Significance
The majority of biological sugars and their derivatives contain higher abundances of the “d” mirror-image forms relative to the “l” forms. For example, nucleic acids are composed of only d sugars. Carbonaceous meteorites can potentially assist in understanding the long-sought origin of such phenomena; They preserve a record of the earliest (∼4.5 Gy) chemical processes in the Solar System. To date, there have been no systematic studies of d/l (i.e., enantiomer) ratios of meteoritic sugar derivatives. In multiple meteorites, we demonstrate that rare and common sugar acids contain large excesses of the d enantiomer. Such data indicate that early meteoritic compounds may have influenced the enantiomer profile of subsequent biological sugars and their derivatives.
Keywords: carbonaceous meteorites, sugar acids, enantiomer excesses, aldonic acids, polyols
Abstract
Biological polymers such as nucleic acids and proteins are constructed of only one—the d or l—of the two possible nonsuperimposable mirror images (enantiomers) of selected organic compounds. However, before the advent of life, it is generally assumed that chemical reactions produced 50:50 (racemic) mixtures of enantiomers, as evidenced by common abiotic laboratory syntheses. Carbonaceous meteorites contain clues to prebiotic chemistry because they preserve a record of some of the Solar System’s earliest (∼4.5 Gy) chemical and physical processes. In multiple carbonaceous meteorites, we show that both rare and common sugar monoacids (aldonic acids) contain significant excesses of the d enantiomer, whereas other (comparable) sugar acids and sugar alcohols are racemic. Although the proposed origins of such excesses are still tentative, the findings imply that meteoritic compounds and/or the processes that operated on meteoritic precursors may have played an ancient role in the enantiomer composition of life’s carbohydrate-related biopolymers.
The organic phase of carbonaceous meteorites comprises an insoluble “macromolecular” material (1–3), a complex mixture of largely uncharacterized solvent-extractable compounds (4), as well as discrete (identified) soluble organic compounds such as amino acids (3, 5) nucleobases (6, 7), and sugar derivatives (8). Analyses of this prebiotic organic carbon have been important in understanding its early Solar System synthesis and history: Characteristics of the organic phase suggest that it consists of both products and survivors of interstellar/presolar grain irradiation (9) and subsequent encapsulation and aqueous reactions (10, 11) in asteroids “parent bodies.”
Several identified meteoritic organic compounds are chiral: They can exist as two nonsuperimposable mirror image compounds called enantiomers, commonly designated d and l. To date, most chiral meteoritic compounds are reported to be racemic mixtures, i.e., their d and l enantiomers are equal in abundance (3). Racemic compounds are expected in nature because typical abiotic synthetic processes are (historically) thought to occur in the absence of asymmetric influences. Racemic mixtures are equated with pristine/uncontaminated abiotic samples when discussing meteoritic compounds. However, some meteoritic amino acids that are rare on Earth, i.e., they are not constituents of proteins and therefore less likely to be contaminants, have been confirmed to contain l enantiomer excesses (EE) (3, 12, 13). The origins of such excesses are unknown.
Sugars, aldehydes, or ketones that contain multiple carbon hydroxyl (carbon alcohol) groups, are also chiral and were likely necessary for the origin of life. In the majority of extant biological sugars and derivatives (“polyols”), the d enantiomers are significantly more abundant than the l enantiomers (we will note exceptions in Results and Natural Occurrence of Relevant Sugar Derivatives and Enantiomers). For example, only d-ribose, d-2′-deoxyribose, and d-glucose are found in polymers such as nucleic acids and polysaccharides. This exclusive use of only one enantiomer is referred to as homochirality, and its biological origin has been the subject of much research.
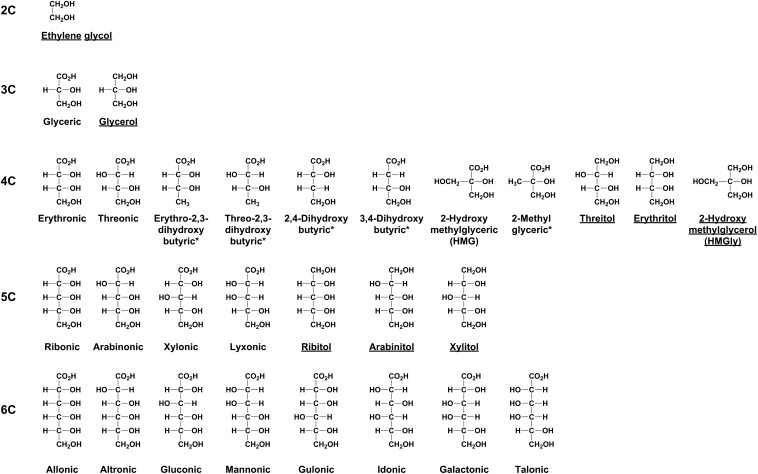
Meteoritic sugar derivatives, the subject of this work, include sugar acids and sugar alcohols (Fig. 1). Previously, we identified several polyols in the Murchison and Murray meteorites by gas chromatography mass spectrometry (GC-MS) (8): Only molecular identifications were reported. Here we describe the results of GC-MS enantiomer analysis of a large suite of meteoritic polyols (Fig. 1) from several carbonaceous meteorites of “type 2” classification (see Materials and Methods). This includes all straight-chained mono sugar acids (aldonic acids) of three carbons (3C) to six carbons (6C), 4C deoxy sugar acids, and chiral (and nonchiral) analyses of the majority of 2C–5C sugar alcohols. We found that aldonic acids, both rare and common, contain significant d EE that increase with increasing carbon number. In addition to rare compounds, we used characteristic markers of extraterrestrial organic compounds, isotope ratios (13C/12C), and comparisons to biological polyols (potential contaminants) to verify the indigenous nature of the anomalous d/l ratios. The magnitudes of the meteoritic aldonic acid EE might relate to the beginnings of life’s biological EE.
Fig. 1.
Structures of sugar derivatives analyzed in this work. The compounds are composed of two to six carbons, 2C−6C. Sugar alcohols are commonly defined by the corresponding (parent) sugars, e.g., ribitol is the reduced form of ribose. Deoxy sugar acids (*) are acids with at least one carbon not bonded to an −OH group. Sugar alcohols are underlined.
Results
Sugar Acid Enantiomeric Excesses and Racemic Sugar Alcohols.
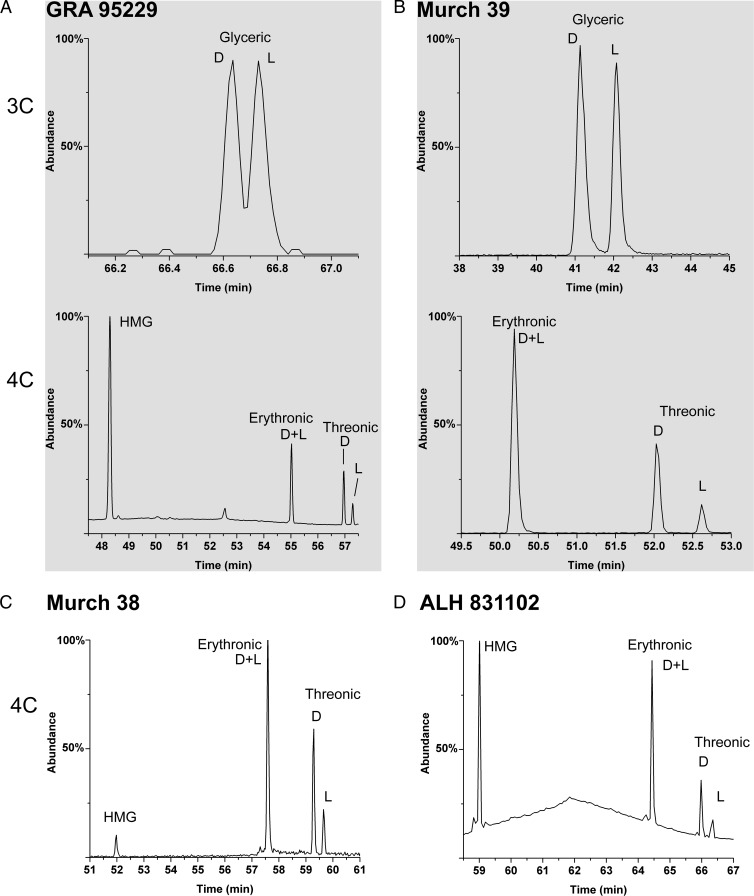
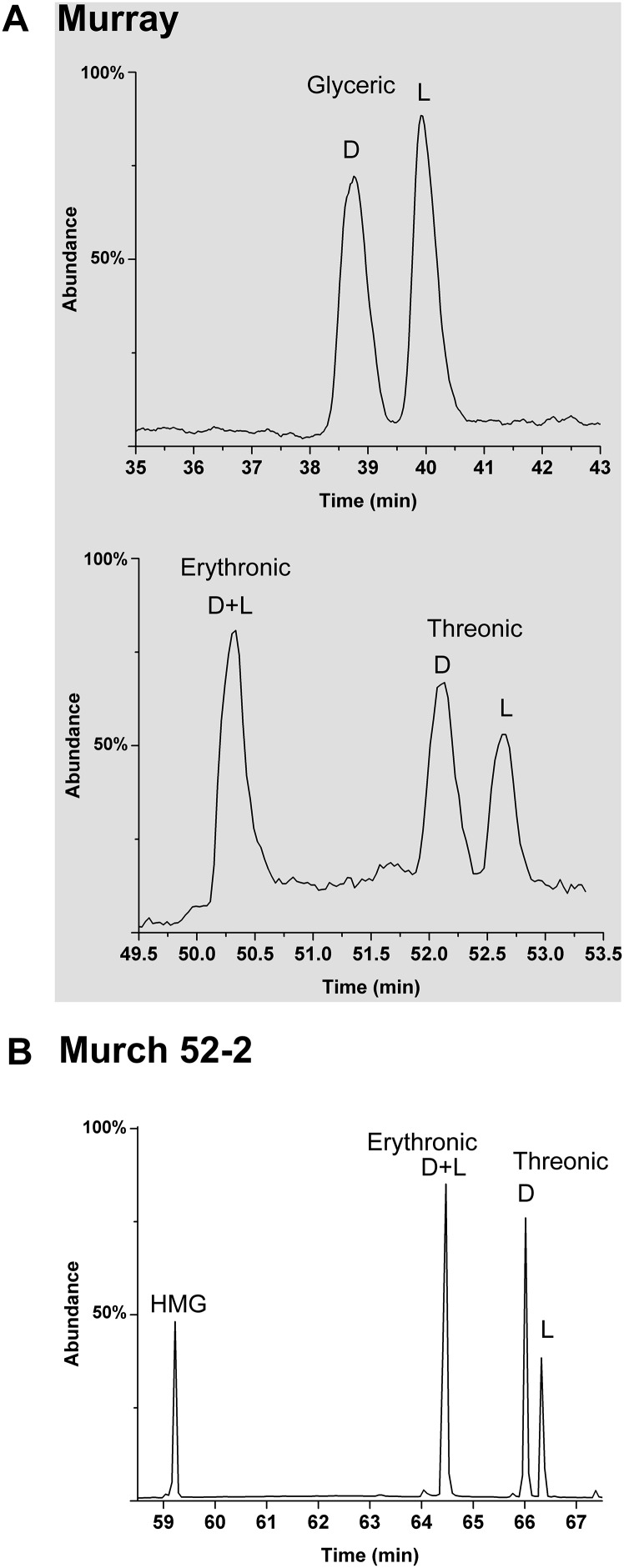
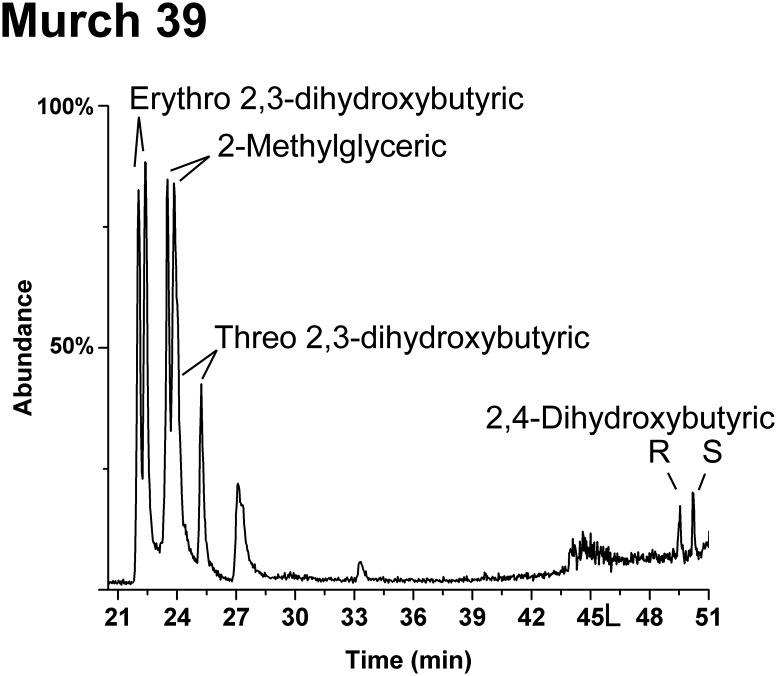
