Significance
Pain after disease/damage of the nervous system is predominantly treated with opioids, but without exploration of the long-term consequences. We demonstrate that a short course of morphine after nerve injury doubles the duration of neuropathic pain. Using genetic and pharmacological interventions, and innovative Designer Receptor Exclusively Activated by Designer Drugs disruption of microglia reactivity, we demonstrate that opioid-prolonged neuropathic pain arises from spinal microglia and NOD-like receptor protein 3 inflammasome formation/activation. Inhibiting these processes permanently resets amplified pain to basal levels, an effect not previously reported. These data support the “two-hit hypothesis” of amplification of microglial activation—nerve injury being the first “hit,” morphine the second. The implications of such potent microglial “priming” has fundamental clinical implications for pain and may extend to many chronic neurological disorders.
Keywords: TLR4, P2X7R, danger signals, DAMP, opioid-induced hyperalgesia
Abstract
Opioid use for pain management has dramatically increased, with little assessment of potential pathophysiological consequences for the primary pain condition. Here, a short course of morphine, starting 10 d after injury in male rats, paradoxically and remarkably doubled the duration of chronic constriction injury (CCI)-allodynia, months after morphine ceased. No such effect of opioids on neuropathic pain has previously been reported. Using pharmacologic and genetic approaches, we discovered that the initiation and maintenance of this multimonth prolongation of neuropathic pain was mediated by a previously unidentified mechanism for spinal cord and pain—namely, morphine-induced spinal NOD-like receptor protein 3 (NLRP3) inflammasomes and associated release of interleukin-1β (IL-1β). As spinal dorsal horn microglia expressed this signaling platform, these cells were selectively inhibited in vivo after transfection with a novel Designer Receptor Exclusively Activated by Designer Drugs (DREADD). Multiday treatment with the DREADD-specific ligand clozapine-N-oxide prevented and enduringly reversed morphine-induced persistent sensitization for weeks to months after cessation of clozapine-N-oxide. These data demonstrate both the critical importance of microglia and that maintenance of chronic pain created by early exposure to opioids can be disrupted, resetting pain to normal. These data also provide strong support for the recent “two-hit hypothesis” of microglial priming, leading to exaggerated reactivity after the second challenge, documented here in the context of nerve injury followed by morphine. This study predicts that prolonged pain is an unrealized and clinically concerning consequence of the abundant use of opioids in chronic pain.
Recent reports are critical of the lack of controlled, long-term studies to support the dramatic escalation of opioid treatment for chronic pain over the past decade (1–5). Although one long-term concern is that there may be no benefit, another is that opioid treatment could have negative consequences for pain. For example, opioids are documented to paradoxically induce nociceptive sensitization [opioid-induced hyperalgesia (OIH)], both in the presence and absence of a pain condition (6, 7). With only one exception (8), OIH has been observed in chronic pain populations and is amplified by the preexisting pain condition (9–16). However, the mechanistic interactions between OIH and the pathophysiology of chronic pain are enigmatic, in part due to the absence of preclinical studies. Furthermore, the duration of OIH in either chronic pain populations or laboratory animals has never been assessed after discontinuation of opioid treatment; rather, pain was only assessed concurrently with, or within a few hours after, opioid administration. There would be major implications for how pain transitions to a chronic state if opioid treatment were to prolong the course of pain long after opioid cessation.
We predicted that opioid treatment would increase the magnitude and/or duration of long-term neuropathic pain, based on three interrelated lines of evidence: (i) Spinal microglial reactivity is triggered after peripheral nerve injury, in part via spinal release of danger-associated molecular patterns (DAMPs) that initiate glial Toll-like receptor 4 (TLR4) signaling (17). Chronic pain is gated by TLR4 in preclinical models, as the ensuing production of neuroexcitatory, immune mediators amplify nociceptive signaling in the spinal dorsal horn (17, 18); (ii) spinal microglial reactivity is also triggered by nonstereoselective opioid activation of TLR4 that promotes spinal release of neuroexcitatory immune mediators (7, 19, 20); and (iii) an immunological phenomenon termed glial “priming” has been described (21, 22), wherein a primary immune challenge (hit 1) confers a heightened neuroinflammatory response to secondary challenge (hit 2). It therefore follows that neuropathic pain after peripheral nerve injury (hit 1) may be exacerbated and prolonged by opioid treatment (hit 2). However, it has not been previously anticipated that opioids could contribute to chronic pain.
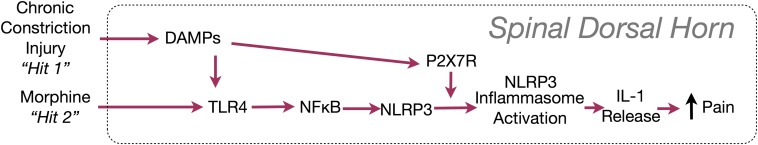
In addition, the superimposition of peripheral nerve injury and opioid treatment may activate a unique mechanism never previously implicated in spinal cord, in opioid treatment, or for pathological pain—namely, activation of the NOD-like receptor protein 3 (NLRP3) inflammasome, a protein complex that activates interleukin-1β (IL-1β), a “gatekeeper of inflammation” (summarized in Fig. S1) (23, 24). TLR4 signaling primes the inflammasome by increasing the expression of NLRP3 and pro–IL-1β (25). A second signal, such as the purinergic receptor P2X7R—engaged by morphine and after peripheral nerve injury (7, 17, 26)—leads to the association of NLRP3, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1, allowing proteolytic activation of IL-1β (25, 27). Therefore, the aim of the present study was to test whether morphine treatment after peripheral nerve injury prolonged neuropathic pain in rats and whether the prolonged pain was mediated by spinal NLRP3 inflammasomes. Our data implicate the two superimposed challenges as both immunological in nature and as contributors to persistent neuropathic pain.
Fig. S1.
IL-1β release after inflammasome activation and proposed induction after chronic constriction injury and morphine.
Results
Morphine Induces Persistent Nociceptive Sensitization After Peripheral Nerve Injury.
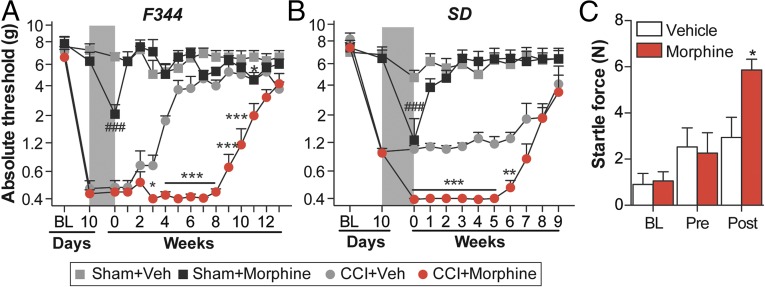
To assess whether morphine could induce persistent sensitization under conditions of established neuropathic pain, morphine or saline was administered for 5 d (5 mg/kg, twice daily), beginning 10 d after sciatic chronic constriction injury (CCI) or sham surgery.* Morphine treatment significantly prolonged CCI-allodynia in the Fischer 344 (F344) strain (Fig. 1A) and increased the magnitude of CCI-allodynia in the Sprague–Dawley (SD) rat strain (Fig. 1B). The 5-d morphine regimen induced only mild and transient mechanical allodynia in sham-operated rats (Fig. 1 A and B), a recognized feature of opioid abstinence (30). The empirical observation that morphine increased the vigor and speed of hindpaw withdrawal to the von Frey filaments in SD rats was supported by increased startle (converted to force; N) to a 0.2-mA shock (Fig. 1C). These data implicate morphine in the prolongation and amplification of neuropathic pain.
Fig. 1.
Repeated morphine increases the magnitude and duration of CCI-allodynia. (A and B) Morphine/saline (5 d; shaded area) was administered 10 d after CCI/sham surgery, and absolute thresholds for mechanical allodynia were quantified in F344 (A) and SD (B) rats. (C) Startle force to 0.2-mA foot shocks at baseline (BL), after CCI but before morphine (predose), and 5 wk after the conclusion of morphine dosing (5 wk). *P < 0.05; **P < 0.01; ***P < 0.001 (relative to CCI+saline); ###P < 0.001 (relative to sham+saline). Data are presented as mean ± SEM; n = 6 or 7 per group.
Morphine-Induced Persistent Nociceptive Sensitization Is Independent of Opioid Receptors.
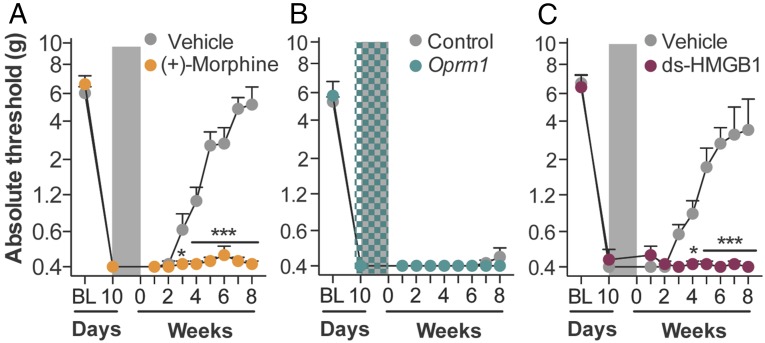
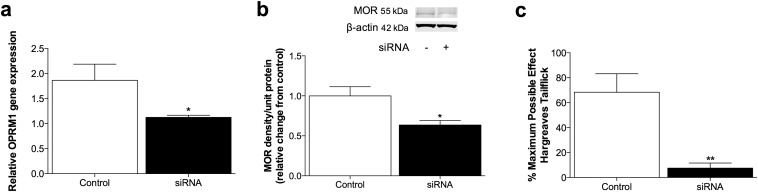
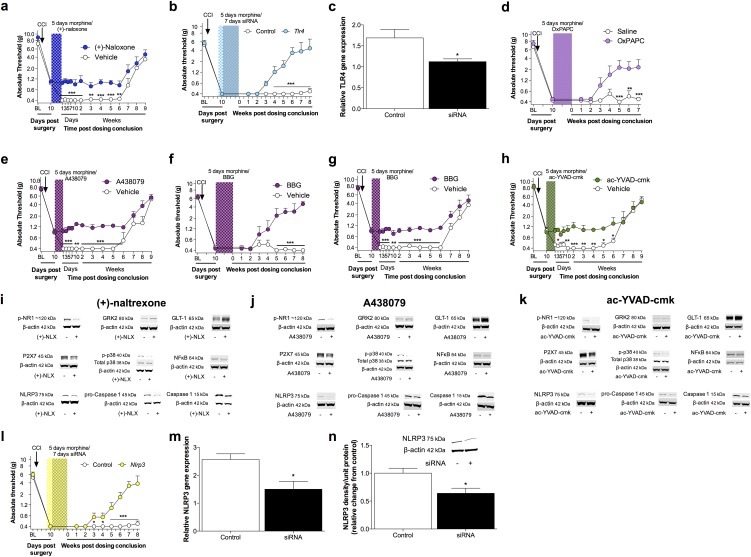
To determine whether opioid receptors mediated persistent sensitization, the μ-, κ-, and δ-opioid receptor-inactive stereoisomer (+)-morphine (31) was administered in lieu of (−)-morphine. (+)-morphine recapitulated persistent sensitization (Fig. 2A), demonstrating that this effect can occur independently of classical opioid receptors. In support, knockdown of spinal Oprm1 (encoding for the μ-opioid receptor) failed to prevent the development of morphine-induced persistent sensitization (Fig. 2B), despite knockdown of the target mRNA and protein sufficient to impair (−)-morphine analgesia (Fig. S2 A and B). Because both morphine isomers are TLR4 agonists (7, 19, 20), the role of this innate immune receptor was assessed by substituting (−)-morphine with the structurally distinct TLR4 agonist disulfide high mobility group box-1 (ds-HMGB1) (32). Persistent sensitization was recapitulated with ds-HMGB1 (Fig. 2C). Therefore, mechanisms of central immune signaling were investigated to explain morphine-induced persistent sensitization.
