anti-RNA polymerase α monoclonal antibody (sc-101597; Santa Cruz Biotechnology) diluted 1:2,000. HRP-conjugated goat anti-mouse secondary antibody (Qiagen) diluted 1:3,000 and the chemiluminescent substrate Luminata Classico (Millipore) were used for detection.
Purification of VexL-His6.
A 5-L LB culture was inoculated at 1:100 using an overnight culture of E. coli Top10 harboring pWQ791, which encodes (the periplasmic) VexL-His6 with its signal sequence. The culture was grown at 37 °C until OD600 = 0.3 was reached. The temperature then was shifted to 20 °C. When the culture OD600 reached 0.6, VexL-His6 expression was induced by adding 0.002% (wt/vol) l-arabinose, and growth was continued for 16 h. Cells were collected by centrifugation at 5,000 × g for 20 min and were resuspended in buffer A [100 mM Tris⋅HCl (pH 8.0) containing 500 mM sucrose] at a ratio of 0.07 mL buffer A per 1 OD600 unit-equivalent of cells. The cell suspension was left on ice for 5 min; then lysozyme (100 µg/mL final concentration) and EDTA (1 mM final concentration) were added. The cell suspension was incubated on ice for 20 min, MgSO4 (20 mM final concentration) was added, and the resulting spheroplasts were removed by centrifugation at 12,000 × g for 10 min at 4 °C. The supernatant containing the periplasmic contents was dialyzed for 16 h in 3,500-MWCO membrane against buffer B [20 mM Tris⋅HCl (pH 7.5) containing 300 mM NaCl and 10 mM MgCl2]. The dialyzed supernatant was clarified by centrifugation at 72,000 × g for 1 h. Then imidazole was added to 10 mM, and the resulting solution was loaded onto a 3-mL nickel-nitrilotriacetic acid column. The column was washed successively with 10 column volumes each of buffer C [20 mM Tris⋅HCl (pH 7.5) containing 350 mM NaCl] and buffer C supplemented with 25 mM and 50 mM imidazole. Protein was eluted in buffer C supplemented with 300 mM imidazole (final concentration) and was concentrated using a Vivaspin 20 column (30,000 MWCO) (GE Healthcare). Protein was exchanged into buffer lacking imidazole using a PD10 column (GE Healthcare Life Sciences). Protein concentration was estimated from A280 values and a theoretical extinction coefficient of 44,725 M/cm.
Purification of VexE-His6.
A 1-L LB culture (supplemented with 100 µg/mL ampicillin) was inoculated at 1:100 with overnight cultures of E. coli harboring pWQ787 (VexE) or pWQ789 (VexEH466A). Cultures were grown at 37 °C to an OD600 of ∼0.5; then plasmid-encoded gene expression was induced with 0.02% (wt/vol) arabinose. After additional growth for 3 h, cells were collected by centrifugation at 5,000 × g for 10 min. The cell pellet was resuspended in 100 mL of buffer D [10 mM sodium phosphate (pH 7.0) containing 100 mM NaCl] supplemented with four cOmplete mini EDTA-free protease inhibitor tablets (Roche) and imidazole to give a final concentration of 10 mM. Cells were lysed using an EmulsiFlex homogenizer (Avestin), and unbroken cells were removed by centrifugation at 4,000 × g for 20 min. The resulting supernatant was treated with 5 µL Benzonase Nuclease (Millipore) with stirring for 15 min at 20 °C. Cell membranes were removed by centrifugation at 100,000 × g for 1 h. The supernatant was mixed with 2 mL of nickel-nitrilotriacetic acid resin on a nutator for 1 h. The resin was loaded onto a column and was washed sequentially with 10 column volumes of buffer D containing imidazole to give step concentrations of 25 mM, 50 mM, and 75 mM. VexE then was eluted with buffer D containing 250 mM imidazole. Fractions containing VexE were dialyzed against buffer A using a 3,500-MWCO dialysis membrane and were concentrated to 500 µL using a Vivaspin 20 concentrator (50,000 MWCO). Protein concentration was determined by A280 using theoretical extinction coefficient of 92,610 M/cm.
