Significance
Disease surveillance systems largely focus on infectious diseases with high mortality, whereas less severe diseases often go unreported. Using chicken pox as an example, we demonstrate that Internet queries can be used as a proxy for disease incidence when reporting is lacking. We established that Google Trends accurately reflected clinical cases in countries with surveillance, and thus population-level dynamics of chicken pox. Then, we discovered robust seasonal variation in query behavior, with a striking latitudinal gradient on a global scale. Next, we showed that real-time data-mining of queries could forecast the timing and magnitude of outbreaks. Finally, our analyses revealed that countries with government-mandated vaccination programs have significantly reduced seasonality of queries, indicating vaccination efforts mitigated chicken pox outbreaks.
Keywords: chicken pox, Internet search, disease dynamics, forecast modeling, vaccination
Abstract
Public health surveillance systems are important for tracking disease dynamics. In recent years, social and real-time digital data sources have provided new means of studying disease transmission. Such affordable and accessible data have the potential to offer new insights into disease epidemiology at national and international scales. We used the extensive information repository Google Trends to examine the digital epidemiology of a common childhood disease, chicken pox, caused by varicella zoster virus (VZV), over an 11-y period. We (i) report robust seasonal information-seeking behavior for chicken pox using Google data from 36 countries, (ii) validate Google data using clinical chicken pox cases, (iii) demonstrate that Google data can be used to identify recurrent seasonal outbreaks and forecast their magnitude and seasonal timing, and (iv) reveal that VZV immunization significantly dampened seasonal cycles in information-seeking behavior. Our findings provide strong evidence that VZV transmission is seasonal and that seasonal peaks show remarkable latitudinal variation. We attribute the dampened seasonal cycles in chicken pox information-seeking behavior to VZV vaccine-induced reduction of seasonal transmission. These data and the methodological approaches provide a way to track the global burden of childhood disease and illustrate population-level effects of immunization. The global latitudinal patterns in outbreak seasonality could direct future studies of environmental and physiological drivers of disease transmission.
Childhood infectious diseases continue to be a major global problem, and surveillance is needed to inform strategies for the prevention and mitigation of disease transmission. Our ability to characterize the global picture of childhood diseases is limited, because detailed epidemiological data are generally nonexistent or inaccessible across much of the world. Available data suggest that recurrent outbreaks of acute infectious diseases peak within a relatively consistent, but disease-specific, seasonal window, which differs geographically (1–5). Geographic variation in disease transmission is poorly understood, suggesting substantial knowledge gains from methods that can expand global epidemiological surveillance. Seasonal variations in host–pathogen interactions are common in nature (6). In humans, the immune system undergoes substantial seasonal changes in gene expression, which is inverted between European locations and Oceania (7). The regulation of seasonal changes in both disease incidence and immune defense is known to interact with environmental factors, such as annual changes in day length, humidity, and ambient temperature (8). Accordingly, quantification of global spatiotemporal patterns of disease incidence can help to disentangle environmental, demographic, and physiological drivers of infectious disease transmission. Furthermore, the recognition of the regional timing of outbreaks would establish the groundwork for anticipating clinical cases and, when applicable, initiating public health interventions.
Because childhood disease outbreaks are often explosive and short-lived (9), temporally rich (i.e., weekly, monthly) data are needed to understand their dynamics. Similarly, to establish the recurrent nature of outbreaks that occur at annual or multiannual frequencies, long-term data are needed. Thus, ideal disease incidence data have both high temporal resolution and breadth (i.e., frequent observations over many years). Over the past decade, the Internet has become a significant health resource for the general public and health professionals (10, 11). Internet query platforms, such as Google Trends, have provided powerful and accessible resources for identifying outbreaks and for implementing intervention strategies (12–14). Research on infectious disease information-seeking behavior has demonstrated that Internet queries can complement traditional surveillance by providing a rapid and efficient means of obtaining large epidemiological datasets (13, 15–18). For example, epidemiological information contained within Google Trends has been used in the study of rotavirus, norovirus, and influenza (14, 15, 17, 18). These tools offer substantial promise for the global monitoring of diseases in countries that lack clinical surveillance but have sufficient Internet coverage to allow for surveillance via digital epidemiology.