Fig. 2.
Opioid receptors do not mediate morphine-induced persistent sensitization. (A) The opioid-receptor inactive (+)-morphine or saline (5 d; shaded area) was administered 10 d after CCI, and absolute thresholds for mechanical allodynia were quantified in F344 rats. (B) Oprm1 siRNA (7 d, beginning 8 d after CCI; green hatched bar) and morphine (5 d, beginning 10 d after CCI; shaded area) were administered, and absolute thresholds for mechanical allodynia were quantified in F344 rats. (C) The TLR4 agonist ds-HMGB1 or saline (5 d; shaded area) was administered 10 d after CCI, and absolute thresholds for mechanical allodynia were quantified in F344 rats. *P < 0.05; ***P < 0.001 (relative to CCI+saline). Data are presented as mean ± SEM; n = 6 per group.
Fig. S2.
(A and B) Expression of Oprm1 mRNA (A) and mu opioid receptor protein (B) after 2 d Oprm1 siRNA. (C) Thermal analgesia at 30 min after intrathecal morphine (50 μg), after 7 d of Oprm1 or missense control siRNA. *P < 0.05; **P < 0.01. Data are presented as mean ± SEM; n = 6 per group.
Central Immune Signaling Mediates Morphine-Induced Persistent Nociceptive Sensitization.
Morphine nonstereoselectively activates innate immunity, inducing production of the “gatekeeper of inflammation” and neuroexcitatory cytokine IL-1β (7, 20, 23, 33, 34). Therefore, IL-1 receptor antagonist (IL-1ra) was intrathecally administered to test whether spinal IL-1 mediated morphine-induced persistent sensitization. Such a result would be congruent with the results using (+)-morphine described above. Intrathecal IL-1ra infusion during morphine administration prevented persistent sensitization (Fig. 3A), whereas acute intrathecal IL-1ra during the period of persistent sensitization significantly attenuated mechanical allodynia, in F344 rats (Fig. 3B) (for parallel data in SD rats, see Fig. S3). Inhibition of TNF and IL-6, cytokines that can be regulated by IL-1β (23), also attenuated morphine-induced persistent sensitization in F344 rats (Fig. 3H) (for parallel SD data, see Fig. S1). These data indicate that the initiation and maintenance of morphine-induced persistent sensitization are dependent on proinflammatory cytokine signaling.
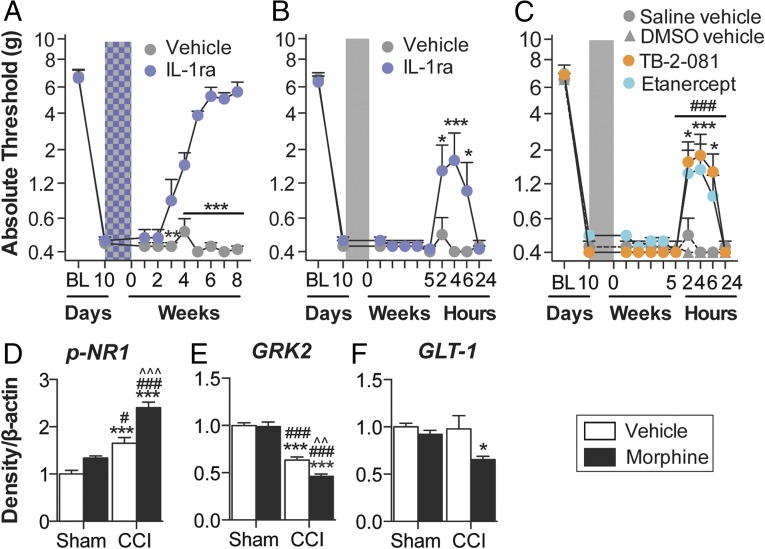
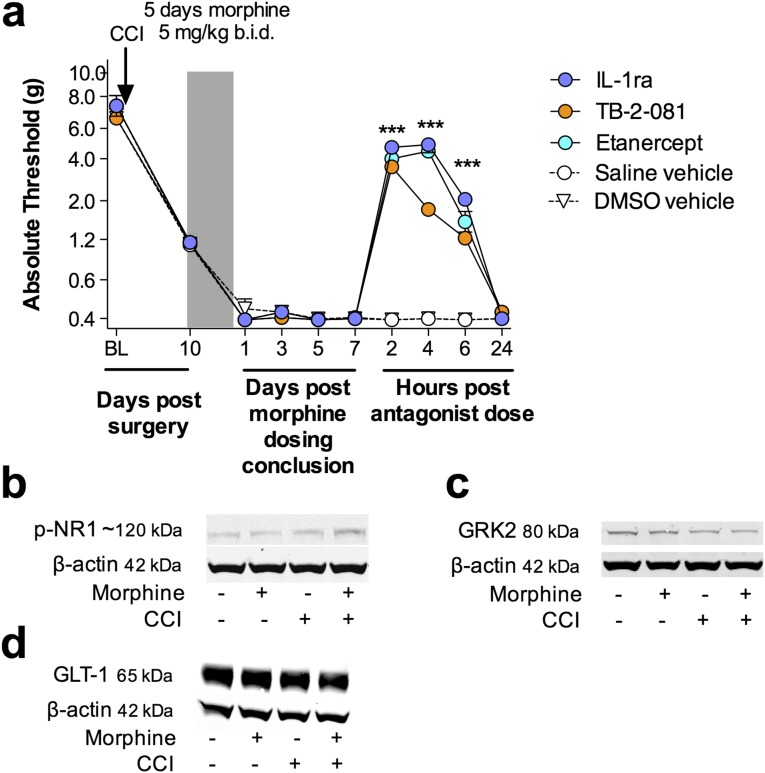
Fig. 3.
Morphine-induced persistent sensitization is mediated by central immune signaling. (A) IL-1ra (blue hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia were quantified in F344 rats. Morphine (5 d; shaded area) was administered 10 d after CCI surgery, (b) IL-1ra, (c) etanercept or TB-2–081 were intrathecally administered 5 wk after morphine conclusion, and absolute thresholds for mechanical allodynia quantified in F344 rats. Ipsilateral lumbar dorsal spinal cords were collected from CCI/sham F344 rats, 5 wk after morphine/saline administration and phospho-NR1 (D), GRK2 (E), and GLT-1 (F) protein levels were quantified. *P < 0.05; **P < 0.01; ***P < 0.001 [relative to vehicle (A–C) and relative to sham+saline (D–F)]; #P < 0.05; ###P < 0.001 [TB-2-081 vs. vehicle (C) and relative to sham+morphine (D–F); ^^P < 0.01; ^^^P < 0.001 [relative to CCI+saline[ (D–F)] . Data are presented as mean ± SEM; n = 5–7 per group.
Fig. S3.
(A) Morphine (5 d; shaded area) was administered 10 d after CCI surgery, inhibitors against IL-1, -6, and TNF were intrathecally administered 1 wk after morphine conclusion, and absolute thresholds for mechanical allodynia were quantified in SD rats. (B–D) Ipsilateral lumbar dorsal spinal cords were collected from CCI/sham F344 rats, 5 wk after morphine/saline administration and (b) phospho-NR1 (B), GRK2 (C) and GLT-1 (D) protein levels were quantified. Representative blots are presented. ***P < 0.001. Data are presented as mean ± SEM; n = 6 per group.
There are several known mechanisms by which IL-1β may increase the excitability of second-order nociceptive projection neurons, including phosphorylation of postsynaptic NR1 NMDA receptor subunits (35), and down-regulation of both the astrocyte glutamate transporter GLT-1 (36) and neuronal G protein-coupled receptor kinase 2 (GRK2; an enzymatic regulator of the homologous desensitization of many G protein-coupled receptors that protects against overstimulation) (37). The respective levels of these proteins were assessed in the ipsilateral lumbar dorsal horn during the period of persistent sensitization in F344 rats (5 wk after the conclusion of morphine or saline administration). Phospho-NR1 was elevated, whereas GRK2 and GLT-1 were decreased by the superimposition of CCI and morphine (Fig. 3 D–F). These data provide biochemical validation of the prolonged allodynia presented in Fig. 1A and additional supportive evidence that morphine-induced persistent sensitization was dependent on IL-1β signaling.
Morphine-Induced Persistent Sensitization Is Associated with Spinal Cord Inflammasome Activation in Microglia.
