Significance
Cochlear hair cells are the receptors of the inner ear, detecting sound stimuli by to-and-fro motion of their sensory hair bundle to open mechano-electrical transduction (MT) channels at the tips of the hairs. During embryonic development, mechanically sensitive ion channels appear transiently in mouse cochlear hair cells before acquisition of the normal adult MT channel. These channels are located on the hair-cell apical plasma membrane, are stretch-activated by membrane deformation, and have similar but not identical properties to normal MT channels. It will be important to establish whether the two types of channel possess the same protein core or are structurally distinct, as this may provide a clue to the functional significance of these supernumerary mechanically sensitive channels.
Keywords: hair cells, mechanotransducer channels, calcium imaging, cochlea, transmembrane channel-like protein
Abstract
Cochlear hair cells normally detect positive deflections of their hair bundles, rotating toward their tallest edge, which opens mechanotransducer (MT) channels by increased tension in interciliary tip links. After tip-link destruction, the normal polarity of MT current is replaced by a mechanically sensitive current evoked by negative bundle deflections. The “reverse-polarity” current was investigated in cochlear hair cells after tip-link destruction with BAPTA, in transmembrane channel-like protein isoforms 1/2 (Tmc1:Tmc2) double mutants, and during perinatal development. This current is a natural adjunct of embryonic development, present in all wild-type hair cells but declining after birth with emergence of the normal-polarity current. Evidence indicated the reverse-polarity current seen developmentally was a manifestation of the same ion channel as that evident under abnormal conditions in Tmc mutants or after tip-link destruction. In all cases, sinusoidal fluid-jet stimuli from different orientations suggested the underlying channels were opened not directly by deflections of the hair bundle but by deformation of the apical plasma membrane. Cell-attached patch recording on the hair-cell apical membrane revealed, after BAPTA treatment or during perinatal development, 90-pS stretch-activated cation channels that could be blocked by Ca2+ and by FM1-43. High-speed Ca2+ imaging, using swept-field confocal microscopy, showed the Ca2+ influx through the reverse-polarity channels was not localized to the hair bundle, but distributed across the apical plasma membrane. These reverse-polarity channels, which we propose to be renamed “unconventional” mechanically sensitive channels, have some properties similar to the normal MT channels, but the relationship between the two types is still not well defined.
Ion channels sensitive to mechanical deformation of the cell membrane are widely distributed in vertebrates and are integral to the function of specialized mechanoreceptors, such as those in the sensory neurons of the skin, vasculature, or inner ear. The molecular structure of these mechanically gated channels has been the subject of much recent research and speculation (1–4) because they are the last category of vertebrate ion-channel (after voltage-gated and ligand-activated channels) evading characterization. Although success was achieved by assigning piezo2 as a transduction channel in many cutaneous touch receptors (3), uncertainty persists about the composition of the mechanotransducer (MT) channel in hair cells of the inner ear (2, 4). There, the MT channels reside in the stereociliary (hair) bundle, where they are activated by deflections of the bundle toward the taller edge of the staircase, and so increasing tension in the oblique extracellular tip links connecting adjacent stereocilia. Transmembrane channel-like protein isoforms 1 and 2, TMC1 and TMC2 (5), have been suggested as candidates for the hair-cell transducer channel (6, 7) because mutations of each alters ion conduction through the MT channels (7–9). Nevertheless, it is unclear whether deleting both Tmc1 and Tmc2 completely abolishes mechanotransduction (7), or merely prevents the MT channels being targeted to the stereociliary tips, so they cannot be gated by tip-link tension (10). In Tmc1:Tmc2 double mutants, or after destruction of the tip links, the normal MT current is lost, only to be replaced by a mechanically sensitive current evoked by stimuli of reverse polarity (10–12). Whether the channels underlying these reverse-polarity currents are the same as those in unperturbed cells is uncertain (2, 13). Two questions arise about them: where are they located on the hair cell, and do they only appear under pathological circumstances, as in destruction of tip links or mutations causing loss of transduction? Here we show that during late embryonic development of the inner ear, reverse-polarity MT currents occur in both cochlear and vestibular hair cells and precede the acquisition of the normal-polarity MT current, as was described in zebrafish hair cells (14). We also show that under conditions that generate “reverse-polarity” currents, stretch-sensitive channels emerge on the apical membrane of the hair cells with properties akin to the reverse-polarity channels.
Results
Reverse-Polarity MT Currents.