Here, we focused on one common childhood disease, chicken pox, as a study system because it would allow us to validate Internet query data using clinical data from the small number of geographically distinct countries that report cases, and to address the impact of varicella zoster virus (VZV) vaccination on outbreaks. Chicken pox, a highly contagious disease caused by VZV, has low mortality but exceptionally high morbidity, with most unvaccinated children infected by the age of 15 y in developed countries (19, 20). The burden of VZV extends beyond chicken pox, because a VZV infection causes fluid-filled blisters, which eventually burst, creating the opportunity for infection from various invasive bacterial pathogens (e.g., group A streptococcal infections) (21). Chicken pox is not included in the WHO global monitoring system for vaccine-preventable diseases (22), meaning there are few countries that report clinical cases. In the United States, a country that immunizes against VZV, chicken pox was historically a notifiable disease. A lapse in national surveillance in 1981–2001 compromised the ability of researchers to examine the long-term disease dynamics and the impact of immunization (3, 23, 24). Although the clear symptomatology of chicken pox makes the disease readily observable at the individual level, the lack of reporting makes VZV transmission dynamics cryptic at the population level and obscures its spatiotemporal patterns.
The VZV vaccine is on the WHO list of essential medicines, which specifies the most important medicines needed for basic health systems (25), and is available as either the stand-alone VZV vaccine or the measles, mumps, rubella, and varicella vaccine. However, the United States, Germany, Canada, Uruguay, Australia, and regions of Spain and Italy are among the few locations that have included VZV vaccination in their childhood immunization schedules for multiple years (26–30). Short-term surveillance studies in select locations of the United States have demonstrated that moderate levels of vaccine coverage were able to reduce chicken pox incidence dramatically (31, 32), partially through the effect of herd immunity (33). However, the effects of VZV vaccination on morbidity and mortality remain poorly understood (29) because global chicken pox report rates are low [e.g., the US rate is estimated to range from to 20% (34)]. Therefore, the public health community is faced with a scarcity of chicken pox data to inform VZV vaccination policy. In certain locations (e.g., Madrid), VZV immunization has ceased, possibly due to the lack of information regarding the effects of immunization that can be used to assess health gains and economic feasibility. Clearly, there remains a lack of research, especially in countries that have recently introduced the VZV vaccine into the childhood immunization schedule (29, 35).
In this study, we took advantage of the extensive data available in Google Trends to study the global seasonal transmission of chicken pox, using the following procedures:
-
i)
We data-mined chicken pox information-seeking behavior using language-specific Google queries of “chicken pox” from 36 countries spanning five continents over an 11-y period (SI Appendix, Table S3), and characterized the seasonality of outbreaks.
-
ii)
We then validated Google Trends data using detailed time series of clinical cases from five countries within four continents.
-
iii)
We confirmed that statistical models have a profound ability to forecast outbreaks in unvaccinated and vaccinated populations.
-
iv)
Finally, we verified the impact of VZV vaccination on the seasonality of chicken pox outbreaks.
Seasonality of Chicken Pox Information Seeking and Validation
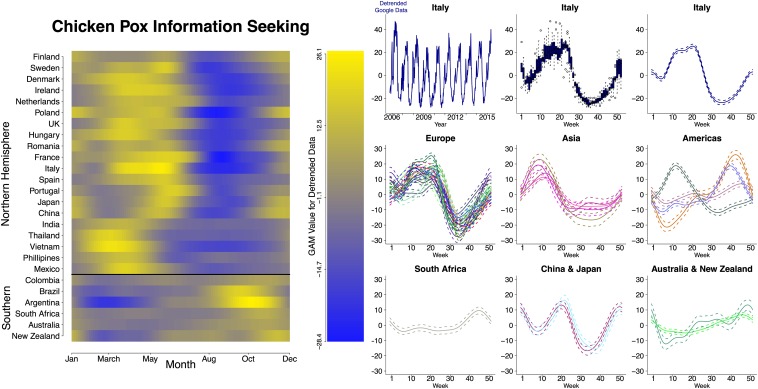
We detected significant seasonality of the Google Trends data (36) in 27 of the 34 countries for which weekly data were available. Each geographic region in our study displayed distinct seasonal patterns of information-seeking behavior (Fig. 1). A strong latitudinal pattern was clearly discernible (Fig. 1), with peaks early in the year in the Northern Hemisphere and later in the Southern Hemisphere, corresponding to springtime outbreaks worldwide. These spring peaks agree with the seasonal timing found in historical datasets and previous studies of chicken pox (1, 3, 37–39) (SI Appendix, Fig. S8). European countries were mostly unimodal, with a peak in March–May, but some had an additional smaller peak in late December. Several countries in the Southern Hemisphere, including Australia, New Zealand, South Africa, and those countries located in South America, had a single discernible peak at various times of the year. China and Japan had bimodal peaks, which occurred in March–May and December–January, balanced by deep summer troughs occurring in July–August. Other Asian countries had a single peak that occurred in February–March, punctuated by a relatively shallow trough.
Fig. 1.