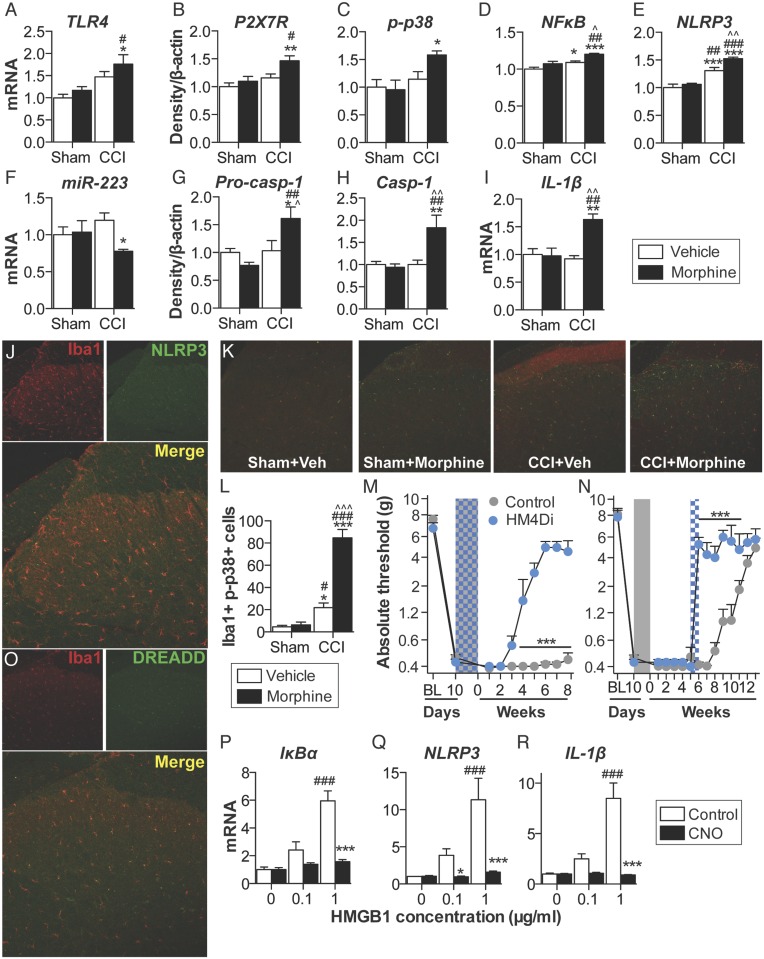
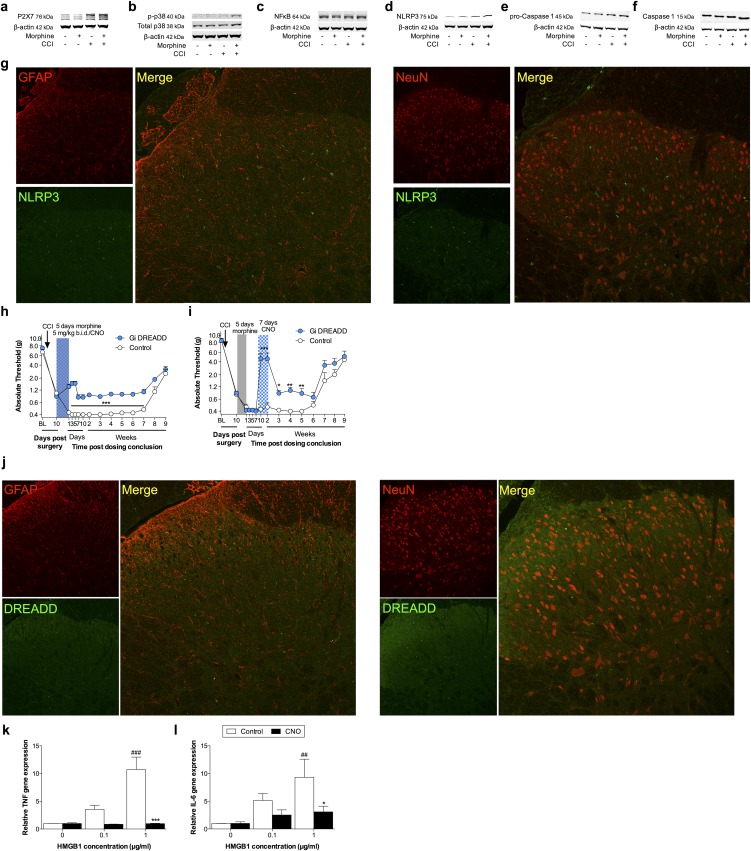
Inflammasomes regulate IL-1β activation in peripheral immune cells (Fig. S1), yet it is not known whether parallel mechanisms exist in the spinal cord (24). Thus, expression of inflammasomes was quantified in the ipsilateral lumbar dorsal horn during the period of persistent sensitization in F344 rats (5 wk after the conclusion of morphine or saline administration). TLR4 mRNA and P2X7R protein levels, which represent the respective first (priming) and second (activation) signals, were elevated by the combination of CCI and morphine, relative to sham and saline control (Fig. 4 A and B). Phosphorylated p38 and the p65 subunit of NF-κB [which are responsible for NLRP3 and IL-1β transcription (25)], as well as NLRP3, were elevated by the combination of CCI and morphine, relative to sham and saline control (Fig. 4 C–E). Expression of a negative regulator of NLRP3, microRNA-223 (miR-223) (38), was decreased by the combination of CCI and morphine, relative to sham and saline control (Fig. 4F). The precursor enzyme procaspase-1, its active form caspase-1, and the product IL-1β mRNA were elevated by the combination of CCI and morphine, relative to sham and saline control (Fig. 4 G–I). These biochemical data support the behavioral attenuation of morphine-induced persistent sensitization by IL-1ra and demonstrate that expression of the NLRP3 inflammasome by microglia is associated with such persistent sensitization.
Fig. 4.
Repeated morphine after CCI amplifies inflammasome activation in microglia. (A–I) Ipsilateral lumbar dorsal spinal cords were collected from F344 rats that had undergone sham or CCI surgery, 5 wk after morphine/saline administration, and respective levels of P2X7R (A), TLR4 (B), phospho-p38/total ERK ratio (C), NF-κB (p65 subunit) (D), NLRP3 (E), miR-223 (F), procaspase-1 (G), caspase-1 (H), and IL-1β (I) quantified. (J) NLRP3 colocalization with Iba1 in the ipsilateral lumbar dorsal horn. (K) Phospho-p38 colocalization with Iba1 in the ipsilateral lumbar dorsal horn. (L) DREADD colocalization with Iba1 in the lumbar dorsal horn. (M and N) F344 rats were transfected with intrathecal inhibitory Gi or control DREADDs, and morphine (5 d; shaded area) was administered 10 d after CCI and absolute thresholds for mechanical allodynia were quantified in F344 rats. CNO (blue hatched bar) was coadministered with morphine (5 d) (M) or 5 wk after morphine dosing had concluded (CNO dosed for 7 d) (N), and absolute thresholds for mechanical allodynia were quantified. (O–Q) Gene expression in BV-2 cells expressing the Gi DREADD after 4 h incubation with a concentration range of HMGB1, and 0 μM (control) or 50 μM CNO. *P < 0.05; **P < 0.01; ***P < 0.001 [relative to sham+saline (A–I and K), relative to vehicle (M and N), and relative to control (O–Q)]; #P < 0.05; ##P < 0.01; ###P < 0.001 [relative to sham+morphine (A–I and K) and relative to 0 μg (O–Q)]; ^P < 0.05; ^^P < 0.01; ^^^P < 0.001 (relative to CCI+saline). Data are presented as mean ± SEM; n = 6 or 7 per group.
Lumbar dorsal spinal NLRP3 was colocalized with the microglia marker Iba1 (Fig. 4J), but not GFAP (astrocytes) or NeuN (neurons) (Fig. S4A). Furthermore, the combination of CCI and morphine increased the number of reactive lumbar dorsal spinal microglia (Iba1+ and phospho-p38+), relative to all other conditions, when assessed 5 wk after the conclusion of morphine or saline administration (Fig. 4K). Therefore, the role of microglia in mediating morphine-induced persistent sensitization was functionally assessed. Current pharmacological methods to attenuate microglial reactivity lack selectivity, whereas the introduction of cellular debris to the local environment by depletion methods may present an immune stimulus in the central nervous system (CNS) (17). Therefore, we developed an inhibitory (Gi) Designer Receptor Exclusively Activated by a Designer Drug (DREADD) (39) under a CD68 promoter that was intrathecally transfected via an AAV9 vector. Transfection of the Gi or control constructs occurred before experimental manipulation, to ensure that microglia would form the majority of CD68+ cells in the spinal cord (40, 41). Gi-linked signaling was predicted to attenuate microglial reactivity because activation of the M4 muscarinic receptor [the Gi DREADD progenitor (39)] inhibits Ca2+ influx in parasympathetic neurons (42), a process associated with decreased proinflammatory cytokine production in microglia (43, 44). DREADD expression was restricted to Iba1+ cells in the lumbar dorsal spinal cord (Fig. 4L), and not those expressing GFAP or NeuN (Fig. S4B). DREADDs were activated with the selective, biologically inert ligand clozapine-N-oxide (CNO). Intrathecal CNO infusion during morphine administration prevented morphine-induced persistent sensitization in F344 rats expressing the Gi DREADD (Fig. 4M). Intrathecal infusion of CNO at 5 wk after the conclusion of morphine administration [which is within the period of persistent sensitization induced by morphine, because mechanical allodynia resolved in saline-treated CCI rats by this time (Fig. 1A)] reversed morphine-induced persistent sensitization in F344 rats expressing the Gi DREADD (Fig. 4N) (for parallel SD data, see Fig. S4C). Inhibition of proinflammatory signaling by Gi DREADDs was confirmed in vitro by using a Gi DREADD-transfected BV-2 microglia cell line. HMGB1—a DAMP released spinally in chronic pain models (17, 45)—increased the expression of gene transcripts encoding IκBα (a negative regulator induced by NF-κB), NLRP3, and IL-1β in a concentration-dependent manner (Fig. 4 O–Q). Such increases in gene expression were attenuated by coincubation with 50 μM CNO (Fig. 4 O–Q). Similar results were found for expression of gene transcripts encoding TNF and IL-6 (Fig. S4D). These data demonstrate that expression of the NLRP3 inflammasome by microglia is associated with morphine-induced persistent sensitization and that the initiation and maintenance of such persistent sensitization is dependent on microglial reactivity.
Fig. S4.