CD Spectroscopy.
Purified VexE and VexEH466A proteins were dialyzed into 5 mM sodium phosphate (pH 7.0) using 3,500-MWCO dialysis membranes. The protein concentration was diluted to 0.016 mg/mL in 5 mM sodium phosphate (pH 7.0). CD spectra were collected on a Jasco J-815 spectropolarimeter using quartz cuvettes with a 1-mm path length at 20.04 °C. Six spectra were collected for each sample and were averaged, and the spectrum of 5 mM sodium phosphate was subtracted.
Immunofluorescence Microscopy.
Five milliliters of LB media inoculated at 1:100 from 5-mL overnight cultures and were grown at 37 °C until OD600 = 0.5 was reached. One OD600 unit-equivalent of cells was collected by centrifugation at 5,000 × g for 10 min. Cells were washed three times in 1 mL PBS and were incubated with 100 µL mouse Vi antigen-specific monoclonal antibody (P2B1G2/A9) (20) diluted 1:100 in PBS containing 1% (wt/vol) BSA (PBS-BSA). The cells then were washed three times with PBS and were incubated with 100 µL rhodamine red-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) diluted 1:50 in PBS-BSA. The labeled cells were washed three times and were resuspended in 100 µL PBS. Ten microliters of the cell suspension were applied to a 2% (wt/vol) agarose pad and were imaged on a Zeiss Axiovert 200 microscope using the 100× objective. Images were processed using Volocity software (PerkinElmer). To permeabilize cell membranes, 1 OD600 unit-equivalent of cells were fixed in 5% (vol/vol) formaldehyde in PBS for 16 h at 4 °C. Cells were washed three times and were resuspended in 100 µL PBS. Ten microliters of cell suspension were applied to poly-l-lysine–coated glass slides and were incubated at 20 °C for 10 min. The immobilized cells were incubated with 10 µL lysozyme (0.5 mg/mL), 10 mM EDTA, and 25 mM Tris⋅HCl (pH 8.0) for 15 min and then with 0.1% (vol/vol) Triton X-100 in PBS for 15 min. Slides were blocked with PBS-BSA for 15 min and were labeled with 10-µL aliquots of antibodies, mounted in Vectashield, and imaged as above.
Luminance Assay.
Luciferase assays were performed as previously described (33), using S. Typhi and mutant derivatives transformed with pNLP15, which contains the spy (Spheroplast protein Y) promoter upstream of the luxCDABE cassette. Cells were inoculated at 1:100 in 5 mL LB and were grown until OD600 = 0.5 was reached. At this time, 200-µL aliquots of culture were transferred in triplicate into 96-well plates for luminance and OD600 assays. Data are shown as CPS/OD600; values represent the mean ± SE of three independent experiments.
Purification of Vi Antigen.