(Left) Global seasonality of chicken pox outbreaks measured using Google Trends as a proxy for chicken pox dynamics. Countries are organized by geographic region and latitude. Latitudinal variation in seasonal chicken pox information-seeking behavior was observed for countries with wavelet-confirmed significant seasonality. The seasonality was estimated by fitting a general additive model (GAM) to the detrended Google data from each country. GAM values using week number as the predictive variable for Google data are shown in the heat map and correspond to the GAM curves to the right. (Right) Data processing and regional GAM values. (Top Row) Data processing steps: Detrended Google data for Italy (Top Left) and box-and-whisker plot of Google data for Italy (Top Center) in which the first to third quartiles are shown in solid color with whiskers representing 95% confidence intervals. All other panels represent GAM values using week number as the predictive variable for Google data in each country. European countries include Finland, Sweden, Denmark, Ireland, Netherlands, Poland, the United Kingdom, Hungary, France, Romania, Italy, Spain, and Portugal. Asian countries include Vietnam, India, Thailand, and the Philippines. Americas include Mexico (with a peak in week 10), Colombia, Brazil, and Argentina.
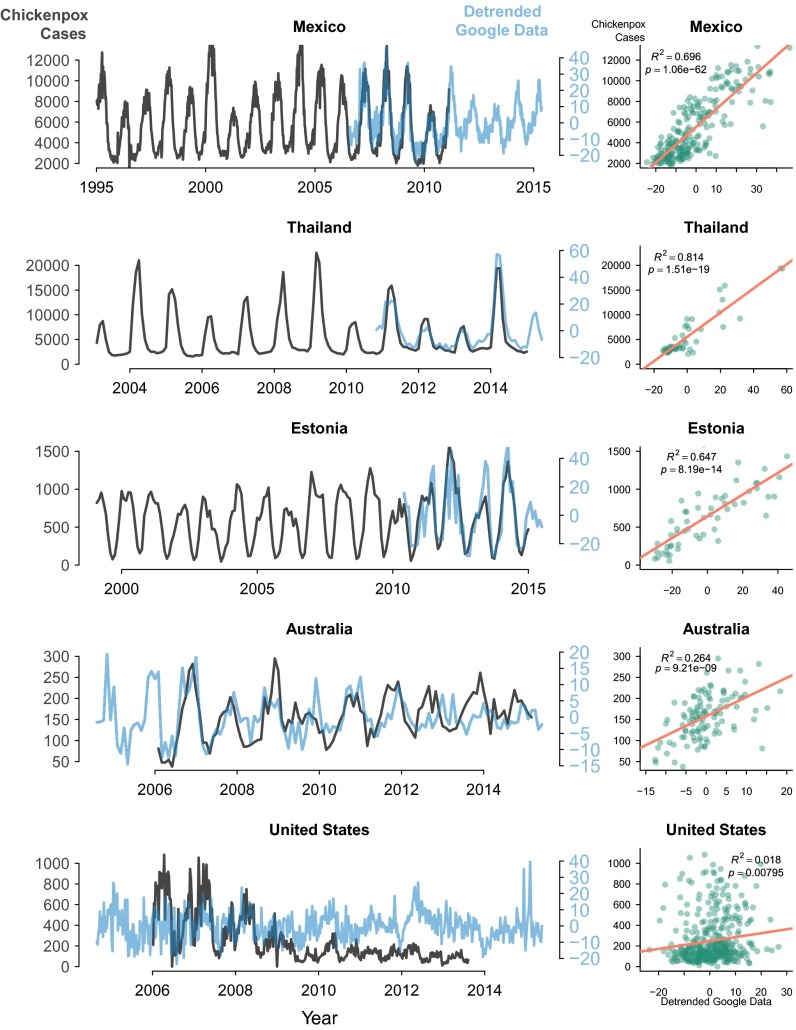
To validate Google Trends as a reasonable proxy for chicken pox dynamics, we compared clinical data with Google Trends data from five countries. We found that in the three countries lacking VZV vaccination (Mexico, Thailand, and Estonia), chicken pox information seeking was significantly correlated with reported cases of chicken pox, with 0.70, 0.81, and 0.65, respectively (Fig. 2). The correlation was reduced, but still significant, in Australia ( 0.26), which implemented nationwide immunization in 2005. In the United States, which has actively vaccinated since 1995, the association between information seeking and reported chicken pox cases was low ( 0.018). To understand the patterns in these countries better, we investigated the context of the language-specific Google searches of chicken pox. We compared the context of top searches containing the term chicken pox to determine whether they were searches for disease, symptoms, or treatment (i.e., indicative of disease in the household/community) or for vaccination (i.e., not necessarily related to disease incidence).
