Significance
Water is the major limiting factor for plant productivity in the field, and current water use in agriculture is not sustainable. Under water limitations, plants can ameliorate the carbon for water exchange leading to higher water use efficiencies. The plant hormone abscisic acid is activated in response to drought and regulates the water status and acclimation responses. Here we show in the model species Arabidopsis that abscisic acid receptors can be explored to constitutively increase the water use efficiency without impinging on growth potential. The finding may support future efforts to generate “more crop per drop” required for a more sustainable water use in agriculture.
Keywords: carbon assimilation, drought resistance, water deficit, water productivity, water use efficiency
Abstract
Plant growth requires the influx of atmospheric CO2 through stomatal pores, and this carbon uptake for photosynthesis is inherently associated with a large efflux of water vapor. Under water deficit, plants reduce transpiration and are able to improve carbon for water exchange leading to higher water use efficiency (WUE). Whether increased WUE can be achieved without trade-offs in plant growth is debated. The signals mediating the WUE response under water deficit are not fully elucidated but involve the phytohormone abscisic acid (ABA). ABA is perceived by a family of related receptors known to mediate acclimation responses and to reduce transpiration. We now show that enhanced stimulation of ABA signaling via distinct ABA receptors can result in plants constitutively growing at high WUE in the model species Arabidopsis. WUE was assessed by three independent approaches involving gravimetric analyses, 13C discrimination studies of shoots and derived cellulose fractions, and by gas exchange measurements of whole plants and individual leaves. Plants expressing the ABA receptors RCAR6/PYL12 combined up to 40% increased WUE with high growth rates, i.e., are water productive. Water productivity was associated with maintenance of net carbon assimilation by compensatory increases of leaf CO2 gradients, thereby sustaining biomass acquisition. Leaf surface temperatures and growth potentials of plants growing under well-watered conditions were found to be reliable indicators for water productivity. The study shows that ABA receptors can be explored to generate more plant biomass per water transpired, which is a prime goal for a more sustainable water use in agriculture.
Plants are ferocious consumers of water, and plant transpiration is the dominant vector for water mobilization from terrestrial surfaces to the atmosphere (1). Plant transpiration is sustained by efficient water uptake through the root systems, which can comprise 500 m2 of root surface and 500 km in combined length even in a single barley plant. Water is the major factor limiting crop productivity in the field (2). Thus, more than two-thirds of the fresh water resources used globally are channeled into agriculture, thereby contributing to potential social conflicts over water (3).
Whereas the gas exchange of CO2 and water vapor at the stomatal pore is a physical process controlled by both the ratio in partial pressure gradients and gas diffusivities (4, 5), terrestrial plants are able to capture carbon more efficiently under water deficit. Both short-term leaf gas exchange measurements and 13C isotope discrimination analyses revealed increases of the instantaneous water use efficiency (insWUE) and intrinsic WUE (iWUE), respectively, by a factor of 1.5–2.5 in wheat and other species (6, 7). The underlying mechanisms, however, are not fully elucidated. Gains in WUE have been found to be associated with trade-offs in growth potential (8–10). WUE is controlled by genes regulating stomatal density and size (11–13). Stomatal aperture is, in turn, controlled by abscisic acid (ABA) signaling (14).
Water deficit generates a hydraulic signal in plants that is rapidly spread over long distances and induces the stress signal ABA (15). Subsequently, ABA mediates rapid responses including stomatal closure and long-term adjustments such as the down-regulation of growth and promotion of senescence (16, 17). ABA perception recruits an ABA-binding REGULATORY COMPONENT OF ABA RECEPTOR (RCAR)/PYRABACTIN RESISTANCE 1 (PYR1)/PYR1-LIKE (PYL) and an associated protein phosphatase of type 2C (PP2C) (18, 19). Administration of ABA and ABA agonists (20, 21), ectopic expression of RCARs (17, 18, 22–24), as well as reduced expression of PP2Cs (25) have been shown to minimize plant transpiration and, in some cases, to enhance survival under water deficit. It is clear that plants with strongly reduced transpiration are limited in CO2 uptake and, hence, in growth and biomass formation. Despite the potential drought resistance of these plants, the growth-restricted trait is not attractive for breeders. A trait providing high WUE without impinging on growth potential would be desirable. Such a trait would confer water productivity by allowing more plant growth and yield per water unit. However, the very existence of such a trait is debated (8, 9).
The central role of ABA in controlling the water status of plants prompted us to investigate whether ABA receptors can be explored to generate water-productive plants. We reasoned that the different facets of ABA responses are likely controlled by specific receptor complexes. The combinatorial complexity of 14 ABA RCAR/PYR/PYL receptors and their interactions with nine PP2C coreceptors hampers the dissection of distinct functions for receptor complexes. Therefore, we examined all ABA receptors of Arabidopsis for their potential to mediate a water-productive trait. Plants overexpressing the receptor RCAR6 combined up to 40% increased WUE with high growth rates, i.e., are water productive. Water productivity was associated with maintenance of net carbon assimilation by compensatory increases of leaf CO2 gradients, thereby sustaining plant growth.
Results
Increased WUE of Arabidopsis Accessions Under Water Deficit.
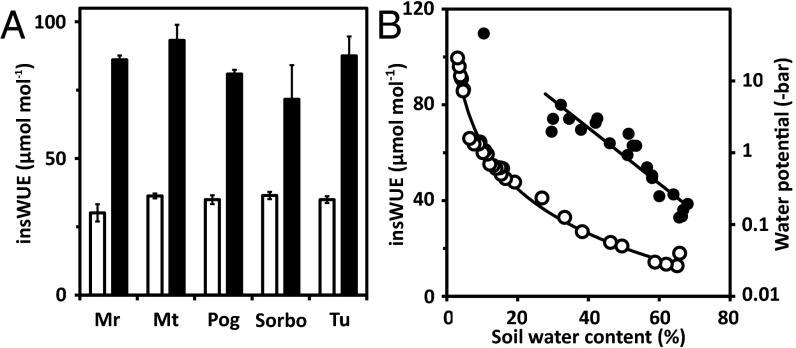
WUE varies in different accessions of Arabidopsis thaliana (26). Under water deficit, an approximately doubling of WUE has been reported in the accessions Columbia (Col-0) and Landsberg (11). We analyzed additional Arabidopsis accessions from different continents and climatic environments for this change in WUE by conducting gas exchange measurements on whole plants. Plants growing under well-watered conditions were compared with plants exposed to water deficit. All accessions analyzed responded by an approximately twofold increase in insWUE imposed by ∼30% residual water content of the soil (Fig. 1A). The analysis confirms the general WUE response of plants if water becomes limiting. Exposing Col-0 plants to progressively drying soil revealed a continuous rise of insWUE negatively correlated with water availability (Fig. 1B). The question we attempted to address was whether enhanced ABA signaling by ABA receptors can establish constitutively enhanced WUE without affecting growth performance.
Fig. 1.
Water use efficiency of Arabidopsis accessions in response to water deficit. (A) The insWUE, i.e., ratio of net CO2 assimilation and stomatal conductance, were compared among the accession Mr-0 (Italy), Mt-0 (Libya), Pog-0 (Canada), Sorbo (Tadjikistan), and Tu-0 (Italy) under well-watered conditions, 66.7 ± 1.2% soil water content (vol/vol; white column), and under water deficit (39 ± 3% soil water content, black column). Whole leaf rosette measurements at photon flux density 150 μmol⋅m−2⋅s−1, 50% humidity, n = 3 with three technical replicates. (B) insWUE of wild type cv. Columbia (Col-0) affected by soil water content (vol/vol, filled circle). The dependence of the water potential Ψ (open circle, single measurements) on soil water content is given.
Overexpression of ABA Receptors Affects Growth and Leaf Temperatures.