Following a variety of manipulations culminating in loss of normal hair-cell transduction, a mechanically sensitive current emerges that is apparently activated by hair bundle deflections of polarity opposite to those opening MT channels in normal cells (10–12, 15, 16). Because the reverse-polarity currents are insensitive to tip-link destruction by submicromolar Ca2+ buffered with BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] (10, 12), they must be activated by an unconventional route; however, why this should require negative deflections of the hair bundle is puzzling. To investigate the origin of the unorthodox stimulus polarity, we varied the orientation of the fluid jet stimulator with respect to the bundle’s axis of symmetry (Fig. 1). With the conventional mode of stimulation, when the fluid jet was placed on the modiolar side, the outward phase of the sinusoidal fluid flow pushed the V-shaped bundle of the outer hair cells (OHC) toward its vertex to activate the normal MT current, but after exposure to BAPTA the current was now activated by inward fluid flow, thus sucking on the apical membrane and bundle (Fig. 1A). Here, the current responded to the reverse polarity of bundle motion. When the fluid jet was repositioned on the opposite side of the bundle (Fig. 1B), the normal MT current was now evoked by inward fluid flow, pulling the V-shaped bundle toward its vertex; but after BAPTA treatment, the reverse-polarity current was still activated by inward fluid flow, again sucking the apical membrane. When the fluid jet was positioned orthogonally to the bundle’s optimal axis (Fig. 1C), the normal MT current occurred on both the inflow and outflow of the fluid jet, each generating a component that would deflect one or other wing of the V-shaped hair bundle toward its apex. However, after BAPTA treatment, the OHC only responded to inward fluid flow, which sucked on the apical membrane. In this condition, as with the other reverse-polarity responses (e.g., Fig. 1A, 4 min after BAPTA), there was sometimes a residual normal-polarity current but this component was always less than one-tenth of the reverse polarity, and moreover disappeared with time (Fig. 1, 30-min and 35-min traces). Similar results were seen in five other OHCs following exposure to submicromolar extracellular Ca2+ buffered with BAPTA. The MT current was 0.98 ± 0.14 nA before BAPTA and 0.58 ± 0.25 nA reverse polarity after BAPTA (mean ± SD, n = 10) when stimulating from the neural side; 0.95 ± 0.11 nA before BAPTA and 0.51 ± 0.25 nA of the same polarity after BAPTA (mean ± SD, n = 6) when stimulating from the abneural side; and 0.63 ± 0.18 nA of two-harmonic before BAPTA and 0.35 ± 0.18 nA after BAPTA (mean ± SD, n = 6) when stimulating orthogonally. Similar results with respect to stimulus polarity were also seen in OHCs from Tmc1:Tmc2 double mutants, with reverse-polarity current amplitudes of 0.85 ± 0.33 nA (mean ± SD, n = 11). A conclusion from these experiments is that the reverse polarity is only opposite to the polarity before BAPTA if the bundle is deflected from its neural side, and general use of the term is misleading. Indeed, the results imply that reverse-polarity channels are activated by suction irrespective of where the stimulus is applied.
Fig. 1.
Reverse-polarity MT currents evoked by suction of hair-cell apical membrane. (A) With the fluid jet on the modiolar side of the OHC hair bundle, positive fluid flow displaces the bundle toward its taller edge evoking MT current in control. After BAPTA exposure, reverse-polarity current appears on the opposite phase evoked by negative fluid flow. (B) With fluid jet on the kinociliary side of bundle, displacement toward its taller edge by inward fluid flow elicits MT current in control, but after BAPTA, current appears on the same phase as control. (C) With fluid jet orthogonal to the bundle’s optimal axis, control MT current has two-harmonic waveform because of alternate stimulation of each wing of the V-shaped bundle but after BAPTA, current still evoked by negative fluid flow. Top trace is displacement monitor, positive denoting outward fluid flow; holding potential −84 mV.
Stretch-Activated Channels on the Apical Membrane.
Because the reverse-polarity channels respond to bundle suction but do not require tip links, it is possible some or all are located on the OHC apical membrane around the base of the bundle. To examine this possibility, cell-attached patch recordings were made on the apical membrane. These recordings revealed the presence of stretch-activated channels in wild-type mice after BAPTA perfusion (Fig. 2) and in Tmc1:Tmc2 double mutants (Fig. 3). For BAPTA perfusion, no such channels were ever recorded before BAPTA application but, a few minutes after BAPTA, stretch-activated channels appeared and increased in number over 20 min (Fig. 2B). These channels had a conductance of 61.0 ± 5.6 pS (mean ± SD, n = 5) when the saline in the patch pipette contained 1.5 mM Ca2+ (Fig. 2C) and 94.3 ± 13.5 pS (n = 4) in 0.07 mM Ca2+ saline (Fig. 2D). The channel’s current–voltage relationship was linear, with reversal potential at 0 mV, suggesting a cation channel. A contribution from chloride was eliminated because channel events persisted when chloride was substituted by gluconate in the patch-electrode solution. Similar to the macroscopic reverse-polarity currents (10), the stretch-activated channels were blocked by Ca2+ and by FM1-43. Including 5 μM FM1-43 in the electrode solution blocked the channels at negative but not positive potentials, thus recapitulating effects on both the normal and reverse-polarity MT currents (9, 17). Stretch-activated channels with similar properties were also seen in OHCs of Tmc1:Tmc2 double mutants (Fig. 3), with slope conductance of 60.4 ± 7.8 pS (mean ± SD, n = 6) in 1.5 mM Ca2+ saline and 92.0 ± 2.0 pS (n = 4) in 0.07 mM Ca2+ saline. There was no significant difference between unitary conductances observed in Tmc1:Tmc2 double mutants or after BAPTA perfusion in either Ca2+ concentration.