Enantiomer analyses of the smallest (3C) meteoritic sugar acid, glyceric acid, reveal a consistent racemic (50:50 ratio of d to l enantiomers) profile (Fig. 2 and Fig. S1). This is the case for all interior meteorite samples and indicates abiotic origins; in contrast, biologically abundant glyceric acid (i.e., a potential contaminant) is overwhelmingly the d enantiomer, as discussed in Natural Occurrence of Sugar Derivatives. The meteoritic 4C sugar acids show a significant (and also consistent) contrast: Threonic acid is only observed to possess large d EE. Such excesses, in multiple samples, are 33–55% (Table 1 and Fig. 2) despite threonic acid being significantly less abundant in biology than glyceric acid—and therefore less of a potential contaminant. Enantiomer analyses of erythronic acid [and arabinonic acid (5C)] have been more of an analytical challenge than other aldonic acids (14): Different analytical methods have been developed only recently (see SI Materials and Methods). However, a measurement of Murchison erythronic acid also demonstrated a substantial excess of the d enantiomer (54%, Table 1). As a marker of indigenous abiotic synthesis, it is significant that the rare and nonbiological branched 4C acid, 2-hydroxymethylglyceric acid (HMG) (Fig. 1), is observed in all meteorite extracts containing threonic acid and erythronic acid (Fig. 2), with the possible exception of the Murray meteorite (see Natural Occurrence of Sugar Derivatives). In addition, the 4C deoxy sugar acids are present and racemic (Fig. S2).
Fig. 2.
GC-MS analysis of 3C and 4C sugar acids and enantiomers from various meteorites. (A) Racemic glyceric acid (Upper) and 4C acids including a d-excess in threonic acid (Lower) from GRA 95229. (B−D) Analyses from the indicated meteoritic samples: Murch 39 (B), glyceric acid (Upper) and 4C acids (Lower); Murch 38 (C), 4C acids; and ALH 831102 (D), 4C acids; the rare branched compound HMG is ubiquitous (see Results). Erythronic acid also contains a d EE (Table 1). All compounds were analyzed as isopropyl ester/trifluoroacetyl ester (i-Pr/TFA) derivatives (see SI Materials and Methods).
Fig. S1.
Additional meteoritic 3C and 4C sugar acid analyses. (A) Glyceric acid and 4C acids from the Murray meteorite. Murray has a much longer residence time on Earth than Murchison, possibly resulting in more contamination of l-threonic acid. HMG was not identified in Murray; this may be due to a general lower abundance of compounds in this sample. (B) HMG and 4C acids from a sample of Murchison 52. (separate from that in Fig. 4C)
Table 1.
Enantiomer ratios and abundances of sugar acids of carbonaceous meteorites
| Number of carbons/compound | Percent d EE* | Meteorite† | Abundance, pmol/g (meteorite)‡ | Biological occurrence of acid (and parent sugar)§ |
| 3C acid | ||||
| Glyceric | 0 | All | 80 × 103 (M) | d, Common |
| 4C acids | ||||
| Erythro-2,3-DHB | 0 | M39, M47 | 1 × 103 (M39) | Rare |
| Threo-2,3-DHB | 0 | M39, M47 | 365 (M39) | Rare |
| 2,4-DHB | 0 | M39, M47 | 149 (M39) | Rare |
| 2-Methylglyceric | 0 | M39, M47 | 1 × 103 (M39) | Rare |
| Threonic | 43, 55, 33, 47, 34 | G, M39, M52, M38, ALH | 4 × 103 (M), 32(G) | Common |
| Erythronic | 54 | M39 | 4 × 103 (M), 32(G) | d, common |
| HMG | — | G, M39, M53, M57, LAP | 120 (G) | Rare |
| 5C acids | ||||
| Lxyonic | 61, (d) | M39, (M52) | 502 (M39) | Rare |
| Ribonic | 57 | M39 | 589 (M39) | d, common |
| Xylonic | 82, (d) | M39, (M52) | 889 (M39) | d, common |
| Arabinonic | 60, 47 | M39, M52 | 963 (M39) | l, common |
| 6C acids | ||||
| Allonic | (d) | M39 | tr (M39) | Rare |
| Idonic | (d) | GRA06100 | tr (GRA06100) | Rare |
| Gulonic | (d/l) | M39 | tr (M39) | Rare |
| Talonic | (d) | M39 | 29 (M39) | Rare |
| Galactonic | (d) | M39, GRA06100 | 92 (M39) | d, common |
| Gluconic | (d) | M39, GRA06100 | 273 (M39) | d, common |
| Mannonic | (d/l) | M39 | 743 (M39) | d, common |
ALH, ALH 83102; DHB, dihydroxybutyric; Erythro-2,3-DHB, erythro-2,3-dihydroxybutyric acid; G, GRA95229; LAP, LAP 02333; M39, Murchison 39; M52, Murchison 52, etc.; Threo-2,3-DHB, threo-2,3-dihydroxybutyric acid.
HMG is a nonchiral compound. (d) Indicates the finding of only the d enantiomers of these acids (both enantiomers were searched for). (d/l) indicates the compound is present but the d/l ratio could not be determined. The enantiomer ratios of DHB, Erythro-2,3-DHB, Threo-2,3-DHB, and 2-methylglyceric acid were also measured in a second aliquot of M39 (M39-2) with better enantiomer resolution than shown in Fig. 2. The 5C acids, d-xylonic and d-lyxonic, were also seen in a second meteorite, M52. The d enantiomers of (rare) altronic acid and talonic acid may be present in trace amounts in the GRA 06100 meteorite.
Glyceric acid is racemic (or near racemic) in all examined interior meteorite samples; crust samples are discussed in Discerning Extraterrestrial from Biological Compounds. M38 was acid hydrolyzed.
Not measured in all meteorites. Abundances are approximate; listed values include both enantiomers in chiral compounds. Glyceric acid, erythronic acid, and threonic acid abundances are from Murchison (M) in previous work (8); tr Indicates trace amounts but positive identifications.
Both d- and l-threonic acid are less common in biology than d-erythronic acid. Neither d- nor l-idose, the parent sugar of idonic acid, are known to occur in nature (20).
Fig. S2.
The 4C deoxy sugar acids from the same sample and GC-MS run of Murchison 39 as that in Figs. 2B and 4A. A small amount of 3,4-dihydroxybutyric acid might be present and coelute with the second enantiomer of 2-methylglyceric acid.
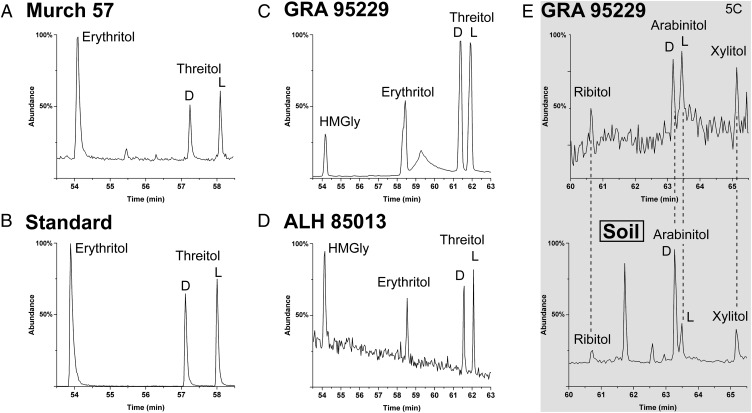
Among the comparable 4C sugar alcohols, only threitol is chiral (ethylene glycol and glycerol are included in this study to compare relative abundances). Fig. 3 shows that, in contrast to the 4C sugar acids, threitol is apparently racemic in multiple meteorites (Table S1). In addition, a rare branched 4C compound is also consistently present in this group: hydroxymethylglycerol (HMGly). Both threitol and HMGly are often found in the same meteorite sample extracts as the anomalous threonic acid.
Fig. 3.
GC-MS analysis of 4C and 5C sugar alcohols including enantiomer separation of d and l threitol from various meteorites. Meteorites analyzed are (A) Murchison (Murch) 57, (B) equimolar standards of erythritol and threitol (total, d+l) run under the same GC parameters as Murch 57, (C) GRA 95229, and (D) ALH 85013. The rare hydroxymethylglycerol (HMGly), the reduced analog of HMG (Fig. 1), was observed in most meteorite samples. (E) 5C Sugar alcohols from GRA 95229 (Upper) compared with corresponding compounds in soil/dust (Lower). The soil/dust sample was extracted for 20 h under an ambient (air) atmosphere. All compounds were analyzed as TFA derivatives.
Table S1.