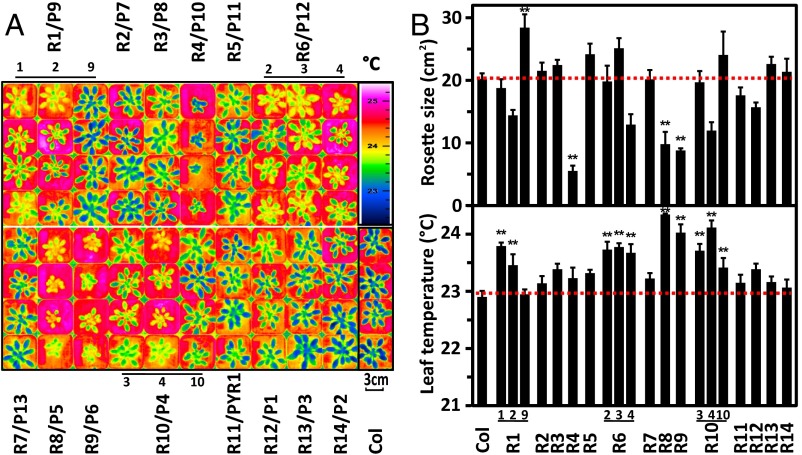
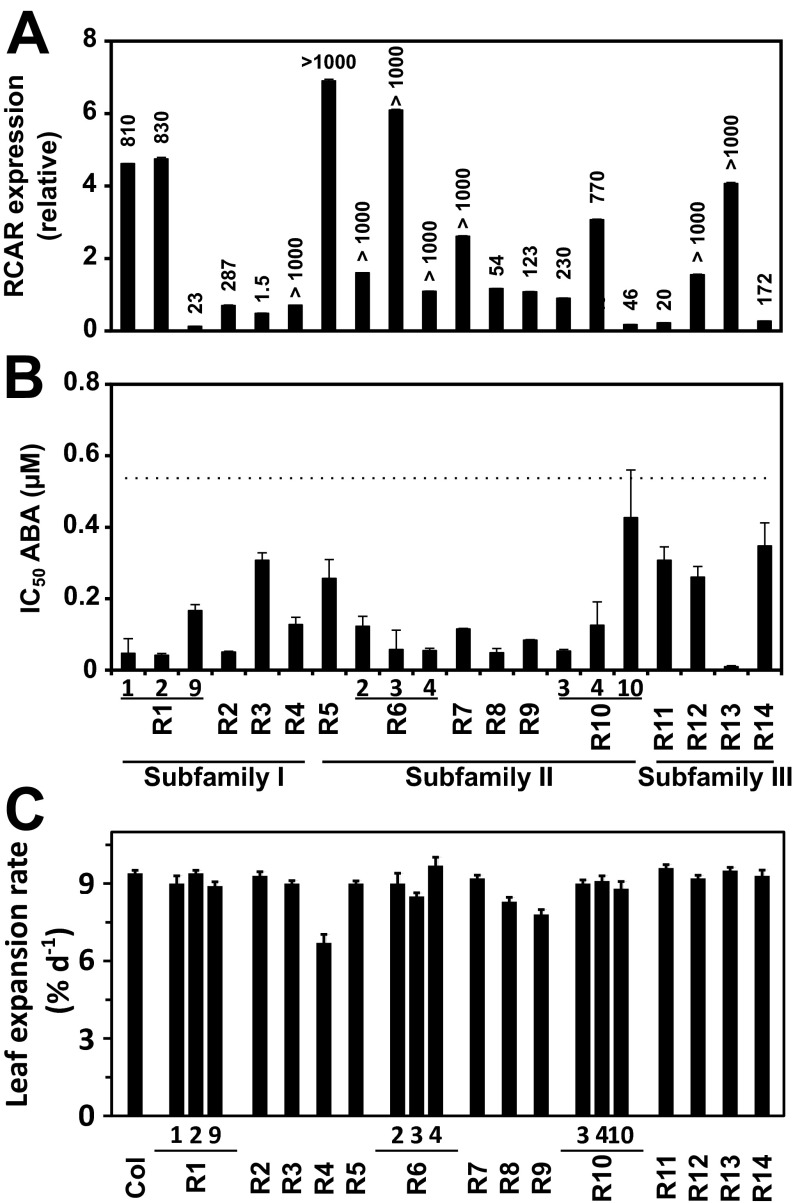
Arabidopsis plants with enhanced expression of single RCAR receptors were generated. Three to five independent homozygous lines for each RCAR member were prescreened for their capacity to grow comparably to the parental line while showing elevated leaf surface temperatures as a first indication for reduced transpiration. For each line, four plants were scored under well-watered conditions. In the case that ectopic expression of individual RCARs generated a single plant line that fulfilled the criteria, three independent lines were selected; if not, only one prototype line was chosen. The selected plants lines were again analyzed for confirmation of the phenotypes by thermal imaging of leaves and determination of the rosette size (Fig. 2 A and B). In addition, the level of RCAR expression and the ABA sensitivity during seed germination was assessed in these RCAR lines (Fig. S1 A and B). The phenotypic assessment revealed increased leaf surface temperatures for the RCAR8 and RCAR9 line associated with severely reduced development of the leaf rosette. Others, such as the RCAR11–RCAR14 lines, showed no significant or marginal elevation of leaf temperatures. However, the Arabidopsis lines RCAR1-1, RCAR6-3, and RCAR10-3 combined increased leaf temperatures with no or minor growth trade-offs (Fig. 2B). The maximum leaf expansion rates of most lines examined were in the range of 9.2% growth per day, however, lines overexpressing RCAR4, RCAR8, and RCAR9 showed reduced growth rates (Fig. S1C).
Fig. 2.
Overexpression of ABA receptors affects growth and leaf temperatures. Arabidopsis Col-0 lines overexpressing the 14 different ABA receptors RCARs (R1–R14)/PYR1/PYLs (P1–P13) under the constitutive viral promoter 35S were homozygous for the transgene. Independent lines are denoted by subscript numbers. (A) Thermogram of the plants grown for 40 d under short day (8 h of light, photon density of 150 μmol⋅m−2⋅s−1) and under well-watered conditions (Ψ ≥ −0.02 bar). Four plants were grown per line in separate pots at randomized positions and the thermal pictures were arranged in groups after imaging. (B) Leaf area as projected rosette size and leaf temperatures from data shown in A. The threshold value of Col-0 is indicated by a dotted line. n = 4 biological replicates per line, ± SEM, **P < 0.001 compared with wild type.
Fig. S1.
Analysis of Arabidopsis lines overexpressing individual ABA receptors. (A) Transcript abundance of the ectopically expressed ABA receptor shown relative to ubiqutin10 expression (columns; ±SD) and to the expression in wild type indicated on Top. The analysis was performed three times with different biological material and gave similar results. The data depict one experiment with three technical replicates. (B) Germination assay of Arabidopsis RCAR lines. The IC50 value for ABA is given for each line (3 × 30 seeds per line and ABA concentration, five different concentrations). The IC50 value for Columbia was 0.56 ± 0.13 μM ABA and is signified by the dotted line. Three independent experiments were conducted. (C) Maximum linear expansion rates of leaves for Col-0 and ABA receptor lines growing under well-watered conditions. The values are given in percentage of increase per day.
RCAR Lines with Higher Water Use Efficiency.