Twelve-liter LB cultures (supplemented with 100 µg/mL 2,3-dihydroxybenzoic acid) were inoculated at 1:100 from overnight cultures of CWG1238 or CWG1239 and were grown at 37 °C to an OD600 of 1.0. For cell-associated Vi antigen, cells were collected by centrifugation at 10,000 × g for 10 min, and polysaccharides were extracted from lyophilized cells by the hot aqueous phenol method (30). The aqueous supernatant was dialyzed into water using a 3,500-MWCO dialysis membrane, and LPS was collected by centrifugation of the dialysate at 100,000 × g for 16 h. For CWG1238 preparations, Vi antigen sedimented with LPS. For CWG1239, Vi antigen remained in the supernatant, which was lyophilized. To collect Vi antigen secreted into culture supernatants, hexadecyltrimethylammonium bromide was added to the cell-free culture medium at a final concentration of 0.1% (wt/vol). The mixture was stirred for 1 h, and precipitated Vi antigen was collected by centrifugation at 10,000 × g. The sedimented material was resuspended in 50 mL of 1-M CaCl2. Ethanol was added to a final concentration of 80% (vol/vol), and the mixture was mixed on a nutator for 20 min. The precipitate was collected by centrifugation at 3,000 × g for 20 min and was washed three times with 50 mL ethanol and once with 50 mL acetone. The Vi antigen was collected by centrifugation at 3,000 × g for 20 min after each washing step. The pellet was resuspended in 20 mL acetone and was dried using a rotary evaporator. Dried Vi antigen was resuspended in 50 mL of 20 mM Tris⋅HCl (pH 8.0) containing 2 mM MgCl2. DNase and RNase (Roche) were added to a final concentration of 10 µg/mL, along with 10 µL of Benzonase Nuclease (Millipore), and the mixture was incubated at 37 °C for 3 h. Proteinase K (Invitrogen) was added to a final concentration of 20 µg/mL and was incubated for 2 h at 55 °C. Then 50 mL of Tris⋅HCl-buffered phenol (pH 8.0) was added, and the mixture was inverted for 20 min at 20 °C. The mixture then was centrifuged at 3,000 × g for 15 min, and the aqueous phase was removed and placed in a 3,500-MWCO dialysis membrane and dialyzed against water for 2 d, before lyophilization. One hundred milligrams of dried Vi antigen was resuspended in 2 mL of water and applied to a Sephadex G-200 column (25 ×750 mm) at a flow rate of 50 µL/min. The column elution buffer consisted of 10 mM Tris⋅HCl (pH 8) containing 0.25% (wt/vol) sodium deoxycholate, 200 mM NaCl, 1 mM EDTA, and 0.02% (wt/vol) sodium azide. Fractions that contained Vi antigen were pooled, dialyzed against the same buffer without deoxycholate, followed by water, and were lyophilized. LPS and Vi antigen elution were monitored by SDS/PAGE and were visualized with silver staining and immunoblotting, respectively.
Preparation of Vi Antigen Oligosaccharides and NMR Analysis.
Thirty milligrams of purified Vi antigen were resuspended in 500 µL of 100% trifluoroacetic acid and were incubated at 100 °C for 16 h. Trifluoroacetic acid was evaporated with air, and the dried material was resuspended in water and lyophilized twice. This material was resuspended in 1 mL of water and was applied to a Sephadex G-50 superfine column (25 × 750 mm) coupled to a Smartline 2300 refractive index detector (Knauer), with an elution flow rate of 0.6 mL/min in aqueous 1% (vol/vol) acetic acid, 0.4% (vol/vol) pyridine. The void volume was collected, concentrated using a rotary evaporator, and lyophilized. To remove O-acetyl groups, purified Vi antigen was treated with 15% (wt/wt) ammonium hydroxide at 37 °C for 24 h. Saponified polysaccharide was purified by gel filtration chromatography as described above. NMR experiments were performed at the NMR facility in the University of Guelph Advanced Analysis Center. Ten milligrams of Vi antigen were deuterium exchanged by lyophilizing twice from 99.9% D2O and were examined as a solution in 99.96% D2O. 13C NMR spectra were collected at 25 °C on a 600 MHz UltraShield spectrometer (Bruker), equipped with a cryoprobe. Data were analyzed using Bruker TopSpin software. Sodium 3-trimethylsilylpropanoate-2,2,3,3-d4 provided an internal standard (δH 0, δC −1.6).
MS of the Vi Antigen Glycolipid Terminus.