Enantiomer ratios and abundances of sugar alcohols in carbonaceous meteorites
| Number of carbons/compound | % EE* | Meteorite† | Abundance,‡ pmol/g | Biological occurrence§ |
| 2C alcohol | ||||
| Ethylene glycol | — | M39 | 320 × 103 | Rare |
| 3C alcohol | ||||
| Glycerol | — | All | 160 × 103 (M39) | Common |
| 4C alcohol | ||||
| Erythritol | — | G, M39, LAP | 26 (G), 14 (ALH) | Common |
| d-threitol | 0 | G, M53, M57, LAP | 32 (G), 14 (ALH) | Common (21, 22) |
| l-threitol | 0 | G, M53, M57, LAP | 32 (G), 14 (ALH) | Rare (22) |
| HMGly | — | G, M53, M57, LAP | 14 (G), 31 (ALH) | Rare |
| 5C alcohol | ||||
| Ribitol | — | G | tr | Common |
| d-arabinitol | 0 | G | tr | Common (55) |
| l-arabinitol | 0 | G | tr | Rare (55) |
| Xylitol | —- | G | tr | Common |
ALH, ALH 85013; G, GRA 95229; LAP, LAP 02333; M39, Murchison 39; M52, Murchison 52, etc.
Dashes indicate a nonchiral compound; “0” for threitol does not exclude possible small l EE in Murchison. Erythritol possesses a plane of symmetry and therefore does not exhibit enantiomerism.
Glycerol was observed in all meteorites. Ethylene glycol is likely to be present in multiple meteorites but was not specifically searched for in all cases.
Ethylene glycol and glycerol abundances from Murchison in previous work (8); tr Indicates trace amounts but positive identifications.
Although found in nature, d-threitol is less common than erythritol.
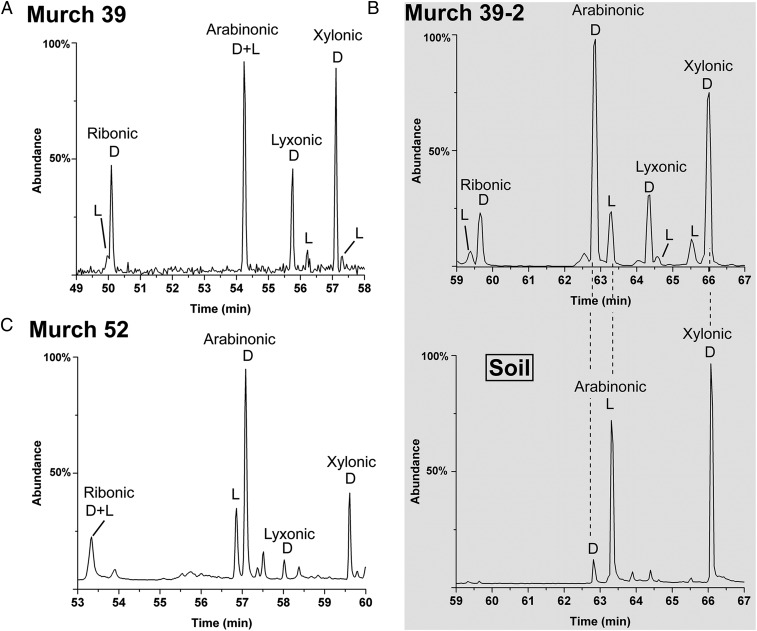
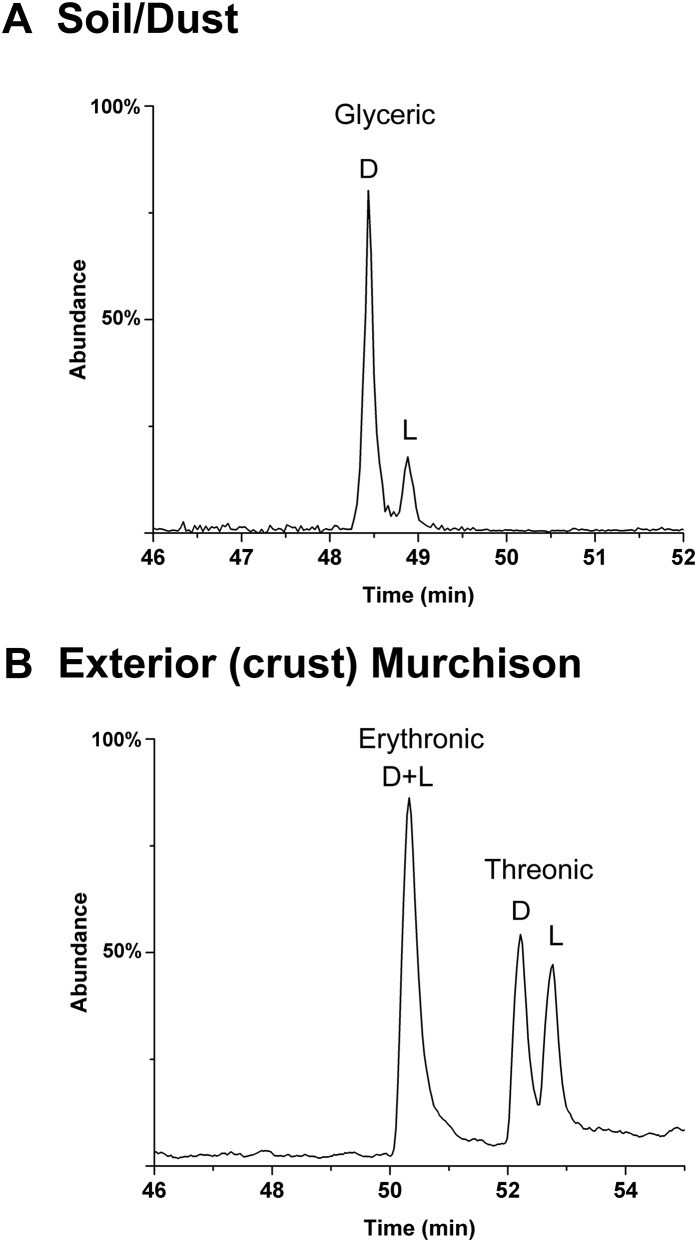
In contrast to the 4C acids, the relatively low abundances of the 5C acids (Fig. 1 and Table 1) often did not allow for their simultaneous detection in the same meteorite sample: Usually, arabinonic acid and/or xylonic acid could be identified. However, two samples of Murchison had sufficient abundances for the simultaneous analysis of all four acids (Fig. 4). All, including the biologically rare lyxonic acid, displayed much larger d EE than the 4C acids, up to 82% (Table 1). The chromatographic separation of d/l arabinonic acid, achieved more recently than other acids, also possesses a large (and comparable) d EE (Table 1 and Fig. 4 B and C). The presence of a d EE in this compound is significant because l-arabinonic acid (and l-arabinose) is by far the more common enantiomer in biology, as exemplified in a microbial (soil/dust) sample (Fig. 4B, Lower). In addition, the 5C acids apparently attained equilibrium proportions on the meteorite parent body, lending further evidence toward the indigenous nature of this suite (see Natural Occurrence of Sugar Derivatives).
Fig. 4.
The 5C sugar acid enantiomer analysis of the Murchison meteorite. (A) A sample of Murch 39 (same sample as in Fig. 2B) analyzed immediately after extraction and preparation. (B) Murch 39-2, a separate portion of the Murch 39 extract, was stored until methods were developed to (reliably) separate enantiomers of arabinonic acid (Upper). The corresponding compounds in a sample of soil/dust (from the same sample as in Fig. 3E) (Lower). The change in d-l elution order of xylonic acid enantiomers from A was due to use of a different GC column (see SI Materials and Methods). A possible trace amount of lyxonic acid is present in the soil/dust sample; this would likely be due to the breakdown/oxidation of the common 6C sugar, galactose (see homologous relationships in Fig. S5) as none of the rare parent sugar of lyxonic acid, lyxose, was found. (C) The 5C acids of Murch 52. Methods for the consistent enantiomer separation of ribonic acid were not yet developed. No l enantiomers of lyxonic acid or xylonic acid were seen—likely due to overall low abundances. The change in d-l elution order of arabinonic acid enantiomers from that of B was due to use of a different GC column and compound derivative. Murch 39 samples were analyzed in their i-Pr/TFA forms; Murch 52 compounds were analyzed in ethyl esters (Et/TFA) forms (see SI Materials and Methods for derivatization details).
Meteoritic 5C sugar alcohols also show a large decrease in abundance relative to their 4C homologs (Table S1). Therefore, the d/l ratio of arabinitol (the only chiral 5C sugar alcohol) cannot yet be determined with confidence. Still, in sample GRA 92229, arabinitol appears to be both nearly racemic, and its abundance is not significantly greater than those of the other 5C sugar alcohols (Fig. 3E); in contrast, microbial 5C profiles typically show that d-arabinitol dominates l-arabinitol, ribitol, and xylitol (Fig. 3E, Lower).
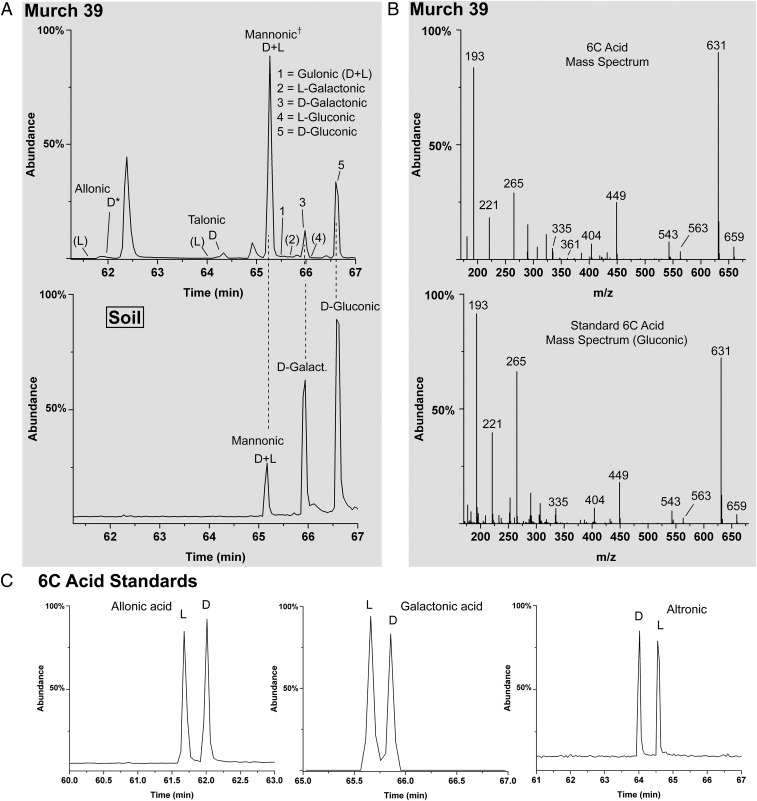
Simultaneous identification of all eight meteoritic straight-chained 6C aldonic sugar acids (Fig. 1) in one sample has not been achieved due to low abundances. However, six of the eight members of the 6C group were identified in a sample of the Murchison meteorite (Fig. 5A), and at least three were identified in GRA 06100 (Table 1). Although we are attempting to measure this group in larger samples, current results of their enantiomer analysis apparently reveal a similarity to their smaller (4C and 5C) homologs: Only d EE are observed. In fact, only the d enantiomers of the 6C acids were seen; therefore a quantification of d/l excesses could not be done. However, if the 6C group is consistent with the trend of the 4C and 5C acids, i.e., increasing d EE with increasing carbon number, one would expect to eventually observe d excesses larger than those of the 5C group (e.g., Fig. 6). In addition to the presence of rare 6C sugar acids in meteorites, possibly significant is the relative abundances within this suite: Gluconic acid is not the most abundant (Table 1). A couple of factors, the ubiquitous nature and large relative abundance of glucose and gluconic acid in organisms (i.e., possible sample contaminants) combined with the ability of several microorganisms to oxidize glucose to gluconic acid (15), suggest that gluconic acid would dominate the meteoritic 6C acids if microbial contamination was significant (e.g., Fig. 5A, Lower). In addition, gluconic acid was also not the most abundant 6C acid in the GRA 06100 meteorite. A laboratory study of 6C aldonic acids under alkaline conditions might reveal whether the meteoritic profile in Fig. 5A is one of equilibrium proportions, i.e., an equilibrium of acids where mannonic>gluconic>galactonic; equilibrium is likely for the meteoritic 5C acids, discussed in Natural Occurrence of Sugar Derivatives.