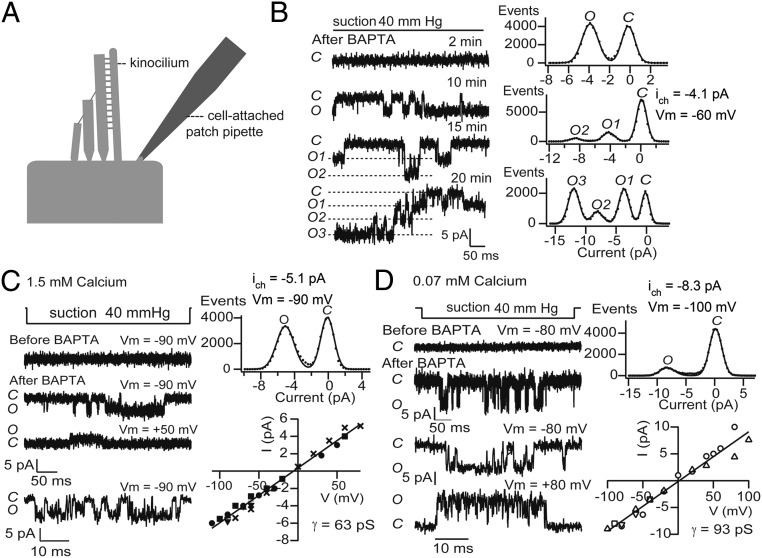
Fig. 2.
Stretch-activated channels on the OHC apical membrane. (A) Location of cell-attached patches on apical plasma membrane near insertion of kinocilium. (B) Cell-attached records at various times after exposure to BAPTA saline when stretch-sensitive channels appeared, with channel number increasing over 20 min. All records from the same cell. Amplitude histograms for 10, 15, and 20 min; holding potential, Vm = −60 mV, 1.5 mM Ca2+ in electrode solution. (C, Left) Voltage dependence of stretch-activated channels, records shown; (Right) amplitude histogram for Vm = −90 mV and current–voltage relationship for this and two other channels. Mean slope conductance, γ = 63 pS fitted to all points; 1.5 mM Ca2+ in electrode solution. (D, Left) Voltage-dependence of stretch activated channels, records; (Right) amplitude histogram for Vm = −100 mV and current–voltage relationship for this and two other channels. Mean slope conductance, γ = 93 pS; 0.07 mM Ca2+ in electrode solution. The Upper trace shows pressure monitor, 40-mmHg suction, which relates to the top records; the Lower trace records on a faster time scale were taken during the applied suction. In C and D, channels were recorded 8 and 10 min, respectively, after BAPTA application.
Fig. 3.
Stretch-activated channels in Tmc1:Tmc2 double knockouts. (A and B) Cell-attached recordings of stretch-activated channels in apical OHC membrane of Tmc1:Tmc2 mutants at (A) −80 mV and (B) +120 mV. (C) Current amplitude histograms at −80 mV and +120 mV. (D) Current–voltage relationships, mean slope conductance = 60 ± 8 pS (n = 6) in 1.5 mM Ca2+ in electrode solution. All are P5 apical OHCs.
Reverse-Polarity MT Currents During Development.
A difficulty with assigning significance to the reverse-polarity MT currents stems from them being recorded only under abnormal circumstances, whether by tip-link disruption or mutations that adversely affect mechanotransduction. Previous work had, however, suggested that this type of current may also be present constitutively during hair-cell development (10, 12, 14, 18). More detailed investigation showed that hair cells first displayed mechanical sensitivity around birth, when it was manifested as a reverse-polarity current, always preceding the normal-polarity current (Fig. 4 and Fig. S1). The reverse-polarity current was maximal in both OHCs and inner hair cells (IHCs) at postnatal day (P) 0, and subsequently declined with increase in the normal MT current. Consequently, over the first few postnatal days, the transducer current for a sinusoidal mechanical stimulus often had a two-harmonic appearance: one component attributable to the normal MT current and the other to the reverse-polarity current (Fig. 4A). In all hair-cell types, the normal MT current developed over about 2 d (time to increase from 10% to 90% of maximum = 2.3 ± 0.4 d), and was half-maximal at 0.1 d in basal OHCs, 2.5 d in apical OHCs, and 2.0 d in apical IHCs (Fig. 4 B, C, and F); the trend, in which transduction at the base of the cochlea develops sooner than at the apex, has been previously reported in both mice and rats (8, 18, 19). Decline in the reverse-polarity current had half-maximal times similar to growth of the normal MT current: 1 d in basal OHCs, 2.4 d in apical OHCs, and 1.6 d in apical IHCs. Reverse-polarity currents were also present in saccular hair cells at embryonic day (E) 16 and E17, but had virtually disappeared by P0 (Fig. S2), by which time normal transduction is fully developed (20). The reverse-polarity current was still present in Tmc1:Tmc2 double mutants when the normal-polarity current was absent (Fig. 4 D and E); it was initially large but declined to a half-maximum at 3.3 d and disappeared by P7 (10).
Fig. 4.