Fig. 2.
Relationship between chicken pox cases and information seeking. (Left) Time series of reported chicken pox cases and information-seeking behavior for chicken pox (i.e., Google Trends data) in Mexico, Thailand, Estonia, Australia, and the United States. Google data were detrended to remove long-term trends and focus on seasonal variation in information seeking. (Right) Relationship between reported cases of chicken pox and chicken pox information seeking when both were available, with applicable and P values. Chicken pox case data from Mexico and the United States were weekly, whereas chicken pox case data from Thailand, Australia, and Estonia were monthly.
The proportion of searches related to disease, rather than chicken pox vaccination or other search contexts, differed between focal countries. In Mexico and Thailand, which do not vaccinate, we classified the relative frequency of the top searches, indicating disease, to be 0.82 and 0.80 (SI Appendix, Fig. S7). In both Australia and the United States, where the VZV vaccine is required, vaccination and other search contexts had a higher relative frequency, whereas disease indicators had a frequency of 0.71 and 0.66, respectively. Interestingly, in the United States, the second highest query involved chicken pox vaccination (SI Appendix, Table S2). Estonia had a low search volume, and the top searches were not available.
These data indicate the value of adding further layers to search terms. By doing so, we identified two patterns in information seeking: (i) the data reflected seasonal dynamics in most countries (Mexico, Thailand, Estonia, and Australia), and (ii) in the United States, where vaccination has long been introduced, the data reflected a shift in the motivation of information seeking. These findings suggest that Google Trends reflect chicken pox dynamics more closely in countries that do not vaccinate vs. countries that do vaccinate, and highlight the dynamic nature of information seeking. Country-specific differences in chicken pox search context likely explains why chicken pox information seeking in Mexico, Thailand, Estonia, and Australia, but not in the United States, strongly reflected reported cases.
Forecasting Outbreaks Using Google Trends
To determine whether the information-seeking behavior observed in Google data, , was able to forecast chicken pox outbreak magnitude and timing, we built and fitted eight different statistical models to forecast chicken pox case data. We evaluated the epidemiological information contained in Google Trends by comparing these Google Trends models with a seasonal null model that did not incorporate Google data (SI Appendix, Figs. S1 and S2 and Table S1). Three models that included the Google data fit better than the null model; however, we focused on the model with the best fit, and refer to it as the Google model hereafter. The null model lacked information seeking in the force of infection parameter, which we defined as the monthly per capita rate at which children aged 0–14 y are infected. To estimate the number of symptomatic VZV infections per month, we multiplied the force of infection with an estimate of the population aged 0–14 y (SI Appendix, Eq. 1). Both the Google and null models were fitted to the case data from a VZV-unvaccinated population (Thailand), which showed robust seasonality, and a VZV-vaccinated population (Australia), which exhibited reduced seasonality. To estimate the number of symptomatic VZV infections each month, , we used Google Trends data from the previous 2 months, and , where t is time in monthly time steps. The chicken pox process model tracked the force of infection, ,
| [1] |
The model also contained environmental stochasticity, , which was drawn from a gamma distribution with a mean of 1 and variance θ. We estimated six parameters for the model: the mean and the phase of the seasonality ( and ω), a parameter scaling the Google Trends data (), the baseline force of infection (), the process noise dispersion parameter (θ), and the reporting dispersion parameter (τ) of a normal distribution, with a mean of 1, from which case reports were drawn. The parameters were estimated using maximum likelihood by iterated particle filtering in the R-package pomp (40, 41).
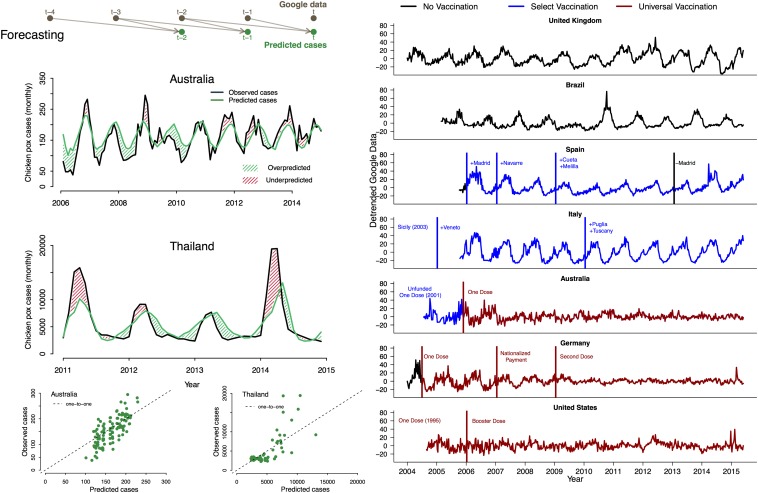
The Google Trends model fit the case data (SI Appendix, Fig. S1) and performed better than the null model (which estimated five parameters) for both Australia and Thailand; null model Akaike information criteria (AICs) were units above Google Trends model AICs in both locations. Because each model was seasonally forced, all models captured the seasonal timing of outbreaks. However, the Google Trends model was able to predict the interannual variation in outbreak size (Fig. 3), whereas the null model could not (SI Appendix, Fig. S1).