Fig. 5.
GC-MS chromatograms and mass spectra of 6C sugar acids; all compounds in this analysis were converted to their i-Pr/TFA derivatives. (A) Murch 39 (Upper) and the corresponding compounds from soil/dust (Lower, from same soil/dust sample as Figs. 4B and 3E); *, in Murch 39, d-allonic acid is of relatively low abundance and is considered a tentative identification; however, the major ions, 631, 563, and 543, are present in the mass spectrum (see B) at the correct GC retention time; †, the enantiomers of mannonic acid coelute under the employed GC conditions. Parentheses indicate that the l enantiomers were not detected; their known retention times are indicated. (B) Mass spectra of a 6C standard (gluconic acid) (Lower) and a 6C sugar acid (Upper) from Murchison. The shown mass spectra are nearly identical to all 6C aldonic acids. (C) Examples of enantiomer separation of standards.
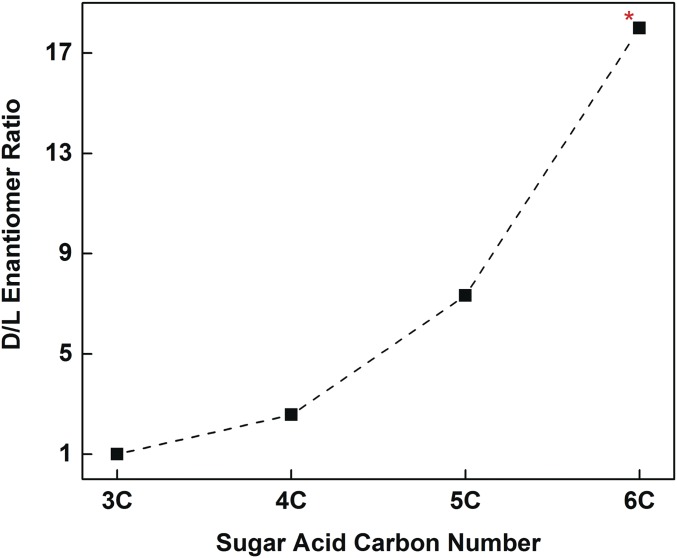
Fig. 6.
Plot of the average d/l ratios of the majority of identified chiral meteoritic sugar acids from Table 1. The 5C values are only from Murch 39; this Murchison fragment was more pristine than Murch 52; *, the 6C value is extrapolated based on the d/l trend of 3C → 4C sugar acids.
Isotope Measurements.
Isotope ratio measurements (13C/12C) were made on some of the most abundant meteoritic sugar acids and glycerol. A measurement of combined (d+l) glyceric acid yielded a value of +60‰ whereas 2-methylglyceric acid was 82‰ (Table S2 and Fig. S3), well above typical values of terrestrial organic compounds (3, 5) and among the highest recorded values for meteoritic compounds. Measurement of glycerol from GRA 95229 gave a value of +5‰, indicating that (at least) a substantial portion of glycerol in this meteorite is indigenous. Isotope measurements on compounds of lower abundances (however, from purified extracts) yielded values of +10‰ for d-threonic acid and +10‰ for combined (d+l) erythronic acid (see SI Materials and Methods). Although these isotope values indicate extraterrestrial origins, significantly more method development is needed for the isotope analysis of individual enantiomers of 4C and higher sugar acids.
Table S2.
13C isotope composition of meteoritic sugar acids and glycerol
| Compound | 13C (‰) | Meteorite |
| Glyceric acid (d+l) | 60 | M30 |
| 2-Methylglyceric acid (d+l) | 82 | M30 |
| Erythro-2,3-dihydroxybutyric acid (d+l) | 18 | M56 |
| Threonic acid (d) | 10* | M53 |
| Erythonic acid (d+l) | 10* | M53 |
| Glycerol | 5 | G |
G, GRA 95229; M, Murchison. Glyceric acid, 2-methylglyceric acid, and erythro-2,3-dihydroxybutyric acid were TMS derivatives; threonic and erythronic were i-Pr/TFA derivatives (see Materials and Methods).
Must be repeated with larger meteorite extracts and method modification.
Fig. S3.
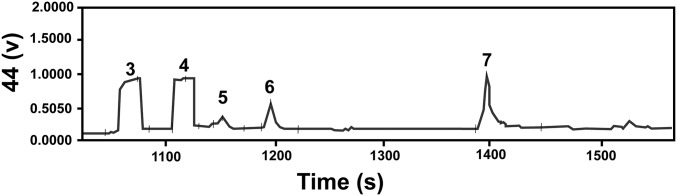
GC-IRMS chromatogram of 13C isotope measurements of d+l glyceric acid (peak 6) and 2-methylglyceric acid (peak 5) after initial purification from a Murchison extract by ion chromatography (both compounds analyzed as TMS derivatives). The β-alanine (peak 7) is an internal standard. Peaks 3 and 4 are pulses of CO2 reference gas.
Discerning Extraterrestrial from Biological Compounds.
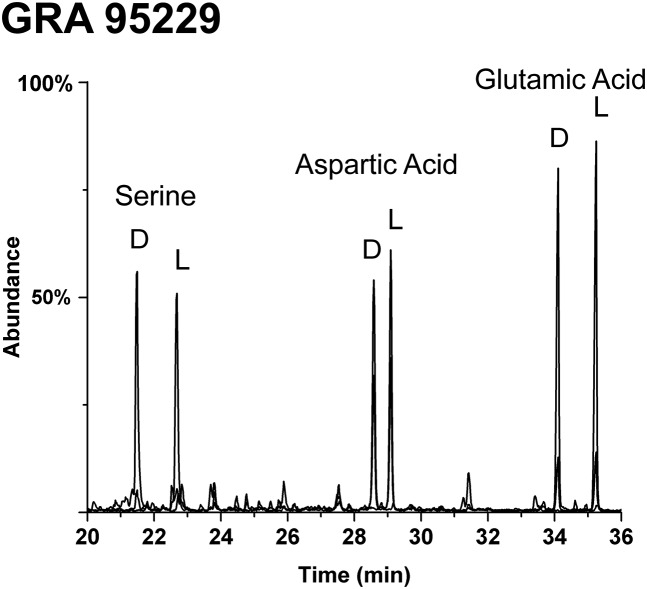
Foremost in discussions of meteoritic organic compounds are markers to discern indigenous products (presolar and/or parent body) versus those of potential contamination (16, 17) in lieu of an ideal direct sampling of asteroids. In this regard, it is significant that in the GRA 95229 meteorite the 3C and 4C sugar acids have very similar enantiomer profiles to those in the much longer-studied Murchison meteorite (Fig. 2). GRA 95229 has been judged to be very pristine due to a lack of significant indicators of contamination (18, 19). We also observed this remarkable cleanliness: Its common protein amino acids, especially serine, are not dominated by the l forms (the usual case with contamination) but instead are racemic (Fig. S4). Furthermore, common sugar contaminants such as l-arabinose, d-xylose, d-glucose, etc., were absent in GRA 95229. Such contaminants would be particularly significant to this study due to their potential oxidization to sugar acids (e.g., Figs. S5 and S6) followed by (less likely) racemization reactions (Fig. S7). It was for this reason that we analyzed the polyol content of dust, soil, and exposed and cultured meteorite crust samples (Figs. S8 and S9): Such samples are more realistic analogs for the contaminants from microorganisms that might affect meteorite samples (normal procedural blanks very rarely show sugar acids).
Fig. S4.
Amino acid enantiomer analysis of meteorite GRA 95229. The sample is from the same extract as that found to have an excess of d-threonic acid and racemic glyceric acid (Fig. 2A). Other protein amino acids (not shown) were also racemic.
Fig. S5.
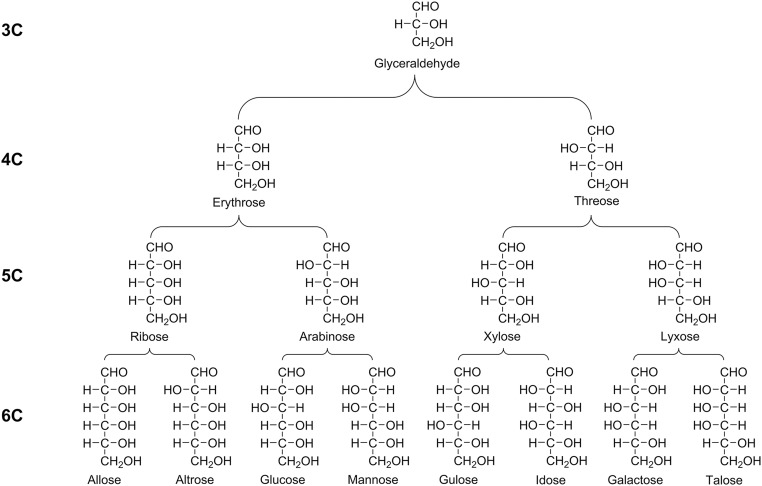
The structural relationships of homologous 3C through 6C straight-chained aldehyde (aldo) sugars (only d enantiomers are shown). Upon mild oxidation or alkaline hydrolysis of an individual sugar, a predictable set of homologous degradation products (sugar acids) is observed. For example, upon oxidation of mannose, observed compounds include mannonic acid (trace amounts) → arabinonic acid → erythronic acid → glyceric acid; for talose, compounds include talonic acid (trace amounts) → lyxonic acid → threonic acid → glyceric acid. Arrows are not definite indicators of the order of product formation. Degradation products are observed to retain the d/l ratio of the starting sugar (Fig. S6).
Fig. S6.
