Significance
Despite numerous publications reporting the accumulation of petroleum hydrocarbons associated with the Deepwater Horizon spill on the seafloor, the mechanisms of their delivery to the seafloor remain unclear. We demonstrate sedimentation of black carbon derived from the in situ burning of surface oil slicks for about 2 mo following the cessation of burning while other contaminants from the spill, including bioactive barium derived from drilling mud, continued to sediment for at least 5 mo after the well was capped. We also show that the episodic sinking of spill-associated substances was mainly mediated by marine particles, especially diatoms. Together, these data demonstrate delivery mechanisms of contaminants from the spill to benthic ecosystems in the deep Gulf of Mexico.
Keywords: Deepwater Horizon, oil spill, hydrocarbon, diatom bloom, drilling mud
Abstract
The 2010 Deepwater Horizon oil spill resulted in 1.6–2.6 × 1010 grams of petrocarbon accumulation on the seafloor. Data from a deep sediment trap, deployed 7.4 km SW of the well between August 2010 and October 2011, disclose that the sinking of spill-associated substances, mediated by marine particles, especially phytoplankton, continued at least 5 mo following the capping of the well. In August/September 2010, an exceptionally large diatom bloom sedimentation event coincided with elevated sinking rates of oil-derived hydrocarbons, black carbon, and two key components of drilling mud, barium and olefins. Barium remained in the water column for months and even entered pelagic food webs. Both saturated and polycyclic aromatic hydrocarbon source indicators corroborate a predominant contribution of crude oil to the sinking hydrocarbons. Cosedimentation with diatoms accumulated contaminants that were dispersed in the water column and transported them downward, where they were concentrated into the upper centimeters of the seafloor, potentially leading to sustained impact on benthic ecosystems.
About 3.2 million barrels of crude oil were released during the Deepwater Horizon (DwH) oil spill into the Gulf of Mexico (GoM) between 20 April 2010 and 15 July 2010 (1–4). The spill was extraordinary in its volume, duration, depth of release (∼1,500 m), and distance traveled, resulting in hundreds of square kilometers of pollution (5, 6). Roughly 5–10% of the spilled oil reached the seafloor (7–9), although the patchy distribution of this deposition combined with its large spatial extent resulted in significant uncertainty in this estimate (10). This deposition of DwH oil compounds was associated with exceptionally high deposition rates of marine organic and inorganic particles (11). Deposition patterns and type are consistent with the hypothesis that marine snow carried a significant fraction of the deposited oil compounds downward (10, 12), a process dubbed the “Dirty Blizzard.”
Marine snow is defined as composite particles > 0.5 mm and consists of phytoplankton, zooplankton feces, feeding structures, mucus, and other debris (13). This material sinks rapidly from the surface ocean (14) and efficiently scavenges suspended or dissolved substances that it encounters (15). By transporting material from the upper to the deep ocean, marine snow acts as a link between the sea surface and the seafloor (16). Different types of marine snow laden with oil (marine oil snow) have been suggested to have transported spilled oil from the surface and from the subsurface plume at ∼1,000 m to the seafloor during the DwH spill (17).
Knowledge of the specific fate and distribution pathways of oil and spill-associated chemicals sheds light on the overall ecosystem response to large oil inputs and the transport mechanisms that remove petrocarbon and other contaminants from the water column. Petrocarbon is defined as petroleum and petroleum transformed by weathering processes, including photooxidation and microbial oxidation, burning, or evaporation. Knowledge of petrocarbon fate is important for litigation purposes and future spill response and restoration planning (18). For example, transport of oil-derived carbon to the seafloor was not one of the mechanisms accounted for in the reckoning of the oil spill calculator (1). However, it will likely be taken into account in the future.
Only weeks after the spill had ended, hydrocarbon from the DwH event was largely undetectable in surface waters (19) and in the water column of the GoM [e.g., cruise Henry Bigelow 02 (14−30 August 2010)]. This may be because their concentrations were below analytical detection limits, or it could have been due to the patchiness of their distribution. We hypothesize (i) that petrocarbon and other contaminants lingering in the water column were adsorbed to fine particles and (ii) that the downward transport of these materials was controlled by the sedimentation of marine snow. To test these hypotheses, we deployed a funnel-shaped sediment trap [Kiel 21 trap, K.U.M. Umwelt und Meerestechnik Kiel GmbH (KUM)] about 7 km SW of the DwH well (28°42.55'N, 88°25.34'W) at ∼1,540 m (Fig. 1), through which the time series of petrochemical sedimentation captured in the sediment trap was examined. This approach allowed us to overcome both analytical limitations and patchiness of distribution because the trap integrated sinking materials over time and space. The second hypothesis was examined by comparing the temporal coherence of pollutant flux with the fluxes of other particles to the deep ocean.
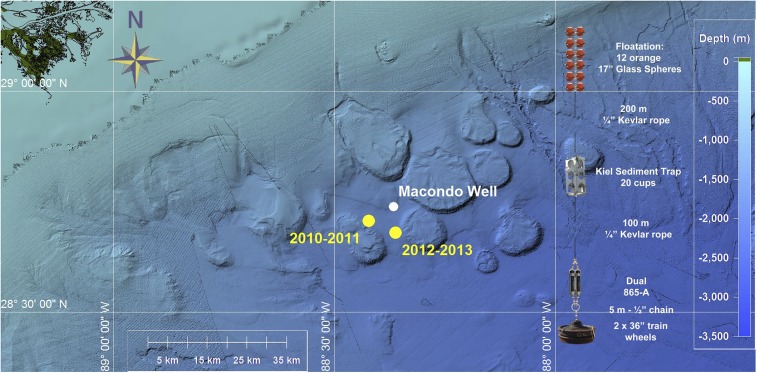
Fig. 1.
The sampling site and schematic of sediment trap array deployed in 2010/2011 and 2012/2013, with NOAA Okeanos Explorer bathymetry data included.
From analysis of these time series samples, we present conclusive evidence that sedimentation of DwH-associated oil components continued for 5 mo after the damaged Macondo well was capped. Sinking material was collected in 20 consecutive 3-wk intervals between 25 August 2010 and 19 October 2011 and fixed in situ with mercuric chloride in a salinity gradient [40 practical salinity unit (PSU)]. Type and abundance of collected materials are determined by particle dynamics in the region of the source water above the trap. After retrieving the trap, samples were examined microscopically, analyzed for dry mass, particulate organic carbon (POC), petroleum hydrocarbons, barium in different particulate phases, black carbon (BC), δ13C-POC, ∆14C-POC, and δ34S-POM isotopic composition, among other parameters (see Methods). Sedimentation patterns in 2010/2011 were compared with those in 2012/2013. This later mooring was deployed about 9 km east of the 2010 trap site (Fig. 1) and collected samples between 28 June 2012 and 22 July 2013.
Results and Discussion
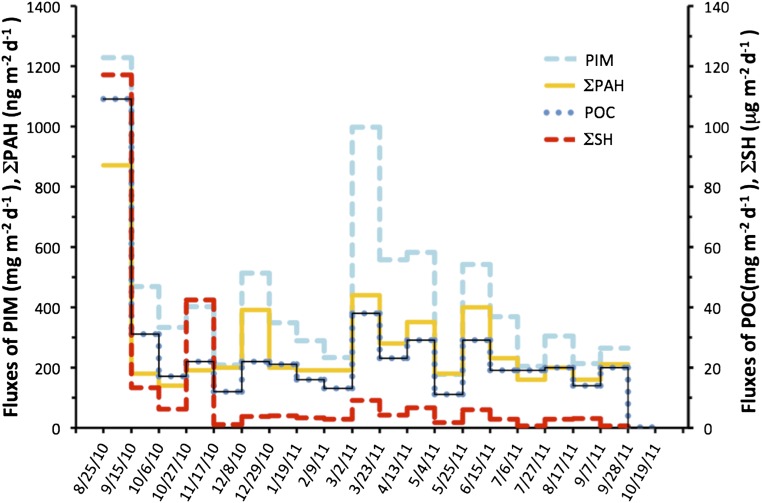
In early September 2010, an exceptionally large sinking event of a Skeletonema sp. (diatom) bloom was observed (Table 1) with a sedimentation rate of POC far in excess of sedimentation rates in the fall of 2011 and 2012 (109 mg POC⋅m−2⋅d−1 vs. ≤20 mg POC⋅m−2⋅d−1, Fig. 2 and Fig. S1). During this event, more than 8 billion diatom frustules per square meter per day, dominated by Skeletonema sp., reached 1,500 m depth, carrying with them ∼120 µg⋅m−2⋅d−1 of the sum of n-alkanes from C22 to C36 (ΣSH) and 8.40 mg⋅m−2⋅d−1 BC, as well as 1.60 mg⋅m−2⋅d−1 barium and 3.70 μg⋅m−2⋅d−1 olefins (Table 1 and Table S1). Barite (barium sulfate) and olefin compounds are key components of drilling mud. Aside from this exceptional 2010 fall sedimentation event, annual peak sedimentation rates of POC were observed in spring (2011, 2013) and were about 50% lower than those of the 2010 fall bloom (Fig. 2 and Fig. S1). Peak fluxes of particulate inorganic matter (PIM) and POC in spring reflect seasonality in the discharge of nutrients and suspended minerals by the Mississippi River (Fig. S2). The biological response to this nutrient input is a phytoplankton bloom (20, 21). Sinking marine snow resulting from such blooms incorporates suspended minerals and other nonsinking particles (15, 22), causing seasonal sedimentation pulses as observed in spring 2011 and spring 2013.
Table 1.