(A–F) Ipsilateral lumbar dorsal spinal cords were collected from F344 rats that had undergone sham or CCI surgery, 5 wk after morphine/saline administration, and respective levels of P2X7R (A), phospho-p38/total p38 ratio (B), NF-κB (p65 subunit) (C), NLRP3 (D), procaspase-1 (E), and caspase-1 (F) quantified. Representative blots presented. (G) Ipsilateral lumbar dorsal spinal cords were collected from F344 rats that had undergone sham or CCI surgery, 5 wk after morphine/saline administration. NLRP3, GFAP, and NeuN immunofluorescence. (H and I) SD rats were transfected with intrathecal inhibitory Gi or control DREADDs, and morphine (5 d; shaded area) was administered 10 d after CCI, and absolute thresholds for mechanical allodynia quantified in F344 rats. CNO (blue hatched bar) was coadministered with morphine (5 d) (H) or 1 wk after morphine conclusion (7 d) (I), and absolute thresholds for mechanical allodynia quantified. (J) Ipsilateral lumbar dorsal spinal cords were collected from F344 rats, 21 d after transfection with control DREADDs. DREADD, GFAP, and NeuN immunofluorescence. (K and L) TNF (K) and IL-6 (L) gene expression in BV-2 cells expressing the Gi DREADD after 4 h incubation with a concentration range of HMGB1, and 0 μM (control) or 50 μM CNO. *P < 0.05; **P < 0.01; ***P < 0.001 (relative to control); ##P < 0.01: ###P < 0.001 (relative to 0 μg). Data are presented as mean ± SEM; n = 6 or 7 per group.
Spinal Cord Inflammasomes Mediate Initiation of Morphine-Induced Persistent Sensitization.
The following experiments were designed to test whether spinal NLRP3 inflammasome activation was causal to the induction of morphine-induced persistent sensitization. Thus, the inflammasome platform was pharmacologically inhibited at several levels during morphine administration and followed by assessment of the behavioral and biochemical consequences for opioid-induced persistent sensitization.
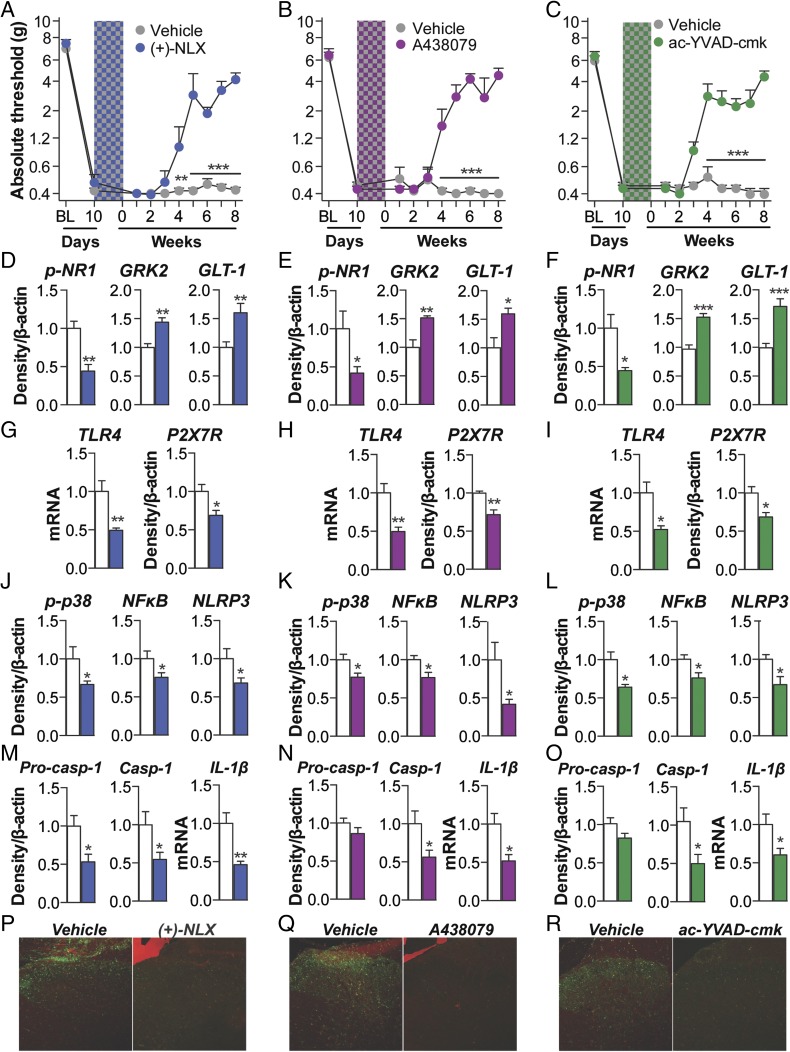
The role of spinal TLR4—activated by both morphine (20) and DAMPs (17)—was explored as the first signal for inflammasome activation. Intrathecal infusion of the TLR4 antagonist (+)-naloxone (46) during morphine administration prevented the development of morphine-induced persistent sensitization in F344 rats (Fig. 5A) (SD data are in Fig. S5A). In support of the pharmacological data, knockdown of spinal Tlr4 (Fig. S5B), as well as TLR2/4 inhibition by oxidized 1-palmitoyl-2-arachidonyl-sn-3-glycero-phosphorylcholine (OxPAPC) (Fig. S5C), also prevented the development of morphine-induced persistent sensitization. Next, the role of spinal P2X7R—also activated by DAMPs (17)—was explored as the second signal for inflammasome activation. Intrathecal infusion of A438079 (47), a selective P2X7R antagonist, during morphine administration prevented the development of morphine-induced persistent sensitization in F344 rats (Fig. 5B) (SD data are in Fig. S5D). In support of the A438079 results, P2X7R inhibition by Brilliant Blue G (48) likewise prevented the development of morphine-induced persistent sensitization in F344 rats and SD rats under identical experimental designs (Fig. S5E). The role of spinal caspase-1 was then explored, because this is the enzyme responsible for the proteolytic activation of IL-1β (25). Intrathecal infusion of N-Ac-Tyr-Val-Ala-Asp-chloromethyl ketone (ac-YVAD-cmk) (49) during morphine administration prevented the development of morphine-induced persistent sensitization in F344 rats (Fig. 5C) (SD data are in Fig. S5F). These data provide evidence that initiation of morphine-induced persistent sensitization is dependent on TLR4, P2X7R, and caspase-1 signaling during morphine administration.
Fig. 5.
Induction of persistent sensitization is dependent on spinal cord inflammasome signaling. (A–C) The TLR4 antagonist (+)-naloxone (blue hatch; 5 d) (A), the P2X7R antagonist A438079 (purple hatch; 5 d) (B), or the caspase-1 inhibitor ac-YVAD-cmk (green hatch; 5 d) (C) was coadministered with morphine (5 d; shaded area) 10 d after CCI surgery, and absolute thresholds for mechanical allodynia were quantified in F344 rats. Ipsilateral lumbar dorsal spinal cords were collected from F344 rats that had undergone CCI surgery, 5 wk after morphine and inhibitor coadministration. (D–F) Respective levels of phospho-NR1, GRK2, and GLT-1 were quantified after treatment with (+)-naloxone (D), A438079 (E), or ac-YVAD-cmk (F). (G–I) Respective levels of P2X7R and TLR4 were quantified after treatment with (g) (+)-naloxone (G), A438079 (H), or ac-YVAD-cmk (I). (J–L) Respective levels of phospho-p38/total ERK ratio, NF-κB (p65 subunit), and NLRP3 were quantified after treatment with (+)-naloxone (J), A438079 (K), or ac-YVAD-cmk (L). (M–O) Respective levels of procaspase-1, caspase-1, and IL-1β were quantified after treatment with (+)-naloxone (M), A438079 (N), or ac-YVAD-cmk (O). (P–R) Reactive lumbar dorsal spinal microglia (Iba1+ and phospho-p38+) after treatment with (+)-naloxone (P), A438079 (Q), ac-YVAD-cmk (R), and respective vehicle controls. (S) Nlrp3 siRNA (7 d, beginning 8 d after CCI; yellow hatched bar) and morphine (5 d, beginning 10 d after CCI; shaded area) were administered, and absolute thresholds for mechanical allodynia were quantified in F344 rats. *P < 0.05; **P < 0.01; ***P < 0.001 (inhibitor vs. control). Data are presented as mean ± SEM; n = 6 or 7 per group.
Fig. S5.
(A) The TLR4 antagonist (+)-naloxone (blue hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia were quantified in SD rats. (B) fTlr4 siRNA (7 d, beginning 8 d after CCI; green hatched bar) and morphine (5 d, beginning 10 d after CCI; shaded area) were administered, and absolute thresholds for mechanical allodynia were quantified in F344 rats. (C) Expression of TLR4 mRNA after Tlr4 siRNA. (D) The TLR2/4 antagonist OxPAPC (purple hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia were quantified in F344 rats. (E) The P2X7R antagonist A438079 (purple hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia were quantified in SD rats. (F and G) The P2X7R antagonist Brilliant Blue G (purple hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia quantified in F344 (F) and SD rats (G). (H) The caspase-1 inhibitor ac-YVAD-cmk (green hatch; 5 d) was coadministered with morphine (5 d; shaded area), 10 d after CCI surgery, and absolute thresholds for mechanical allodynia quantified in SD rats. (I–K) Respective levels of phospho-NR1, GRK2, GLT-1, P2X7R, phospho-p38/total p38 ratio, NF-κB (p65 subunit), NLRP3, procaspase-1, and caspase-1 were quantified after treatment with (+)-naloxone (I), A438079 (J), or ac-YVAD-cmk (K). Representative blots are presented. (L) Nlrp3 siRNA (7 d, beginning 8 d after CCI; yellow hatched bar) and morphine (5 d, beginning 10 d after CCI; shaded area) were administered, and absolute thresholds for mechanical allodynia were quantified in F344 rats. (M and N) Expression of NLRP3 mRNA (M) and protein (N) after Nlrp3 siRNA. *P < 0.05; **P < 0.01; ***P < 0.001 (inhibitor vs. control). Data are presented as mean ± SEM; n = 6 or 7 per group.
Markers of IL-1β–induced neuroexcitation were quantified in the ipsilateral lumbar dorsal quadrant after coadministration of (+)-naloxone, A438079, or ac-YVAD-cmk with morphine (within the period of persistent sensitization in F344 rats; 5 wk after the conclusion of morphine administration). Each inhibitor decreased expression of phospho-NR1, and increased expression of GRK2 and GLT-1, relative to vehicle controls (Fig. 5 D–F). These data provide biochemical support for the prevented allodynia presented in Fig. 5 A–C and of attenuated IL-1β signaling.