Reverse-polarity currents during cochlear development. (A) MT currents for sinusoidal fluid jet stimuli for P0, P2, and P5, wild-type apical OHCs. Note transformation from reverse polarity to biphasic to normal polarity with age. (B) Apical OHCs, reverse-polarity (crosses) and normal-polarity (filled circles) currents with age; mean ±1 SD, number of cells above points. (C) Basal OHCs, reverse-polarity current (crosses) and normal-polarity currents (filled circles) with age. (D) Representative MT current traces for sinusoidal fluid jet stimuli for P0, P2, and P5, Tmc1−/−:Tmc2−/−, apical OHCs. Note only reverse-polarity responses at all ages. (E) Tmc1−/−:Tmc2−/− apical OHCs, reverse-polarity and normal-polarity currents with age. (F) Apical IHCs, reverse-polarity and normal-polarity currents with postnatal age; in all experiments depicted, holding potential = −84 mV.
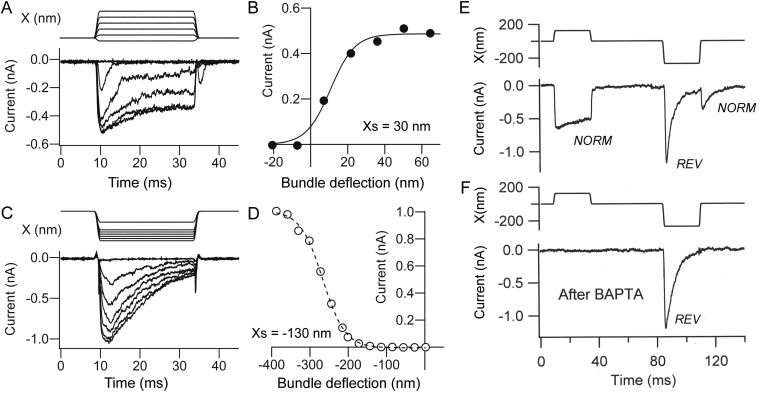
Fig. S1.
Normal-polarity and reverse-polarity currents during development. (A) Normal-polarity MT currents evoked by positive hair bundle displacements in P3 OHC. (B) Current–displacement relationship for records in A fit with single-state Boltzmann equation: I = Imax/(1 + exp(−((X − X0.5)/Xs))) where X0.5 = the half-activation displacement, Xs = rate, and Imax = maximum current; Imax = 0.749 nA, X0.5 = 11 nm, Xs = 7 nm. (C) Reverse-polarity MT currents evoked by negative hair bundle displacements in an E18 apical OHC. (D) Current–displacement relationship for records in C fit with single-state Boltzmann equation with Imax = 1.0 nA, X0.5 = −267 nm, Xs = 29 nm; holding potential = −84 mV. The bundle displacement were derived as usual from photodiode calibrations, but the immediate stimulus for the channel opening is likely not to be bundle displacement per se, but the resulting deformation of the apical membrane. The decay in the current is attributable in part to properties of the channels because the decay time constant is voltage-dependent (10). (E) Positive bundle deflections evoked normal-polarity (NORM) current, negative displacement elicit reverse-polarity current (REV) at onset and normal polarity at positive going stimulus offset. (F) After BAPTA exposure, the normal-polarity current was lost but the reverse polarity persisted. In E and F, P2 apical OHC.
Fig. S2.
Normal-polarity and reverse-polarity currents in saccular hair cells. (A) MT currents in vestibular hair cells (VHC): reverse polarity dominates at E16, and is replaced by normal-polarity current at P0. In this CD1 mouse strain, P0 corresponds approximately to E19. (B) Collected results on amplitudes (mean ± SEM) of reverse-polarity currents (crosses) and normal-polarity currents (filled triangles) as a function of developmental age. Note that by birth, reverse-polarity currents have almost disappeared.
Despite their constitutive nature, the reverse-polarity currents observed developmentally had several properties akin to those mechanically sensitive currents seen in Tmc1:Tmc2 mutants. First, they were insensitive to tip-link destruction with BAPTA, perfusion of which, in five different OHCs, converted a biphasic current into a single component (Fig. S1). Second, stretch-sensitive channels recorded on the top of the hair cell in neonatal animals had a unitary conductance of 64.1 ± 3.6 pS (mean ± SD, n = 3 OHCs) in 1.5 mM Ca2+. This conductance, measured in P0 to P1 wild-type mice at an age where the reverse-polarity current predominates, is not significantly different from stretch-sensitive channels after BAPTA (P = 0.52). Both single channels and reverse-polarity currents were unaffected by 50 μM PPADS (pyridoxalphosphate-6-azophenyl-2′4′-disulfonic acid Na4), a concentration that fully blocks P2X receptor channels (Fig. S3). Third, the reverse-polarity MT currents, like the normal polarity currents, were blocked by FM1-43 (Fig. 5 A–C). Normal polarity currents in P5 to P6 wild-type OHCs were blocked at −84 mV by FM1-43 with an IC50 of 2.8 ± 0.4 μM and Hill coefficient of 1.9 (Fig. 5D), values similar to those previously reported (17). The reverse-polarity currents in Tmc1−/−:Tmc2−/− OHCs were about 10-fold less sensitive to FM1-43 block, with an IC50 of 26 ± 2.3 μM and a Hill coefficient of 1.9, whereas the reverse-polarity currents in P1 wild-type had an IC50 of 29 ± 1.0 μM and a Hill coefficient of 2.0. Because FM1-43 is an open channel blocker, the difference in IC50 between normal- and reverse-polarity channels may partly stem from a difference in their resting open probability as argued for dihydrostreptomycin, another open MT-channel blocker (21). The clearest manifestation of this difference was seen on puffing FM1-43 onto hair bundles (Fig. 5E). In wild-type, all OHCs rapidly fluoresced because of influx of the dye through partially open MT channels. However, in the Tmc1:Tmc2 double mutant, no intracellular fluorescence was visible until the reverse-polarity channels were opened by deflecting the hair bundle.