Fig. 3.
(Left) Forecasting chicken pox cases using Google Trends. (Top Left) Forecasting model schematic, Google Trends data from months and are used to predict chicken pox cases in month t. (Middle Left) Observed and predicted chicken pox cases in Australia (active immunization) and Thailand (no immunization) from 10,000 simulations of the fitted models parameterized with the maximum likelihood estimates; overpredicted (green hash marks) and underpredicted (red hash marks) regions are indicated. (Bottom Left) Model predicted cases vs. observed chicken pox cases along the dotted 1-to-1 line. (Right) Detrended chicken pox information seeking in relation to immunization. Data are weekly; x axes indicate time, and y axes are the detrended Google data (same scale for all panels). Countries with universal (national) immunization are highlighted in red, countries with select (regional or municipal) immunization are highlighted in blue, and countries lacking any mandatory immunization are highlighted in black. (Panels 1 and 2, starting from the top) The United Kingdom and Brazil, two countries with no immunization. (Panels 3 and 4) Spain and Italy, two countries with no universal (national) immunization, but with select regional or municipal immunization. Vertical lines identify the implementation (blue for select, red for national) or termination (black) of immunization efforts. Cities and regions in these panels indicate where these efforts were focused. (Panels 5 and 6) Australia and Germany, two countries that implemented national immunization in 2004. Australia has had the vaccine since 2001, but nationwide immunization was not funded by the government until November 2005. Germany required a single dose for every child in July 2004, provided nationalized payment in 2007, and required a second dose in 2009. (Panel 7) The United States, which has had national immunization since 1995, required a booster dose in 2006.
The Signature of VZV Immunization
We investigated the signature of VZV immunization by examining Google Trends data in countries that actively immunize and countries that do not. Seasonality of information-seeking behavior was much stronger in countries lacking active immunization programs than in countries that include the VZV vaccine as part of the childhood immunization schedule (Fig. 3). Germany, which made the VZV vaccine mandatory in July 2004 (27, 42), had weakening seasonality in information seeking until 2009, when a second VZV booster dose was added to the immunization schedule, drastically reducing information-seeking seasonality (SI Appendix, Figs. S5 and S6). In Australia, where the VZV vaccine was publicly funded in November 2005 (43), the amplitude of information seeking was severely dampened by the end of 2007. In the United States, immunization began in 1995 (31), and Canada required the vaccination starting in 2000 (44). In these two countries, where the VZV vaccine introduction predated Google Trends data, little seasonality was observed in the Google Trends data (Fig. 3 and SI Appendix, Fig. S9). In Spain and Italy, where only a few regions or municipalities require VZV immunization (29), minimal change was observed in the Google Trends dynamics following immunization implementation. However, in Spain, there was a reduction in search amplitude when immunization efforts were at their maximum, likely indicating a reduction in searches following immunization, similar to Germany (SI Appendix, Fig. S6).
Because information-seeking behavior strongly correlates with seasonal outbreaks of chicken pox (Fig. 2), the loss of information seeking seasonality in countries that immunize can signal the loss of recurrent seasonal chicken pox outbreaks, indicating outbreak mitigation driven by VZV immunization. We suggest that if (i) disease transmission is seasonal, as it is for chicken pox and other childhood diseases, and (ii) vaccination reduces disease transmission, then the impact of immunization can be measured as the reduction in seasonal outbreak amplitude. This assumption is justified because vaccination will strongly diminish the transmission rate during the high-transmission season. In the case of chicken pox, the reduction of seasonality in information seeking is likely due to diminished outbreak seasonality seen in clinical data (Fig. 2 and SI Appendix, Fig. S8) and the subsequent shift of information seeking from disease queries to vaccination queries.