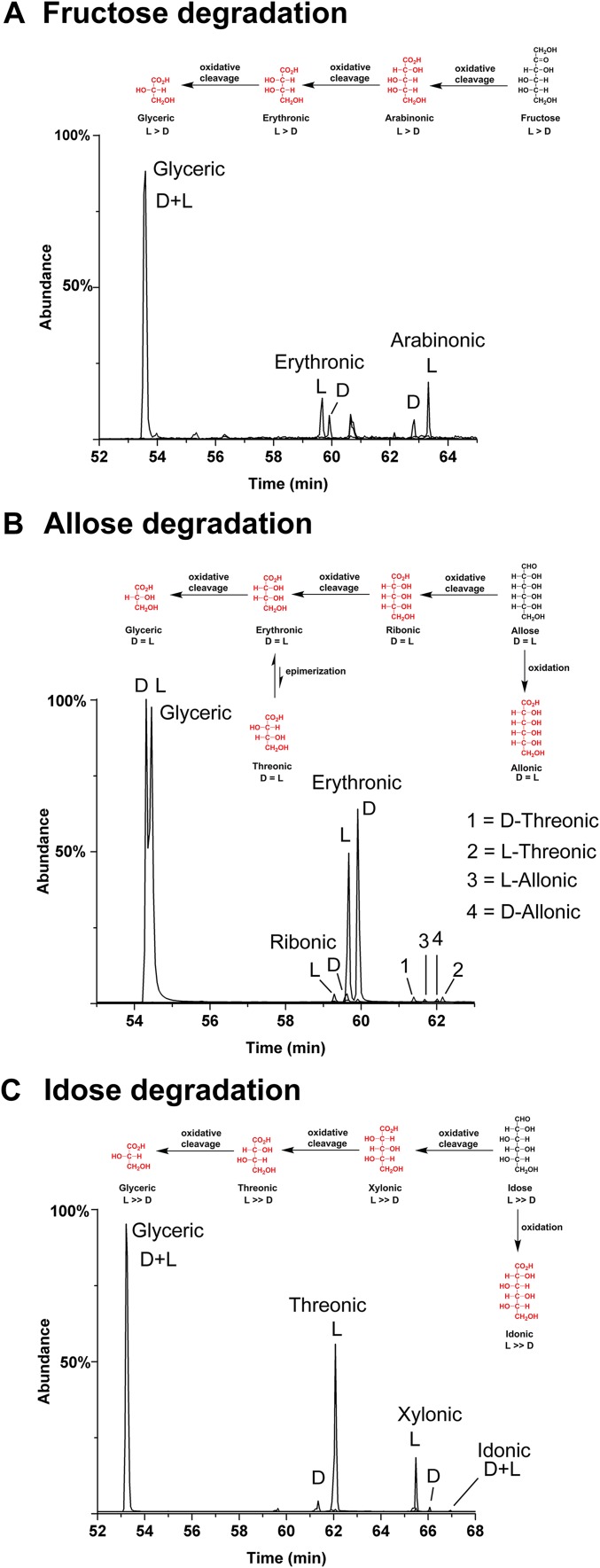
A few examples of degradation products from the oxidation and alkaline hydrolysis of sugar standards: (A) fructose, (B) allose, and (C) idose. The observed reactions in each chromatogram demonstrate that degradation products maintain the initial d/l ratio of the starting sugar. Glyceric acid enantiomers were not consistently resolvable (depending on column condition); the shown chromatograms illustrate a range of compounds.
Fig. S7.
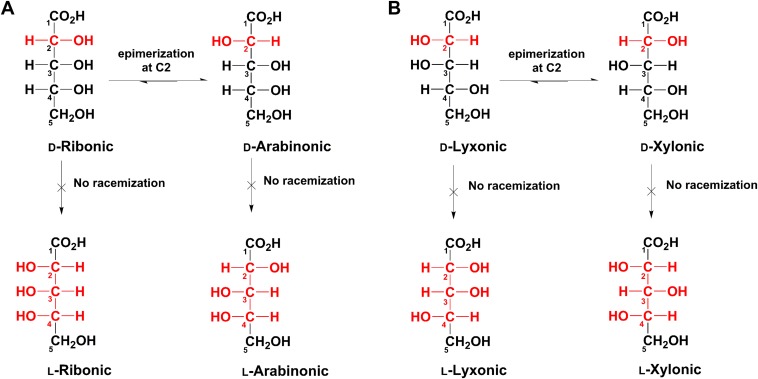
The interconversion and retention of EE of 5C sugar acids under relatively mild alkaline conditions. In each compound, only carbon #2 can (relatively) easily invert configuration thus allowing epimerization (schemes A and B) but not compete racemization.
Fig. S8.
Additional contamination studies. (A) A typical profile of glyceric acid from water-extracted soil/dust (microorganisms)—as compared with racemic glyceric acid observed in the meteorites of this study. (B) The 4C acids in a known contaminated (crust) sample of Murchison; the GC column did not separate the enantiomers of erythronic acid. There is a significant increase in the l/d ratio of threonic acid relative to that of interior samples and a pristine meteorite such as GRA95229 (Table 1 and Fig. 2): The increase in the l enantiomer can be the result of microbial vitamin C metabolism and/or the result of selective microbial consumption of the d enantiomer as shown by laboratory experiments (Fig. S9).
Fig. S9.
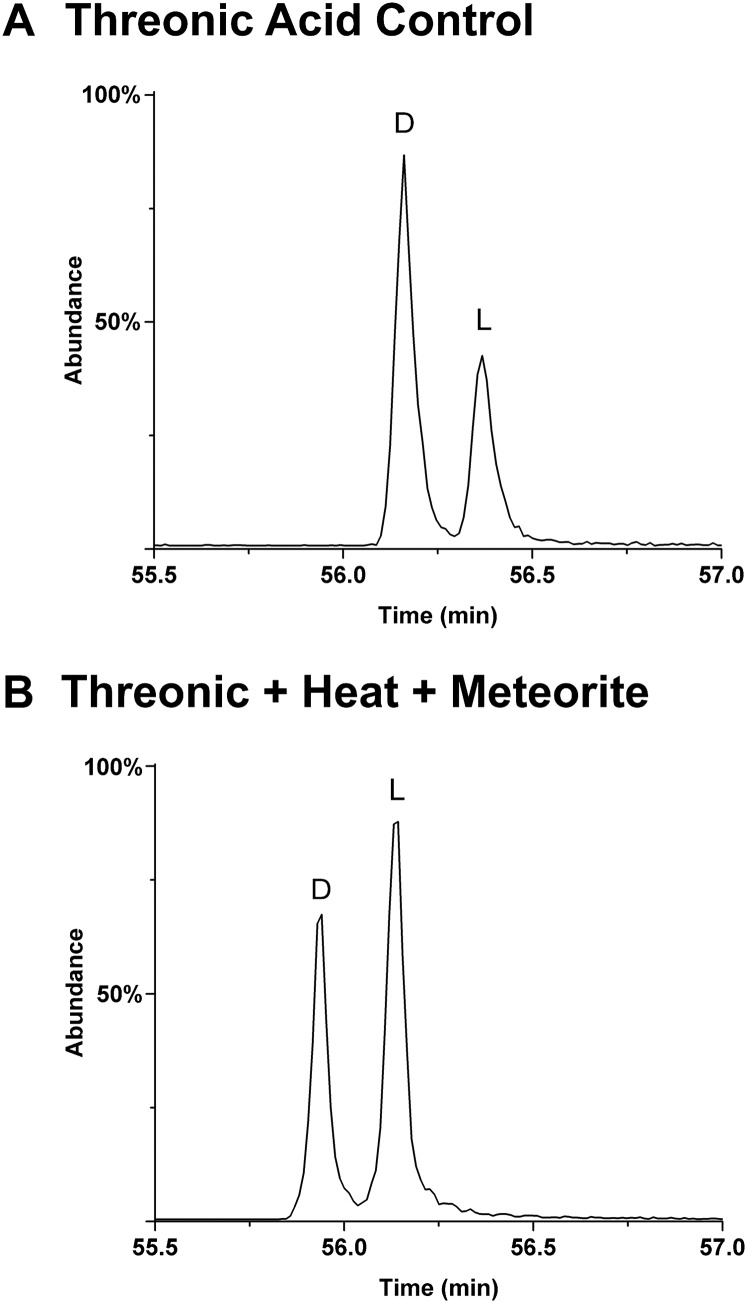
Chromatograms showing microbial action on a standard mixture of dl-threonic acid. (A) Control sample containing only the standards in water. (B) The results of sustained microbial action on the same mixture after addition of a small amount of Murchison meteorite powder and incubation at 37 °C for 5 days. There is clearly preferential consumption of the d enantiomer. Theoretically, such an effect would lead to excesses of the l enantiomer (opposite to the meteoritic results in Table 1) if microbial consumption of meteoritic threonic acid under ambient (dry) meteorite storage conditions was significant; incubation results were similar for other polyol standards. In the case of dl-arabinonic acid, where the l enantiomer is biologically dominant, microbes indeed consumed more of the l enantiomer: ∼2–3 times less l is present (relative to d) after 10 days. However, all observed soil or contaminated meteorite samples show an excess of the l-arabinonic enantiomer (e.g., Fig. 4B, Lower). Such results indicate that preferential microbial consumption of enantiomers are unlikely to be significant, at least in the present results.
However, because contaminants can generally be detected in laboratory materials, reagents, and less pristine meteorite samples, we found it imperative to address and investigate specific markers, sources, and mechanisms of potential contamination. Our studies on the degradation and racemization of potential sugar contaminants combined with previous racemization studies (20) have helped to rule out these mechanisms as significant sources of contamination during sample preparation; see Supporting Information.
Natural Occurrence of Relevant Sugar Derivatives and Enantiomers.
When describing the results of analyses of meteoritic sugar derivatives (above), we noted that d/l ratios of meteoritic compounds such as glyceric acid, arabinonic acid, and arabinitol are either opposite, or different from, those of common biological contamination (e.g., Figs. 3E and 4B). The racemic nature of meteoritic d/l-threitol (Table S1) adds to such differences; although d-threitol can be found in nature (21, 22), a natural or biological occurrence of l-threitol has not been reported (22). The relative abundances of the meteoritic 4C acids, erythronic and threonic, highlight a key difference from their terrestrial analogs: These two compounds are nearly equal in abundance in meteorites (Table 1). However, in all water-extracted soil/dust samples that we have analyzed, erythronic acid is significantly more abundant than threonic acid—consistent with the extensive role of d-erythronic acid in biological processes (however, if hydrolyzed or oxidized, such samples will produce certain levels of both acids and their enantiomers through the reactions/breakdown of other sugars).
In some instances, terrestrial l enantiomers may be found in significant (relative) abundances: l-threonic acid is an oxidation product of l-ascorbic acid (vitamin C) metabolism, as are some other sugar acids, including l-tartaric acid, l-glyceric, etc. (23, 24), and also the sugar l-threose (25). Although very rare, l-lyxonic acid has also been identified as a vitamin C product (24); the rare sugar d-lyxose can be found in a modified form in a limited number of organisms (26). Among the compounds involved in the synthesis of vitamin C are l-gulose, l-gulonic acid, l-galactose, and l-galactonic acid (27). The lack of significant relative abundances of the l enantiomers of sugar acids (from interior meteorite samples), particularly l-arabinonic acid and l-threonic acid, suggests that the observed d EE are likely to be indigenous to the meteorites.