Fig. S3.
Effects of the P2X receptor blocker PPADS (50 μM) on OHC channels and reverse-polarity currents. (A) Examples of stretch-activated channels recorded from apical plasma membrane in the presence of PPADS; below is the amplitude histogram showing single-channel current of 4.9 pA. (B) Current–voltage relationship for channels in A, giving slope conductance of 56 pS. Channels in another cell with PPADS gave slope conductance of 52 pS. (C) Superimposed MT currents from controls (black) and during perfusion with 50 μM PPADS (red) which blocks neither reverse-polarity nor the smaller normal-polarity component. All records from OHCs of P1 cochlear apex.
Fig. 5.
Effects of FM1-43 on normal- and reverse-polarity MT currents. (A) Normal-polarity MT currents in P6 wild-type OHC in the absence and presence of 3 μM FM1-43. (B) Reverse-polarity currents in P1 wild-type OHC in the absence and presence of 30 μM FM1-43. (C) Reverse-polarity MT currents in an OHC of P5 Tmc1:Tmc2 mutant in the absence and presence of 30 μM FM1-43. (D) Dose–response plots under the three conditions: normal (open circle), reverse-polarity wild-type (filled circles), and Tmc1:Tmc2 mutant (crosses) all fit with the Hill equation. Each point is the mean ± SD of 2–5 measurements. Normal-polarity points, IC50 = 2.8 μM, Hill coefficient nH = 1.9; reverse-polarity points, IC50 = 26 μM, nH = 1.8; holding potential = −84 mV in A–D. (E) Images of isolated organ of Corti after puff of FM1-43 in Tmc1:Tmc2 double mutant in unperturbed condition (Upper) and after bundle stimulation (Lower). (Magnification: 500×.) (F) Stretch-activated channels after BAPTA with 5 μM FM1-43 in the patch electrode are blocked at hyperpolarized holding potential (−80, −100 mV) but block is relieved at +150 mV.
Calcium Imaging.
Because the reverse-polarity MT channels, like the normal MT channels, are permeable to Ca2+ (10), fast confocal imaging of Ca2+ influx during mechanical stimulation was used to obtain more information about their location. This approach had previously shown the conventional MT channels to be at the tips of the shorter rows of stereocilia (22). In the present experiments on early neonates (P0–P2), diphasic steps to the hair bundle generated both normal-polarity and reverse-polarity currents on the positive and negative transitions of the stimulus, respectively (Fig. S1). Fluorescence images of hair cells filled with the Ca2+ indicator Fluo-5F were acquired with confocal sections through the middle of the hair bundle or at the cell apex (Fig. 6 A and C). Following a positive stimulus that generated a normal-polarity MT current (Fig. 6A), there was a rapid increase in fluorescence if the region of interest (ROI) was drawn over the hair bundle (Fig. 6B, position 1, red), influx of Ca2+ being triggered by hair-cell hyperpolarization to augment the electrical driving force on the ion. If the ROI was drawn outside the bundle (Fig. 6B, position 2, black), there was only a small delayed increase in fluorescence (ΔF), probably reflecting diffusion from the primary source in the bundle. In response to a negative stimulus evoking a reverse-polarity MT current (Fig. 6 C and D), an increase was observed across the apical membrane (Fig. 6C, position 2); there was no ΔF in the hair bundle (Fig. 6C, position 1) during the reverse-polarity current, only at the end of the stimulus when the positive-going displacement back to the resting position elicited a small normal-polarity current (Fig. 6C, arrow). The ratio of cell–apex fluorescence for the positive and negative stimuli in Fig. 6C was 2.5. Similar distributions of Ca2+ response were observed in one other IHC and two OHCs in which the ratio of cell–apex ΔF for the positive to negative stimuli was 6.5, 9.3, and 3.2 respectively. In another OHC, there was no normal-polarity MT current because of the young age P1, but a reverse-polarity current was present and was associated with a fluorescence increase across the top of the cell. These measurements suggest that the reverse-polarity MT channels are in the apical plasma membrane rather than confined to the hair bundle.
Fig. 6.