Discussion
In this study, we used digital epidemiology of chicken pox to (i) reveal previously unreported seasonal outbreaks on a global scale, which displayed robust latitudinal dependence; (ii) confirm the reliability of the Google data against known clinical cases; (iii) forecast the size of annual outbreaks; and (iv) uncover the population-level effects of routine VZV immunization. The lack of contemporary reporting, due to the relatively benign nature of infections and the increased use of immunization, has made it difficult to decipher modern VZV global epidemiology. Here, we established that information-seeking behavior can be applied to reveal the underlying epidemiology of a childhood disease, chicken pox.
Our analyses reveal profound global patterns of seasonality in chicken pox transmission dynamics. These seasonal patterns are spatially structured: We have demonstrated a latitudinal pattern in the timing of outbreaks, with inverted phases between the Southern Hemisphere and Northern Hemisphere and an apex in the spring. Evidence of the underlying biological basis for seasonality in chicken pox transmission remains an open question. There is a significant latitudinal shift (i.e., near 6 months) in chicken pox outbreak timing from the Northern Hemisphere to the Southern Hemisphere, which suggests an influence of environmental, biological, and/or behavioral drivers that vary with latitude, such as seasonal immunity, environmental factors, and/or school terms. Interestingly, Google Trends also revealed seasonal variation in croup; fifth disease; and hand, foot, and mouth disease; each of these childhood diseases exhibited a unique annual peak with little overlap in their seasonal window of outbreak occurrence. The lack of synchrony among childhood diseases likely indicates that school terms and holidays are not the primary drivers determining outbreak timing (SI Appendix, Fig. S3). We speculate that seasonal information-seeking behavior linked to childhood illnesses reflects biologically based seasonality of host–pathogen interactions (6, 7). Our present data open new possibilities for extensive global analyses, which could disentangle contributions of different seasonal drivers to a broad range of infectious diseases. There is a pressing need for such knowledge because global seasonality is becoming rapidly modified and disrupted through human action, with potentially far-reaching implications for infectious disease transmission (45).
By taking advantage of freely available, real-time Internet search query data, we were able to validate information-seeking behavior as an appropriate proxy for otherwise cryptic chicken pox outbreaks and use those data to forecast outbreaks 1 month in advance. Our modeling approach, which incorporated Google Trends and the knowledge of spring peaks, was better able to forecast outbreaks than models that ignored Google Trends. Although the added value of incorporating Google Trends into the model was particularly clear for Thailand, which does not immunize against VZV, it also held for Australia, a country that vaccinates. These results suggest that information seeking can be used for rapid forecasting when the reporting of clinical cases is unavailable or too slow.
Comparisons of Google Trends data with the reported cases in countries that lacked VZV immunization revealed a significant positive relationship (70%, 81%, and 65% of variation in reported cases explained by variation in Google Trends). However, the relationship significantly decreased in countries that included VZV vaccination in their childhood immunization schedule and displayed either no seasonality or low-amplitude seasonal cycles (e.g., 1.8% in the United States, 26% in Australia). Interestingly, in Italy and Spain, where the VZV vaccine was only required in specific regions or municipalities of the country, no change in seasonal information-seeking behavior was detected in the face of vaccination, implying that widespread immunization is necessary to mitigate seasonal cycles of disease and information seeking. These findings, particularly from the highly vaccinated countries in our data (the United States and Australia) indicate that immunization programs diminish seasonal information-seeking behavior and likely represent decreased seasonality of outbreaks.
Studies of disease transmission at the global level, and the success of interventions, are limited by data availability. Disease surveillance is a major obstacle in the global effort to improve public health, and is made difficult by underreporting, language barriers, the logistics of data acquisition, and the time required for data curation. We demonstrated that seasonal variation in information seeking reflected disease dynamics, and as such, we were able to reveal global patterns of outbreaks and their mitigation via immunization efforts. Thus, digital epidemiology is an easily accessible tool that can be used to complement traditional disease surveillance, and may be the only readily available data source for studying seasonal transmission of nonnotifiable diseases in certain instances. We focused on chicken pox and its dynamics to demonstrate the strength of digital epidemiology for studying childhood diseases at the population level, because VZV is endemic worldwide and the global landscape of VZV vaccination is rapidly changing. Unfortunately, there is still a geographic imbalance of data sources: The vast majority of digital epidemiology data are derived from temperate regions with high Internet coverage. However, because many childhood diseases remain nonnotifiable throughout the developing world, digital epidemiology provides a valuable approach for identifying recurrent outbreaks when clinical data are lacking. It remains an open challenge to extend the reach of digital epidemiology to study other benign and malignant diseases with underreported outbreaks and to identify spatiotemporal patterns, where knowledge about the drivers of disease dynamics is most urgently needed.