Sedimentation rates of dry wt., diatoms, foraminifera, barium, ΣPAH, ΣSH, BC, and the signal of Δ14C-POC and δ34S-POM
| Phase | Cup no. | Start date, mm/dd/yy | Dry wt., mg⋅m−2⋅d−1 | Diatom frustules, 106 m−2⋅d−1 | Foramanifera, m−2⋅d−1 | Barium, mg⋅m−2⋅d−1 | ΣPAH, μg⋅m−2⋅d−1 | ΣSH μg⋅m−2⋅d−1 | BC, mg⋅m−2⋅d−1 | Δ14C-POC, ‰ | δ34S-POM, ‰ |
| 1 | 1 | 8/25/10 | 1618 ± 160 | 8135 | 27 | 1.60 ± 2.17 | 0.87 ± 0.12 | 117.1 ± 13.0 | 8.42 ± 0.15 | −21.4 | 21.9 |
| 2 | 2 | 09/15/10 | 663 ± 46 | 35 | 130 | 0.25 ± 0.02 | 0.18 ± 0.02 | 13.2 ± 1.1 | 0.42 ± 0.08 | +76.1 | 16.7 |
| 3 | 10/06/10 | 426 ± 18 | 62 | 130 | 0.26 ± 0.01 | 0.14 ± 0.02 | 6.2 ± 0.4 | 0.31 ± 0.06 | −80.9 | 14.4 | |
| 4 | 10/27/10 | 519 ± 43 | 28 | 140 | 0.59 ± 0.05 | 0.19 ± 0.02 | 42.4 ± 4.1 | NA | −113.7 | 7.5 | |
| 5 | 11/17/10 | 283 ± 28 | 14 | 198 | 0.22 ± 0.02 | 0.20 ± 0.03 | 1.2 ± 0.1 | NA | −114.3 | 8.4 | |
| 6 | 12/08/10 | 671 ± 15 | NA | 264 | 0.43 ± 0.01 | 0.39 ± 0.04 | 3.8 ± 0.2 | 0.44 ± 0.13 | −141.0 | 9.1 | |
| 3 | 7 | 12/29/10 | 467 ± 16 | NA | 280 | 0.22 ± 0.01 | 0.20 ± 0.02 | 3.9 ± 0.2 | NA | −67.0 | 5.3 |
| 8 | 01/19/11 | 420 ± 24 | NA | 411 | 0.17 ± 0.01 | 0.19 ± 0.02 | 3.3 ± 0.3 | NA | −72.4 | 4.8 | |
| 9 | 02/09/11 | 337 ± 20 | NA | 259 | 0.17 ± 0.01 | 0.19 ± 0.02 | 2.8 ± 0.2 | NA | −94.9 | 2.9 | |
| 10 | 03/02/11 | 1282 ± 28 | 3 | 149 | 0.70 ± 0.02 | 0.44 ± 0.05 | 9.0 ± 0.5 | 1.07 ± 0.02 | −85.1 | 5.0 | |
| 11 | 03/23/11 | 746 ± 35 | NA | 390 | 0.32 ± 0.02 | 0.28 ± 0.03 | 4.3 ± 0.3 | NA | −103.2 | 6.7 | |
| 12 | 04/13/11 | 763 ± 29 | NA | 283 | 0.41 ± 0.02 | 0.35 ± 0.04 | 6.7 ± 0.3 | NA | −53.8 | 6.7 | |
| 13 | 05/04/11 | 248 ± 9 | NA | 229 | 0.13 ± 0.01 | 0.18 ± 0.02 | 1.7 ± 0.1 | NA | −69.9 | 8.5 | |
| 14 | 05/25/11 | 762 ± 54 | NA | 332 | 0.21 ± 0.02 | 0.4 ± 0.05 | 5.9 ± 0.5 | NA | −60.1 | 6.0 | |
| 15 | 06/15/11 | 440 ± 29 | 8 | 381 | 0.19 ± 0.01 | 0.23 ± 0.03 | 2.8 ± 0.2 | NA | −20.2 | 5.1 | |
| 16 | 07/06/11 | 291 ± 7 | NA | 276 | 0.14 ± 0.01 | 0.16 ± 0.03 | 0.6 ± 0.1 | NA | −20.4 | 4.4 | |
| 17 | 07/27/11 | 400 ± 30 | NA | 300 | 0.12 ± 0.01 | 0.20 ± 0.02 | 2.9 ± 0.3 | 0.49 ± 0.027 | −60.9 | 12.4 | |
| 18 | 08/17/11 | 314 ± 50 | 16 | 230 | 0.11 ± 0.02 | 0.26 ± 0.03 | 3.0 ± 0.5 | NA | −67.9 | 14.9 | |
| 19 | 09/07/11 | 384 ± 22 | 28 | 155 | 0.12 ± 0.01 | 0.56 ± 0.06 | 0.7 ± 0.1 | NA | −56.6 | 8.6 |
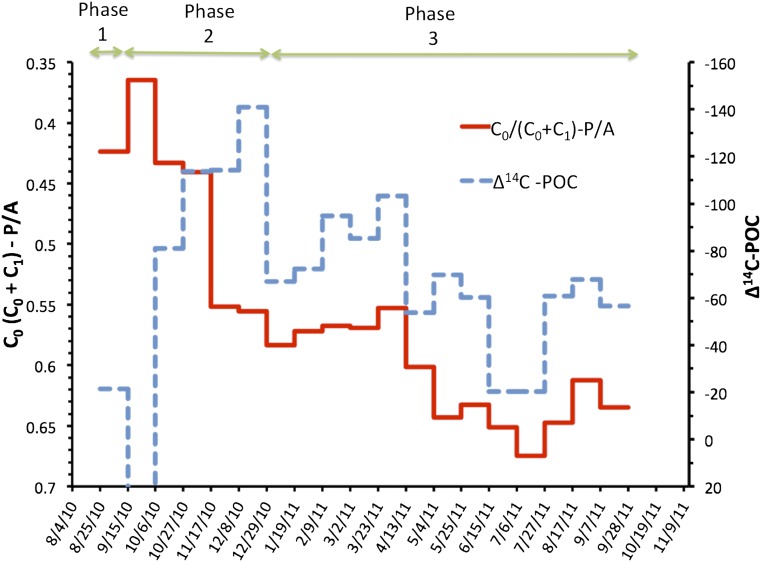
Phase 1 is the period impacted by diatom bloom, phase 2 represents continued cosedimentation of oil spill associated substances, and phase 3 was when sedimentation of oil spill related substances was negligible; NA, no analysis; ± is the SD; uncertainty of ∆14C-POC is 3.0‰, and δ34S-POM is 0.3‰ based on replicate analysis of randomly selected samples. The ∆14C-POC value of cup 2 is positive. See Table S2 for the list of PAH compounds for calculating ΣPAH.
Fig. 2.
Temporal change in sedimentation rates of PIM, POC, the sum of saturated hydrocarbons (ΣSH), and the sum of PAHs (ΣPAH). Estimated uncertainties of sedimentation rates are 10%, and uncertainties of PIM, ΣSH, and ΣPAH can be found in Table 1 and Table S1.
Fig. S1.
Time series of POC fluxes between 2010–2011 and 2012–2013. Uncertainties of POC analysis are typically lower than 10% (Tables S1 and S3).
Table S1.
Sedimentation rates of PIM, POC, C18− olefins, stable isotope composition of organic matter (δ13C-POC and δ15N-POC), three ratios (ΣSH/ ΣPAH, Fl/(Fl+Py), and C0/(C0+C1)-P/A), and concentrations of char and soot
| Cup no. | Midpoint date, mm/dd/yy | PIM, mg⋅m−2⋅d−1 | POC, mg⋅m−2⋅d−1 | C18− olefins, μg⋅m−2⋅d−1 | δ13C-POC, ‰ | δ15N-PON, ‰ | ΣSH/ΣPAH | Fl/(Fl+Py) | C0/(C0+C1)-P/A | Char, mg⋅g−1 | Soot, mg⋅g−1 |
| 1 | 09/04/10 | 1228 ± 181 | 109 ± 2 | 3.70 ± 0.26 | −21.5 | 8.9 | 134.2 ± 24.0 | 0.41 ± 0.02 | 0.42 ± 0.03 | 5.20 ± 0.57 | 0.25 ± 0. 06 |
| 2 | 09/25/10 | 469 ± 6 | 31 ± 0 | 0.00 | −21.6 | 6.6 | 73.9 ± 10.7 | 0.50 ± 0.02 | 0.36 ± 0.02 | 0.63 ± 0.09 | 0.22 ± 0. 05 |
| 3 | 10/16/10 | 334 ± 11 | 17 ± 1 | 0.28 ± 0.02 | −21.9 | 5.8 | 44.8 ± 5.7 | 0.51 ± 0.02 | 0.43 ± 0.03 | 0.73 ± 0.04 | 0.26 ± 0. 07 |
| 4 | 11/06/10 | 402 ± 39 | 22 ± 1 | 0.07 ± 0.01 | −22.1 | 5.4 | 222.5 ± 36.0 | 0.49 ± 0.02 | 0.44 ± 0.03 | NA | NA |
| 5 | 11/27/10 | 208 ± 16 | 12 ± 0 | 0.14 ± 0.01 | −22.2 | 5.0 | 5.8 ± 1.0 | 0.50 ± 0.02 | 0.55 ± 0.03 | NA | NA |
| 6 | 12/18/10 | 513 ± 5 | 22 ± 1 | 0.00 | −22.1 | 4.4 | 9.7 ± 1.1 | 0.45 ± 0.02 | 0.56 ± 0.03 | 0.65 ± 0.12 | 0.20 ± 0. 01 |
| 7 | 01/08/10 | 348 ± 19 | 21 ± 0 | 0.00 | −22.6 | 3.7 | 19.8 ± 2.4 | 0.45 ± 0.02 | 0.58 ± 0.03 | NA | NA |
| 8 | 01/29/11 | 288 ± 35 | 16 ± 0 | 0.00 | −22.2 | 3.6 | 17.9 ± 2.5 | 0.45 ± 0.02 | 0.57 ± 0.03 | NA | NA |
| 9 | 02/19/11 | 234 ± 19 | 13 ± 0 | 0.00 | −22.4 | 3.5 | 15.2 ± 2.1 | 0.46 ± 0.02 | 0.57 ± 0.03 | NA | NA |
| 10 | 03/12/11 | 998 ± 30 | 38 ± 1 | 0.00 | −21.8 | 4.4 | 20.3 ± 2.4 | 0.46 ± 0.02 | 0.57 ± 0.03 | 0.84 ± 0.10 | 0.20 ± 0. 04 |
| 11 | 04/02/11 | 558 ± 19 | 23 ± 0 | 0.00 | −21.9 | 4.4 | 15.5 ± 2.0 | 0.47 ± 0.02 | 0.55 ± 0.03 | NA | NA |
| 12 | 04/23/11 | 582 ± 37 | 29 ± 1 | 0.00 | −21.5 | 8.7 | 19.0 ± 2.4 | 0.47 ± 0.02 | 0.60 ± 0.04 | NA | NA |
| 13 | 05/14/11 | 177 ± 5 | 11 ± 0 | 0.00 | −21.9 | 4.8 | 9.7 ± 1.2 | 0.52 ± 0.02 | 0.64 ± 0.04 | NA | NA |
| 14 | 06/04/11 | 542 ± 66 | 29 ± 1 | 0.00 | −22.2 | 4.0 | 14.8 ± 2.2 | 0.51 ± 0.02 | 0.63 ± 0.04 | NA | NA |
| 15 | 06/25/11 | 369 ± 7 | 19 ± 0 | 0.00 | −22.2 | 4.4 | 12.6 ± 1.8 | 0.48 ± 0.02 | 0.65 ± 0.04 | NA | NA |
| 16 | 07/16/11 | 204 ± 6 | 19 ± 3 | 0.00 | −22.2 | 4.5 | 3.7 ± 0.4 | 0.52 ± 0.02 | 0.67 ± 0.04 | NA | NA |
| 17 | 08/06/11 | 305 ± 7 | 20 ± 0 | 0.00 | −22.3 | 6.1 | 14.6 ± 2.2 | 0.54 ± 0.02 | 0.65 ± 0.04 | 1.21 ± 0.15 | 0.29 ± 0. 06 |
| 18 | 08/27/11 | 212 ± 29 | 14 ± 0 | 0.00 | −22.3 | 5.5 | 18.4 ± 4.6 | 0.53 ± 0.02 | 0.61 ± 0.04 | NA | NA |
| 19 | 09/17/11 | 265 ± 25 | 20 ± 0 | 0.00 | −22.4 | 5.4 | 3.2 ± 0.4 | 0.51 ± 0.02 | 0.63 ± 0.04 | NA | NA |
Uncertainties of δ13C-POC and δ15N-PON are 0.3‰ based on replicate analysis of randomly selected samples; NA, no analysis.