Ca2+ imaging during of normal- and reverse-polarity MT currents. (A) Positive fluid jet step plus diphasic change in membrane potential from HP = −50 mV elicits a normal-polarity current and a fast fluorescence increase, ΔF, on hyperpolarization at ROI-1 (region of interest 1) but not at ROI-2; plane of focus at the middle of bundle. (B) Bright-field images of the IHC bundle (arrow) focused at midbundle (Left) and the base of bundle (Right); FJ, fluid jet; schematic shows top view of IHC defining ROI-1 over the hair bundle and ROI-2 over apex membrane behind the bundle. (C) Negative fluid jet step plus diphasic change in membrane potential from HP = −50 mV elicits a reverse-polarity current and fast fluorescence increase on hyperpolarization at ROI-2 but not at ROI-1. A delayed fluorescence increase at ROI-1 occurs in response to the small normal-polarity current (arrow) at the positive-going offset of the fluid jet; plane of focus at the base of bundle. (D) Pseudocolor fluorescence intensities before (a) and during the reverse-polarity current (b), and during the normal-polarity current (c), each being the average of three images taken at the times indicated in C. Note the ΔF for reverse-polarity current occurs over much of cell apex (b), whereas ΔF for normal-polarity current (b) is confined to the hair bundle. All records from same apical IHC of P1 wild-type.
The small size of the Ca2+ response linked to the reverse-polarity current makes detailed analysis difficult but is to be expected. To a first approximation, the Ca2+ signal will depend on the total charge transfer, which is proportional to the product of the time-integral of the MT current and the Ca2+ permeability of the MT channel. If QR is the ratio of the charge transfers for the normal- and reverse-polarity MT currents, and FR is the ΔF ratio for the normal and reverse-polarity MT currents, then the ratio of Ca2+ permeabilities, PCaR, for the two channel types is given by PCaR = FR/QR, assuming no change in the channel’s Cs+ permeability. For the cell of Fig. 6C, the predicted PCaR was 2.52/0.7 = 3.6; the mean PCaR value for four cells (two IHCs and two OHCs) was 3.8 ± 0.5. Ca2+ selectivity values have been directly determined from measurements of reversal potentials, which gave PCa/PCs = 6.1 for apical wild-type OHCs and PCa/PCs = 1.8 for the reverse-polarity channels (8, 9), their ratio being 3.4.
Discussion
Location of Reverse-Polarity MT Channels.
Three lines of evidence indicate that the reverse-polarity MT channels (10) occur mainly in the apical plasma membrane of the OHC: stimulating the bundle from different orientations, recording stretch-activated channels and imaging Ca2+ influx. The results of varying the orientation of the fluid-jet stimulator (Fig. 1) argue that the mechanically sensitive channels seen after BAPTA treatment and in Tmc1:Tmc2 double mutants, unlike normal MT channels, do not retain sensitivity to a specific axis of hair bundle stimulation. This can be most simply explained if they are stretch-sensitive channels gated by deformation of the plasma membrane overlying the hair bundle or the cell apex around the base of the bundle. These channels should not, therefore, be referred to as “reverse-polarity” channels—which is a misnomer—but as “unconventional” mechanically sensitive channels. Ca2+ influx measurements through these unconventional mechanically sensitive channels confirmed they were in the apical membrane and not confined to the tips of the stereocilia. This notion was endorsed by recording stretch-activated channels in the apical membrane with properties similar to those of Tmc1: Tmc2 double mutants inferred from whole-cell recordings. Thus, both are cation channels with a conductance in 1.5 mM Ca2+ of ∼60 pS (9, 10), they are increased about 1.5-fold to 90 pS on reducing external Ca2+ to a level comparable to that found in endolymph (10), and they are blocked by FM1-43. Although stretch-activated cation channels have been recorded from OHCs, none exactly match the attributes seen here. Those previously reported in the OHC basolateral membrane were 150 pS K+-selective channels (23, 24), 30 pS voltage-dependent cation channels (24), and channels passing both anions and cations (25). There has been a report of ion channels on the OHC apical membrane recorded using a scanning ion conductance probe (26). OHCs possess purinergic P2X receptors on their apical and stereociliary membranes (27), but the standard P2X receptor inhibitor, PPADS (28), had no effect on the unconventional currents or channels (Fig. S3).
Unconventional MT Currents During Development.
Although the existence of unconventional MT channels after tip-link destruction or in Tmc1:Tmc2 double mutants is indisputable (10–12, 29), their physiological role and relationship to the normal MT channels gated by tension in the tip-links, is more uncertain. A possible clue is that they are present in all hair-cell classes—cochlear inner and outer, and vestibular—during maturation of the transduction machinery. We found the unconventional channels exhibit an inverse relationship to the normal MT channels: they appear in OHCs and IHCs during the late embryonic period, their density peaks around birth, and then declines with development of the normal MT current. To our knowledge, this is the first report of a systematic acquisition then disappearance of the reverse-polarity channels in wild-type animals without any genetic or chemical manipulation. Based on their properties (single-channel conductance, block by FM1-43 and Ca2+ selectivity), the unconventional MT currents recorded during development in wild-type mice and those seen under abnormal conditions (in mutants or after tip-link destruction) are manifestations of the same ion channel. Reverse-polarity MT currents were previously found to occur embryonically (12), but no subsequent pattern of development or relationship to the normal MT current was evident in that work, and it was further claimed that the reverse-polarity current persisted until at least P10, which we never saw. One hypothesis is that the unconventional channels are associated with the small microvilli that carpet the top surface of hair cells behind the hair bundle, and their transient neonatal appearances are linked. However, the currents are gone by P4, well before the microvilli disappear at P5–P7 (see supplemental figure 4B in ref. 6).