Expression of inflammasomes was quantified in the ipsilateral lumbar dorsal quadrant within the period of persistent sensitization in F344 rats (5 wk after the conclusion of morphine administration). (+)-naloxone, A438079 and ac-YVAD-cmk each decreased expression of receptors mediating inflammasome priming (TLR4) and activation (P2X7R ) (Fig. 5 G–I). Furthermore, each inhibitor decreased expression of phospho-p38 and p65 NF-κB, and, consequently, NLRP3 (Fig. 5 J–L). Each inhibitor decreased expression of procaspase-1, caspase-1, and IL-1β mRNA (with the exception of procaspase-1 expression, which was not altered by (+)-naloxone at this timepoint) (Fig. 5 M–O). In support of a role for microglia in morphine-induced persistent sensitization, the number of reactive lumbar dorsal spinal microglia (Iba1+ and phospho-p38+) was attenuated by (+)-naloxone, A438079, and ac-YVAD-cmk, relative to vehicle controls (Fig. 4 P-R). Together, these data demonstrate that activation of microglia and spinal cord inflammasomes is dependent on TLR4, P2X7R, and caspase-1 signaling during morphine administration and reveal underlying biochemical and molecular changes likely responsible for the behavioral effects.
Finally, the role of NLRP3 activation in the initiation of morphine-induced persistent sensitization was confirmed by knockdown of spinal Nlrp3, which prevented prolonged allodynia in F344 rats (Fig. 5S). Knockdown of the target mRNA and protein was verified (Fig. S5G). By intrathecally inhibiting the first (TLR4) and second (P2X7R) signals, as well as NLRP3 and caspase-1, during morphine administration, these affirmative data demonstrate a causal role for spinal NLRP3 inflammasomes in the initiation of morphine-induced persistent sensitization.
Spinal Cord Inflammasomes Mediate the Maintenance of Persistent Sensitization.
Because NLRP3 inflammasome expression remained elevated within the period of morphine-induced persistent sensitization (5 wk after the conclusion of morphine administration) (Fig. 4), we tested whether such expression was causal to the maintenance of persistent sensitization. Thus, the inflammasome platform was pharmacologically inhibited within the period of persistent sensitization (5 wk after the conclusion of morphine administration for F344 rats). Inhibition was accompanied by assessment of the behavioral and biochemical consequences for opioid-induced persistent sensitization.
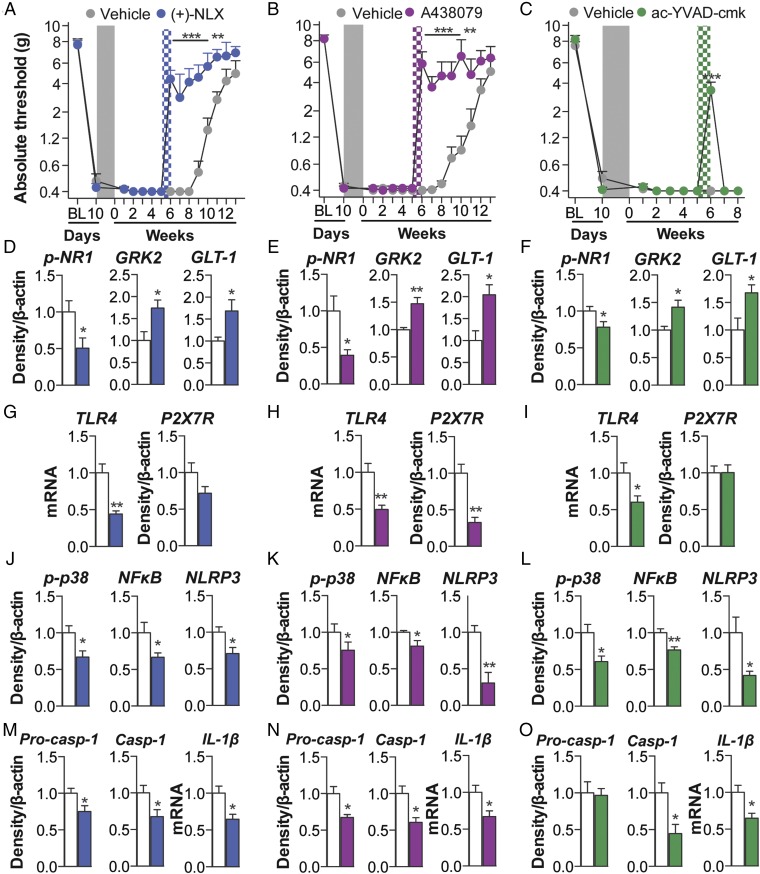
The role of TLR4 was explored as the first signal for inflammasome activation. Intrathecal infusion of (+)-naloxone starting 5 wk after morphine administration enduringly reversed established morphine-induced persistent sensitization in F344 rats (Fig. 6A) (SD data are in Fig. S6A). The role of P2X7R was explored as the second signal for inflammasome activation. Intrathecal infusion of A438079 starting 5 wk after morphine administration enduringly reversed established morphine-induced persistent sensitization in F344 rats (Fig. 6B) (SD data are in Fig. S6B). In support, Brilliant Blue G also reversed morphine-induced persistent sensitization in F344 rats and SD rats under identical experimental designs (Fig. S6C). The role of caspase-1 was then explored, because it is the enzyme that is responsible for the proteolytic activation of IL-1β. Intrathecal infusion of ac-YVAD-cmk beginning 5 wk after morphine administration reversed morphine-induced persistent sensitization in F344 rats (Fig. 6C) (SD data are in Fig. S6D). These data demonstrate that maintenance of morphine-induced persistent sensitization is dependent on sustained TLR4, P2X7R, and caspase-1 signaling.
Fig. 6.
Maintenance of persistent sensitization is dependent on inflammasome signaling. (A–C) The TLR4 antagonist (+)-naloxone (blue hatch; 5 d) (A), the P2X7R antagonist A438079 (purple hatch; 5 d) (B), or the caspase-1 inhibitor ac-YVAD-cmk (green hatch; 5 d) (C) was administered 5 wk after morphine (5 d, administered 10 d after CCI; shaded area), and absolute thresholds for mechanical allodynia were quantified in F344 rats. Ipsilateral lumbar dorsal spinal cords were collected from F344 rats, 1 d after the conclusion of inhibitor treatment. (D–F) Respective levels of phospho-NR1, GRK2, and GLT-1 were quantified after treatment with (+)-naloxone (D), A438079 (E), or ac-YVAD-cmk (F). (G–I) Respective levels of P2X7R and TLR4 were quantified after treatment with (+)-naloxone (G), A438079 (H), or ac-YVAD-cmk (I). (J–L) Respective levels of phospho-p38/total ERK ratio, NF-κB (p65 subunit), and NLRP3 were quantified after treatment with (+)-naloxone (J), A438079 (K), or ac-YVAD-cmk (L). (M–O) Respective levels of procaspase-1, caspase-1, and IL-1β were quantified after treatment with (+)-naloxone (M), A438079 (N), or (ac-YVAD-cmk (O). *P < 0.05; **P < 0.01; ***P < 0.001 (inhibitor vs. control). Data are presented as mean ± SEM; n = 6 or 7 per group.
Fig. S6.
(A) The TLR4 antagonist (+)-naloxone (blue hatch; 7 d) was administered 5 wk after morphine (5 d, administered 10 d after CCI; shaded area), and absolute thresholds for mechanical allodynia were quantified in SD rats. (B) The P2X7R antagonist A438079 (purple hatch; 7 d) was administered 5 wk after morphine (5 d, administered 10 d after CCI; shaded area), and absolute thresholds for mechanical allodynia were quantified in SD rats. (C and D) The P2X7R antagonist Brilliant Blue G (purple hatch; 7 d) was administered 5 wk after morphine (5 d, administered 10 d after CCI; shaded area) and absolute thresholds for mechanical allodynia quantified in F344 (C) and SD rats (D). (E) The caspase-1 inhibitor ac-YVAD-cmk (green hatch; 5 d) was administered 5 wk after morphine (5 d, administered 10 d after CCI; shaded area), and absolute thresholds for mechanical allodynia were quantified in SD rats. (F–H) Respective levels of phospho-NR1, GRK2, GLT-1, P2X7R, phospho-p38/total p38 ratio, NF-κB (p65 subunit), NLRP3, procaspase-1, and caspase-1 were quantified after treatment with (+)-naloxone (F), A438079 (G), or ac-YVAD-cmk (H). Representative blots are presented. *P < 0.05; **P < 0.01; ***P < 0.001 (inhibitor vs. control). Data are presented as mean ± SEM; n = 6 or 7 per group.
Markers of IL-1–induced neuroexcitation were quantified in the ipsilateral lumbar dorsal quadrant after reversal of morphine-induced persistent sensitization by (+)-naloxone, A438079, or ac-YVAD-cmk. Each inhibitor decreased expression of phospho-NR1, and increased expression of GRK2 and GLT-1, relative to vehicle controls (Fig. 6 D–F). These data provide biochemical support for the reversed allodynia presented in Fig. 6 A–C and of attenuated IL-1β signaling.
Expression of inflammasomes was quantified in the ipsilateral lumbar dorsal quadrant 1 d after the conclusion of inhibitor infusion (43 d after the conclusion of morphine administration) in F344 rats. (+)-naloxone, A438079, and ac-YVAD-cmk each decreased expression of receptors mediating inflammasome priming and activation TLR4 and P2X7R (Fig. 6 G–I). Furthermore, each inhibitor decreased expression of phospho-p38 and p65 NF-κB, and, consequently, NLRP3 (Fig. 6 J–L). Each inhibitor decreased expression of procaspase-1, caspase-1, and IL-1β mRNA (Fig. 6 M–O). There were three exceptions, where (+)-naloxone did not decrease expression of P2X7R or procaspase-1, and ac-YVAD-cmk did not decrease expression of P2X7R or procaspase-1 at this time point. These data demonstrate that the sustained activation of inflammasomes is dependent on TLR4, P2X7R, and caspase-1 signaling after morphine administration. Furthermore, this affirmative dataset demonstrates a causal role for spinal inflammasomes in the maintenance of morphine-induced persistent sensitization.