The presence and abundances of the rare 4C branched and deoxy acids and the rare 4C sugar alcohols (Fig. 1) indicate extraterrestrial origins: Their abundances are not markedly lower than those of the more biologically common compounds (erythronic acid and erythritol), and all chiral compounds in this group are racemic (Table 1 and Table S1). HMG (Fig. 1) could have at least partial origins in the oxidation of its parent sugar, 2-hydroxymethylglyceraldehyde: This compound is known to be produced in (abiotic) formose reactions (28) and might have originated in such, or related (29), reactions on the meteorite parent body.
The relative abundances among the meteoritic 5C sugar acids from one sample (Murchison 39, Fig. 4A) might also indicate indigenous aqueous processing. Previous research (30) with laboratory standards of the same 5C acids has shown that epimer pairs (Fig. S7) reach equilibrium in solutions of strong base (KOH, T ≈ 100–140 °C, several hours); the ribonic/arabinonic ratio was found to be 0.63, and lyxonic acid/xylonic acid was 0.53. The ratios of the same meteoritic epimer pairs are nearly identical, 0.62 and 0.5, respectively, indicating attainment of equilibrium in a longer period due to the relatively mild conditions of pH and temperature in type 2 carbonaceous meteorites. The relatively fast and gentle meteorite extraction conditions of the present study would not allow such equilibration.
Although simulating the exact history of possible contaminants in a given meteorite is unlikely, certain types of analyses, samples, and analogs are useful. We used local dust/soil mixtures as analogs for, at least some, expected contaminants. Although all meteorite powders were subjected to quick and gentle extraction (see Materials and Methods), dust/soil samples were subjected to conditions from gentle to relatively harsh. Some (below) underwent short periods of water extraction; another, shown in Figs. 3E, 4B, and 5A, underwent no oxidation or alkaline hydrolysis but an extended period (20 h) of stirring in water under ambient air, i.e., it was exposed to oxygen; others underwent alkaline hydrolysis and oxidation. All were meant, among other things, to determine which sugar acids might be produced by decomposition of ambient sugars—some of which likely already happened to the soil portion of the samples, given the years of exposure of soils to the elements.
Analyses show the expected, more biologically abundant, compounds and enantiomer ratios: high d to l glyceric acid (Fig. S8A), high l to d arabinonic acid, abundant d-xylonic acid (Fig. 4B, Lower), and high d to l arabinitol (Fig. 3E, Lower). Unoxidized sugars are, of course, major components of such samples, principally l-arabinose, d-xylose, d-glucose, d-galactose, and d-mannose. We often observed the production of acids such as erythronic, threonic, and glyceric from such sugars when dust/soil samples received harsher treatments (e.g., Fig. S6). Importantly, we have never observed the rare sugar lyxose or the rare 6C sugars in such samples. Also, as shown in Figs. S8B and S9, we have ruled out significant effects of long-term microbial contamination on the enantiomer profiles of the present (interior) samples.
Discussion and Conclusions
The majority of carbonaceous meteorites are thought to be fragments of asteroids (10, 31). There is ample evidence for the formation of meteoritic compounds from simpler precursors during asteroidal aqueous alteration (12). At least one example from the present set of compounds is likely to be ethylene glycol. A partial origin of this abundant meteoritic compound could well be its facile formation in aqueous solution (32) from interstellar ethylene oxide—a cyclic precursor molecule and constituent of interstellar grains (33, 34). However, before later aqueous reactions on asteroid parent bodies, small molecules bound to interstellar/presolar grains (the building blocks of asteroids and comets) were subjected to radiative (9) and other processing in the forming Solar System.
Previously, it was postulated that a portion of meteoritic sugar derivatives could be the result of photolysis of precursors on interstellar grains (8). Recent analysis of a suite of soluble compounds from CR meteorites led researchers to conclude that a fraction of the compounds (including sugar alcohols) were formed before any possible onset of aqueous alteration in the parent bodies and could therefore have origins in preaccretion environments: Some of the meteorites showed little or no evidence of the action of liquid water (12). Consistent with these findings, recent laboratory experiments designed to simulate the low-temperature irradiation of organic precursors on interstellar grains produced a suite of larger polyols, including multiple sugars (35, 36). In the case of formaldehyde, a precursor to polyols, simulations using IR detection have shown that formaldehyde/NH3/H2O mixtures start to form compounds down to at least 40 K, i.e., temperatures relevant to interstellar/presolar grains (37). In addition, this work did not use radiation or other energy sources commonly used in such simulations: Sample warming was the only initiator of synthesis. More recent work, using irradiation (UV and electron), has shown in situ synthesis of organic molecules in mixtures starting at 5 K, i.e., the reactions mixtures were not warmed in preparation for analysis (by laser ablation/ionization mass spectrometry) (38).
The exposure of grains (and their carbon molecules) to interstellar forces offers a possible explanation for the EE observed in some of the meteoritic polyols. Primordial and ubiquitous forces such as radiation and magnetism preceded complex chiral compounds and may have contributed to the earliest EE. In the laboratory, two methods using these forces have been confirmed to produce EE in compounds. One is photolysis of enantiomers by circularly polarized UV light (UV CPL) (39). UV CPL is also suggested as a possible interstellar mechanism for the production of EE (40, 41). Laboratory experiments with UV CPL have demonstrated a slight preferential destruction of one enantiomer, which resulted in relatively small but reversible EE (depending on the direction of the CPL) from an initially racemic mixture of amino acids (39). When performed at low (i.e., interstellar) temperatures, UV CPL is reported to induce EE during the actual synthesis of compounds (42).
A second method involves magnetochiral (inducing enantioselectivity with magnetism + light) techniques and is also suggested as a possible interstellar agent of EE (43). Theoretical calculations suggest the necessity of including transition or rare-Earth elements and the asymmetric application of the two forces to a sample; the incident magnetic field and light must be in parallel (or antiparallel) directions. In a demonstration of magnetochiral effects, a racemic (and premade) complex (K/Cr/oxalate) was simultaneously exposed to intense unpolarized light and a parallel magnetic field of ≤15 T; small (∼10−4) but reversible EEs (depending on the direction of the magnetic field) were temporarily created (43). Although the EE produced in both of the above experiments were small, a general scenario for the origin of the large EE observed in meteoritic sugar acids (and other meteoritic compounds) might involve the creation of small initial EE on interstellar/presolar grains. These initial EE could then influence the continued organic synthesis on grains, as well as while grains conglomerate into larger bodies, and, finally, during periods of aqueous activity in asteroids (with increasing EE at each stage).
However, if the combination of radiation and magnetism contributed to EE in organic compounds in the early Solar System, then, as described above, parallel magnetic and light directions were likely required. In this regard, if the Solar System formed as part of a star cluster, as is the case for most stars (44, 45), the Sun's disk of icy material was likely irradiated by a neighboring star (46). Recent observations of the external irradiation of multiple young (protoplanetary) disks by single massive (O/B) stars show that the entirety of a disk can be irradiated (47). In a study applying such a scenario to our own protoplanetary disk, calculations demonstrated that irradiation by a neighboring (O/B) star might have produced significantly more organic material in the disk than what would have been produced by irradiation from the Sun (46).
Such external irradiations are unidirectional for a given planetary disk (47). If any simultaneous (and sufficient) magnetic fields, internal to the disk (but also possibly from the external source), were also parallel to the irradiation, the conditions demonstrated in the above magnetochiral laboratory experiment would be satisfied and could possibly result in EE in subsequent compounds. UV CPL from a neighboring star could also result in net EE in disk organic compounds. Calculations also show (9) that any ambient (from all directions) external irradiation on the protoplanetary disk combined with periodic warming of disk grains will result in significant organic production; complex organic material was therefore suggested to be a much more general feature of planetary disks and not necessarily dependent on nearby massive (O/B) stars (9). Within a single planetary disk, such physical forces may or may not be asymmetrical.
All of the present meteoritic sugar acids with EE are diastereomers (i.e., they contain more than one chiral carbon). Likewise, a few diastereomeric meteoritic amino acids have recently been found to contain EE (12). Due to the fact that such compounds normally retain enantiomer asymmetry under mild conditions (Fig. S7), they may be illustrative of EE preserved for ∼4.5 Gy. In this regard, they may give further hints that, in the early Solar System, whatever asymmetric forces produced EE may have been more significant than once believed. For example, it has been shown that the actual largest and ubiquitous source of meteoritic organic carbon, the macromolecular carbon, also contains a (unknown) source of asymmetry (48). If, as suggested, the polyols in this work resulted partially from formose reactions, then they (or their higher homologs) may be constituents of this unknown source of asymmetry, given that the macromolecular carbon is also similar to formaldehyde reaction products (49).
In summary, we find that all 4C−6C straight-chained aldonic acids (excluding deoxy acids) in multiple carbonaceous meteorites possess d EE that increase with increasing carbon number. Observations that support evidence of the extraterrestrial origins of this phenomenon include the presence of rare compounds: an excess of d arabinonic acid, d- and l-lyxonic acid (with d/l ratios similar to the other 5C sugar acids), rare (d-only) 6C acids, and branched nonbiological 4C compounds. In contrast, comparable (and biological) sugar alcohols and rare deoxy sugar acids are racemic in some of the same extracts as acids that possess EE. Other evidence of abiotic chemistry includes the apparent equilibrium proportions of the 5C acids and high 13C/12C ratios in some compounds. Polyol homologs or their synthetic processes may also contribute to the unknown source of asymmetry (EE) in meteoritic macromolecular carbon.
Asymmetric meteoritic compounds may have either played a direct role in the formation of the first homochiral biological polymers or influenced the chirality of their subsequent syntheses. It now seems possible that the EE of two meteoritic classes of compounds, sugar acids (d) and amino acids (l), qualitatively match the excesses of the corresponding classes in extant biology. However, although we have used criteria (rare compounds/enantiomer and isotope ratios) in attempts to discern extraterrestrial from Earth material in the present samples, it is critical that current (50) and future space missions capable of enantiomer analysis examine and/or return samples of carbonaceous material to verify laboratory enantiomer measurements of meteoritic compounds.
Materials and Methods
Meteorite Samples Analyzed (Classification)−.
The following samples were analyzed (51): Murchison (CM2), Murray (CM2), GRA 95229 (CR2), Alan Hills (ALH) 83102 (CM2), ALH 85013 (CM2), LaPaz Icefield (LAP) 02333 (CM2), and GRA 06100 (CR2). The designation “2” indicates that a meteorite has experienced significant hydration (among other properties). In general, type 2 meteorites contain the highest amounts of soluble organic compounds. All meteorites except Murchison and Murray were obtained from the Antarctic collection at NASA's Johnson Space Center.
Extraction and Preparation of Meteorite Samples.