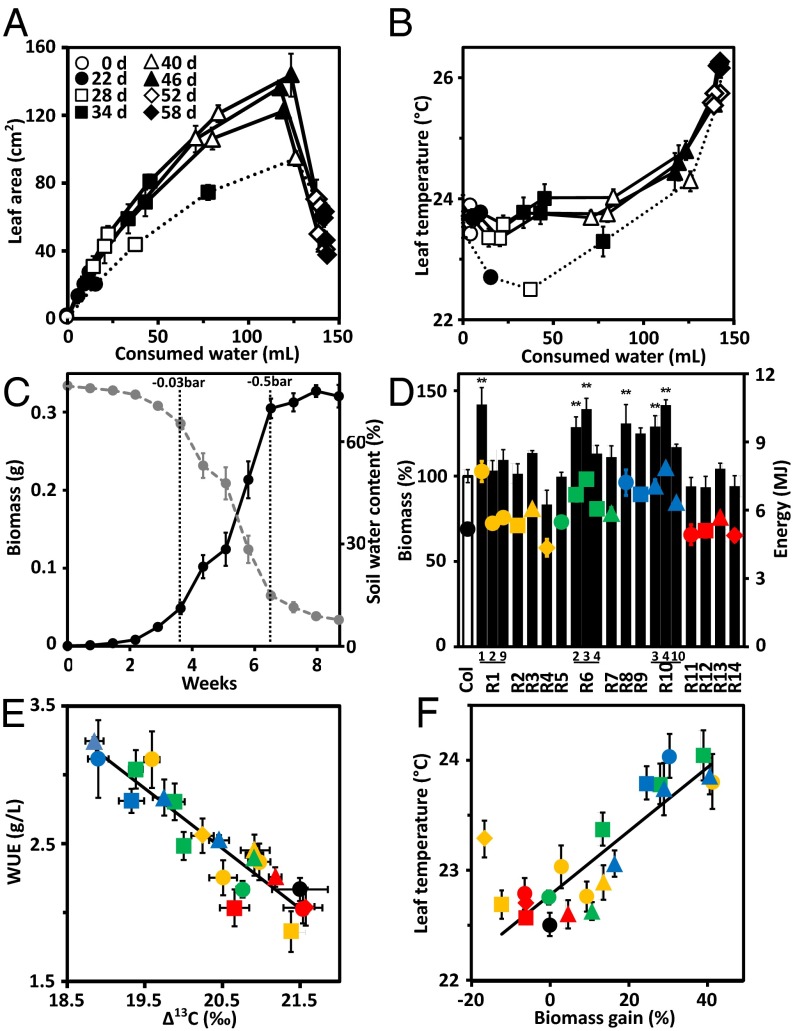
Analysis of three independent RCAR6 lines for water consumption over time indicated a reduced transpiration at similar leaf rosette growth (Fig. 3A) and elevated leaf temperatures of the ABA receptor lines compared with Col-0 (Fig. 3B). The experiment was conducted by withholding watering and minimizing evaporation at an early developmental stage of the plantlets, 18 d after germination with a total leaf area smaller than 0.5 cm2. The soil volume and soil water content per planting pot were identical for each single plant. The experiment started under well-watered conditions with a soil water content of 75% (vol/vol) and was terminated 8 wk after onset when the plant-available water in the soil was consumed and the residual water content in the soil was below 10%. Under this drought regime, the biomass of wild-type plants increased from 0.001 to 0.32 g and most of the biomass build-up occurred under mild to severe water deficit as a simulation of mounting water deficit in the field (Fig. 3C). Symptoms of mild water deficit were observed at water potentials Ψ < −0.03 bar, indicated by changes of stomatal aperture evidenced by increases in leaf surface temperatures. Severe water deficit (Ψ < −0.5 bar) resulted in wilting and shrinkage of leaves. All selected RCAR lines were subjected to this drought regime and analyzed for the maximum accumulation of dry matter and the calorimetric yields (Fig. 3D). The same amount of water was available to every plant; hence, higher biomass values of RCAR lines compared with the wild type reflect higher WUE, i.e., more carbon gain per water unit. Indeed, lines overexpressing RCAR1, RCAR6, RCAR8, RCAR9, and RCAR10 (Fig. 3D) had increased biomass production; some showed increases up to 40% in both dry matter and calorimetric values. Those ABA receptors belong to subclass I and II (18, 19). The plant lines of subclass III receptors, RCAR11–RCAR14, showed no significant gains in biomass.
Fig. 3.
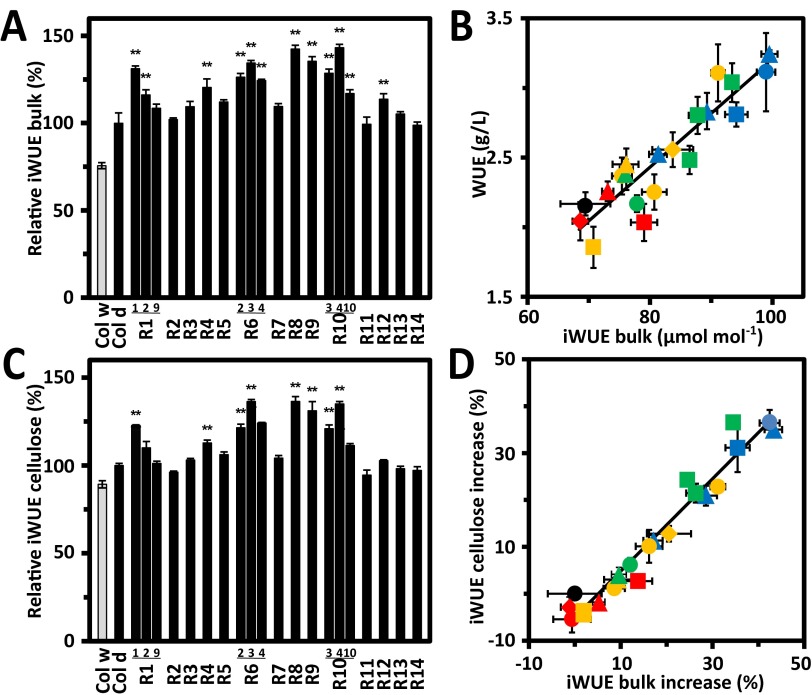
Efficient water use conferred by ABA receptor expression. Water consumption in relation to (A) growth and (B) leaf surface temperatures of three independent RCAR6-overexpressing lines (solid graphs) compared with Col-0 (dotted line) growing on drying soil. Single plantlets (n = 4 per line) were grown for 18 d before the discontinuation of watering at soil water potential Ψ = −0.02 bar. At the onset of drought (day 0) water consumption per plant was recorded for 58 consecutive days (58 d). Plants were grown under a short-day light regime (8 h, photon density of 150 μmol⋅m−2⋅s−1) to keep them at vegetative developmental stage. Growth is expressed as increase in leaf area of the projected rosette. Wilting of leaves caused a reduction in rosette size. (C) Accumulation of biomass (dry weight, n = 6) in Col-0 and changes in soil water content (gray line) during a drought experiment as shown in A as mean ± SEM. The dotted vertical lines indicate threshold Ψ values for mild and severe water deficit, respectively. (D) Above-ground biomass and calorimetric yield of RCAR1–RCAR14 lines (R1–R14) at the end of drought treatment as in A. Biomass of Col-0 was 0.31 ± 0.01 g dry weight per plantlet and set to 100% (column). The energy content of the plants at harvest is indicated by symbols; Col-0 (black filled circle), subclade I comprising members RCAR1–4 lines (yellow), subclade II with RCAR5–7 (green) and RCAR8–10 (blue), and subclade III with RCAR11–14 (red). (E) Association of 13C discrimination in bulk above-ground biomass with WUE (biomass produced per water consumed) as shown in D. (F) Association of biomass gain under drought with leaf surface temperatures of lines under well-watered conditions taken from 7-wk-old plants. Symbols in E and F are as in D. n = 4 biological replicates per data point, mean ± SEM, **P < 0.001 compared with wild type (one-way ANOVA).
Stomatal limitation of CO2 assimilation affects 13C discrimination (Δ13C), which is an indicator of iWUE (4, 5). Hence, Δ13C provides an assessment of WUE independent of the WUE analysis by gravimetry. The values for gravimetrically determined WUE and Δ13C of total aboveground biomass (Fig. 3E) showed a clear association (R ± 0.92, P < 0.001) for all plant lines. The Δ13C-deduced iWUE (Fig. S2A) confirmed an approximate 40% increase of iWUE from 69 μmol carbon per mole water in wild type to a maximum value of 93 and 99 μmol⋅mol−1 in two RCAR lines, RCAR6-3 and RCAR10-4, respectively, and correlated with biomass-based WUE (Fig. S2B). The 13C/12C composition of cell wall material integrates water relations during growth, which prevails during periods of less severe water deficit. Δ13C of the cellulose fraction indicated an iWUE up to 36% (±1%, P < 0.001) higher in RCAR plants than wild type (Fig. S2 C and D). Leaf surface temperatures of RCAR-overexpressing plants under well-watered conditions correlated positively with biomass gain under water-limiting conditions, with the exception of RCAR4 (Fig. 3F, R = 0.91, P < 0.001). The RCAR4 line had markedly impaired growth and not all plant-accessible water was consumed at harvest. Taken together, the data support an increased WUE conferred by ectopic expression of distinct ABA receptors leading to greater net carbon assimilation per unit of water transpired.