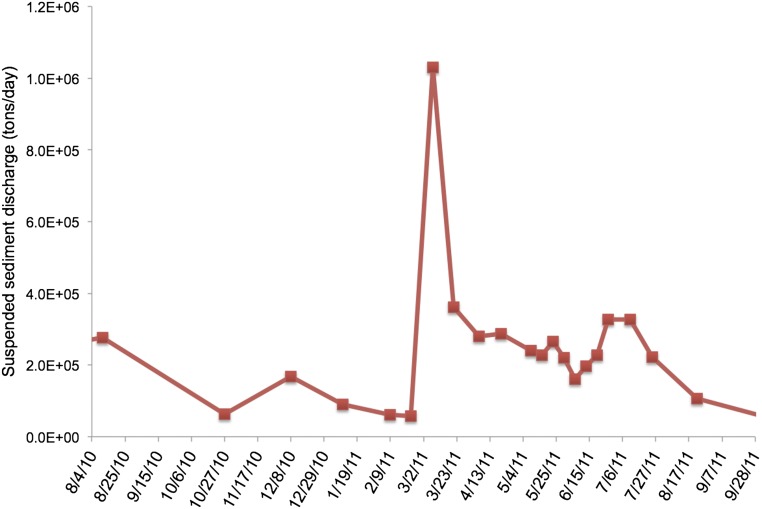
Fig. S2.
Discharge of suspended sediment (tons per day) at St. Francisville USGS station.
In August 2010, an anomaly of MODIS (Moderate Resolution Imaging Spectroradiometer) fluorescence line height, used as a proxy for phytoplankton biomass, was observed in the northeastern GoM, suggesting the occurrence of large phytoplankton blooms related to the DwH spill (21). Skeletonema sp., a common diatom in the GoM, thrives in the presence of crude oil and is known to be associated with oil extraction activities (23). At bloom concentrations, Skeletonema sp. coagulates and forms rapidly sinking marine snow aggregates (24), which may be facilitated by the presence of oil and BC. Oil products can promote aggregation and subsequent efficient sedimentation of marine particles (25), because oil-containing aggregates are larger and more cohesive than those formed in the absence of oil (17). In particular, BC from fossil fuel combustion is very surface-active (26) and promotes the coagulation of marine particles (27).
Two BC components (soot and char) can be formed through incomplete combustion. BC sources in the GoM in 2010 include in situ burning (ISB) of crude oil (Supporting Information) and atmospheric deposition from boat traffic related to various activities (e.g., skimming). Soot formed in the gas phase from both ISB and boat traffic will be distributed farther afield (28), whereas char, which is the burn residue left on surface waters after ISB, will stay in the water column and be subject to deposition (29). From late April to mid-July, about 400 controlled ISBs were conducted within 24 km of the well (29, 30), removing ∼220,000–310,000 barrels of oil (1). Production rates of char, the burn residues, are about 5% (±4%) of the mass of crude oil burned, depending on burn temperature, thickness, and surface area of crude oil on the water surface, etc. (31, 32). Assuming that 5% of burned oil was transformed into char and that all produced char was deposited within 24 km of the well (approximately the area where ISB took place) results in an input of 0.9–1.3 g⋅m−2. Sedimentation of char in association with the diatom bloom represents 15–20% of this input (see Supporting Information for details). After the bloom sedimentation, char concentration decreased to background levels (Table S1), indicating a residence time of BC in the water column of about 2 mo, similar to what has been observed elsewhere (33).
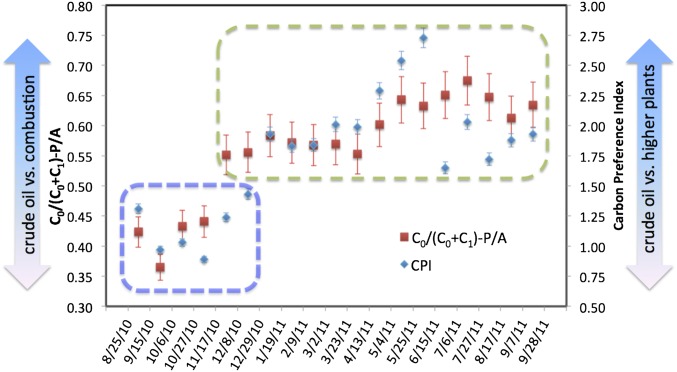
The hydrocarbons that sank together with the diatom bloom were predominantly petroleum-derived. The carbon preference index (CPI), which is a mathematical expression of the odd-over-even predominance between n-C24 and n-C36 (see Supporting Information for CPI equation) of the Macondo crude oil is ∼1 (34). In contrast, surface sediments collected from sites unaffected by DwH or natural oil seeps have a CPI of ∼2.5 (Supporting Information). A CPI value of about 1.3 in the first sample indicates the dominance of alkanes from crude oil. In addition, the sensitive polycyclic aromatic hydrocarbon (PAH, Table S2) source indicator C0/(C0+C1)-P/A (phenanthrene/anthracene), was below 0.5 (35, 36), confirming that crude oil was the dominant source of sinking hydrocarbons in late summer to early fall 2010 (Supporting Information). In contrast to the 2010 samples (Fig. 3), the trap samples from 2012 all had a PAH source indicator value of greater than 0.8 (Table S3). A C0/(C0+C1)-P/A ratio of 0.5 delineates the transition between unburned (<0.5) and combusted petroleum (>0.5) (36, 37). This indicator is sensitive to weathering of oil as well, because its value decreases as fresh crude oil weathers (38). The decrease causes the difference between weathered crude oil and combustion sources (i.e., pyrogenic PAHs) to be more pronounced (i.e., larger) with the passage of time. The ratio of another independent PAH source indicator Fl/(Fl+Py) (where Fl is fluoranthene and Py is pyrene) is about 0.4 in the first sample, below the transition value of 0.5 (36, 39) (Supporting Information). This also confirms the dominance of petroleum-derived sources during this period (25 August through 15 September).
Table S2.
List of PAH compounds quantified
| Compound no. | PAH compounds |
| 1 | Acenaphthene |
| 2 | Acenaphthylene |
| 3 | Fluorene |
| 4 | Phenanthrene |
| 5 | Anthracene |
| 6 | Fluoranthene |
| 7 | Pyrene |
| 8 | Benzo[a]anthracene |
| 9 | Chrysene |
| 10 | Benzo[b]fluoranthene |
| 11 | Benzo[k]fluoranthene |
| 12 | Benzo[a]pyrene |
| 13 | Indeno[1,2,3-cd]pyrene |
| 14 | Dibenzo[ah]anthracene |
| 15 | Benzo[ghi]perylene |
| 16 | 2,6-dimethylnaphthalene |
| 17 | 2-methylphenanthrene |
| 18 | 1-methylphenanthrene |
| 19 | 3-methylphenanthrene |
| 20 | 4-methylphenanthrene |
| 21 | 1,3-,2,10-,3,9-,3,10-dimethylphenanthrene |
| 22 | 1,6- and 2,9- dimethylphenanthrene |
| 23 | 1,7-dimethylphenanthrene |
| 24 | 2,3-dimethylphenanthrene |
| 25 | 2,6-dimethylphenanthrene |
| 26 | 2,7-dimethylphenanthrene |
| 27 | 3,6-dimethylphenanthrene |
| 28 | 1-methylpyrene |
| 29 | 4-methylpyrene |
| 30 | 6-methylbenzo[a]anthracene |
ΣPAH is the sum of all quantified PAH compounds.
Fig. 3.
Relative hydrocarbon contributions from crude oil in settled materials, as revealed by hydrocarbon source indicators. The blue box, in which CPI is <1.6 and C0/(C0+C1)P/A ratio is <0.5, highlights the samples with significant sources from crude oil (petrocarbon), and the green box marks samples with low or negligible contributions from crude oil. The separation into two periods (blue and green boxes) is artificial and may be seen as a conservative estimate for the timespan during which appreciable amounts of oil components from the DwH spill sank. Before December 2010, petrogenic sources dominated; thereafter, noncrude oil hydrocarbon sources (e.g., higher plants for saturated hydrocarbons and combustion for PAHs) dominated.
Table S3.
Sedimentation rates of POC, ΣSH, ΣPAH, and isotopic signature (δ13C-POC, Δ14C-POC) of settled material in summer 2012
| Cup no. | Midpoint date, mm/dd/yy | POC, mg⋅m−2⋅d−1 | ΣPAH, μg⋅m−2⋅d−1 | C0(C0+C1)-P/A | ΣSH, μg⋅m−2⋅d−1 | CPI | δ13C-POC, ‰ | Δ14C-POC, ‰ |
| 1 | 07/07/12 | 14.6 ± 0.1 | 0.13 ± 0.02 | 0.82 ± 0.05 | 1.50 ± 0.12 | 1.60 ± 0.05 | −20.9 | −32.6 |
| 2 | 07/25/12 | 12.4 ± 0.3 | 0.11 ± 0.02 | 0.83 ± 0.05 | 0.79 ± 0.05 | 2.00 ± 0.06 | −20.8 | −41.4 |
| 3 | 08/12/12 | 15.3 ± 0.7 | 0.10 ± 0.01 | 0.82 ± 0.05 | 0.75 ± 0.04 | 2.50 ± 0.08 | −20.8 | −35.4 |
| 4 | 08/30/12 | 19.0 ± 3.3 | 0.16 ± 0.03 | 0.86 ± 0.05 | 0.75 ± 0.07 | 2.30 ± 0.07 | −20.8 | −73.1 |
The CPI of normal alkanes is >1.5 in all four samples, consistent with negligible sedimentation of hydrocarbons from crude oil. Higher (less negative) δ13C-POC and Δ14C-POC confirm that, in 2012, sinking material was dominated by more recent primary production and less fossil carbon compared with summer 2011 samples. Uncertainties of δ13C-POC and Δ14C-POC are 0.3‰ and 3.0‰, based on replicate analysis of randomly selected samples.