Procedures for the extraction and isolation of bulk fractions of compounds (including ion exchange chromatography) and purification of individual compounds are similar to those of previous analyses (8, 52, 53). Interior portions of each meteorite were taken and the water extraction of all listed meteorite samples were done under very mild extraction conditions: None were heated above room temperature, to avoid decomposition, racemization, or epimerization of any possible susceptible compounds. A typical extraction consisted of a brief (∼3 min) stirring and/or sonication of a meteorite powder in water. The sample was then centrifuged, and the water was removed and frozen; this procedure was repeated multiple times, and the separate water extractions were combined in preparation for further processing. Sample Murchison 39 (Murch 39) was extracted under a helium atmosphere to further guard against possible oxidation of any carbonyl compounds. The water extract of Murch 38 (Table 1) was subsequently acid hydrolyzed with trifluoroacetic acid. Subsequent desalting and fractionation of compounds by acidity is similar to previous work (52). After centrifugation to remove insoluble matter, a slightly acidified and concentrated supernatant was applied to cation exchange resins: typically AG 50-X4 resin, 200–400 mesh, H+ form (Bio-Rad). Eluents were then concentrated, made slightly alkaline, added to anion exchange resins, and eluted with aqueous acid solutions to fractionate compounds by binding affinity (to the resin). The form (i.e., counter ion) of the anion exchange resin for some samples (including Murch 39) was acetate, again, to minimize possible side reactions of possible labile compounds—in this case, carbonyl compounds (54). See Supporting Information for complete material and methods.
SI Materials and Methods
Derivatization of Compounds for GC-MS.
All compounds in this work required derivatization to increase their volatility for analysis by GC-MS. The most common derivatives were i-Pr/TFA and Et/TFA. Such derivatizations and analyses were very similar to those in previous work (14). No racemizations of compounds were observed. Enantiomer analysis of all compounds was done in their straight-chain forms; samples were brought to alkaline pH and dried (rotary evaporation) to open any possible lactones (cyclic structures) (14). For isotope analyses—by GC isotope ratio mass spectrometry (GC-IRMS)—some compounds, i.e., glyceric acid, 2-methylglyceric acid, and erythro-2,3-dihydroxybutyric acid (Table S1), were analyzed as their trimethylsilyl (TMS) derivatives with methods (including compound purification) nearly identical to those in previous work (53). Complete procedural blanks were performed: Of all sugar derivatives, only trace amounts of glycerol are commonly observed; sugar acids > 3C have not been observed.
GC-MS.
Two GC-MS units in electron impact mode were used for analyses: a Finnigan GCQ GC interfaced to a Finnigan GCQ ion trap mass spectrometer and an Agilent 6890N GC interfaced to an Agilent 5973 or 5975 Mass Selective Detector (quadrupole mass spectrometer). Both GCs were used in splitless mode. GC columns used were a Varian Corp. (Chrompack) Chirasil Dex-CB (25 m × 0.25 mm × 0.25 μm) or 50 m × 0.25 mm × 0.25 μm; an Agilent HP-Chiral-20B —30 m (or 50 m) × 0.25 mm × 0.2 μm. Column combinations were often used in series. For example, results in Fig. 4B were obtained with a Chiralsil Dex+HP-Chiral while separations in Fig. 4 A and C were achieved with a single Chirasil Dex. Typical GC conditions were injector temperature = 200 °C and helium flow rate constant at 1 mL/min. MS conditions were quadrupole temperature (Agilent), 150 °C; carrier gas, helium; transfer line, 180–200 °C; and electron voltage, 70 ev. In our early enantiomer analyses of the sugar derivatives, it was observed that, in some instances, when a Chirasil Dex-CB column is relatively new, the enantiomers of arabinonic acid and erythronic acid will separate. However, the most consistent enantiomer separation of these acids was eventually obtained with the use of HP chiral columns and i-Pr/TFA derivatization.
Purification of Compounds by Ion Chromatography in Preparation for Isotope Measurements.
Ion chromatography purifications of individual meteoritic compounds from mixtures were very similar to previous methods (53): A portion of a solution from the anion exchange column was filtered, dried, reconstituted with water or buffer, and injected directly into an ion chromatograph (IC) with conductivity detection (Dionex Corp.). Compounds were usually eluted with a sodium carbonate (Na2CO3)/sodium bicarbonate (NaHCO3) buffer (typically at 2 mL/min) through a 10 × 100 mm “Star Ion” column (Phenomenex Corp.). Around the retention time of a specific compound, the eluent from the IC was collected in an individual container for further processing.
Isotope Analysis.
Isotopic measurements of individual compounds were also done using procedures similar to those applied previously for isolation of organic acids from the Murchison meteorite (53). For GC-IRMS, helium flow was usually ∼1.0 mL/min. For some measurements, a Finnigan high-temperature conversion interphase III and Mat Delta+-XL IRMS were used. Oxidation was at 960 °C with a ceramic oxidation reactor bearing NiO/CuO/Pt wires. Isotopically calibrated n-alkane standards (Chiron AS) were analyzed frequently throughout the analyses to confirm the efficiency of combustion catalysts in the oxidation reactor. Data were analyzed with Finnigan ISODAT software. Standard CO2 δ13C was −41.72 ‰ vs. Vienna Pee Dee Belemnite. The δ notation expresses the isotopic ratios as follows: δ (‰) = (Rsample − Rstandard/Rstandard) × 103. Erythronic acid and threonic acid (i-Pr/TFA derivatives) were measured with a trace GC Ultra GC connected to a Delta Plus Advantage isotope ratio mass spectrometer through a GC/C-III interface. Compounds were separated on a Chirasil Dex-CB column. Typical GC-MS conditions are described above.
Degradation of Possible Sugar Contaminants.
In general, aldonic acids can be derived from the degradation of sugars; thus we subjected standard sugars, each with a specified d/l ratio, to a treatment of oxidation and alkaline hydrolysis. Among other effects, such treatments resulted in the decomposition of sugars to smaller sugar acids along homologous lines (e.g., Fig. S5). Although meteorite samples underwent significantly milder treatment during preparation (see Materials and Methods), our intent was to observe how the degradation product profiles of potential contaminant sugars compare with meteorite samples. The treatments included all 5C and 6C aldoses and fructose. Fig. S6 gives examples of the GC-MS separation of product aldonic acids from just three such oxidations: allose, idose, and fructose. In all cases, including those not shown, glyceric acid, erythronic acid, or threonic acid are the dominant sugar acid products. In the vast majority of experiments, a small amount of the corresponding 5C and 6C acids are also formed; no 6C acid appeared in the case of fructose oxidation. In all cases, the d/l ratios of degradation products maintain, with high fidelity, the d/l ratio of the initial sugar. For example, idose containing a large l excess yields products of the same l/d ratios (Fig. S6). Oxidation of 5C sugars give analogous results; if l-arabinose, biologically abundant and therefore a potentially significant contaminant, was present and oxidized during meteorite extraction, then, out of several samples, one would expect at least some portion to show significant abundances of l-arabinonic acid, l-erythronic acid, and l-glyceric acid. This is never the case: Interior meteorite samples consistently show racemic or near-racemic glyceric acid and significant d-only EE in all other aldonic acids (Table 1). As summarized in Fig. 6, meteoritic aldonic acid EE (d) decrease with decreasing carbon number; this is clearly not the case with the products of a potential contaminant subjected to harsher conditions than meteorite extracts (e.g., Fig. S6).
Racemization of Contaminants.
Glyceric acid would appear to be the most likely aldonic acid to racemize (i.e., invert configuration, d ⇌ l) due to the possession of only one chiral carbon (Fig. 1). Therefore, consideration might be given to racemization of possible contaminant d-glyceric acid as accounting for the racemic glyceric acid mixtures observed in the present meteorite samples (Fig. 2 and Fig. S1). However, in the laboratory, even after several months at temperatures of 22−70 °C under alkaline conditions (aqueous Na2CO3), we observed no racemization of this acid. It should be noted that the pH of such solutions (∼11) is higher than that of the original meteorite parent body, as evidenced by the pH of typical meteorite water extracts (∼7–9.5), and such treatments are also much harsher than those of the present meteorite extractions (see Materials and Methods), further lessening the chances of racemization during sample preparation. It is also possible that sugar contaminants might racemize and/or oxidize to sugar acids, e.g., d-glyceraldehyde → d+l glyceric acid, l-arabinose → l-arabinonic acid, and d-xylose → d-xylonic acid. Glyceraldehyde oxidizes more readily than larger sugars due to its inability to form a stable cyclic structure. Therefore, complete racemization/oxidation of a contaminant d-glyceraldehyde could theoretically result in a racemic mixture of glyceric acid in meteorite samples. However, the mentioned pristine profile of meteorite GRA95229 (see Discussion) and the relatively high 13C/12C ratio of glyceric acid in Murchison (Table S2) argue against significant contamination. The racemization of larger sugars has been the subject of much previous research: Results have shown that even under the most ideal laboratory reaction conditions, it is difficult to obtain the desired racemization products (epimers) in significant yields (20). Under the present gentle and fast sample extraction conditions (see Materials and Methods), such side reactions are expected to be insignificant. In contrast to glyceric acid, the meteoritic 4C, 5C, and 6C sugar acids are diastereomers, i.e., they contain more than one chiral center (Fig. 1). If reaction conditions are sufficiently alkaline (but not excessively), these compounds can undergo racemization (or “epimerization”), predominately at carbon #2 (Fig. S7). Therefore, theoretically, the most likely contaminant sugar acids could have epimerized to the most structurally similar meteoritic isomers, e.g., d-xylonic acid → d-lyxonic acid or l-arabinonic acid → l-ribonic acid. However, much more harsh and/or prolonged periods of treatments are required for significant conversions of this type. We have not observed formation of epimers using standards under sample preparation conditions. Fig. S7 also illustrates why the observed EE of the 4C, 5C, and 6C acids may have originated very early in the history of these compounds: Their initial d/l ratios may have been “locked in” due to the unlikely subsequent conversion of d to l (or vice versa) of an individual compound. In each compound shown in Fig. S7, only carbon #2 can (relatively) easily invert configuration—allowing epimerization (scheme A and B). Therefore, because a “d” or “l” enantiomer is determined by the configuration at carbon #4, conversion to enantiomers is quantitatively prevented. This implies that any initial meteoritic EE of such acids with more than one chiral carbon (diastereomers) were likely to be retained, given the apparently mild parent body conditions of the present meteorites.
Acknowledgments
We thank Joseph Solvason, Rhonda Franklin, Carol Elland, and Lorna Jones for assistance with the manuscript; Mary Noe and Winslow Briggs for comments on the manuscript; and Art Weber and Scott Sandford for helpful discussions. We thank Samantha Yim, Jeremy Lanoiselee, Mastewal Abate, Novelle Kimmich, Josh Sarinana, Cynthia Aysio, Ben Fasbinder, Patricia Chang, Malika Carter, Chris Reed, and David Macon for assistance with laboratory analyses at various times and Veda Bartlow for assistance with syntheses of deoxy sugar acids. We thank Donna Kleiner and Lisa Sewell for library resources; The NASA Johnson Space Center Antarctic Meteorite Program for Antarctic meteorites; and the Arizona State University Center for Meteorite Studies, Sandra Pizzarello, and Ted Bunch for samples of the Murchison meteorite. This work was partially supported by the NASA Exobiology and Evolutionary Biology Program. A.C.R. is supported by an appointment to the NASA Postdoctoral Program at Ames Research Center administered by Universities Space Research Association through a contract with NASA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603030113/-/DCSupplemental.