Fig. S2.
Intrinsic water use efficiencies of Arabidopsis RCAR lines. (A and B) Δ13C-based iWUE of bulk above-ground biomass and (C) of extracted cellulose fractions from the experiment shown in Fig. 3D for RCAR lines (R1–R14) relative to Col-0 exposed to drought (Col d, iWUE = 80 μmol⋅mol−1 in C).The value for wild-type Col-0 grown under well-watered conditions (Ψ > −0.03 bar) is shown as additional reference (gray column, Col w). (B) Correlation of gravimetrically determined WUE and Δ13C-based iWUE (R = 0.92, P < 0.001). (D) Correlation of differences in iWUE of RCAR lines in relation to wild type (filled black circle) from bulk above-ground biomass and extracted cellulose fractions (R = 0.96, P < 0.001). Symbols denote the RCAR lines and wild type as in Fig. 3D. n = 4 biological replicates per data point, mean ± SEM, **P < 0.001 compared with wild type.
Water Productivity Associated with Enhanced CO2 Gradients.
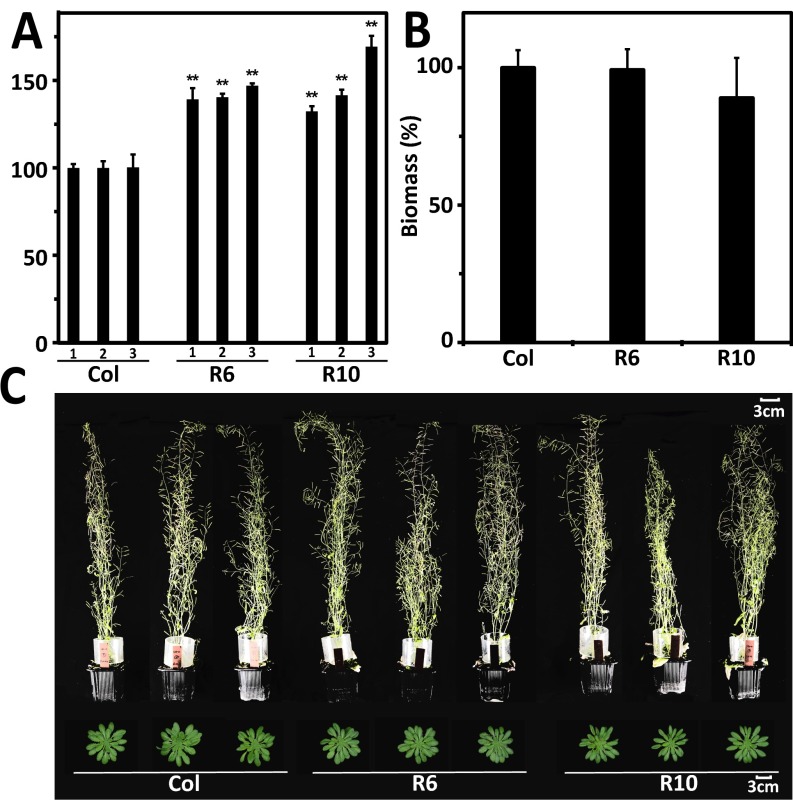
The RCAR6-3 and RCAR10-3 lines combined enhanced water efficiency with no growth penalty (Figs. 2 and 3A), whereas RCAR6-4 and RCAR10-4 lines showed somewhat reduced growth under well-watered conditions. The RCAR10-4 line performed better than RCAR10-3 under drought, which might be attributed to the higher receptor expression in RCAR10-4 (Fig. S1A). All four lines showed high ectopic RCAR expression. For analysis of the physiological basis of water productivity, we chose RCAR6-3 and RCAR10-4, the latter as a candidate for higher WUE combined with some penalties in growth potential. Both of these selected Arabidopsis lines consistently performed better in reiterated and independently conducted experiments. In comparison with the wild type, biomass gains of 39–46% and 32–69% for the RCAR6 and RCAR10 line, respectively, were found under drought (Fig. S3A). There was no significant growth difference detectable between plants of the RCAR6 line and wild type grown under well-watered conditions (Fig. S3 B and C) and final biomass yield differed by 1% (±7%, n = 28, P = 0.7).
Fig. S3.
Biomass production under well-watered growth regimes or drought. (A) RCAR6-3 (R6) and RCAR10-4 (R10) consistently had a higher biomass yield than Col-0 under progressively drying soil (see Fig. 3 for drought condition). The mean above-ground biomass of Col-0 was 0.31 g, 0.31 g, and 0.27 g in three independent experiments (numbered 1–3) and the yield of RCAR lines is expressed relative to Col-0 (n = 4 per data point, mean ± SEM, **P < 0.001 compared with wild type). (B) The dry weight of above-ground biomass of Arabidopsis wild type Col-0 (n = 28), RCAR6-3 (R6, n = 28), and RCAR10-4 (R10, n = 4) was determined after 4 mo of growth under well-watered and long-day regimes (16 h of light per day). Above-ground biomass of Col-0 was 1.41 ± 0.11 g and set to 100%. (C) Representative pictures of the three lines at the age of 8 wk (Upper row) and of rosettes 7 wk (Lower) grown under long-day and short-day, respectively, and under well-watered conditions.
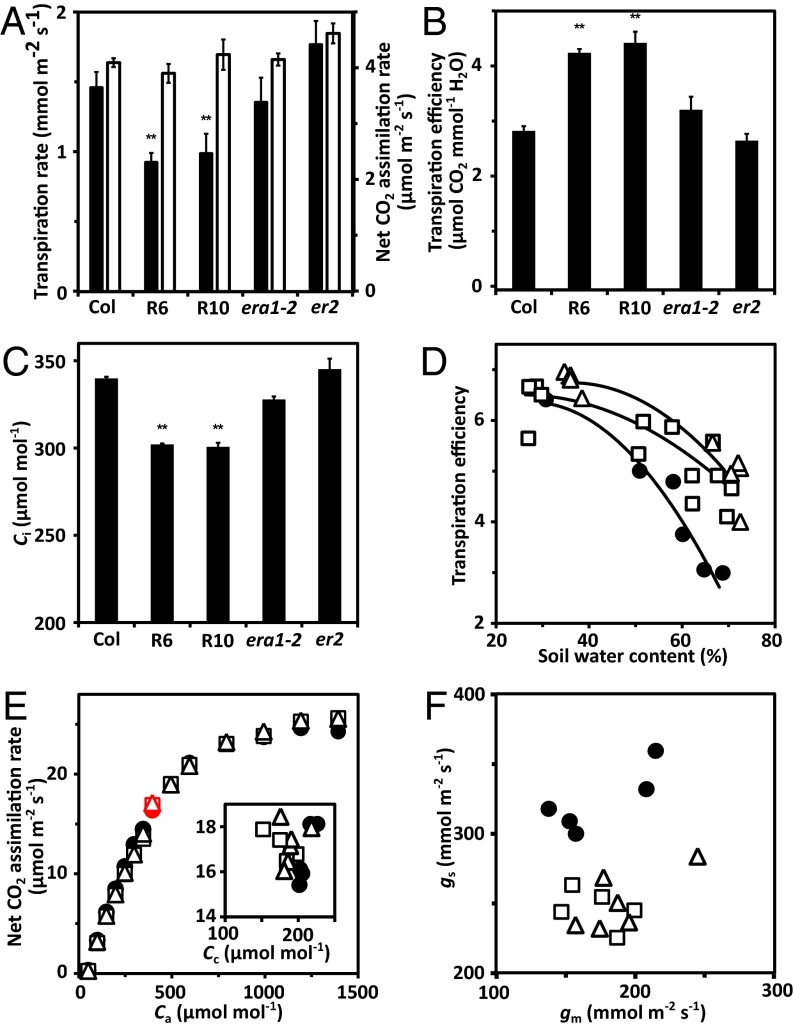
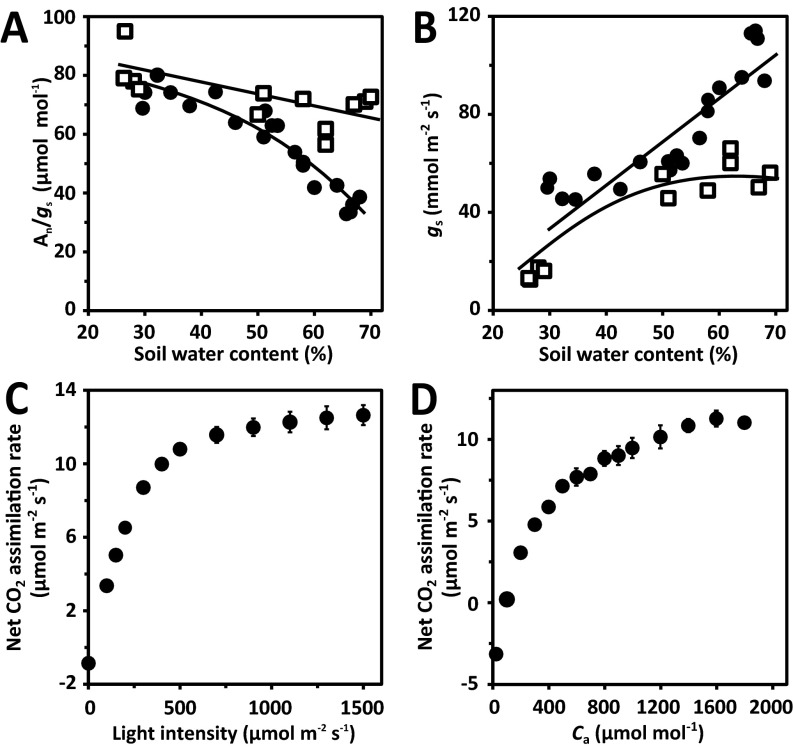
Under well-watered conditions, gas exchange measurements of whole plants revealed lowered transpiration rates at similar net CO2 assimilation rates compared with the wild type, leading to higher transpiration efficiencies of both RCAR lines (Fig. 4 A and B). The Arabidopsis erecta mutant has increased transpiration rates (11), whereas the farnesyltransferase-deficient era1-2 mutant is considered drought resistant (27). The erecta and era1 mutants were found to have higher and slightly lower transpiration compared with the wild type, respectively, and inverse transpiration efficiencies (Fig. 4 A and B). The ratio of CO2 influx and water vapor efflux of plants is determined by the partial