The molecular distribution of alkanes can indicate the origin of the settling petroleum hydrocarbons, because lighter oil components (< C5) dissolved en route to the ocean surface (40, 41), and components between C5 and C13 were largely evaporated or degraded (34, 41). The absence of C13 and lighter alkanes in our trap samples may thus imply that sinking petroleum hydrocarbons were from the surface rather than from the subsurface plume (7). However, weathering and consumption of lighter alkanes within the deep-sea plume (42) may have a similar effect, and it is thus not possible to unambiguously determine if the petroleum-derived carbon in the trap samples stemmed from the surface or from the deep plume or both. Incorporation of oil into diatom aggregates during their formation has been shown experimentally (17). Scavenging of oil from the deep plume by settling marine snow is another possible mechanism for the inclusion of oil in marine snow. The net effect of removing dispersed or diluted oil from the water and transporting it to the seafloor is the same in both scenarios.
There was no indication that the petroleum hydrocarbons that were collected in the sediment trap stemmed from resuspended sediments from below the trap or from natural seeps. Planktonic foraminifera were observed in settled material during 2010; however, the absence of benthic species indicates the lack of substantial resuspension during this time period (Supporting Information). This observation is consistent with physical indicators; resuspension to more than 100 m above the seafloor requires high current speeds, but, between August and December 2010, the northern GoM was free of large storm events [National Hurricane Center, National Oceanic and Atmospheric Administration NOAA)]. Furthermore, resuspended sediments as a single source could not generate the highly varied temporal patterns of SH, PAH, and BC observed in this study. Natural seeps are also unlikely to be the major petroleum hydrocarbon source in these samples. There are no natural seeps identified within 2 km of the trap site, and only three seeps were identified within 5 km. Due to dissolution, evaporation, degradation, mixing, and dispersion, petroleum hydrocarbons from these small seeps were unlikely to be a measurable source of petrocarbon in the trap. In fact, the absence of measurable amounts of petroleum hydrocarbons in 2011 (Fig. 2) and 2012 trap samples (Table S3) emphasizes that the influence of oil from natural seeps was minimal, assuming that natural seepage proceeded at a relatively constant rate.
The petrocarbon detected in the deep sediment trap indicates that these contaminants had lingered in the water for at least 40 d (15 July to 25 August). This can be due to the slow deposition rate of small particles (e.g., clay), to which those petroleum hydrocarbons, especially those > C13, adsorb due to their low solubility (43). In the open sea, fine suspended particulate matter settles very slowly (<0.01 mm⋅s−1) (44) and thus remains in the surface layers for extended time periods (45, 46). However, those particles can be scavenged by marine snow consisting of diatoms, feces, feeding structures, or detritus, therewith cosedimenting rapidly (47, 48). Marine snow can settle at 50 to >200 m⋅d−1 (12, 14).
The blizzard of sinking diatom aggregates sedimented not only petroleum hydrocarbons but also components of the drilling mud, as indicated by the high fluxes of barium and olefins (Table 1 and Fig. 4). Water-based drilling mud, which contains barite, olefins, and clay bentonite, was used during the DwH event for “top kill” and “static kill” techniques. The molecular distribution of olefin compounds enriched in our trap samples before December 2010 (Table S1 and Fig. 4) exhibited a dominance of C16 and C18 (Fig. 5), identified by the 2D gas chromatograph (GC×GC) time-of-flight (TOF) mass spectrometer, which unambiguously traces olefins stemming from drilling mud (49, 50). These olefins fell below the detection limit in 2011. When water-based drilling mud is released into the marine environment, the majority of mud solids form a plume that settles quickly to the seafloor, but ∼10% of its mass, especially the fine clay and barite particles, remains suspended and disperses (51). Clay, such as bentonite, has a high adsorption capacity for hydrocarbons (52); thus it is reasonable to assume that olefins would adsorb to these clay particles. Due to their extremely low solubility, olefins remain associated with the clay particles after the release of the drilling mud into the water. Similar to other small particles, clay particles likely remained suspended in the water for weeks to months, until the occurrence of a large sedimentation event (e.g., diatom bloom) transferred them to the seafloor.
Fig. 4.
Concentrations of barium and olefin, two drilling mud-related chemicals, in settled materials. The gray area depicts background concentration for barium in the northern GoM. See Table 1 for definition of three phases. Uncertainties of barium and olefin can be found in Table 1 and Table S1.
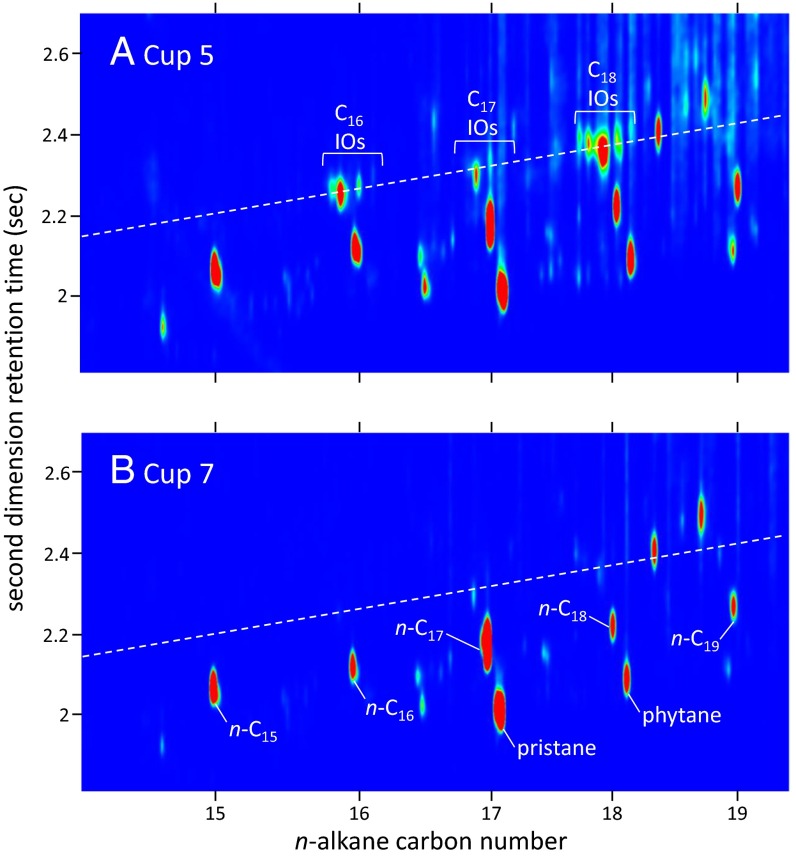
Fig. 5.
Partial GC×GC TOF chromatograms of ions of alkene (55 + 69 + 83) in (A) cup 5 and (B) cup 7, in which data are displayed in color contour plots, with blue, yellow, and red representing low, medium, and high signal strength, respectively. The isomer mixture and pattern of C16 to C18 internal olefins (IOs) indicates that drilling-mud olefins are present in cup 5 but not in cup 7.
An anomaly of dissolved barium, attributed to drilling mud application, was observed in late May at ∼1,100 m depth in the water column about 6.3 km southwest of the well (53), but it is unexpected that high fluxes of barium arrived in the sediment trap in September as far away as 7.5 km from the well site. Due to its high density, barite (BaSO4) was assumed to have sedimented quickly and remain near the application sites (51). In trap material, however, barium was consistently enriched until January 2011, suggesting that sinking marine snow collected barium from the water for up to 4 mo after drilling mud was last used (August). After January, barium concentrations fell to background levels (Supporting Information). These unexpected elevated fluxes of barium in trap samples were partially due to the existence of fine barite particles in drilling mud. Electron microprobe analysis confirmed such fine barite particles dominated in cup 1 but not in cup 14 (Fig. S3). Barite particles < 5 μm comprise about 10% of 4.1 specific gravity grade barite (54). Elevated barium flux during the sedimentation of the diatom bloom was also due to barium associated with the Al−Si phase, which includes both clay and biologically produced opal frustules (Supporting Information).
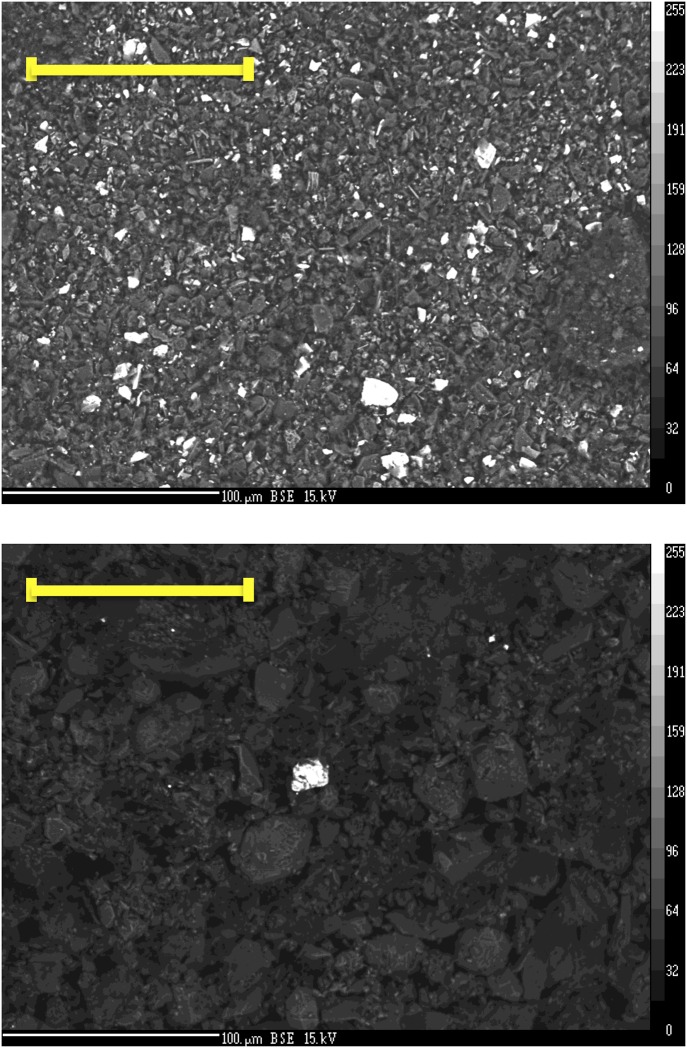
Fig. S3.