Discussion
We discovered that a brief course of morphine treatment, administered upon expression of neuropathic pain, drives persistent sensitization for months after cessation of morphine. This persistent sensitization is (i) not dependent on opioid receptor signaling; (ii) correlated with increased expression of the ipsilateral spinal lumbar dorsal inflammasome and localized to microglia; (iii) initiated by morphine-induced spinal NLRP3 inflammasome activation, a protein structure that had not previously been identified in the spinal cord or linked to pain; and (iv) maintained by spinal inflammasome activation.
Mild OIH was induced in pain-free, previously opioid-naïve subjects, but resolved within days, as reported in clinical and laboratory animal studies (6, 7). However, we discovered that morphine interacts with neuropathic pain pathophysiology to potently prolong this allodynia. We implicated the dorsal spinal NLRP3 inflammasome in morphine-induced persistent sensitization, discovering that this signaling platform has a triumvirate of previously undocumented roles in: the spinal cord, a neuropathic pain model, and enhancement of its activity by morphine (24). Dorsal spinal NLRP3 inflammasomes mediate the initiation of morphine-induced persistent sensitization, because inhibition of TLR4, P2X7R, caspase-1, or IL-1 during morphine administration prevents prolonged allodynia. Maintenance of morphine-induced persistent sensitization is also dependent on this pathway, because inhibition of TLR4, P2X7R, caspase-1, or IL-1 reversed prolonged allodynia, an effect that was sustained after TLR4 or P2X7R antagonism. It should be noted that the role of TLR4 in OIH has been challenged (50, 51), although these data do not preclude a role for this receptor in morphine-induced persistent sensitization. Furthermore, TLR4 is posited to exclusively regulate male pain behaviors (26, 52). However, ongoing studies indicate that morphine-induced persistent sensitization also occurs in female rodents.
Expression of NLRP3 induced by persistent sensitization was localized to microglia, cells that also express TLR4 and P2X7R (17). The contribution of microglia to the induction and maintenance of morphine-induced persistent sensitization was confirmed by selectively inhibiting these cells with a Gi DREADD (Fig. 4). The novel application of DREADD technology represents an important technical advance, because putative microglial inhibitors (e.g., minocycline, ibudilast, or propentofylline) have activity at other CNS cells, including neurons (17, 53). Expression of DREADDs before neuropathic pain induction prevented injury-induced recruitment of monocyte-derived cells from contributing to the observed effects. Although we predict that Gi-linked signaling inhibits Ca2+ influx in microglia to attenuate proinflammatory cytokine production (43, 44), the precise mechanisms are the subject of ongoing investigation. Because microglial activity has not been selectively manipulated in any prior study, these data, to our knowledge, are the first to unequivocally implicate microglia in a pathological pain state.
The mechanism(s) by which inflammasomes remained activated after cessation of morphine is an avenue for further investigation. Initial activation of inflammasomes may have induced several adaptations that create a positive feedback loop at TLR4 and P2X7R. One adaptation may be disrupted glutamate homeostasis, due to IL-1β–mediated down-regulation of GLT-1 (Fig. 3F). Elevated glutamate may trigger ATP release from glia (54, 55), as well as excitotoxicity and subsequent DAMP release (17). ATP and reactive oxygen species released after glial P2X7R activation (56, 57), as well as additional DAMPs released as a consequence of HMGB1-induced excitotoxicity (58), may also maintain inflammasome signaling. However, whether spinal cord inflammasomes remain activated in the absence of morphine by reactive oxygen species and/or DAMP signaling at TLR4 and P2X7R, as part of a positive feedback loop, requires future examination.
The implications of the present study are striking in light of the “two-hit” model of glial priming and exaggerated neuroinflammation. Firstly, this model may provide a basis for understanding how opioids exaggerate pain in preclinical models of peripheral inflammation and surgery (59, 60), as well as clinically after thoracotomy (61, 62). Secondly, opioids superimposed on CNS neuroinflammation may have far-ranging consequences beyond pain. For example, opioids may also serve as a second hit for glia primed by aging or inflammation/trauma and may lead to cognitive decline in the elderly (63), postoperative cognitive decline (64), and impaired recovery of motor function after spinal cord injury (65, 66). Whether the mechanistic underpinnings revealed in the current series of studies will prove to generalize to such opioid-related phenomena remains to be defined. Finally, the implications of the present studies may extend beyond opioids as the second hit. A broad range of repeated neuroinflammatory challenges not only induce a transition from acute to persistent pain (60, 67, 68), but also induce behaviors that are comorbid with pain, including cognitive impairment (69), depression (70), and anxiety (71). Therefore, our data provide a rationale to examine whether the ubiquitous management of chronic pain with opioids contributes to the incidence of such pain, and potentially pain comorbidities—a hypothesis not previously considered or tested.
In summary, the mechanisms underlying the transition from acute to chronic pain are poorly understood (17, 72, 73). We discovered that a short course of morphine administered upon expression of neuropathic pain remarkably doubled the duration of CCI-allodynia. This process was dependent upon dorsal spinal microglial reactivity and NLRP3 inflammasomes. These findings comport with prior demonstrations that repeated immune challenges induce a transition from acute to chronic pain (60, 67, 68), which may also underpin pain comorbidities (69–71). An evaluation of the long-term consequences of opioid treatment for chronic pain will identify whether this phenomenon manifests clinically. Our data suggest a unique strategy to prevent and reverse the deleterious long-term effects of opioid treatment without compromising morphine analgesia; μ-opioid receptor-mediated analgesia can be maintained, while simultaneously eliminating inflammasome-mediated persistent sensitization.
Materials and Methods
SI Materials and Methods provides complete experimental methods. It includes subjects, drugs, RNA interference, surgery, catheter implantation, mechanical allodynia, shock sensitivity, and thermal analgesia testing, in vitro Gi DREADD transfection and stimulation, RT-PCR, Western blotting, and immunohistochemistry. Methods for statistical analysis are also included.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder.
SI Materials and Methods
Subjects.
Pathogen-free adult male F344 and SD rats (n = 6 or 7 rats per group for each experiment; 10–12 wk old on arrival; Harlan Labs) were used in all experiments. Rats were housed in temperature-controlled (23 °C ± 3 °C) and light-controlled (12-h light:dark cycle; lights on at 07:00 h) rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder.
Drugs.
The (−)-morphine was gifted by the NIDA drug depositary. (+)-morphine, (+)-naloxone and TB-2-081 (3-O-formyl-20R,21-epoxy-resibufogenin) were gifted by Kenner Rice (National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism, Bethesda). IL-1ra was gifted from Amgen, and etanercept (Amgen) was a generous gift from Nancy Sajben, Scripps Memorial Hospital, La Jolla, CA. ds-HMGB1 (HMGBiotech), A438079 (Tocris Bioscience), Brilliant Blue G (BBG; Sigma-Aldrich), ac-YVAD-cmk (Cayman Chemical), OxPAPC (Invivogen), and CNO (Enzo Life Sciences) were obtained commercially. DREADDs pAAV-CD68-hM4D-mCherry (Gi) and pAAV-CD68-EGFP (control) plasmids were generated as follows: The CD68 promoter/enhancer was PCR amplified from pcDNA3-CD68 promoter/enhancer (Addgene no. 34837) and cloned into pAAV-hM4D-mCherry and pAAV-EGFP by using Mlu I and Sal I cuts sites. Both plasmids were packaged in serotype 9 by the University of North Carolina Vector Core Facility. Sterile saline (0.9%) was the vehicle for all drugs, except TB-2-081 [3% (vol/vol) dimethyl sulfoxide (DMSO); Sigma-Aldrich], ac-YVAD-cmk [3% (vol/vol) DMSO; Sigma-Aldrich], OxPAPC (suspended in chloroform at a concentration of 1 mg/mL, vortexed and evaporated under a stream of nitrogen gas, and diluted in saline on the experimental day), and CNO [1.5% (vol/vol) DMSO; Sigma-Aldrich]. Where applicable, drugs were prepared and are reported as free base concentrations.
RNA Interference.
siRNAs were purchased from Life Technologies and are summarized in Table S1. siRNA or missense control (0.24 μg/μL in DNase-free water) was mixed at a 1:1 ratio with RNAiMAX transfection reagent [6% (vol/vol) in PBS; Life Technologies], according to manufacturer instructions.
Table S1.
siRNA specifications
| Gene | siRNA sequence (5′-3′) |
| Tlr4 | Sense: GGUGUUGGAUUUUACGAAUtt |
| Antisense: AUUCGUAAAAUCCAACACCag | |
| Oprm1 | Sense: GCUGAUCACGAUUCCAGAAtt |
| Antisense: UUCUGGAAUCGUGAUCAGCgc | |
| Nlrp3 | Sense: GAAUGAACGUGUUCCAGAAtt |
| Antisense: UUCUGGAACACGUUCAUUCtc |
CCI.
Neuropathic pain was induced by using the mild (28) and classic (29) CCI models of sciatic nerve injury. CCI was performed at the midthigh level of the left hindleg as described (28). In brief, animals were anesthetized with isoflurane. The shaved skin was treated with Nolvasan, and the surgery was aseptically performed. Either one (SD rats) or four (F344 rats) sterile chromic gut sutures (cuticular 4-0 chromic gut; Ethicon) were loosely tied around the gently isolated sciatic nerve. Animals were monitored postoperatively until fully ambulatory before return to their home cage.
Acute and Chronic Catheter Implantation.
Construction and implantation of the acute and indwelling intrathecal catheters was based on described methods (74). In brief, intrathecal operations were conducted under isoflurane anesthesia by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was inserted such that the proximal catheter tip lay over the lumbosacral enlargement. For acute intrathecal drug delivery, catheters were removed immediately after drug delivery. For indwelling catheters, the needle was removed and the catheter was sutured to the superficial musculature of the lower back. The catheters were 17 cm in length and were attached to a preloaded osmotic minipump where appropriate (Alzet; 2001).
Intrathecal and s.c. Drug Administration.