pressure differences of both gases at the stomatal pore (4, 5). Hence, increased transpiration efficiencies at comparable net CO2 assimilation rates require a steeper CO2 gradient at a given water pressure difference. Indeed, both RCAR lines had intercellular CO2 levels lowered by about 38 μmol⋅mol−1 leading to ∼59% enhanced CO2 gradients at the stomata (Fig. 4 C and D). As a consequence, the transpiration efficiency was higher in the RCAR6 and RCAR10 lines by ∼49% and 55% (P < 0.001), respectively (Fig. 4B). Higher transpiration efficiencies of RCAR lines were found irrespective of the soil water content (Fig. 4D). The ratio of net CO2 assimilation to stomatal conductance gs, which reflects the partial CO2 pressure gradient at the stomatal pore, increased with reduced soil water content in wild type but remained high and fairly constant in the RCAR6-3 line (Fig. S4A).
Fig. 4.
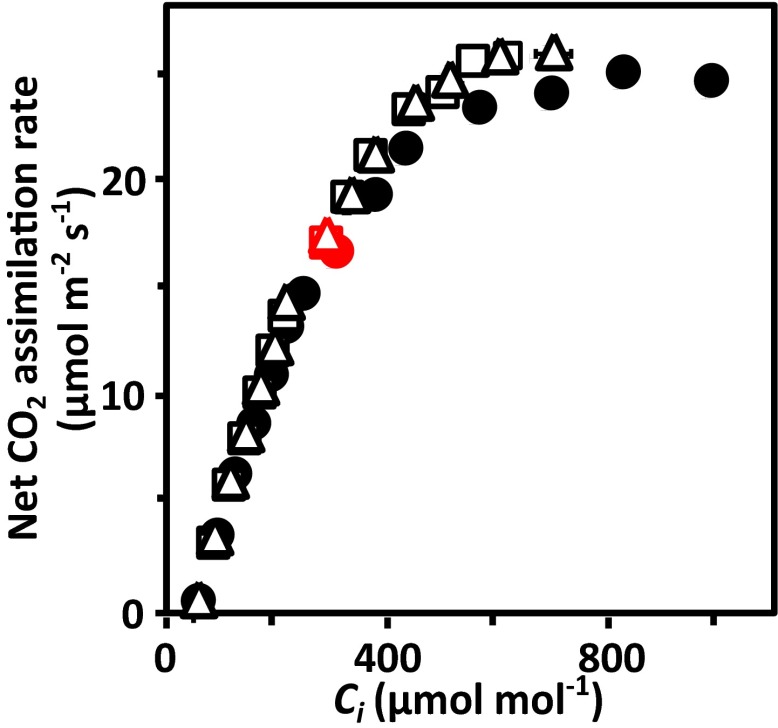
Reduced transpiration and maintenance of CO2 assimilation in RCAR6-3 and RCAR10-4 lines. Whole plant gas exchange analysis at well-watered conditions (A–C) and during drought (D). (A) Transpiration (black bars) and net CO2 assimilation rates (open bars) of RCAR6 (R6) and RCAR10 (R10) lines in comparison with Col-0 and gas exchange mutants era1-2 and er2 at water potential Ψ > −0.03 bar and external CO2 concentration Ca of 420 μmol⋅mol−1. (B) Transpiration efficiency, i.e., ratio of rates for net CO2 assimilation and transpiration (mmol CO2 mol H2O−1) and (C) intercellular CO2 concentration Ci of the same plants as in A. (D) Transpiration efficiency affected by soil water content in RCAR6 (open squares), RCAR10 (open triangles), and wild type (filled circles); single whole plant measurements with 10 technical replicates per data point (D), **P < 0.001 compared with wild type. (E) Analysis of net CO2 assimilation rates in response to external CO2 concentrations (Ca, ambient Ca in red) and (F) mesophyll (gm) and stomatal conductance (gs) in single leaves at high photosynthetic photon flux density (1,500 μmol⋅m−2⋅s−1) and soil Ψ > −0.03 bar. The CO2 concentration in chloroplasts (Cc; Inset) and conductance rates were analyzed at ambient Ca (400 μmol⋅mol−1). Labeling of plant lines is as in D. (E) n ≥ 5 with six technical replicates. (Inset and F) Data of single measurements.
Fig. S4.
Gas exchange analysis of wild-type Col-0 and RCAR6 overexpressing plant. (A and B) Analysis of the leaf rosette of Arabidopsis plants for the relation of the soil water content (vol/vol) and (A) the ratio of net CO2 assimilation (An) and stomatal conductance (gs), which reflects the partial CO2 pressure gradient at the stomatal pore, and (B) gs in Col-0 (filled circle) and the RCAR6-3 line (open square). (C and D) Single leaves of Arabidopsis were analyzed for net CO2 assimilation rates at (C) different light intensities and ambient CO2 (Ca = 400 μmol⋅mol−1) as well as for (D) different atmospheric CO2 levels (Ca) and a constant photon flux of 150 μmol⋅m−2⋅s−1, both at well-watered conditions (Ψ > −0.03 bar). In C, leaves were preadjusted to saturating light intensities (1,500 μmol⋅m−2⋅s−1) for 2 h before the analysis. (A and B) Single plant measurements with 10 technical replicates per data point. (C and D) n = 3 biological replicates per line, four technical replicates per data point, mean ± SEM.
Similarly, gs in wild-type plants was lowered to one-half by a reduction of soil water content from ∼70% to 50%, whereas gs in the RCAR6-3 line was largely unaffected but consistently lower than wild type, possibly reflecting an enhanced ABA response (Fig. S4B). The gas exchange analyses were conducted on entire plants at ambient CO2 level and photon density of 150 μmol⋅m−2⋅s−1; both parameters are limiting photosynthesis (Fig. S4 C and D).