MS experiments were performed at the Mass Spectrometry Facility in the University of Guelph Advanced Analysis Centre. LC-MS analyses were performed on an Agilent 1200 high-performance liquid chromatograph interfaced with an Agilent UHD 6530 QTOF mass spectrometer. A C18 column (Agilent Poroshell 120, EC-C18, 50 × 3.0 mm, 2.7 µm) was used for chromatographic separation with the following solvents: aqueous 0.1% (vol/vol) formic acid (A), and 0.1% (vol/vol) formic acid in acetonitrile (B). The mobile phase gradient was as follows: 10% B held for 1 min and then increased to 100% B in 29 min followed by column wash at 100% B for 5 min, and 20 min reequilibration. The flow rate was maintained at 0.4 mL/min. The MS electrospray capillary voltage was maintained at 4.0 kV and the drying gas temperature at 250 °C with a flow rate of 8 L/min. Nebulizer pressure was 30 psi, and the fragmentor was set to 160 V. Nitrogen was used as both the nebulizing and drying gas. The mass-to-charge ratio was scanned across the range of 100–3,000 m/z using 2 GHz (extended dynamic range) in negative-ion mode. The acquisition rate was two spectra/s. The instrument was externally calibrated with the ESI Tune Mix (Agilent). The sample injection volume was 10 µL. Data were analyzed using Agilent Qualitative Analysis Software.
High-Pressure Freezing, Freeze Substitution, and Electron Microscopy.
S. Typhi and mutant derivatives were grown in 1 L of LB medium supplemented with 100 µg/mL 2,3-dihydroxybenzoic acid at 37 °C until the OD600 reached 0.4. Cells were collected by centrifugation at 4,000 × g for 5 min and were washed in 1 mL fresh growth medium. Two microliters of cell culture were mixed with 1 µL of 250 mM sucrose and then were frozen immediately using a Leica EM HPM100 high-pressure freezer. Cryofixed cells were transferred to vials containing 1 mL substitution medium (1% OsO4, 0.1% uranyl acetate in acetone) and were placed in a Leica AFS2 freeze substitution unit for substitution under controlled temperatures. Following substitution, cells were washed three times in 100% HPLC-grade acetone and were infiltrated with 10–15% (vol/vol) Epon 812 in acetone overnight. Samples were further infiltrated with 25% (vol/vol) Epon 812 in acetone for 3 h, followed by 50% (vol/vol) Epon 812 in acetone overnight. The Epon/acetone mix was exchanged with fresh 50% (vol/vol) Epon 812 in acetone, and the acetone was allowed to evaporate overnight before samples were embedded in 100% Epon 812 and polymerized at 60 °C for 48 h. Ultrathin sections were cut using a Reichert Ultracut E ultramicrotome and were placed on 100-mesh platinum/copper grids for viewing. Ultra-thin sections were negatively stained with 2% (wt/vol) uranyl acetate for 7 min, washed with water, and then stained with Reynold’s lead citrate for 3 min. Images were acquired using an FEI Tecnai G2 F20 transmission electron microscope at 200kV coupled to a bottom-mount Gatan 4 k × 4 k CCD camera in the Molecular and Cellular Imaging Facility at the University of Guelph Advanced Analysis Center.
Growth Curves.
Viable cell counts were performed using E. coli Top10 harboring pBAD24 (vector), pWQ787 (VexE), or pWQ789 (VexEH466A). Five-milliliter overnight cultures were used to inoculate 100 mL LB medium (in 500 mL flasks), supplemented with 100 μg/mL ampicillin, to give an initial OD600 of 0.01. Cultures were incubated at 37 °C with shaking at 200 rpm. At 2.5 h l-Arabinose was added to the culture medium to a final concentration of 0.02% (wt/vol). Aliquots of cells were removed each hour, and dilutions were plated on LB agar supplemented with 100 μg/mL ampicillin chloramphenicol.
Purification and Characterization of Lipid A.
Lipid A was isolated from E. coli BKT09 harboring pWQ284 (vector), pWQ794 (vexE), pWQ795 (lpxL), or pWQ796 (lpxM). Two hundred milliliters of LB medium supplemented with 0.2% (wt/vol) l-arabinose, and 37 µg/mL chloramphenicol were inoculated at 1:100 from 5-mL overnight cultures. Cultures were incubated at 30 °C until the culture OD600 reached 1.0; then cells were collected by centrifugation at 4,000 × g for 20 min. Lipid A was isolated by chloroform-methanol extraction and mild acid hydrolysis as previously described (34). The resulting dried lipid A was resuspended in 400 µL 4:1 (vol/vol) chloroform-methanol. This solution was diluted 5:1 (vol/vol) in 50% (vol/vol) aqueous isopropanol immediately before manual infusion into a Bruker AmaZon SL ion trap mass spectrometer at the Mass Spectrometry Facility in the University of Guelph Advanced Analysis Centre. MS electrospray capillary entrance and exit voltages were set to 4 kV and 140 V, respectively. Nitrogen was used as the drying gas and was maintained at 300 °C. The mass-to-charge ratio was scanned across the range of 700–1,800 m/z in negative-ion mode. Data were analyzed using Bruker DataAnalysis 4.2 software and are presented as deconvoluted (neutral-charge) spectra.