Electron microprobe backscattered electrons (EM-BSE) images of the residue phase of cups 1 and 14. (Upper) EM-BSE image of cup 1, in which the size of barite particles (bright) mainly ranges from 1 μm to 5 μm. (Scale bar: 100 μm.) (Lower) EM-BSE image of cup 14, in which fine barite particles were largely absent, and only one large barite particle (∼20 μm) is visible. (Scale bar: 100 μm.)
In addition, barium was incorporated into biogenic particles, extending its residence time in the water as well. Using a sequential leaching approach modified from Eagle et al. (55, 56), we found that the Ba:Ca ratio in the carbonate phase in cup 1 is ∼3 times that in cup 14, implying the incorporation of extra Ba into calcium carbonate tests and shells (Supporting Information). The Ba:Ca ratios in planktonic shells are correlated with the Ba levels in the water (57, 58). Our results suggest that the application of barite in drilling mud can increase the dissolved barium concentration in the water, leading to its increased incorporation into planktonic shells (e.g., foraminifera and pteropods). Considering the widespread use of drilling mud at hundreds of ocean drilling sites around the world, the environmental implications (59, 60) of such an unexpectedly long residence time of barium in the water column is significant and worthy of further investigation.
The concurrence of elevated sedimentation rates of drilling mud components and oil products, including petroleum hydrocarbons and BC, with diatoms (POC, diatom abundance) suggests that rapidly sinking diatom aggregates scavenged suspended substances and particles from the spill and mediating measures, effectively cleansing the water column. Cosedimentation of petrocarbons with organic matter has been observed under nonspill conditions as well (47, 48, 61, 62). The presence of fresh marine phytoplankton during the bloom sedimentation is indicated by intact cells, the modern ∆14C-POC signature, which reflects the relative contributions of crude oil and modern carbon (e.g., phytoplankton), and in the marine source signatures of δ34S-POM and δ13C-POC (Table 1 and Table S1).
The mass sedimentation event in early September 2010 was followed by a temporary decrease in the sedimentation of oil and drilling mud markers between mid-September and October (Table 1). Despite the persistent low rates of POC and PIM sedimentation until March 2011 (Fig. 2), sedimentation of DwH-related substances (e.g., barium, olefins, and ΣSH) increased again in late October 2010 (Fig. 4). Additionally, the relative contributions of the spill-related substances to total settled material were transiently low directly after the mass sedimentation event. For example, the barium contribution to sinking material decreased from about 1,000 mg⋅kg−1 of dry weight during the bloom sedimentation event to 400 mg⋅kg−1 directly thereafter (Fig. 4), but then rebounded to about 1,200 mg⋅kg−1 in late October. We hypothesize that the short-lived decrease in the relative contribution of barium to total sinking matter immediately after the mass sedimentation event reflected a transient decrease of barium concentration in the water column near the trap caused by the “scrubbing effect” of the large diatom flux event. However, diatom blooms are spatially patchy (21), and lateral advection and mixing of polluted waters that did not experience a diatom bloom, as well as an influx of organisms with Ba-rich shells, would have led to the renewed increase in concentrations of the spill-related contaminants in late October.
The Δ14C-POC signal is consistent with a transitory variation in the contribution of oil spill contaminants to sinking matter directly after the fall bloom (Fig. S4): During the diatom sedimentation event (phase 1, see Table 1), the fossil fuel signal was masked by the large amount of newly assimilated phytoplankton carbon that sank, resulting in a more modern overall value (Δ14C-POC = −21‰). The Δ14C-POC signal increased further (+76‰) in the second half of September 2010, as fossil carbon (Fig. S4) was temporarily cleansed from the water column, but phytoplankton still sank, albeit at low concentrations (Table 1). A value of +40‰ or greater is indicative of modern carbon fixed at the surface (63). The subsequent rapid shift to Δ14C-POC representing more fossil carbon in October (−80‰) to the end of December (−140‰), reflects the renewed increase in the relative contribution of oil components to total settled material, as oil constituents increased again in source water (phase 2). In 2011, Δ14C-POC became successively more modern and trended with the PAH source indicator (C0/(C0+C1)-P/A), as contributions of crude oil to total organic carbon flux and to petroleum hydrocarbons simultaneously decreased with time (Fig. S4, phase 3). The δ34S-POM values of +14‰ to +21‰ confirmed initial algal inputs and then declined to values below +10‰ as these became less prominent, consistent with the dynamics suggested by Δ14C-POC data (Table 1).
Fig. S4.
Comparison of the time series of ∆14C-POC and the PAH source indicator. Uncertainty of ∆14C-POC is 3.0‰, and uncertainty of C0/(C0+C1)-P/A is about 6% (Table 1 and Table S1).
Scavenging and subsequent sedimentation of oil-spill-related substances is not only determined by their concentrations in the water but also by their respective physicochemical characteristics, including their affinities to sinking particles. As an example, sedimentation rates of BC, which is highly surface-active, declined first, in September, whereas sinking crude oil-derived hydrocarbons declined by late December 2010. Although olefins and barium both stemmed from the same source, olefin sedimentation declined earlier than barium, which was incorporated into planktonic shells. The observed temporal sequence of loss of oil spill contaminants in sinking matter is consistent with the interpretation that these substances all originated from a single event, the DwH oil spill.
After mid-January 2011, no unequivocal sedimentation of oil-spill-related substances was observed. PAH and SH continued to sediment, but the source indicators used suggest that they were predominately derived from noncrude oil sources (e.g., riverine input). The CPI and C0/(C0+C1)-P/A ratios exceeded 1.7 and 0.55, respectively, similar to values observed in the trap in summer 2012 (Table S3) and in sediments at a reference site that was not impacted by the spill or by natural oil seeps. Principal component analysis based on hopane and sterane biomarker ratios also confirmed that those 2011 trap samples were distinctly different from the Macondo oil (Fig. S5 and Table S4). Olefins were absent and sedimentation of barium declined to background levels, as those found in unaffected sediments in the northern GoM (64). ∆PO14C, although variable, possibly due to riverine input (65–67), remained around −90‰ to −50‰, indicating greater relative importance of modern carbon with less fossil input (Supporting Information).
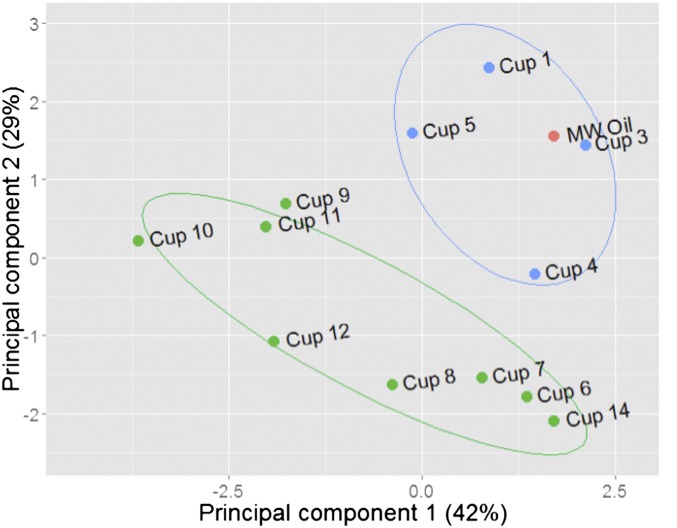
Fig. S5.
Principal component analysis of eight biomarker ratios given in Table S4. Scores of the first two principal components of Macondo Well oil and trap samples are shown. Macondo Well oil (red point) falls within the same 68% confidence ellipse (blue) as cups 1–5 (blue points). Some cups were not included due to low mass.
Table S4.