At saturating light and modulating different CO2 concentrations of the air, the net CO2 assimilation rates of leaves from RCAR6, RCAR10, and wild-type plants were quite similar (Fig. 4E). At ambient CO2 levels (400 μmol⋅mol−1) there was no significant difference (P > 0.3), which supports a comparable CO2 flux from air to chloroplasts under these conditions. The CO2 diffusion path from air to the site of photosynthesis, the chloroplast stroma, is controlled by the stomatal conductance gs and mesophyll conductance gm that control CO2 entry from the atmosphere into the intercellular space and the passage from there into chloroplasts, respectively. Changes in mesophyll conductance for CO2 affect the CO2 flux and a higher mesophyll conductance may sustain photosynthesis under CO2 limiting conditions (28). The net CO2 assimilation was similar among the plant lines in response to variation of the intercellular CO2 level (Fig. S5) even under CO2 starvation conditions, as generated by artificially low CO2 concentrations in the air (<150 μmol⋅mol−1). Analysis of the mesophyll conductance revealed variable values among leaves of a single line (Fig. 4F) but did not detectably differ among the lines (P > 0.9). The mesophyll conductance was somewhat lower than the stomatal conductance. The calculated CO2 concentration in chloroplasts of the RCAR lines was indistinguishable (Fig. 4E). The plastidic CO2 level in leaves of the RCAR6 line was ∼177 ± 8 μmol⋅mol−1, whereas the levels were higher by ∼30 μmol⋅mol−1 in the wild type. As a consequence, the CO2 gradient between ambient air and chloroplasts differed by 16% (P = 0.01) between leaves in RCAR6 and wild type: ∼217 μmol⋅mol−1 versus 189 μmol⋅mol−1 in Col-0 leaves. This increase was sufficient to counterbalance the 24% reduction in stomatal conductance and thereby sustaining net CO2 influx (Fig. 4E and F).
Fig. S5.
Net CO2 assimilation rates in response to intercellular CO2 concentrations, Ci. Single leaves of RCAR6-3 (open squares), RCAR10-4 (open triangles), wild type (filled circles) were measured at high photosynthetic photon flux density (1,500 μmol⋅m−2⋅s−1) and well-watered conditions (Ψ > −0.03 bar). The net CO2 assimilation rates at ambient CO2 concentration (400 μmol⋅mol−1) and the corresponding Ci value are highlighted in red (n ≥ 5 biological replicates per line, two technical replicates per data point, mean ± SEM).
Shoot Versus Root Contribution to Water Use Efficiency.
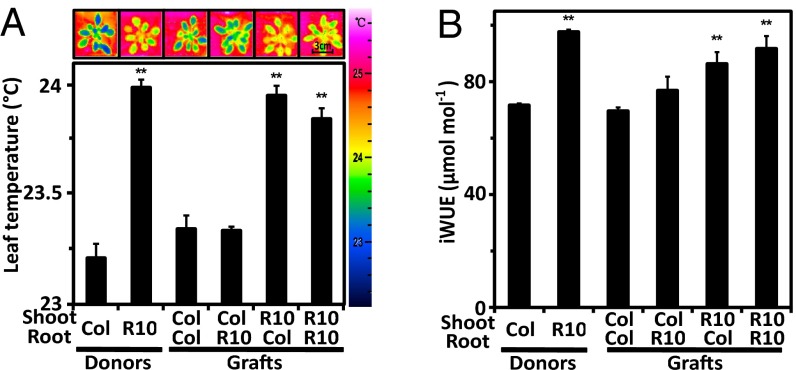
Water deficit triggers a plethora of physiological changes, in which ABA plays a key role by mediating osmoregulation at the whole plant level, photosynthetic adjustments, and root-specific responses such as the stimulation of root growth for exploring soil moisture (29). Reciprocal grafts of above- and below-ground organs between the RCAR10 line and wild type were performed to decipher which organ part contributes to WUE. Analysis of leaf temperatures indicated a shoot-prone reduction in transpirational cooling of the ABA receptor-expressing plants consistent with enhanced ABA signaling in guard cells (Fig. 5A). The 13C discrimination analysis of above-ground biomass from the various grafted plants grown for 10 wk also supported a major effect by RCAR10 shoots for increasing iWUE. The carbon isotopic signatures also tentatively supported a contribution of the root system to enhance WUE (Fig. 5B). Taken together, the findings emphasize the dominant role of the shoot in ameliorating carbon for water exchange.
Fig. 5.
Organ-mediated changes in WUE analyzed by grafting of the RCAR10-4 line and wild type. (A) Leaf surface temperature of donor lines, self-grafted donor lines, and reciprocal graftings of roots and shoots by thermal imaging of 6-wk-old plants at soil water potential Ψ ≥ −0.03 bar. (B) iWUE based on 13C analysis of above-ground biomass collected from 10-wk-old plants exposed to progressive drought as described in Fig. 3. n ≥ 4, two technical replicates, mean ± SEM; **P < 0.001 compared with wild type.
Discussion
It is well established that terrestrial plants can improve WUE under water-restricted conditions. Whether this response is necessarily linked to reduced biomass production and which signals increase WUE under these conditions has not been fully elucidated. In this study, we analyzed the ABA receptor family of Arabidopsis for its potential to provide a water-productive trait by mediating a constitutively increased WUE with no or marginal impacts on growth potentials.
Our findings support the conclusion that activation of ABA signaling is able to increase WUE. Because ABA is induced under drought, ABA and concomitantly triggered ABA responses are likely sufficient to mediate a more efficient carbon capture under water deficit. In our analysis, we were able to separate increased WUE from trade-offs in growth potentials and showed that ABA receptors can be used to generate water-productive plants. An Arabidopsis line overexpressing the ABA receptor RCAR6, and analyzed in detail, showed a constitutively elevated WUE without significant reduction in growth under well-watered conditions. Water productivity was associated with reduced transpiration and maintenance of carbon assimilation as well as growth. Stomatal size and densities were not different in the RCAR6 line from the wild type, indicating that the observed changes in transpiration reflect ABA signaling-mediated changes in apertures of stomata. This conclusion is in line with other studies in which ectopic ABA receptor expression caused ABA-hypersensitive stomatal responses (18, 22–24).
Reduced stomatal conductance found in the RCAR6 and RCAR10 lines was compensated by increased CO2 gradients across the stomatal pore that allowed for maintenance of the CO2 influx. As a consequence, whole plant transpiration efficiencies were increased in the high WUE lines under well-watered conditions to levels induced in Arabidopsis accessions under water deficit. The mesophyll conductance did not differ among the RCAR6-3 and RCAR10-4 lines and wild type under light saturating conditions. Light saturation ensures maximal photosynthetic CO2 demand and may uncover differences in net CO2 assimilation. However, such differences were not observed as reported for gas-exchange–restricted plants (30) despite the lower chloroplastidic CO2 levels in the water-productive plants. Even at lowered environmental CO2 concentrations that exacerbate CO2 deficiencies for the carboxylation reaction, leaves of the RCAR lines showed comparable net CO2 assimilation rates. Hence, the water-productive RCAR lines were able to sustain net CO2 assimilation by a slightly (10%) increased CO2 gradient between ambient air and the chloroplast. The physiological adjustments of water-productive plants might also involve specific long-term adjustments of shoot and root organs (29, 31) for optimizing water use. The finding of higher WUE conferred by the root system overexpressing RCAR10 tentatively supports such a role.
Constitutively increased transpiration efficiencies mediated by reduced transpiration of RCAR overexpressing lines requires ABA receptors capable of modulating stomatal apertures in response even to low ABA levels in the nanomolar range, as present under well-watered conditions (32). However, ABA receptors that are exceedingly sensitive to ABA and too efficient in mediating stomatal closure would curb net CO2 assimilation and growth. The dynamics in water status within a plant’s life and concomitant changes in endogenous ABA concentrations by a factor of 40 and more (32) may add to the selectivity of ABA receptors capable of mediating water productivity. Hence, the delicate balance of net CO2 assimilation versus transpiration at varying ABA levels, and water status of plants likely imposes major constraints on the suitability of ABA receptors to confer water productivity. In addition, the transfer DNA (T-DNA) insertion site and posttranscriptional silencing induced by the high ectopic RCAR expression levels could influence RCAR abundance, the regulation of its abundance under water deficit, and thus affect stomatal aperture and trade-offs in photosynthesis. Hence, our analysis provides a proof of concept that certain RCARs are suitable to improve water productivity of plants but the study is not exhaustive enough to clearly exclude other RCAR members.