Acknowledgments
Plasmids pGVXN158 containing the viaB locus, pNLP15 containing the spy promoter fused to the luxCDABE cassette, and E. coli BKT09 were generous gifts from Dr. Michael Wetter, Dr. Tracy Raivio, and Dr. Russell Bishop, respectively. We thank Prof. Ayub Qadri for the gift of monoclonal antibodies raised to Vi antigen; Drs. Dyanne Brewer and Armen Charchoglyan for technical assistance with MS; Mrs. Valerie Robertson and Dr. Andy Lo for technical support with NMR spectroscopy; Dr. Michaela Strüder-Kypke and Mr. Robert Harris for freeze substitution and electron microscopy; Dr. Colin Cooper for sequencing A. denitrificans; and Dr. Iain Mainprize for the generation of a wecA mutant in E. coli. This work was supported by funding from Canadian Institutes of Health Research. C.W. holds a Canada Research Chair, and S.D.L. is the recipient of a Canada Graduate Scholarship from the National Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KT99772).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524665113/-/DCSupplemental.
References
- 1.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CM, Roberts IS. Capsular polysaccharides and their role in virulence. Contrib Microbiol. 2005;12:55–66. doi: 10.1159/000081689. [DOI] [PubMed] [Google Scholar]
- 3.Keestra-Gounder AM, Tsolis RM, Bäumler AJ. Now you see me, now you don’t: The interaction of Salmonella with innate immune receptors. Nat Rev Microbiol. 2015;13(4):206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 4.Hone DM, et al. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988;56(5):1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klugman KP, et al. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;2(8569):1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- 6.Willis LM, et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci USA. 2013;110(19):7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis LM, Whitfield C. KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci USA. 2013;110(51):20753–20758. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013;378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Heyns K, Kiessling G. Strukturaufklärung des vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carbohydr Res. 1967;3(3):340–353. German. [Google Scholar]
- 10.Virlogeux I, Waxin H, Ecobichon C, Popoff MY. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology. 1995;141(Pt 12):3039–3047. doi: 10.1099/13500872-141-12-3039. [DOI] [PubMed] [Google Scholar]
- 11.Wetter M, et al. Molecular characterization of the viaB locus encoding the biosynthetic machinery for Vi capsule formation in Salmonella Typhi. PLoS One. 2012;7(9):e45609. doi: 10.1371/journal.pone.0045609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snellings NJ, Johnson EM, Kopecko DJ, Collins HH, Baron LS. Genetic regulation of variable Vi antigen expression in a strain of Citrobacter freundii. J Bacteriol. 1981;145(2):1010–1017. doi: 10.1128/jb.145.2.1010-1017.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 15.Lewin TM, Wang P, Coleman RA. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry. 1999;38(18):5764–5771. doi: 10.1021/bi982805d. [DOI] [PubMed] [Google Scholar]
- 16.Six DA, Carty SM, Guan Z, Raetz CR. Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis. Biochemistry. 2008;47(33):8623–8637. doi: 10.1021/bi800873n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyedarabi A, et al. Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase. Biochemistry. 2010;49(3):539–546. doi: 10.1021/bi901503g. [DOI] [PubMed] [Google Scholar]
- 19.Anderson MS, Raetz CR. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987;262(11):5159–5169. [PubMed] [Google Scholar]
- 20.Qadri A, Ghosh S, Talwar GP. Monoclonal antibodies against two discrete determinants on Vi capsular polysaccharide. J Immunoassay. 1990;11(2):235–250. doi: 10.1080/01971529008053271. [DOI] [PubMed] [Google Scholar]