For acute intrathecal drug delivery, the catheters were preloaded with drugs at the distal end in a total volume and delivered over 20–30 s once the catheter was in position. Acute intrathecal doses were as follows, (−)-morphine: 50 μg in 10 μL; IL-1ra: 100 μg in 10 μL; TB-2-081: 10 μg in 10 μL; etanercept: 100 μg in 10 μL; DREADDs: 1.2 × 1013 vector genome in 8 μL (injected 2 wk before surgery). Intrathecal osmotic minipump infusions were as follows, ds-HMGB1: 60 ng/h; IL-1ra: 5 μg/h; (+)-naloxone: 60 μg/h; A438079: 30 ng/h; BBG: 30 ng/h; ac-YVAD-cmk: 1 μg/h; OxPAPC: 20 μg/h; CNO: 20 μg/h. Drugs were infused for 5.5 d (prevention studies) or 7 d (reversal studies). When coadministered with morphine, osmotic minipumps were implanted the afternoon before morphine dosing began to allow minipump equilibration. Because poor stability precluded the use of osmotic minipumps for siRNA dosing, daily injections of Tlr4, Oprm1, Nlrp3 or missense control siRNA (1.2 μg in 10 μL) were performed for 7 d, beginning 2 d before morphine administration to allow sufficient knockdown before morphine administration. (−)-Morphine and (+)-morphine were administered s.c. at 5 mg/kg per mL, twice daily. The human morphine dose equivalency is 114 mg/d (75) and was selected to fall at the lower end of the 100–200 mg/d range considered as “moderate” by the American Pain Society and American Academy of Pain Medicine (76). Respective equivolume vehicles were used as controls.
Mechanical Allodynia.
Testing was conducted blind with respect to group assignment. Rats received at least three 60-min habituations to the test environment before behavioral testing. The von Frey test (77) was performed at the distal region of the heel in the hindpaws, within the region of sciatic innervation as previously described in detail (78, 79). Assessments were made before surgery (baseline), before morphine administration (day 10 after surgery) and at regular intervals after the conclusion of morphine administration (days or weeks after dosing conclusion). Assessments for cytokine inhibitor studies were made at 0, 2, 4, 6, and 24 h after a single intrathecal dose. A logarithmic series of 10 calibrated Semmes–Weinstein monofilaments (von Frey hairs; Stoelting) were applied randomly to the left vs. right hindpaws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer-designated 3.61 (0.40 g) to 5.18 (15.14 g) filaments. The behavioral responses were used to calculate absolute threshold (the 50% probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method (80, 81), as described previously (79, 82).
Shock Sensitivity Test.
Somatosensory reactivity to shock was observed in specialized chambers obtained commercially (Kinder Scientific). Each isolation chamber contained a rat holder (SM2001 Ð Kinder Scientific) held in place onto a platform load cell that detected cage displacement. Scrambled electric shock was administered through a metal grid (SMG-R Ð Kinder Scientific) connected to a programmable animal shocker (scrambled output; SMSCK Ð Kinder Scientific) inserted into each rat holder. Rats were placed into the holders and 50 ms shocks were administered beginning at 0 (no shock), increasing to 0.2 mA in 0.02-mA increments, and then decreasing down to 0 mA. Shocks were presented at a 60-s interval, and the series was repeated twice with a 5-min interseries interval. Sensitivity to shock was inferred by average displacement of the load cell (shock elicited startle) averaged across a total of four trials. Displacement was recorded by a controlling computer for 200 ms beginning with the initial presentation of each electric shock and converted to force (N) using known standards.
Thermal Analgesia.
Testing was conducted blind with respect to group assignment. Rats received at least three 60-min habituations to the test environment on separate days before behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the plantar surface of each hind paw and tail were assessed using a modified Hargreaves test (83). Briefly, baseline withdrawal values were calculated from an average of two consecutive withdrawal latencies of the tail and the left and the right hind paws, measured at 15 min intervals. Latencies at baseline ranged from 8 to 10 s, and a cutoff time of 20 s was imposed to avoid tissue damage. Nociceptive assessments were made 30 min after remote morphine delivery via indwelling intrathecal catheters. Withdrawal latencies were converted to percentage of maximum possible effect using the following equation: (test − baseline/20 s cutoff − baseline) × 100.
In Vitro Gi DREADD Transfection and Stimulation Assay.
Mouse microglia BV-2 cells were grown in DMEM supplemented with 10% (vol/vol) FBS. Cells were seeded at a density of 1 × 104 cells/mL in 35 mm dish. When cells reach ∼60% confluence, the medium was aspirated and 0.5 mL of pLenti6.3-CMV hM4Di lenti-virus medium (multiplicity of infection = 104) and 8 μg/mL polybrene were added for transfection. After 24 h transfection, the virus was removed and the cells were cultured in DMEM supplemented with 10% (vol/vol) FBS for additional 24 h. The pLenti6.3-CMV hM4Di positive cells were then selected by DMEM supplemented with 10% (vol/vol) FBS and 10 μg/mL puromycin. Positive colonies were picked up and were further cultured. The pLenti6.3-CMV hM4Di positive cells were further confirmed by V5 tag Western blotting.
pLenti6.3-CMV hM4Di positive cells were seeded into 96 well plates at a density of 5 × 104 cells/mL in DMEM supplemented with 10% (vol/vol) FBS. After 24 h, cells were washed and incubated with a concentration range of ds-HMGB1 (0, 0.1, and 1 μg/mL), together with 0 or 50 μM of CNO, in serum-free DMEM for 4 h. The cells were then collected by centrifugation for RT-PCR.
RT-PCR.
RT-PCR was performed on L4/5 spinal ipsilateral dorsal quadrants obtained from rats that had been transcardially perfused with saline under sodium pentobarbital anesthesia, and on BV-2 cells from the Gi DREADD stimulation assay. Total RNA was isolated using a standard method of phenol:chloroform extraction (84). cDNA amplification was performed using Quantitect SYBR Green PCR kit (Qiagen) in iCycler iQ 96-well PCR plates (Bio-Rad) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Primer sequences (GenBank, National Center for Biotechnology Information; www.ncbi.nlm.nih.gov) are displayed in Table S2. Each sample was measured in duplicate by using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (GAPDH) using the ∆∆CT method (85). GAPDH was not significantly different between treatments.
Table S2.
PCR primer specifications
| Gene | Primer sequence (5′-3′) |
| Rat Gapdh | F: AGGGACAATCTCACACAGG |
| R: GACTCAACCTTCCTCTCCA | |
| Rat Tlr4 | F: TCCCTGCATAGAGGTACTTC |
| R: CACACCTGGATAAATCCAGC | |
| Rat Mir223 | F: TCTGGCCTTCTGCAGTGTTA |
| R: CTGATAAGCATGAGCCACAC | |
| Rat Il1b | F: GAAGTCAAGACCAAAGTGG |
| R: TGAAGTCAACTATGTCCCG | |
| Mouse Gapdh | F: GGAGAAACCTGCCAAGTATG |
| R: GTCATTGAGAGCAATGCCAG | |
| Mouse Nfkbia | F: CACCAACTACAATGGCCACA |
| R: GCTCCTGAGCGTTGACATCA | |
| Mouse NLRP3 | F: CTCAAAACCAACCAGAACTT |
| R: TGTCTAATTCCAGCATCTGT | |
| Mouse Il1b | F: TGCTGTCGGACCCATATGAG |
| R: ATCCACACTCTCCAGCTGCA | |
| Mouse Tnf | F: CCCTCACACTCAGATCATCT |
| R: TGTCTTTGAGATCCATGCCG | |
| Mouse Il6 | F: GAAAAGAGTTGTGCAATGGC |
| R: TATGGTACTCCAGAAGACCA |
Western Blotting.
Western blot analyses were performed on L4/5 spinal ipsilateral dorsal quadrants obtained from rats that had been transcardially perfused with saline under sodium pentobarbital anesthesia. Tissue was sonicated, on ice, in 50 mM Tris buffer containing 100 mM 6-amino-n-caproic acid, 1 mM EDTA, 5 mM benzamidine, 0.2 mM phenylmethyl sulfonyl fluoride (in 100% ethanol), and protease inhibitors. After extraction, proteins were subjected to NuPAGE Bis-Tris (4–12%) gel electrophoresis under reducing conditions (Invitrogen) and then transferred to nitrocellulose membranes electrophoretically (Bio-Rad). Nonspecific binding sites on the membrane were blocked with Odyssey Blocking Buffer [50% (vol/vol); LI-COR Biosciences] in TBS containing 0.1% Tween-20, 0.05% Tris-Chloride, and 0.03% 5 M NaCl (TBS-T) for 1 h at 22–24 °C. Membranes were subsequently incubated with primary antibodies in Odyssey Blocking buffer containing 0.1% Tween-20 overnight at 4 °C. The membranes were then washed with PBS containing 0.1% Tween-20, and probed with appropriate IRDye secondary antibodies (LI-COR Biosciences) in Odyssey Blocking buffer containing 0.1% Tween-20 for 1 h at 22–24 °C, protected from light. After washing with PBS containing 0.1% Tween-20, membranes were scanned on an Odyssey Infrared Imaging System (LI-COR Biosciences). Where necessary, membranes were stripped with a NewBlot Stripping Buffer according to manufacturer instructions (LI-COR Biosciences), and reprobed with an antibody against loading control protein. Primary antibodies and dilution ratios used were: rabbit P2X7R 1:400 (Alomone Labs), rabbit p38 1:1,000 (Cell Signaling), rabbit phospho-p38 1:1,000 (Cell Signaling), rabbit p65/NFκB 1:500 (Merck Millipore), rabbit NLRP3 1:500 (LifeSpan Biosciences), mouse caspase-1 1:200 (Santa Cruz), rabbit phospho-NRI 1:500 (Merck Millipore), mouse GRK2 1:2,000 (Thermo Fisher Scientific), and guinea pig GLT-1 1:5,000 (Merck Millipore). Mouse β actin 1:100,000 (Sigma-Aldrich) was used against loading control protein. Secondary antibodies used were: Goat anti-mouse IRDye 680RD 1:15,000 (LI-COR Biosciences), goat anti-rabbit IRDye 800CW 1:15,000 (LI-COR Biosciences), and donkey anti-guinea pig IRDye 800CW 1:15,000 (LI-COR Biosciences). Bands were quantified by using Image Studio (LI-COR Biosciences).
Immunohistochemistry.