Subclade I receptors showed a variable result; the RCAR1 overexpressing plants were more water productive in one of three lines. The variability in growth might be attributed to RCAR1/PYL9-mediated induction of leaf senescence found in a recent study by Zhao et al. (17). Such a phenotype was not observed in an earlier characterization of RCAR1 overexpressing plants (18). Zhao et al. (17) had been using overexpression of the Arabidopsis ABA receptors and identified RCAR1 to mediate drought resistance. Whereas that study focused on severe drought scenarios and senescence-related processes, our analysis has addressed the contribution of RCAR members to water productivity. For instance, expression of the high ABA affinity receptor RCAR4, also a subclade I member, resulted in stunted growth. Such a trait might be beneficial to provide drought resilience but the water productivity of the RCAR4 line was found to be low in our study. However, most ABA subclade II receptors, comprising RCAR5–RCAR10, contributed to water productivity under drought. In contrast, subclade III receptors, which are considered to be dimeric and less affine receptors (33) that include the prototype PYR1/RCAR11 (19), did not.
Water shortage is an environmental challenge encountered by most perennial plants even in the tropics (34, 35). Several plant species have developed specialized water-storing organs, as exemplified by succulents. Others, such as resurrection plants, are truly drought tolerant (36). However, most plants do not conserve water and initiate adaptive responses only when water becomes scarce. This physiological response is possibly due to selection forces in nature, where a species would not benefit from water productivity but face trade-offs associated with reduced transpiration, including higher leaf temperatures and possible constraints on nutrient and CO2 uptake. This situation would change, however, for crop plants where water-saving strategies in monocultures increase water productivity and sustain biomass production under water-limiting conditions.
Both the general response of terrestrial plants to increase WUE under water deficit and the conservation of ABA receptor components in dicots and monocots (37, 38) imply that water-productive plants can be generated with crops. Such crops would combine elevated WUE and high yields similar to plants cultivated under deficit irrigation (39). The plants engineered for water productivity, however, would offer the advantage that no technical equipment is necessary for establishing enhanced WUE and saving soil-borne water to better withstand periods of drought. The identification of ABA receptors for providing water productivity will aid in breeding programs and biotechnical efforts to reduce unsustainable water withdrawal (40) and to generate “more crop per drop” (35, 39, 41).
Methods
Details on materials and methods, including the plant material and chemicals, drought assay, analysis of 13C discrimination and intrinsic water use efficiency, as well as other physiological analyses and statistical tools used are provided in SI Methods.
SI Methods
Plant Material and Chemicals.
Chemicals were obtained from Sigma-Aldrich (www.sigmaaldrich.com), J. T. Baker (www.avantormaterials.com), and (S)-ABA from CHEMOS (www.chemos.de). The Arabidopsis accession Mr-0, Mt-0, Pog-0, Sorbo, Tu-0, and Col-0 and the mutants er2, and era1-2 in the Col genetic background were provided by the Nottingham Arabidopsis Stock Center. RCAR1–14 overexpressors were established by transferring an expression cassette for RCARs under control of the viral 35S promoter into Arabidopsis and selecting for homozygous lines as carried out for RCAR1 (18). Homozygous RCAR-overexpressing lines of the T3 generation were prescreened for reduced transpiration rates combined with minor growth trade-offs compared with wild type. In the case of ectopic expression of individual RCARs generating plants that possibly fulfilled these criteria, three independent lines were selected for further analysis. If not, only one prototype line was chosen and further characterized.
RCAR transcript abundance was determined by RT-PCR analysis (Bio-Rad, CFX96) using ubiquitin 10 as reference and target-specific primers (42). RNA extraction and subsequent cDNA biosynthesis from 12-d-old seedlings was performed as described (42). Plants were grown in soil (Classic Profi Substrate Einheitserde Werkverband) at 22 °C under short-day conditions with 8 h of light (photosynthetic photon flux density: 150 µmol⋅m−2⋅s−1) and ∼50% humidity during light period, unless otherwise stated, in plant growth cabinets (E15, Conviron).
Drought Assay.
Arabidopsis wild-type and T3 and T4 transgenic plants were subjected to drought stress by withholding watering for 4–6 wk. Single seedlings were grown in 200-mL pots containing calibrated amounts of soil (33.4 g dry weight ± 0.5 g) for 18 d under well-watered condition by maintaining soil moisture near field capacity (70–75% soil volumetric water content, ≥ −0.02 bar water potential) before covering the soil surface to prevent evaporation as described (43). Each experiment contained at least four replicates of Columbia and single RCAR lines at randomized positions in the cabinet. Repetitions of the experiment were conducted independently over the course of 16 mo. The relative soil water content (vol/vol) and the soil water potential were determined gravimetrically and by using a pF meter (Umwelt Geräte Technik), respectively. Projected leaf area of the Arabidopsis rosettes was analyzed by using Photoshop Elements software (Adobe). Under well-watered conditions (soil water potential Ψ > −0.03 bar), the logarithmic growth phase was used to derive the maximal linear leaf expansion rate as the square root function of the daily growth rate of the projected leaf area. Above-ground material was harvested for determination of biomass and calorimetric yields after drying the material for 3 d at 60 °C to achieve constant weight.
Physiological Analysis.
Germination assays were performed in the presence of various ABA levels, as reported (44). Grafting experiments were conducted as described (44). Gas exchange measurements were carried out for analysis of carbon assimilation rates, intercellular CO2 levels (ci), and stomatal conductance (gs) in the whole plant configuration and at single leaf level using the GFS-3000 gas exchange system equipped with custom-built cuvettes and the provided software (Heinz Walz). Mesophyll conductance (gm) and CO2 concentration in plastids of leaves of comparable developmental stage and age (leaf number 16 ± 3, 2 wk after leaf emergence) were determined by using a 2-cm2 cuvette and applying the constant J method at saturating light condition (photosynthetic photon flux density: 1,500 µmol⋅m−2⋅s−1) as described (45). The energy content was determined for each Arabidopsis line by combusting aliquots of dried plant material (four biological replicates, two technical repetitions) under high oxygen atmosphere using a Calorimeter C2000 (IKA) calibrated with benzoic acid provided by the supplier. Thermal imaging (InfraTec) was carried out on whole plant trays containing 24 pots at randomized positions and within the environmentally controlled plant growth cabinets.
13C Analysis and Intrinsic Water Use Efficiency.
Carbon isotope composition (δ13C) of whole-shoot biomass and extracted cellulose (46) was analyzed using an elemental analyzer (NA 1110; Carlo Erba Instruments) interfaced (ConFlo II, Finnigan MAT) with a continuous-flow isotope ratio mass spectrometer (Delta Plus; Finnigan MAT), EA-CF-IRMS. Each sample was measured against standard CO2 calibrated with an isotope standard (International Atomic Energy Agency CH6, accuracy of calibration ± 0.06‰ SD). The precision of sample repeats was 0.08‰ (SD) for bulk biomass samples and 0.13‰ for cellulose and included deviations by subsampling, cellulose extraction, and EA-CF-IRMS measurement. 13C discrimination (Δ13C, in ‰) was calculated as Δ13C = (δ13Cair − δ13Cplant)/(1 + δ13Cplant/1,000) (4). Intrinsic WUE has been defined as: iWUE = A/gs = 0.625 Ca (1 − Ci/Ca), where A is the net CO2 assimilation rate and gs the stomatal conductance (5). The factor 0.625 gives the ratio of the diffusivities of CO2 and H2O in air, and Ca and Ci designate the CO2 concentrations in ambient air and intercellular space. In the growth cabinet, Ca was 422 ± 12 μmol⋅mol−1 with an approximated δ13Cair value of −9.7‰. The “simplified” Farquhar model based on nonlimiting mesophyll conductance was applied to estimate Ci/Ca: Δ13C = a + (b − a) Ci/Ca. The term a (4.4‰) denotes the fractionation of 13CO2 relative to 12CO2 during diffusion through the stomatal pores, and b (27.6‰), the net fractionation during carboxylation reactions.