Biomarker ratios in trap samples and of the Macondo Well (MW) oil
| Samples | DiaC27βαS/ DiaC27βαR | DiaC27βαR/ DiaC29βαR | DiaC27βαR/ C29αααS | C29αββ(R+S)/C29ααα(R+S) | C29ααα(S)/ C29αββ(R+S) | C29ααα(S)/ sum(αββ steranes) | C27αββ(S)/ sum(αββ steranes) | Ts/Tm |
| Cup 1 | 1.28 ± 0.14 | 1.03 ± 0.24 | 2.25 ± 0.20 | 1.15 ± 0.26 | 0.53 ± 0.17 | 0.18 ± 0.04 | 0.15 ± 0.03 | 1.10 ± 0.04 |
| Cup 3 | 1.54 ± 0.17 | 0.91 ± 0.21 | 1.66 ± 0.15 | 1.58 ± 0.36 | 0.43 ± 0.14 | 0.14 ± 0.03 | 0.25 ± 0.05 | 1.14 ± 0.05 |
| Cup 4 | 1.37 ± 0.15 | 0.87 ± 0.20 | 1.06 ± 0.10 | 2.01 ± 0.46 | 0.35 ± 0.11 | 0.19 ± 0.04 | 0.08 ± 0.02 | 1.29 ± 0.05 |
| Cup 5 | 1.24 ± 0.14 | 0.75 ± 0.17 | 1.43 ± 0.13 | 1.11 ± 0.26 | 0.49 ± 0.16 | 0.19 ± 0.04 | 0.14 ± 0.03 | 1.00 ± 0.04 |
| Cup 6 | 1.27 ± 0.14 | 0.56 ± 0.13 | 1.13 ± 0.10 | 2.13 ± 0.49 | 0.29 ± 0.09 | 0.15 ± 0.03 | 0.04 ± 0.01 | 1.02 ± 0.04 |
| Cup 7 | 1.63 ± 0.18 | 0.91 ± 0.21 | 0.82 ± 0.07 | 1.55 ± 0.36 | 0.33 ± 0.11 | 0.16 ± 0.03 | 0.07 ± 0.01 | 0.76 ± 0.03 |
| Cup 8 | 1.39 ± 0.15 | 0.53 ± 0.12 | 0.92 ± 0.08 | 1.85 ± 0.43 | 0.38 ± 0.12 | 0.19 ± 0.04 | 0.06 ± 0.01 | 0.70 ± 0.03 |
| Cup 9 | 1.47 ± 0.16 | 0.74 ± 0.17 | 0.86 ± 0.08 | 0.98 ± 0.23 | 0.63 ± 0.20 | 0.25 ± 0.05 | 0.08 ± 0.02 | 1.01 ± 0.04 |
| Cup 10 | 1.45 ± 0.16 | 0.47 ± 0.11 | 0.66 ± 0.06 | 0.89 ± 0.20 | 0.68 ± 0.22 | 0.3 ± 0.06 | 0.1 ± 0.02 | 0.69 ± 0.03 |
| Cup 11 | 1.15 ± 0.13 | 0.46 ± 0.11 | 0.63 ± 0.06 | 1.11 ± 0.26 | 0.52 ± 0.17 | 0.24 ± 0.05 | 0.08 ± 0.02 | 1.18 ± 0.05 |
| Cup 12 | 1.52 ± 0.17 | 0.39 ± 0.09 | 0.65 ± 0.06 | 1.13 ± 0.26 | 0.55 ± 0.18 | 0.19 ± 0.04 | 0.06 ± 0.01 | 0.92 ± 0.04 |
| Cup 14 | 1.95 ± 0.21 | 0.77 ± 0.18 | 0.85 ± 0.08 | 1.86 ± 0.43 | 0.29 ± 0.09 | 0.16 ± 0.03 | 0.06 ± 0.01 | 1.14 ± 0.05 |
| MW oil | 1.3 ± 0.14 | 0.65 ± 0.15 | 2.00 ± 0.18 | 1.7 ± 0.39 | 0.37 ± 0.12 | 0.18 ± 0.04 | 0.18 ± 0.03 | 1.40 ± 0.06 |
About 2,272 µg⋅m−2 ΣSH sank in excess of background values between late August and December 2010, equivalent to the downward transport of about 85 (±7.04) mg⋅m−2 of crude oil (Supporting Information). Assuming a petrocarbon footprint of 8,400 km2 (8), the ΣSH sedimentation between late August and December 2010 was about 7.2 (± 0.6) × 108 grams, which is ∼0.14 (± 0.01)% of the total amount of crude oil released, and 0.8–8.1% of petrocarbon flux to the seafloor estimated by Chanton et al. (8) using 14C data in surface sediments, and 0.7–6% of the flux estimated by Valentine et al. (7) (see Supporting Information for details of the estimation). The fact that sedimentation of a single diatom bloom at a time when oil concentrations in the water were considered negligible carried to depth on the order of 5% of the sunken oil compounds suggests that just a few marine snow sedimentation events during and after the spill could easily explain the observed deposition of oil and marine particles to the seafloor.
In conclusion, the persistent deposition of the spill-associated substances in the 5 mo after the spill is evidence that oil spill and drilling mud-derived substances must have lingered in the water column long after their release ended, although oil visible at the sea surface disappeared within weeks following capping (19). Barium remained in the water column for months and even entered pelagic food webs. The sedimentation of these spill-related substances was mediated by marine snow, specifically, by a diatom bloom. The oil and BC likely promoted aggregation of marine particles, leading to more effective sedimentation and the resultant transport of exceptionally large amounts of oil-derived material, some 1.6–2.6 × 1010 g (8), to the seafloor. Scavenging of these substances from the water column by sinking marine snow provides a mechanism for previously dispersed oil to reaccumulate at the seafloor. These same mechanisms must have been in effect before the leak was capped, leading to unprecedented accumulation rates at the seafloor and providing an explanation for DwH’s unexpectedly strong impacts on benthic organisms, including fish and coral in deep waters (68–71).
Methods
Site Description and Sampling Method.
The trap was moored at 28°42.55'N, 88°25.34'W in 1,538 m water depth about 105 m above the seafloor. This site was chosen for its proximity to the DwH well head (∼7.4 km SW) and because it positioned the trap under the subsurface plume observed at around a 1,000-m depth to the southwest of the incident site. The deployment site is characterized by a smooth flat bottom of mostly muddy sediments located to the west of Gloria Dome and just to the east on the NE side of Biloxi Dome. For comparison, data from a deployment at 28°40.78'N, 88°21.68'W, about 5 nautical miles east of the initial trap sites (Fig. 1) in 1,655 m water depth and about 80 m above the seafloor, were analyzed. Settling material was collected in four consecutive cups between 28 June 2012 and 8 September 2012 as well as in 18 cups between 12 September 2012 and 22 July 2013. Bathymetry data in Fig. 1 are from NOAA Okeanos Explorer cruises EX1105, EX1202L2, and EX1202L3 and from the General Bathymetric Chart of the Oceans.
The opening of the almost 2-m-high funnel-shaped Kiel Sediment trap (KUM; www.kum-kiel.de/home/) has a 0.5-m2 collection surface, which is covered with a hexagonal lattice grid baffle that reduces washout. The multibottle turntable allows for the collection of 20 individual samples in 300-mL polypropylene bottles. Samples were collected over 21-d intervals between 25 August 2010 and 19 October 2011. Before deployment, sample bottles were filled with filtered seawater to which NaCl (pro analysis, Fisher Scientific) was added to a final salinity of ∼40 PSU and the preservative HgCl2 at a final concentration of ∼0.14%. Upon retrieval, cups were stored and transported dark and cold (4 °C) until processing and analysis. First, cups were gently mixed, and, after material was allowed to resettle for 7 d, sample material was split repeatedly using a Folsom Plankton splitter. During splitting, artificial seawater was used for rinsing. One 1/8 split was used for hydrocarbon analysis, 1/16 split was used for isotope measurements, and appropriate fractions of 1/128 splits were used for the determination of dry weight, POC, barium, and microscopical enumeration of phytoplankton and foraminifera. On average, dry weight measurements between different splits varied by <6%. Depending on analysis, split samples were transferred into appropriate containers, e.g., precombusted glass bottles (at 450 °C overnight) for hydrocarbon analysis. Due to the unexpected nature of the spill, only one trap was deployed. Sedimentation rates in 2012–2013, which are presented for comparison, were measured with a similar time series sediment trap, a funnel-shaped McLane trap moored at 28°40.78'N, 88°21.68'W. Sample processing and analysis were generally the same as for the first 2010/2011 trap.
Analysis.
Dry weight, PIM, and POC.
Quadruplicate sample aliquots were filtered onto preweighed and precombusted (450 °C for 4–6 h) 25-mm glass microfiber filters (GF/F), briefly rinsed with Milli-Q (MQ) water, and dried at 60 °C before reweighing for dry weight determination (dry wt.). PIM was determined from one duplicate set of dry wt. filters, reweighing a third time after combustion at 450 °C for 4–6 h to remove organic matter. The filters for determination of POC were fumed with 10% HCl to remove inorganic carbon and analyzed using a carbon, hydrogen, nitrogen (CHN) elemental analyzer (model C 44OHA by Control Equipment, now Exeter Analytical).
Isotopes.
Before carbon isotope analysis, samples were dried, ground, treated with 10% HCl to remove carbonates, rinsed, and freeze-dried. Samples were then analyzed for percent organic carbon (%C), δ13C-POC, and Δ14C-POC. The first two analyses were performed on a Carlo Erba elemental analyzer coupled to a Delta XP Thermo Finnegan isotope ratio mass spectrometer. Results are presented relative to Vienna Pee Dee Belemnite (VPDB) [δ13C = (Rsam/Rstd − 1) × 1,000, where R = 13C/12C]. Samples for ∆14C-POC analysis were combusted (72) and purified CO2 prepared as graphite targets were analyzed by accelerator mass spectrometry at the University of Georgia (UGA) Center for Applied Isotope Studies (73). Values are reported according to the Δ notation put forth in ref. 74. The Δ notation normalizes the radiocarbon content of a sample to a nominal δ13C value (−25‰) and the year that the sample was collected. The scale is linear and starts at −1,000‰ when a sample has essentially 0% modern carbon, which would represent petroleum residue (75, 76). Analytical reproducibility was on the order of 3‰. The Δ14C content of dissolved inorganic carbon in the open Gulf was +41 ± 3‰ (63). Plankton in 2010 and 2011 was +23 ± 20‰. The greater variability in the plankton was due to the admixture of fossil carbon, the same thing we are measuring here. Measurement variability is small relative to the signal we are measuring. In parallel, δ13C-POC was also determined in a CHN elemental analyzer (Finnigan Delta Plus Advantage) from subsamples processed as for POC (filtering onto precombusted 25-mm GF/F filters and fuming these with 10% HCl). Results from both δ13C-POC determinations agreed well, and averages were used.
Samples for sulfur isotope analysis (δ34S-POM) were first combusted with an elemental analyzer (S 4010). SO2 gases were separated with a 0.8-m GC column (100 °C) and analyzed with a continuous flow isotope ratio mass spectrometer (Delta Plus XP) (77). Final determination of 34S-POM was based on ions 64 and 66, using a dial reactor configuration (78) with the second reactor full of quartz chips to buffer 18O contribution to the SO2. Approximately 5 mg of niobium pentoxide was amended to each sample to improve combustion. No correction for oxygen isotope contribution was made. At least three isotopic reference materials were interspersed with samples for calibration. Sulfur isotopic ratios are reported in per mill relative to Vienna Canyon Diablo Troilite (VCDT) by assigning a value of −0.3 per mill to International Atomic Energy Agency (IAEA) S-1 silver sulfide (79).
The sequential leaching experiment.
We conducted a sequential leaching analysis for trap samples 1 and 14, following an approach modified from Gonneea and Paytan (56). Five steps are listed as follows: (i) 13 mL 4N acetic acid was added to 0.5−1 g of samples, and samples were then shaken overnight; (ii) 13 mL of 10% (wt/wt) sodium hypochlorite was added, and then the samples were placed in a 50 °C water bath overnight; (iii) 13 mL of 0.2 N hydroxylamine in 25% acetic acid was added, and the samples were placed in 80 °C water bath overnight; (iv) 12 mL of 1:2 40% hydrofluoric acid (HF):1 N nitric acid was then added and shaken overnight; (v) samples were heated to ∼230 °C and dried down to small volume, and then nitric acid, perchloric acid, and concentrated HF were added. The walls were washed down with nitric acid, followed by addition of 2.5 mL of 5% boric acid (wt/vol, aqueous) and then 7 mL MQ water. Between each step, samples were rinsed three times with 5 mL water. The water from the three rinses after each step was combined. Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure barium and calcium levels, after dilution where necessary. The trap 1 sample and a GoM sediment sample were analyzed twice to evaluate the uncertainties of this method. The results of the repeated measures for total barium levels are consistent (±6%), and values of the National Institute of Standards and Technology (NIST) 1643e standard are within 1% of the certified amount.