References
- 1.Cody G, Alexander CMO’D. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim Cosmochim Acta. 2005;69(4):1085–1097. [Google Scholar]
- 2.Alexander CMO’D, et al. Deuterium enrichments in chondritic macromolecular material-Implications for the origin and evolution of organics, water and asteroids. Geochim Cosmochim Acta. 2010;74(15):4417–4437. [Google Scholar]
- 3.Pizzarello S, Cooper GW, Flynn GJ. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta D, Leshin LA, McSween HY Jr, editors. Meteorites and the Early Solar System II. Univ Arizona Press; Tucson, AZ: 2006. pp. 625–651. [Google Scholar]
- 4.Schmitt-Kopplin P, et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc Natl Acad Sci USA. 2010;107(7):2763–2768. doi: 10.1073/pnas.0912157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsila JE, Charnley SB, Burton AS, Glavin DP, Dworkin JP. Compound specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteorit Planet Sci. 2012;47(9):1517–1536. [Google Scholar]
- 6.Stoks PG, Schwartz AW. Basic nitrogen-heterocyclic compounds in the Murchison meteorite. Geochim Cosmochim Acta. 1982;46(3):309–315. [Google Scholar]
- 7.Callahan MP, et al. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA. 2011;108(34):13995–13998. doi: 10.1073/pnas.1106493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper G, et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature. 2001;414(6866):879–883. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 9.Ciesla FJ, Sandford SA. Organic synthesis via irradiation and warming of ice grains in the solar nebula. Science. 2012;336(6080):452–454. doi: 10.1126/science.1217291. [DOI] [PubMed] [Google Scholar]
- 10.McAdam MM, Sunshine JM, Howard KT, McCoy TM. Aqueous alteration on asteroids: Linking the mineralogy and spectroscopy of CM and CI chondrites. Icarus. 2015;245:320–332. [Google Scholar]
- 11.Bunch TE, Chang S. Carbonaceous chondrites. 2. Carbonaceous chondrite phyllosilicates and light-element geochemistry as indicators of parent body processes and surface conditions. Geochim Cosmochim Acta. 1980;44(10):1543–1577. [Google Scholar]
- 12.Pizzarello S, Schrader DL, Monroe AA, Lauretta DS. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc Natl Acad Sci USA. 2012;109(30):11949–11954. doi: 10.1073/pnas.1204865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glavin DP, Dworkin JP. Enrichment of the amino acid l-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci USA. 2009;106(14):5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper G, Sant M, Asiyo C. Gas chromatography-mass spectrometry resolution of sugar acid enantiomers on a permethylated beta-cyclodextrin stationary phase. J Chromatogr A. 2009;1216(40):6838–6843. doi: 10.1016/j.chroma.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 15.Shindia AA, El-Sherbeny GA, El-Esawy AE, Sheriff YM. Production of gluconic acid by some local fungi. Mycobiology. 2006;34(1):22–29. doi: 10.4489/MYCO.2006.34.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oró J, Tornabene T. Bacterial contamination of some carbonaceous meteorites. Science. 1965;150(3699):1046–1048. doi: 10.1126/science.150.3699.1046. [DOI] [PubMed] [Google Scholar]
- 17.Steele A, et al. Imaging of the biological contamination of meteorites: A practical assessment. Lunar Planet Sci Conf. 1999;XXX:Abstr 1321. [Google Scholar]
- 18.Martins Z, Alexander CMO, Orzechowska GE, Fogel ML, Ehrenfreund P. Indigenous amino acids in primitive CR meteorites. Meteorit Planet Sci. 2007;42(12):2125–2136. [Google Scholar]
- 19.Pizzarello S, Huang Y, Alexandre MR. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc Natl Acad Sci USA. 2008;105(10):3700–3704. doi: 10.1073/pnas.0709909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angyal S. The Lobry de Bruyn-Alberda van Ekenstein transformation and related reactions. In: Stütz A, editor. Glycoscience, Topics in Current Chemistry. Vol 215. Springer; Berlin: 2001. pp. 1–14. [Google Scholar]
- 21.Walters KR, Jr, Pan Q, Serianni AS, Duman JG. Cryoprotectant biosynthesis and the selective accumulation of threitol in the freeze-tolerant Alaskan beetle, Upis ceramboides. J Biol Chem. 2009;284(25):16822–16831. doi: 10.1074/jbc.M109.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkinshaw JH, Stickings CE, Tessier P. Biochemistry of the wood-rotting fungi: 5. The production of d-threitol (l-erythritol) by Armillaria mellea (Vahl) Quélet. Biochem J. 1948;42(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- 23.Loewus FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52(2):193–210. [Google Scholar]
- 24.Kanfer J, Ashwell G, Burns JJ. Formation of l-lyxonic and l-xylonic acids from L-ascorbic acid in rat kidney. J Biol Chem. 1960;235(9):2518–2521. [PubMed] [Google Scholar]
- 25.López MG, Feather MS. The production of threose as a degradation product from l-ascorbic acid. J Carbohydr Chem. 1992;11(6):799–806. [Google Scholar]
- 26.Khoo KH, et al. Chemistry of the lyxose-containing mycobacteriophage receptors of Mycobacterium phlei/Mycobacterium smegmatis. Biochemistry. 1996;35(36):11812–11819. doi: 10.1021/bi961055+. [DOI] [PubMed] [Google Scholar]
- 27.Valpuesta V, Botella MA. Biosynthesis of l-ascorbic acid in plants: New pathways for an old antioxidant. Trends Plant Sci. 2004;9(12):573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Partridge RD, Weiss AH, Todd D. Branched-chain carbohydrate structures resulting from formaldehyde condensation. Carbohydr Res. 1972;24(1):29–44. [Google Scholar]
- 29.Cleaves HJ., II . A hypothesis for a unified mechanism of formation and enantioenrichment of polyols and aldaric, aldonic, amino, hydroxy and sugar acids in carbonaceous chondrites. In: Egel R, Lankenau D-H, Mulkidjanian AY, editors. Origins of Life: The Primal Self-Organization. Springer; Berlin: 2011. pp. 37–55. [Google Scholar]
- 30.Kalman M, Sokolowski J, Szafranek J. Kinetics and mechanism for the epimerization of aldopentonic acid potassium-salts in aqueous alkali. J Carbohydr Chem. 1987;6(4):587–592. [Google Scholar]
- 31.Ostrowski DR, Lacy CHS, Gietzen KM, Sears DWG. IRTF spectra for 17 asteroids from the C and X complexes: A discussion of continuum slopes and their relationships to C chondrites and phyllosilicates. Icarus. 2011;212(2):682–696. [Google Scholar]
- 32.Rebsdat S, Mayer D. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2012. Ethylene Glycol. [Google Scholar]
- 33.Dickens JE, et al. Detection of interstellar ethylene oxide (c-C2H4O) Astrophys J. 1997;489(2):753–757. doi: 10.1086/304821. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda M, et al. Survey observations of c-C2H4O and CH3CHO toward massive star-forming regions. Astrophys J. 2001;560(2):792–805. [Google Scholar]
- 35.Nuevo M, Sandford SA, Cooper G. Sugar and sugar derivatives in residues produced from the UV irradiation of astrophysical ice analogs. Lunar Planet Sci Conf. 2016;XLVII:Abstr 1278. [Google Scholar]
- 36.Meinert C, et al. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science. 2016;352(6282):208–212. doi: 10.1126/science.aad8137. [DOI] [PubMed] [Google Scholar]
- 37.Schutte WA, Allamandola LJ, Sandford SA. Formaldehyde and organic molecule production in astrophysical ices at cryogenic temperatures. Science. 1993;259(5098):1143–1145. doi: 10.1126/science.11540093. [DOI] [PubMed] [Google Scholar]
- 38.Henderson BL, Gudipati MS. Direct detection of complex organic products in ultraviolet (Lyα) and electron-irradiated astrophysical and cometary ice analogs using two-step laser ablation and ionization mass spectrometry. Astrophys J. 2015;800(1):66. [Google Scholar]
- 39.Flores JJ, Bonner WA, Massey GA. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J Am Chem Soc. 1977;99(11):3622–3625. doi: 10.1021/ja00453a018. [DOI] [PubMed] [Google Scholar]
- 40.Bailey J, et al. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science. 1998;281(5377):672–674. [PubMed] [Google Scholar]
- 41.Kwon J, et al. Near-infrared circular polarization survey in star-forming regions: Correlations and trends. Astrophys J Lett. 2014;795(1):L16. [Google Scholar]
- 42.Modica P, et al. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys J. 2014;788(1):79. [Google Scholar]
- 43.Rikken GLJA, Raupach E. Enantioselective magnetochiral photochemistry. Nature. 2000;405(6789):932–935. doi: 10.1038/35016043. [DOI] [PubMed] [Google Scholar]
- 44.Raḿirez I, et al. Elemental abundances of solar sibling candidates. Astrophys J. 2014;787(2):154. [Google Scholar]
- 45.Levison HF, Duncan MJ, Brasser R, Kaufmann DE. Capture of the Sun’s Oort cloud from stars in its birth cluster. Science. 2010;329(5988):187–190. doi: 10.1126/science.1187535. [DOI] [PubMed] [Google Scholar]
- 46.Throop HB. UV photolysis, organic molecules in young disks, and the origin of meteoritic amino acids. Icarus. 2011;212(2):885–895. [Google Scholar]
- 47.Mann RK, et al. ALMA observations of the Orion proplyds. Astrophys J. 2014;784(1):82. [Google Scholar]
- 48.Kawasaki T, et al. The distribution of chiral asymmetry in meteorites: An investigation using asymmetric autocatalytic chiral sensors. Geochim Cosmochim Acta. 2006;70(21):5395–5402. [Google Scholar]
- 49.Kebukawa Y, Kilcoyne ALD, Cody GD. Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys J. 2013;771(1):19. [Google Scholar]
- 50.Goesmann F, et al. COSAC prepares for sampling and in situ analysis of cometary matter from comet 67P/Churyumov–Gerasimenko. Planet Space Sci. 2014;103:318–330. [Google Scholar]
- 51.The Meteoritical Society 2015 Meteoritical Bulletin Database. Available at www.lpi.usra.edu/meteor/. Accessed May 13, 2016.
- 52.Cooper GW, Cronin JR. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim Cosmochim Acta. 1995;59(5):1003–1015. doi: 10.1016/0016-7037(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 53.Cooper G, Reed C, Nguyen D, Carter M, Wang Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc Natl Acad Sci USA. 2011;108(34):14015–14020. doi: 10.1073/pnas.1105715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rendleman JA, Hodge JE. Complexes of carbohydrates with aluminate ion. Aldose-ketose interconversion on anion-exchange resin (aluminate and hydroxide forms) Carbohydr Res. 1979;75:83–99. [Google Scholar]
- 55.Ahmed Z. Production of natural and rare pentoses using microorganisms and their enzymes. Electron J Biotechnol. 2001;4(2):1–9. [Google Scholar]