Lumbar spinal cords were obtained from rats that had been transcardially perfused with saline, followed by 4% (wt/vol) paraformaldehyde/0.1 M phosphate buffer (pH 7.4), under sodium pentobarbital anesthesia. After immersion postfixation in 4% (wt/vol) paraformaldehyde/0.1 M phosphate buffer (pH 7.4) for an additional 24 h, the lumbar spinal cords were dehydrated in 30% (wt/vol) sucrose with 0.1% azide at 4 °C until slicing. Samples were freeze-mounted in optimal cutting temperature embedding medium, and cut at 20 μm frozen sections. Sections were permeabilized with 0.01 M PBS and 0.5% Triton X-100, and incubated overnight in 2.5% (wt/vol) BSA blocking solution. Sections were washed and then incubated at 4 °C for 24 h (or 48 h for phospho-p38) in 2% (vol/vol) normal goat serum together with primary antibodies at the following dilution ratios: rabbit Iba1 1:100 (Abcam), mouse Iba1 1:100 (Abcam), rabbit GFAP 1:500 (Abcam), rabbit NeuN 1:500 (Abcam), mouse NLRP3 1:200 (Antibodies-online), and rabbit phospho-p38 1:400 (Cell Signaling). After washing, sections were incubated at room temperature for 2 h in 2% normal goat serum together with secondary antibodies at the following dilution ratios: goat Alexa Fluor 594 1:250 (Life Technologies) and goat Alexa Fluor 488 1:250 (Life Technologies). Fluorescent photomicrographs (20x; Zeiss microscope, LSM 510) of ipsilateral lumbar dorsal horn sections were captured by using Zen 2009 imaging software (Zeiss).
Statistics.
Mechanical allodynia was analyzed as the interpolated 50% thresholds (absolute threshold). Where appropriate, one-way ANOVAs followed by Tukey’s post hoc test or paired t tests were used to confirm that there were no baseline differences in absolute thresholds between treatment groups. Differences between treatment groups were determined using repeated measures two-way ANOVA, with CCI, morphine, inhibitor or time as main effects, followed by Sidak’s post hoc test. A repeated measures two-way ANOVA, with morphine and time as main effects, followed by Sidak’s post hoc test was used to determine statistical significance between groups for the shock sensitivity test. Integrated Densities for Western blots and relative mRNA expression levels were analyzed by two-way ANOVA and Sidak’s post hoc test or unpaired t test, as appropriate. P < 0.05 was considered significant.
Acknowledgments
This work was supported by the American Pain Society Future Leaders in Pain Research Grants Program (P.M.G.); National Health and Medical Research Council CJ Martin Fellowship ID 1054091 (to P.M.G.); American Australian Association Sir Keith Murdoch Fellowship (P.M.G.); National Natural Science Foundation of China Grant 21543013 (to X.W.); Natural Science Foundation of Jilin Province Grant 20160101211JC (to X.W.); and NIH Grants DE021966, DA023132 (to L.R.W.), DA017204 (to D.J.U. and B.L.R.), U01MH105892 (to B.L.R.), and GM101279 (to H.H.Y.). The work of the Chemical Biology Research Branch was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
The authors declare no conflict of interest.
*The duration of mechanical allodynia after classic CCI (four sutures around the sciatic nerve) (28) is shorter in the F344 rat strain, relative to the SD rat strain (29). Therefore, the potential for morphine to increase the duration of CCI-allodynia was assessed by using F344s. Conversely, both rat strains exhibit near maximal allodynia with classic CCI, so an increase in the magnitude of allodynia was not testable under this condition. Moving to a mild CCI (one suture around the sciatic nerve) (27) induced submaximal allodynia in SD rats, whereas F344s were still maximal on this measure. Therefore, the effect of morphine on the magnitude of CCI-allodynia was assessed using SDs.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602070113/-/DCSupplemental.
References
- 1.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 2.Manchikanti L, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3) Suppl:ES9–ES38. [PubMed] [Google Scholar]
- 3.Daubresse M, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870–878. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, et al. 2014 The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain. (Agency for Healthcare Research and Quality, Rockville, MD). Available at www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141007.pdf)
- 5.Franklin GM. American Academy of Neurology Opioids for chronic noncancer pain: A position paper of the American Academy of Neurology. Neurology. 2014;83(14):1277–1284. doi: 10.1212/WNL.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 6.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 7.Grace PM, Maier SF, Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache. 2015;55(4):475–489. doi: 10.1111/head.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu LF, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: A double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153(8):1583–1592. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Hooten WM, Lamer TJ, Twyner C. Opioid-induced hyperalgesia in community-dwelling adults with chronic pain. Pain. 2015;156(6):1145–1152. doi: 10.1097/j.pain.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hina N, Fletcher D, Poindessous-Jazat F, Martinez V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: An observational case-control study. Eur J Anaesthesiol. 2015;32(4):255–261. doi: 10.1097/EJA.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 11.Basaria S, et al. Effects of testosterone replacement in men with opioid-induced androgen deficiency: A randomized controlled trial. Pain. 2015;156(2):280–288. doi: 10.1097/01.j.pain.0000460308.86819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzan E, Eisenberg E, Treister R, Haddad M, Pud D. A negative correlation between hyperalgesia and analgesia in patients with chronic radicular pain: Is hydromorphone therapy a double-edged sword? Pain Physician. 2013;16(1):65–76. [PubMed] [Google Scholar]
- 13.Doehring A, Oertel BG, Sittl R, Lötsch J. Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. Pain. 2013;154(1):15–23. doi: 10.1016/j.pain.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143(1-2):65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139(2):431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain. 2006;7(1):43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson MR, et al. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA. 2012;109(16):6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112(1):7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 24.de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34(3):369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorge RE, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18(4):595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini A, et al. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015;29(6):2450–2461. doi: 10.1096/fj.14-268714. [DOI] [PubMed] [Google Scholar]
- 28.Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE. A novel animal model of graded neuropathic pain: Utility to investigate mechanisms of population heterogeneity. J Neurosci Methods. 2010;193(1):47–53. doi: 10.1016/j.jneumeth.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86(1-2):56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 31.Pert CB, Snyder SH. Opiate receptor: Demonstration in nervous tissue. Science. 1973;179(4077):1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212(1):5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167(3):880–893. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson MR, et al. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang RX, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135(3):232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62(7):1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleibeuker W, et al. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22(2):200–208. doi: 10.1016/j.bbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Bauernfeind F, et al. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189(8):4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 39.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27(45):12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuevas J, Adams DJ. M4 muscarinic receptor activation modulates calcium channel currents in rat intracardiac neurons. J Neurophysiol. 1997;78(4):1903–1912. doi: 10.1152/jn.1997.78.4.1903. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi Y, et al. Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci. 2011;31(48):17370–17382. doi: 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): Suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23(11):4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agalave NM, et al. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155(9):1802–1813. doi: 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Hutchinson MR, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28(1):20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson DW, et al. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49(12):3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 48.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58(1):82–88. [PubMed] [Google Scholar]
- 49.Rabuffetti M, et al. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J Neurosci. 2000;20(12):4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrini F, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl⁻ homeostasis. Nat Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattioli TA, et al. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One. 2014;9(5):e97361. doi: 10.1371/journal.pone.0097361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation. 2013;10:148. doi: 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grace PM, et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience. 2014;280:299–317. doi: 10.1016/j.neuroscience.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu GJ, Kalous A, Werry EL, Bennett MR. Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol Pharmacol. 2006;70(3):851–859. doi: 10.1124/mol.105.021436. [DOI] [PubMed] [Google Scholar]
- 55.Queiroz G, Meyer DK, Meyer A, Starke K, von Kügelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91(3):1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- 56.Ficker C, et al. Astrocyte-neuron interaction in the substantia gelatinosa of the spinal cord dorsal horn via P2X7 receptor-mediated release of glutamate and reactive oxygen species. Glia. 2014;62(10):1671–1686. doi: 10.1002/glia.22707. [DOI] [PubMed] [Google Scholar]
- 57.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26(5):1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal. 2014;21(12):1726–1740. doi: 10.1089/ars.2013.5349. [DOI] [PubMed] [Google Scholar]
- 59.Célérier E, González JR, Maldonado R, Cabañero D, Puig MM. Opioid-induced hyperalgesia in a murine model of postoperative pain: Role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology. 2006;104(3):546–555. doi: 10.1097/00000542-200603000-00023. [DOI] [PubMed] [Google Scholar]
- 60.Loram LC, et al. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain Behav Immun. 2012;26(8):1256–1264. doi: 10.1016/j.bbi.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Gulik L, et al. Remifentanil during cardiac surgery is associated with chronic thoracic pain 1 yr after sternotomy. Br J Anaesth. 2012;109(4):616–622. doi: 10.1093/bja/aes247. [DOI] [PubMed] [Google Scholar]
- 62.Salengros JC, et al. Different anesthetic techniques associated with different incidences of chronic post-thoracotomy pain: Low-dose remifentanil plus presurgical epidural analgesia is preferable to high-dose remifentanil with postsurgical epidural analgesia. J Cardiothorac Vasc Anesth. 2010;24(4):608–616. doi: 10.1053/j.jvca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Puustinen J, et al. Use of CNS medications and cognitive decline in the aged: a longitudinal population-based study. BMC Geriatr. 2011;11:70. doi: 10.1186/1471-2318-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50–59. doi: 10.1097/01.JGP.0000229792.31009.da. [DOI] [PubMed] [Google Scholar]
- 65.Hook MA, et al. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179(2):281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hook MA, et al. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J Neurotrauma. 2009;26(5):741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hains LE, et al. Prior laparotomy or corticosterone potentiates lipopolysaccharide-induced fever and sickness behaviors. J Neuroimmunol. 2011;239(1-2):53–60. doi: 10.1016/j.jneuroim.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hains LE, et al. Pain intensity and duration can be enhanced by prior challenge: Initial evidence suggestive of a role of microglial priming. J Pain. 2010;11(10):1004–1014. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrientos RM, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Fenn AM, et al. Immune activation promotes depression 1 month after diffuse brain injury: A role for primed microglia. Biol Psychiatry. 2014;76(7):575–584. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164(2):231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 72.Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(Suppl 1):i69–i85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- 73.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26(4):360–367. doi: 10.1097/WCO.0b013e32836336ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90(1):81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 75.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 76.APS-AAPM 2009 Clinical Guideline for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. Available at americanpainsociety.org/uploads/education/guidelines/chronic-opioid-therapy-cncp.pdf. Accessed April 4, 2016.
- 77.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 78.Chacur M, et al. A new model of sciatic inflammatory neuritis (SIN): Induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94(3):231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 79.Milligan ED, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21(8):2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvey LO., Jr Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- 81.Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61(1):87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- 82.Milligan ED, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861(1):105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 83.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 84.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 85.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]