Measurement of total Ba level in all trap samples.
For measuring the total Ba level in trap samples, about 10 mg of material was filtered onto 0.6-µm polycarbonate (PC) filters (47 mm) that had been prerinsed with, first, 40 mL of 10% HCL and, then, 200 mL of MQ water. Samples were digested using 0.5 mL concentrated HNO3 (Seastar Baseline) + 0.3 mL HF (Ultrex) + 0.2 mL H2O2 (ACS reagent grade). Samples were microwaved in sealed Teflon containers for 5 min, cooled, and then microwaved another 5 min. Next, 2.4 mL of 5% boric acid (ACS reagent grade) was added, and the sample was microwaved for another 5 min. On cooling, the samples were diluted 20× in 1% HNO3 and analyzed for Ba, Al, and Fe using a Thermo Fisher Element 2 ICP-MS operated in medium resolution. Calibration was by external standards, and 2 ppb In was added to the diluted samples as an internal standard.
Phytoplankton and foraminifera enumeration.
Subsamples were investigated using an inverted microscope (IM 35; Zeiss) according to the Utermöhl method (80). A minimum of 50–100 phytoplankton cells of the dominant groups were enumerated at four magnifications (100×, 160×, 250×, and 400×) using phase contrast microscopy. Only the dominant, identifiable cells were considered; for diatoms, intact cells (plasma containing frustules), empty frustules, and resting spores were counted separately. Totals are presented.
Planktonic foraminifera were picked wet from 1/16 or 1/32 splits after wet sieving at 100 µm. All foraminifera were picked with a micropipette, speciated, and counted. Total numbers of foraminifera counted per sample ranged from 9 (August 25 sample, 1/32 split) to 270 (January 11 sample, 1/16 split), with an average of 154 foraminifera per sample.
Analysis for hydrocarbons and olefins.
Trap sediments and overlaying water fixed with HgCl2 were transferred to plastic centrifuge containers and centrifuged at 3,400 × g for 10 min. Water was decanted, and the sediments were transferred to ashed aluminum trays and then dried in an oven at 40 °C under a flow of air filtered through Florisil until dryness. Dry sediments were weighed, ground, and then extracted using 70:30 dimethyl chloride:methanol by Accelerated Solvent Extraction system 200 (ASE 200; previously Dionex, now Thermo Fisher). Six hundred nanogram 17β(H),21β(H)-Hopane (catalog no. 07562; Sigma Aldrich) was added to the extraction cell before extraction. Extracts were evaporated to about 1 mL under a gentle flow of N2, then an alumina gel liquid chromatograph column was used to purify hydrocarbons (39). Samples were concentrated to ∼200 mL, and then transferred to GC vials and spiked with 0.5 ppm deuterated PAH standard mix, which consists of five separate standards: Acenaphthylene-D8 (catalog no. DLM-2204–1.2; Cambridge Isotope Labs), Indeno[1,2,3-cd]pyrene-D12 (catalog no. DLM-2148–1.2; Cambridge Isotope Labs), Benzo[a]pyrene-D12 (catalog no. S-431; SPEX), Anthracene-D10 (catalog no. 48863; Supelco), and Terphenyl-D14 (catalog no. 48418; Supelco).
Saturated hydrocarbon and olefins analyses were performed on a comprehensive GC×GC TOF mass spectrometer (LECO): An Rxi-1ms column (20 m length, 0.18 mm i.d., 0.18-μm film thickness; Restek) was used in the first dimension, and a BPX50 column (1 m length, 0.1 mm i.d., 0.1-μm film thickness; SGE) was used in the second dimension (49). Primary oven temperature was held at 60 °C for 12 min and then ramped from 60 °C to 350 °C at 1.5 °C⋅min−1. The secondary oven was set to be 30 °C hotter than the primary oven, and the modulator was set to be 55 °C hotter than the primary oven. The TOF detector signal was sampled at a data acquisition rate of 200 spectra per second. The transfer line was held at a constant temperature of 310 °C, and the source temperature was set at 200 °C. The detector voltage was 1,677 V, the mass scan range was 50–300 amu, and electron energy was 70 eV. Front inlet temperature was 300 °C, initially splitless and then changed to a split ratio of 20:1. The carrier flow was constant at 1.5 mL⋅min−1.
PAH analysis was performed on a Varian GC-MS (now Agilent) with an option of large volume injection to achieve a lower detection limit. An Rxi-5ms column (30 m length, 0.25 mm i.d., 0.25-μm film thickness; Restek) was installed, and the column oven was held at 50 °C for 5 min, then ramped to 310 °C at 10 °C⋅min−1, then held at 310 °C for 20 min. The front inlet with ability of cryofocusing was programmed to hold at 20 °C for 0.6 min, ramp to 50 °C at 200 °C⋅min−1, and hold at 50 °C for 0.6 min, then ramp to 300 °C at 200 °C⋅min−1, where it remained for the rest of the run. The injector was initially split at a ratio of 20:1, splitless at 1.45 min until 4.25 min when the split ratio was increased to 100:1, finally settling at a split of 20:1 at 7.6 min. A constant carrier flow of 1 mL⋅min−1 was used. The transfer line temperature was 310 °C, the source temperature was 150 °C, and electron energy was 70 eV; 20 μL of each sample was injected per run.
To obtain a reliable and accurate result, quality assurance/quality control was implemented throughout the analysis of organics. Known levels of stable-isotope-labeled internal standards were added to calculate the possible loss of low-molecular-weight hydrocarbons during the purification and concentration steps before analysis. The calibration standards were measured at the beginning and the end of the actual sample measurements. Internal standards were also added to monitor instrument precision. For every 12 samples, NIST standard reference material (SRM) 1648 was processed and analyzed in a manner identical to the trap samples. Only results that fell within certified values were accepted. Recovery rate of low molecular weight hydrocarbons, e.g., naphthalene, was about 50–70%, and recovery of high molecular weight (HMW) hydrocarbons, e.g., chrysene, was 85–115%.
BC analysis.
BC was determined using the Interagency Monitoring of Protected Visual Environments (IMPROVE) protocol (81–83). There is no universally accepted method for measurements of BC in ocean sediment samples (84). The method used here has the advantage of determining levels of both char and soot, which are two BC components. The chemothermal oxidation pretreatment (CTO)-375 method (85), which has frequently been used for marine particles and sediments, includes only analysis of soot, not char. We compared our methods and CTO-375 and found that soot levels determined with both methods significantly correlated (R = 0.84, P < 0.01) (86).
About 150 ± 30 mg ground (<63 µm) and homogenized samples were weighed for acid pretreatments (81, 82). In brief, hydrochloric acid (HCl), HF, and their mixture were used to remove carbonate, metal oxides, and minerals. The remaining residues were filtered through a 47-mm quartz filter (0.4-µm pore size; Whatman) and air-dried in a baking oven at 35 °C. A 0.526-cm2 circular punch from the filters was stepwise heated to 120 °C, 250 °C, 450 °C, and 550 °C in a pure helium environment, and four organic carbon (OC) fractions were produced: OC1, OC2, OC3, and OC4. Then the oven temperature was raised to 550 °C, 700 °C, and 800 °C in a 2% O2/98% He atmosphere, and three elemental carbon (EC) fractions were produced: EC1, EC2, and EC3. In this procedure, the pyrolyzed organic carbon produced in the inert He atmosphere was monitored using a laser to determine the OC/EC split. The IMPROVE protocol defines the sum of all three elemental carbon (EC) fractions minus pyrolyzed organic carbon as BC. EC1 minus pyrolyzed organic carbon was defined as char, and the sum of EC2 and EC3 was defined as soot (87).
Electron microprobe analysis of barite particles.
To examine the particle size and morphology of barite particles, a small fraction of the residue phase samples from the sequential leaching experiment in cup 1 and cup 14 were subjected to electron microprobe (Cameca SX-100) analysis at Rensselaer Polytechnic Institute. Processed sediments were observed by backscattered electron (BSE) imaging, which indicates the material's electron density where image brightness is proportional to mean atomic number. Processed sediments were placed on carbon tape and coated with ∼20 nm of carbon for electrical conductivity. Electron column conditions included 15 keV accelerating voltage and 1-nanoamp beam current, using the 70-μm aperture. Images were acquired with a single 25-s frame time in scanning mode. Barite appears bright white near saturation in BSE images due to the very high electron density of barium.
Comparison of Annual POC Sedimentation Patterns in 2010/2011 with 2012/2013
The 2010 August/September sedimentation event was exceptional, in both magnitude and timing (Fig. S1): It was 2–5 times higher than other peak sedimentation events that were observed in the remainder of 2010 through July 2013. Seasonal peak sedimentation was observed in spring and early summer in 2011 and 2013 and was coupled to Mississippi discharge (Fig. S2). Furthermore, sedimentation rates in August/September in 2011 and 2012 were low, indicating the anomalous nature of the rates observed in August 2010. Comparison of the 2012/2013 trap with one deployed ∼100 m below, 25 m above the seafloor, reveals that sedimentation rates of both traps were within ±2% (upper trap slightly higher, as expected) and that sedimentation patterns were identical. Overall estimated uncertainty of flux rates is ∼10%.
Input of Suspended Particles from the Mississippi River
Discharge of suspended minerals at St. Francisville US Geological Survey (USGS) station (lat 30°45'30“N, long 91°23'45”W, site 07373420) peaked in March 2011 (Fig. S2). Mississippi River discharge contributes nutrients and suspended minerals to the GoM, leading to an annual spring bloom and smaller summer sedimentation pulses at the site of our trap. Data were downloaded from the USGS website.
Calculation of Black Carbon Input
The ISB of crude oil during the DwH incident left a remarkable footprint in the form of burn residues (28). Production rates of residues vary from 1% to 9% of the mass of crude oil burned, depending on the burning parameters, like burn temperature, thickness and surface area of crude oil on the water surface, etc. (31, 32). The fraction of oil with a low boiling point (BP <204 °C) will largely be missing in burn residues due to its rapid evaporation. Burn residues contain some quantity (∼7%) of the middle boiling point fraction (BP from 204 °C to 580 °C), but they are dominated by the high boiling point fraction and by newly formed char (88). Because of preferential loss of light to middle-weight oil components and formation of charred material, burn residues have a higher density than the original oil, and may even be heavier than seawater. The fate of these burn residues, e.g., their settling speeds, depends on their hydrodynamic particle size and hydrological parameters.