The ion channels underlying the developmental reverse-polarity MT current are unidentified, but their persistence in Tmc1:Tmc2 mutants (Fig. 4F) suggest they are not TMC isoforms. The channels’ developmental role may be to sense membrane forces distributed across the top of the hair cell, and Ca2+ influx through them is perhaps linked to proper shaping and maturation of the hair bundle, which ensues over this perinatal period (30). However, the relationship of the unconventional to the normal MT channels is unclear and it is instructive to compare their properties. In apical OHCs, both are cation channels that pass Ca2+ with PCa/PCs = ∼5.8 in normal and ∼1.5 for reverse-polarity channels; both are blocked by external FM1-43 with IC50 of ∼3 μM in normal and 26 μM in unconventional channels; the unitary conductances are similar in apical OHCs, with values in 1.5 mM Ca2+ of 63 pS in normal and 61 pS for unconventional channels. However, in basal OHCs the normal channels are about twofold larger than the reverse-polarity channels, a difference that depends on the presence of TMC1, which may contribute to an external vestibule (9, 31). In both cases the channels are blocked by extracellular Ca2+, lowering its concentration to a few tens of micromolars, increasing channel conductance by 50%. Thus, biophysical attributes of the normal-polarity and unconventional channels are similar but not identical. If the channels had the same pore, such differences could reflect the presence of other subunits in the normal channel, such as LHFPL5 and TMIE (9, 32–34). It will be important to establish whether both have the same protein core or are structurally distinct, as this may provide a clue to the functional significance of the unconventional channels.
Materials and Methods
Preparation.
MT currents were recorded from hair cells in the isolated organ of Corti and saccular macula of mice between E17 to P8 (where P0 = E19 is the birth date) using methods previously described (9, 35). Mutation in the Tmc1 gene was achieved with Tmc1Δ/Δ (B6.129-Tmc1tm1.1Ajg/J; Jackson Lab (6). The Tmc2 mutant (B6.129S5-Tmc2tm1Lex/Mmucd) from the Mutant Mouse Regional Resource Center, University of California, Davis, CA, was used to generate the double-mutants Tmc1−/−:Tmc2−/−. Mice were genotyped from tail clips taken after dissection for the electrophysiology recordings, the experimenter being blind to the genotype. Mice were killed by decapitation using methods approved by the Institutional Animal Care and Use Committees of the University of Wisconsin–Madison and Stanford University, according to current National Institutes of Health guidelines. Excised cochlear turns, low-frequency apex, or high-frequency base (∼0.2 or 0.75 of the distance along the cochlea from the apex), were immobilized under strands of dental floss in a recording chamber on a Leica DLFS fixed-stage microscope and viewed through a 63×, 0.9 NA, long working-distance water-immersion objective. The chamber was perfused with artificial perilymph of composition: 152 mM NaCl, 6 mM KCl, 1.5 mM CaCl2, 2 mM Na-pyruvate, 8 mM d-glucose, and 10 mM Na-Hepes, pH 7.4, osmolarity ∼320 mOsm/L at room temperature, 21–23 °C. Flow through a local puffer pipette (tip diameter, 8 μm) driven by a Picospritzer (General Valve) was used to control the solution around the hair bundle. In some experiments, PPADS (Sigma-Aldrich) was added to block P2X receptor channels (28).
Electrophysiology and Stimulation.
Most whole-cell recordings were made with patch electrodes filled with: 130 mM CsCl, 3 mM MgATP, 10 mM Tris phosphocreatine, 1 mM EGTA, 0.5 mM GTP, 0.5 mM cAMP, 10 mM Cs-Hepes, pH 7.2, ∼295 mOsm/L, and connected to an Axopatch 200B amplifier with 5-kHz output filter. Hair bundles were deflected with a fluid jet (8, 36), driven by a 40-Hz sinusoidal voltage or voltage step to elicit a maximal MT current. In some experiments, the resulting bundle motion was determined by projecting an image of the bundle onto a pair of photodiodes (8, 37). Single stretch-sensitive channels were obtained from cell-attached patch recordings on the hair-cell apical membrane. For these recordings, patch electrodes (resistances ∼4 MΩ) were filled with a extracellular Na-based saline containing either 1.5 mM or 0.07 mM CaCl2 and just before forming a seal, the preparation was perfused with a high K+ solution (140 mM KCl, 0.2 mM CaCl2, 8 mM d-glucose, 10 mM K-Hepes, pH 7.4) to zero the hair-cell resting potential. The resting potential after exposure to the high K+ was measured by attaining whole-cell at the end of a channel recording yielding 1.3 ± 4 mV (n = 4), confirming that the high K+ solution did zero the membrane potential. Suction was applied via the patch pipette and calibrated with a 3460 Pressure Gauge (Control Company). It was not possible to apply multiple controlled stimuli to derive the channel kinetics and therefore ascertain the presence of channel adaptation or inactivation in ensemble averages.
Ca2+ Imaging.