- 21.Weigel PH, et al. Hyaluronan synthase assembles chitin oligomers with -GlcNAc(α1→)UDP at the reducing end. Glycobiology. 2015;25(6):632–643. doi: 10.1093/glycob/cwv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larue K, Ford RC, Willis LM, Whitfield C. Functional and structural characterization of polysaccharide co-polymerase proteins required for polymer export in ATP-binding cassette transporter-dependent capsule biosynthesis pathways. J Biol Chem. 2011;286(19):16658–16668. doi: 10.1074/jbc.M111.228221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kos V, Cuthbertson L, Whitfield C. The Klebsiella pneumoniae O2a antigen defines a second mechanism for O antigen ATP-binding cassette transporters. J Biol Chem. 2009;284(5):2947–2956. doi: 10.1074/jbc.M807213200. [DOI] [PubMed] [Google Scholar]
- 24.Westphal O, Jann K. Bacterial lipopolysaccharides: Extraction with phenol-water and further applications of the procedure. In: Whisler RL, editor. Methods in Carbohydrate Chemistry. Vol 5. Academic; New York: 1965. pp. 83–91. [Google Scholar]
- 25.D’Andrea LD, Regan L. TPR proteins: The versatile helix. Trends Biochem Sci. 2003;28(12):655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Marchler-Bauer A, et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39(Database issue):D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpenahalli MR, Lupas AN, Söding J. TPRpred: A tool for prediction of TPR-, PPR- and SEL1-like repeats from protein sequences. BMC Bioinformatics. 2007;8:2. doi: 10.1186/1471-2105-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouet P, Robert X, Courcelle E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003;31(13):3320–3323. doi: 10.1093/nar/gkg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price NL, Raivio TL. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol. 2009;191(6):1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson JC, O’Brien JP, Brodbelt JS, Trent MS. Isolation and chemical characterization of lipid A from gram-negative bacteria. J Vis Exp. 2013;79(79):e50623. doi: 10.3791/50623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadam SK, Rehemtulla A, Sanderson KE. Cloning of rfaG, B, I, and J genes for glycosyltransferase enzymes for synthesis of the lipopolysaccharide core of Salmonella typhimurium. J Bacteriol. 1985;161(1):277–284. doi: 10.1128/jb.161.1.277-284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundborg M, Widmalm G. NMR chemical shift prediction of glycans: Application of the computer program CASPER in structural analysis. Methods Mol Biol. 2015;1273:29–40. doi: 10.1007/978-1-4939-2343-4_3. [DOI] [PubMed] [Google Scholar]
- 37.Emptage RP, Daughtry KD, Pemble CW, IV, Raetz CR. Crystal structure of LpxK, the 4′-kinase of lipid A biosynthesis and atypical P-loop kinase functioning at the membrane interface. Proc Natl Acad Sci USA. 2012;109(32):12956–12961. doi: 10.1073/pnas.1206072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hone DM, Harris AM, Chatfield S, Dougan G, Levine MM. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine. 1991;9(11):810–816. doi: 10.1016/0264-410x(91)90218-u. [DOI] [PubMed] [Google Scholar]
- 39.Chang AC, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuthbertson L, Kimber MS, Whitfield C. Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proc Natl Acad Sci USA. 2007;104(49):19529–19534. doi: 10.1073/pnas.0705709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinrichs DE, Yethon JA, Amor PA, Whitfield C. The assembly system for the outer core portion of R1- and R4-type lipopolysaccharides of Escherichia coli. The R1 core-specific beta-glucosyltransferase provides a novel attachment site for O-polysaccharides. J Biol Chem. 1998;273(45):29497–29505. doi: 10.1074/jbc.273.45.29497. [DOI] [PubMed] [Google Scholar]