Statistical Analysis.
Data were analyzed by using one-way ANOVA and linear regression analysis with the SPSS version 16.0 software for Windows. Unless otherwise stated, we chose the sample size n = 4 of plant individuals per genotype and treatment. Analysis of large sample size experiments revealed that normal distribution occurred with a SD, σ, of 10%. The sample size was computed with the G*Power 3.1.9.2 software program under the assumption that a difference of 30% (3 × σ) between RCAR-overexpressing lines and the wild type would occur, with presumed values for α and β of 0.05 and 0.1, respectively. The cohort size of four individuals is recommended based on these parameters for an unpaired, two-sided t test.
Acknowledgments
We thank our colleagues for critical reading and constructive comments on the manuscript. Financial support was provided by Deutsche Forschungsgemeinschaft Grants (GR938 and SFB924) and Bayerisches Staatsministerium für Bildung und Kultus, Wissenschaft und Kunst (ForPlanta).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601954113/-/DCSupplemental.
References
- 1.Jasechko S, et al. Terrestrial water fluxes dominated by transpiration. Nature. 2013;496(7445):347–350. doi: 10.1038/nature11983. [DOI] [PubMed] [Google Scholar]
- 2.Boyer JS. Plant productivity and environment. Science. 1982;218(4571):443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 3.Gleick PH, Ajami N. The World's Water Volume 8: The Biennial Report on Freshwater Resources. Island Press; Washington, DC: 2014. [Google Scholar]
- 4.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol. 1989;40:503–537. [Google Scholar]
- 5.Franks PJ, et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013;197(4):1077–1094. doi: 10.1111/nph.12104. [DOI] [PubMed] [Google Scholar]
- 6.Rizza F, et al. Constitutive differences in water use efficiency between two durum wheat cultivars. Field Crops Res. 2012;125:49–60. [Google Scholar]
- 7.Medrano H, Escalona JM, Bota J, Gulías J, Flexas J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann Bot (Lond) 2002;89(Spec No):895–905. doi: 10.1093/aob/mcf079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum A. Drought resistance, water-use efficiency, and yield potential: Are they mutually compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Sciences. 2005;56:1159–1168. [Google Scholar]
- 9.Morison JI, Baker NR, Mullineaux PM, Davies WJ. Improving water use in crop production. Philos Trans R Soc Lond B Biol Sci. 2008;363(1491):639–658. doi: 10.1098/rstb.2007.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney AM, McKay JK, Richards JH, Juenger TE. Direct and indirect selection on flowering time, water-use efficiency (WUE, δ (13)C), and WUE plasticity to drought in Arabidopsis thaliana. Ecol Evol. 2014;4(23):4505–4521. doi: 10.1002/ece3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436(7052):866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- 12.Yoo CY, et al. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell. 2010;22(12):4128–4141. doi: 10.1105/tpc.110.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Marais DL, et al. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc Natl Acad Sci USA. 2014;111(7):2836–2841. doi: 10.1073/pnas.1321429111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christmann A, Grill E, Huang J. Hydraulic signals in long-distance signaling. Curr Opin Plant Biol. 2013;16(3):293–300. doi: 10.1016/j.pbi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Natl Acad Sci USA. 2016;113(7):1949–1954. doi: 10.1073/pnas.1522840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324(5930):1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324(5930):1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto M, et al. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc Natl Acad Sci USA. 2013;110(29):12132–12137. doi: 10.1073/pnas.1305919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, et al. Agrochemical control of plant water use using engineered abscisic acid receptors. Nature. 2015;520(7548):545–548. doi: 10.1038/nature14123. [DOI] [PubMed] [Google Scholar]
- 22.Santiago J, et al. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009;60(4):575–588. doi: 10.1111/j.1365-313X.2009.03981.x. [DOI] [PubMed] [Google Scholar]
- 23.Pizzio GA, et al. The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol. 2013;163(1):441–455. doi: 10.1104/pp.113.224162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Guzmán M, et al. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J Exp Bot. 2014;65(15):4451–4464. doi: 10.1093/jxb/eru219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio S, et al. Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol. 2009;150(3):1345–1355. doi: 10.1104/pp.109.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Easlon HM, et al. The physiological basis for genetic variation in water use efficiency and carbon isotope composition in Arabidopsis thaliana. Photosynth Res. 2014;119(1-2):119–129. doi: 10.1007/s11120-013-9891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282(5387):287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- 28.Flexas J, et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth Res. 2013;117(1-3):45–59. doi: 10.1007/s11120-013-9844-z. [DOI] [PubMed] [Google Scholar]
- 29.Sharp R, Davies W. Root growth and water uptake by maize plants in drying soil. J Exp Bot. 1985;36:1441–1456. [Google Scholar]
- 30.Franks PJ, Doheny-Adams T, Britton-Harper ZJ, Gray JE. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015;207(1):188–195. doi: 10.1111/nph.13347. [DOI] [PubMed] [Google Scholar]
- 31.Hibberd JM, Quick WP. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature. 2002;415(6870):451–454. doi: 10.1038/415451a. [DOI] [PubMed] [Google Scholar]
- 32.Christmann A, Hoffmann T, Teplova I, Grill E, Müller A. Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol. 2005;137(1):209–219. doi: 10.1104/pp.104.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Mogami J, Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Carvalhais N, et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature. 2014;514(7521):213–217. doi: 10.1038/nature13731. [DOI] [PubMed] [Google Scholar]
- 35.Bernacchi CJ, VanLoocke A. Terrestrial ecosystems in a changing environment: A dominant role for water. Annu Rev Plant Biol. 2015;66:599–622. doi: 10.1146/annurev-arplant-043014-114834. [DOI] [PubMed] [Google Scholar]
- 36.Leprince O, Buitink J. Introduction to desiccation biology: From old borders to new frontiers. Planta. 2015;242(2):369–378. doi: 10.1007/s00425-015-2357-6. [DOI] [PubMed] [Google Scholar]
- 37.Hauser F, Waadt R, Schroeder JI. Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol. 2011;21(9):R346–R355. doi: 10.1016/j.cub.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs S, Grill E, Meskiene I, Schweighofer A. Type 2C protein phosphatases in plants. FEBS J. 2013;280(2):681–693. doi: 10.1111/j.1742-4658.2012.08670.x. [DOI] [PubMed] [Google Scholar]
- 39.Monaghan JM, et al. More ‘crop per drop’: Constraints and opportunities for precision irrigation in European agriculture. J Sci Food Agric. 2013;93(5):977–980. doi: 10.1002/jsfa.6051. [DOI] [PubMed] [Google Scholar]
- 40.Gleeson T, Wada Y, Bierkens MF, van Beek LP. Water balance of global aquifers revealed by groundwater footprint. Nature. 2012;488(7410):197–200. doi: 10.1038/nature11295. [DOI] [PubMed] [Google Scholar]
- 41.Elliott J, et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc Natl Acad Sci USA. 2014;111(9):3239–3244. doi: 10.1073/pnas.1222474110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs S, Tischer SV, Wunschel C, Christmann A, Grill E. Abscisic acid sensor RCAR7/PYL13, specific regulator of protein phosphatase coreceptors. Proc Natl Acad Sci USA. 2014;111(15):5741–5746. doi: 10.1073/pnas.1322085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin Z, Franks C, Payton P, Burke J. A simple method to determine transpiration efficiency in sorghum. Field Crops Res. 2008;107:180–183. [Google Scholar]
- 44.Christmann A, Weiler EW, Steudle E, Grill E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007;52(1):167–174. doi: 10.1111/j.1365-313X.2007.03234.x. [DOI] [PubMed] [Google Scholar]
- 45.Flexas J, et al. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol. 2007;175(3):501–511. doi: 10.1111/j.1469-8137.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 46.Brendel O, Iannetta P, Stewart D. A rapid and simple method to isolate pure alpha-cellulose. Phytochem Anal. 2000;11:7–10. [Google Scholar]