Materials and Methods
Google Trends data (Dataset S1) for the language-specific search term for chicken pox were downloaded and tested for seasonality. These data were then compared against reported cases of chicken pox in countries where case reports were available. We then constructed and tested multiple statistical models to determine whether Google Trends data could forecast chicken pox seasonality. Finally, we examined the effect of national immunization campaigns on the seasonal amplitude of Google searches. Further methodological descriptions are included in SI Appendix. This study was done with freely available, deidentified, preexisting data (Datasets S2–S6); thus, no consent was required.
Supplementary Material
Acknowledgments
We thank Fernando Gonzalez-Dominguez and Gilberto Vaughan for providing the chicken pox case reports from Mexico and the Estonia Health Board, Department of Communicable Disease Surveillance and Control, for Estonian chicken pox case reports. K.M.B. would like to thank Mercedes Pascual, the members of her laboratory, and Marisa Eisenberg for helpful comments. Jesus Cantu (Princeton University) translated and categorized chicken pox searches from Mexico, Thailand, Australia, and the United States.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523941113/-/DCSupplemental.
References
- 1.London WP, Yorke JA. Recurrent outbreaks of measles, chickenpox and mumps. I. Seasonal variation in contact rates. Am J Epidemiol. 1973;98(6):453–468. doi: 10.1093/oxfordjournals.aje.a121575. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf CJE, Bjornstad ON, Grenfell BT, Andreasen V. Seasonality and comparative dynamics of six childhood infections in pre-vaccination Copenhagen. Proc R Soc Lond B Biol Sci. 2009;276(1676):4111–4118. doi: 10.1098/rspb.2009.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Panhuis WG, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med. 2013;369(22):2152–2158. doi: 10.1056/NEJMms1215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altizer S, et al. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9(4):467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 5.Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proc Biol Sci. 2006;273(1600):2541–2550. doi: 10.1098/rspb.2006.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Bakker M, Helm B. The influence of biological rhythms on host-parasite interactions. Trends Ecol Evol. 2015;30(6):314–326. doi: 10.1016/j.tree.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Dopico XC, et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson TJ, Prendergast BJ. Photoperiodic time measurement and seasonal immunological plasticity. Front Neuroendocrinol. 2015;37:76–88. doi: 10.1016/j.yfrne.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeling M, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton Univ Press; Princeton, NJ: 2008. [Google Scholar]
- 10.Higgins O, Sixsmith J, Barry M, Domegan C. 2011. A Literature Review on Health Information Seeking Behaviour on the Web: A Health Consumer and Health Professional Perspective, European Centre for Disease Prevention and Control Technical Report, Stockholm (ECDC, Stockholm, Sweden). Available at ecdc.europa.eu/en/publications/Publications/Literature%20review%20on%20health%20information-seeking%20behaviour%20on%20the%20web.pdf. Accessed June 2, 2015.
- 11.Brownstein JS, Freifeld CC, Madoff LC. Digital disease detection--harnessing the Web for public health surveillance. N Engl J Med. 2009;360(21):2153–2155, 2157. doi: 10.1056/NEJMp0900702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryden J, Funk S, Jansen VA. Word usage mirrors community structure in the online social network Twitter. EPJ Data Science. 2013;2(1):1–9. [Google Scholar]
- 13.Salathé M, et al. Digital epidemiology. PLOS Comput Biol. 2012;8(7):e1002616. doi: 10.1371/journal.pcbi.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulth A, Rydevik G, Linde A. Web queries as a source for syndromic surveillance. PLoS One. 2009;4(2):e4378. doi: 10.1371/journal.pone.0004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaman J, Karspeck A. Forecasting seasonal outbreaks of influenza. Proc Natl Acad Sci USA. 2012;109(50):20425–20430. doi: 10.1073/pnas.1208772109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg J, et al. Detecting influenza epidemics using search engine query data. Nature. 2009;457(7232):1012–1014. doi: 10.1038/nature07634. [DOI] [PubMed] [Google Scholar]
- 17.Desai R, et al. Use of Internet search data to monitor impact of rotavirus vaccination in the United States. Clin Infect Dis. 2012;54(9):e115–e118. doi: 10.1093/cid/cis121. [DOI] [PubMed] [Google Scholar]
- 18.Desai R, et al. Norovirus disease surveillance using Google Internet query share data. Clin Infect Dis. 2012;55(8):e75–e78. doi: 10.1093/cid/cis579. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta N, Brewer J. A global perspective of the epidemiology and burden of varicella-zoster virus. Curr Pediatr Rev. 2009;5(4):207–228. [Google Scholar]
- 20.WHO 2014. Background Paper on Varicella Vaccine: SAGE Working Group on Varicella and Herpes Zoster Vaccines. Available at www.who.int/immunization/sage/meetings/2014/april/1_SAGE_varicella_background_paper_FINAL.pdf. Accessed April 20, 2015.