Changes in intracellular Ca2+ concentration were measured with the low-affinity indicator Fluo-5F (Life Technologies). The K+ salt of Fluo-5F was introduced at 0.5–1 mM via the patch pipette with ascorbic acid added as an antioxidant to minimize cell damage by free radicals generated during illumination. The pipette solution was: 95 mM CsCl, 40 mM Cs-ascorbate, 3.5 mM MgATP, 2 mM Cs-pyruvate, 5 mM Tris creatine phosphate, 1 mM EGTA, 10 mM Hepes, pH 7.2. Fluorescence emission was measured with a swept-field confocal (Prairie Technologies) illuminated with a 200-mW argon laser at 488 nm and viewed with a 60× 1.0 numerical aperture water-immersion Olympus objective, scanned at 500 Hz (22). Images were collected and analyzed using Neuroplex software (RedShirtImaging) and ImageJ, the average intensity change ΔF being calculated for small areas overlying the bundle. To eliminate pseudofluorescence changes because of movement artifacts, the mechanical stimulus was preceded by depolarization to a positive potential near the Ca2+ equilibrium potential, which was then switched negative to initiate the Ca2+ influx and associated fluorescence change while the bundle remained displaced (22). Unless otherwise stated, values are quoted as mean ±1 SD, each recording used to construct a mean being from a different animal. Statistical significance was assessed with a two-tailed Student’s t test.
Acknowledgments
The work was funded by National Institutes on Deafness and other Communication Disorders Grants DC01362 (to R.F.) and DC003896 (to A.J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.A.F. is a guest editor invited by the Editorial Board.
See Commentary on page 6594.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601067113/-/DCSupplemental.
References
- 1.Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 2.Fettiplace R, Kim KX. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev. 2014;94(3):951–986. doi: 10.1152/physrev.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87(6):1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao B, Müller U. The elusive mechanotransduction machinery of hair cells. Curr Opin Neurobiol. 2015;34:172–179. doi: 10.1016/j.conb.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurima K, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30(3):277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima Y, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest. 2011;121(12):4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan B, et al. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79(3):504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KX, Fettiplace R. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol. 2013;141(1):141–148. doi: 10.1085/jgp.201210913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol. 2014;144(1):55–69. doi: 10.1085/jgp.201411173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KX, et al. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol. 2013;142(5):493–505. doi: 10.1085/jgp.201311068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alagramam KN, et al. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One. 2011;6(4):e19183. doi: 10.1371/journal.pone.0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcotti W, et al. Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci. 2014;34(16):5505–5514. doi: 10.1523/JNEUROSCI.4086-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effertz T, Scharr AL, Ricci AJ. The how and why of identifying the hair cell mechano-electrical transduction channel. Pflügers Arch. 2015;467(1):73–84. doi: 10.1007/s00424-014-1606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindt KS, Finch G, Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell. 2012;23(2):329–341. doi: 10.1016/j.devcel.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalski N, et al. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27(24):6478–6488. doi: 10.1523/JNEUROSCI.0342-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepanyan R, Frolenkov GI. Fast adaptation and Ca2+ sensitivity of the mechanotransducer require myosin-XVa in inner but not outer cochlear hair cells. J Neurosci. 2009;29(13):4023–4034. doi: 10.1523/JNEUROSCI.4566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21(18):7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27(50):13890–13902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lelli A, Asai Y, Forge A, Holt JR, Géléoc GSG. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol. 2009;101(6):2961–2973. doi: 10.1152/jn.00136.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Géléoc GSG, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;6(10):1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricci A. Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol. 2002;87(4):1738–1748. doi: 10.1152/jn.00574.2001. [DOI] [PubMed] [Google Scholar]
- 22.Beurg M, Fettiplace R, Nam J-H, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12(5):553–558. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasa KH, Li MX, Jia M, Kachar B. Stretch sensitivity of the lateral wall of the auditory outer hair cell from the guinea pig. Neurosci Lett. 1991;133(2):171–174. doi: 10.1016/0304-3940(91)90562-8. [DOI] [PubMed] [Google Scholar]
- 24.Ding JP, Salvi RJ, Sachs F. Stretch-activated ion channels in guinea pig outer hair cells. Hear Res. 1991;56(1-2):19–28. doi: 10.1016/0378-5955(91)90149-4. [DOI] [PubMed] [Google Scholar]
- 25.Rybalchenko V, Santos-Sacchi J. Cl- flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol. 2003;547(Pt 3):873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frolenkov GI, et al. Single-channel recordings from the apical surface of outer hair cells with a scanning ion conductance probe. Assoc Res Otolaryngol Abstr. 2004;27:150. [Google Scholar]
- 27.Housley GD, et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: Implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19(19):8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 29.Kurima K, et al. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Reports. 2015;12(10):1606–1617. doi: 10.1016/j.celrep.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485(1):75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- 31.Beurg M, Xiong W, Zhao B, Müller U, Fettiplace R. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci USA. 2015;112(5):1589–1594. doi: 10.1073/pnas.1420906112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong W, et al. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151(6):1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, et al. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron. 2014;84(5):954–967. doi: 10.1016/j.neuron.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleason MR, et al. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci USA. 2009;106(50):21347–21352. doi: 10.1073/pnas.0911632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci. 2006;26(43):10992–11000. doi: 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc Biol Sci. 1992;249(1325):185–193. doi: 10.1098/rspb.1992.0102. [DOI] [PubMed] [Google Scholar]
- 37.Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]