- 21.Vugia DJ, et al. Invasive group A streptococcal infections in children with varicella in Southern California. Pediatr Infect Dis J. 1996;15(2):146–150. doi: 10.1097/00006454-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 22.WHO 2015 Immunization surveillance, assessment and monitoring: Vaccine-preventable diseases. Available at apps.who.int/immunization_monitoring/diseases/en/. Accessed September 15, 2015.
- 23.CDC Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States 2012-13 season. MMWR Morb Mortal Wkly Rep. 2012;61(32):605–608. [PubMed] [Google Scholar]
- 24.CDC 2015 Conducting Varicella Surveillance. Available at www.cdc.gov/chickenpox/hcp/conducting-surveillance.html. Accessed September 15, 2015.
- 25.WHO 2013 WHO Model Lists of Essential Medicines. Available at www.who.int/medicines/publications/essentialmedicines/en/. Accessed September 15, 2015.
- 26.PHAC 2015 Varicella (Chickenpox). Available at www.phac-aspc.gc.ca/im/vpd-mev/varicella-eng.php. Accessed September 15, 2015.
- 27.Robert Koch-InstitutStand Empfehlungen der Stïndigen Impfkommission (STIKO) am Robert Koch-InstitutStand. Epidemiol Bull. 2004;30:235–254. German. [Google Scholar]
- 28.Siedler A, Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 2010;15(13):19530. [PubMed] [Google Scholar]
- 29.ECDC . Varicella Vaccine in the European Union. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2014. [Google Scholar]
- 30.Quian J, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997-2005. Arch Dis Child. 2008;93(10):845–850. doi: 10.1136/adc.2007.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seward JF, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287(5):606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 32.Baxter R, et al. Impact of vaccination on the epidemiology of varicella: 1995-2009. Pediatrics. 2014;134(1):24–30. doi: 10.1542/peds.2013-4251. [DOI] [PubMed] [Google Scholar]
- 33.Clements DA, Zaref JI, Bland CL, Walter EB, Coplan PM. Partial uptake of varicella vaccine and the epidemiological effect on varicella disease in 11 day-care centers in North Carolina. Arch Pediatr Adolesc Med. 2001;155(4):455–461. doi: 10.1001/archpedi.155.4.455. [DOI] [PubMed] [Google Scholar]
- 34.CDC Evaluation of varicella reporting to the National Notifiable Disease Surveillance System–United States, 1972-1997. MMWR Morb Mortal Wkly Rep. 1999;48(03):55–58. [PubMed] [Google Scholar]
- 35.CDC 2015 Infectious Diseases Related to Travel. Available at wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/varicella-chickenpox. Accessed September 9, 2015.
- 36.Google 2015 Google Trends. Available at https://www.google.com/trends/. Accessed April 20, 2015.
- 37.Australian Government: Department of Health Surveillance of viral pathogens in Australia: Varicella. Commun Dis Intell. 2002;26(4):576–580. [PubMed] [Google Scholar]
- 38.Seward J, Galil K, Wharton M. Epidemiology of Varicella. Cambridge Univ Press; Cambridge, UK: 2000. pp. 187–205. [Google Scholar]
- 39.Vergara-Castañeda A, et al. Epidemiology of varicella in Mexico. J Clin Virol. 2012;55(1):51–57. doi: 10.1016/j.jcv.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 40.King AA, Nguyen D, Ionides EL. Statistical inference for partially observed Markov processes via the R package pomp. J Stat Softw. 2015;69:1–43. [Google Scholar]
- 41.King AA, et al. 2015 pomp: Statistical Inference for Partially-Observed Markov Processes. R package, version 1.2.1.1. Available at kingaa.github.io/pomp. Accessed April 20, 2015.
- 42.Rasch G, Hellenbrand W. Germany adds varicella vaccine to the national vaccination programme. Euro Surveill. 2004;8(31):2511. [Google Scholar]
- 43.Carville KS, Riddell MA, Kelly HA. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine. 2010;28(13):2532–2538. doi: 10.1016/j.vaccine.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 44.Sweet L, Gallant P, Morris M, Halperin SA. Canada’s first universal varicella immunization program: Lessons from Prince Edward Island. Can J Infect Dis. 2003;14(1):41–44. doi: 10.1155/2003/904351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevenson T, et al. Disrupted seasonal biology impacts health, food security and ecosystems. Proc R Soc Lond B Biol Sci. 2015;282(1817):20151453. doi: 10.1098/rspb.2015.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.