Two types of BC, char and soot, can be formed during incomplete combustion. The soot, which forms in the gas phase and typically has particle size < 1 μm, is released to the air, whereas char, which normally has a particle size of >1 μm, remains with burn residues (89). Traffic and atmospheric deposition would mainly produce soot, whereas ISB can produce both char and soot. High levels of char rather than soot were observed in cup 1 (Table S1), indicating that BC in cup 1 mainly comprised char formed in ISB. Due to its small particle size, soot emitted from ISB can be distributed farther in the air.
Approximately 220,000–310,000 barrels of oil were burned, equivalent to 3.2–4.6 × 1010 g of crude oil (28). Assuming that 5% of burned oil became BC and was deposited within 24 km of the well (∼1.8 × 109 m2), which is approximately the area where ISB took place (29), the total BC released in this area would be 900–1,300 mg⋅m−2. Level of excess char (above the background level) in cup 1 was about 170 ± 4 mg⋅m−2, which was 15–20% of the total input of char.
Hydrocarbons and Their Source Analysis
Hydrocarbons in the GoM can be derived from different sources. PAHs and alkanes were used to distinguish between contributions from crude oil and alternative sources.
The CPI was calculated based on the following equation: , where C is the abundance of each n-alkane (90). The CPI of fossil fuels is around 1, whereas the CPI value of fresh, higher plants is 5 or higher. This ratio can thus be used to distinguish between hydrocarbons originating from crude oil and those from higher plants. A CPI of 1.5 was chosen as the upper boundary for crude oil-dominated alkanes. The early diagenesis process can decrease the CPI of higher plants, obscuring the distinction with time. The CPI of sediment from a reference site at lat 30°19'29“N, long 88°23'12”W, which was not impacted by the DwH spill, is 2.5 (±0.1), and the CPI of settling matter was >1.6 in all 2012 traps (Table S3).
C0/(C0+C1)P/A is the ratio between the parental PAHs with a mass of 178 (P/A) and the sum of these two compounds plus their monomethyl homolog compounds (MPa). This ratio is generally used to differentiate between unburned petrogenic sources and combustion sources (91, 92). Four monomethyl Pa compounds (i.e., 2-MP, 1-MP, 3-MP, and 4-MP), which were the major C1-P/A compounds detected in the trap samples, were identified and used for calculating the C0/(C0+C1)P/A values in our study, and a value of ≤0.5 was used to delineate PAH dominated by crude oil. Unlike the CPI, this PAH source indicator is still senstive with regard to changes caused by weathering. After crude oil is released into the environment, P and A, two parental PAH compounds (C0) are preferentially lost relative to their alkylated homologs (C1-P/A) in the early weathering processes (35). Thus, differences in this ratio between crude oil and combustion sources (i.e., pyrogenic PAHs) only become more pronounced (i.e., larger) over time. In samples with C0/(C0+C1)P/A values ≤ 0.5, the ΣSH/ΣPAH ratios are also larger than 30 (Table S1). The ΣSH/ΣPAH ratio is elevated in fresh crude oil (∼420 in NIST SRM 2779—GoM crude oil), whereas it is much less in pyrogenic sources (e.g., ∼11 in NIST SRM1648—urban particulate matter). The elevated ΣSH/ΣPAH ratios in the first several samples confirm the input from crude oil in this period.
Fl/(Fl+Py), the ratio of Fl to the sum of Fl and Py, has been widely used in studies to differentiate between petrogenic and pyrogenic sources (36, 39, 93, 94). A ratio of Fl/(Fl+Py) less than 0.4 is indicative of a petrogenic PAH source. A ratio between 0.4 and 0.5 signals a mixture of petrogenic and pyrogenic sources, whereas a ratio greater than 0.5 is likely dominated by pyrogenic sources (36, 94, 95). However, we found that this ratio is sensitive to input from pyrogenic sources but not sensitive to the introduction of petrogenic sources (93). Poor response to the petrogenic input results from the fact that Fl and Py are only minor petrogenic PAH constituents, amounting to only about 0.04% (±0.03) of total PAH (96). However, Fl and Py abundance in pyrogenic sources can account for up to ∼7.0% (±3.1) of total PAH (97). Thus, minimal mixing of pyrogenic sources with petroleum-derived samples can alter the ratio of Fl/(Fl+Py) in samples dramatically (93). In contrast, phenanthrene-based indices are sensitive to inputs of both petrogenic and pyrogenic sources.
Alkylbenzenes, cycloalkanes, and alkylcyclohexane were also detected in some trap samples and in GoM crude oil Standard (NIST SRM 2779), but these compounds were below the quantification limit (signal:noise < 6) in many of the trap samples. Sterane and hopane ratios (Table S4) have been used to differentiate oil sources (95), but, similarly, some of these biomarkers were below the quantification limit due to low mass of some trap samples. For example, steranes could only be quantified in 12 of the 20 samples. The ratio Ts/Tm of two C27 hopanes, 18α-22,29,30-trisnorneohopane (Ts) and 17α-22,29,30-trisnorhopane (Tm), has been used to differentiate between crude oil and combustion products (71). In the first six trap samples, this ratio ranged from 1.00 to 1.30, and tended to be lower thereafter, although the trend was not clear (Table S4). The Ts/Tm ratio in Macondo oil (SRM 2779) analyzed in our laboratory was 1.40, similar to the ratio reported in other studies (71). The different ratios observed in some trap samples suggest the input of other sources of hopanes (e.g., from combustion of diesel) in addition to the spilled oil. In addition, we also observed the difference in steranes ratios between trap samples and NIST GoM crude oil standard (Table S4), presumably due to changes in biomarker ratios as oil weathers (71, 95, 98) as well as the mixing of other hydrocarbon sources in trap samples. Principal component analysis of these biomarker ratios can be a powerful approach (71) because it could group samples based on a suite of ratios rather than any individual one. Fig. S5 shows the PCA results on eight hopane and sterane ratios in these 12 samples. Samples from 2010 loosely groups with Macondo oil, whereas 2011 samples show a distinctly different distribution of PC scores from Macondo oil (Fig. S5). This result is consistent with CPI and PAH source analysis.
Resuspension of seafloor sediments can be excluded as a source of the hydrocarbons observed in traps between August 2010 and January 2011, because benthic foraminifera, which would be included in resuspended material, were only observed after May 2011 (cup 13, 4–25 May 2011; cup 14, 25 May through 15 June 2011; and cup 18, 17 August through 7 September 2011). Foraminiferal flux was low (∼100 m−2⋅d−1) during summer/fall but averaged 298 shells m−2⋅d−1 in winter and late spring, a seasonal pattern typical for this region (99). Composition was dominated by 12 planktonic species, including warm and cold water assemblages depending on season, but input of benthic species (e.g., Bulimina aculeata) was below 1% in the above-mentioned cups, and completely absent in fall and winter of 2010.
Carbon Source of POC
The ∆14C-POC reflects the relative contribution of fossil (e.g., oil) carbon to fresh (e.g., diatom) carbon, whereas the PAH source indicator [C0/(C0+C1)-P/A] reflects the contribution of fossil carbon to hydrocarbon flux. Patterns of both indicators are in agreement with the proposed flux dynamics (Fig. S4). Although contributions of fossil carbon to hydrocarbon flux were initially high (phase 1, see Table 1), the large fraction of diatom carbon (young carbon) in sinking matter led to an overall young ∆14C-POC age. This initial exceptional sedimentation event transiently removed fossil carbon from the water column above the trap, so that, compared with cup 1, the ∆14C-POC age was younger in cup 2, which continued to collect sinking diatom carbon, albeit at a greatly reduced rate. As oil components were replenished in the source waters of the trap and contributions of fresh marine organic carbon decreased, the ∆14C-POC age increased (phase 2), although absolute contributions of fossil hydrocarbon sedimentation declined. After December 2010, the contribution of fossil carbon to sinking material became small and the overall ∆14C-POC age was dominated by younger (marine and terrestrial) organic carbon (phase 3).
The input from the Mississippi River plume is an important third component of organic material input to the sediment trap, as evidenced by the significant association of POC with PIM (r2 = 0.76, P < 0.001). This has also been noted in Goni et al. publications (65–67). A three component mixing model in development is consistent with that hypothesis. Overall, the 14C data reflect the large phytoplankton bloom observed early in the deployment (+76‰), more negative values indicating greater petrocarbon input following the bloom period (−114 to −141‰), and then a slow recovery indicated by increasing 14C enrichment over the deployment period towards background values (−20 to −60‰).
The Association of Barium with Various Particulate Phases
In marine waters, barium (Ba) can be associated with various particulate phases (56). Ba content in these different phases were measured in trap samples 1 and 14, following a sequential leaching approach modified from Eagle et al. (55). The fluxes of barium as carbonates, organic matter, Fe–Mn hydroxides, terrestrial aluminum-silicates, and barite in cup 1 were 61, 29, 39, 131, and 264 µg·m−2·d−1, respectively, much higher than their corresponding fluxes in cup 14, which were 6, 8, 23, 52, and 160 µg·m−2·d−1. In both samples, the highest Ba fluxes were in the barite phase; electron microprobe backscattered electron (EM-BSE) analysis demonstrated that fine barite particles (between <1 to 5 µm) were very abundant and dominant in trap cup 1, while only a few, larger barite particles (5 to 15 µm) were observed in cup 14 (Fig. S3). The sequential leaching method cannot differentiate between aluminum-silicates (e.g., clays) and opal, making it impossible to evaluate the partitioning of Ba between the Al–Si minerals and opal frustules. Ba can not be incorporated into diatom frustules (100), suggesting negligible direct contribution of Ba from opal in the Al–Si phase. However, fine barite and Al–Si particles (e.g., clay), which individually were too small to sink, cosedimented with the diatom bloom, leading to the high Ba flux in cup 1. Flux of barium enriched carbonates (e.g., planktonic shells) contributed significantly to high flux rates in cup 1 as well. The Ba/Ca ratio in the carbonate phase is significantly higher in trap 1 (0.28%) than in trap 14 (0.08%), which confirms that planktonic shells (the dominant carbonate source in marine waters) were enriched in Ba. Calcium carbonate shells in traps stemmed from foraminifera and pteropods.
Our results suggest that concentrations of barite in the water were elevated until removed by the sedimentation pulse resulting from the diatom bloom and that barium was enriched in the hard parts of pelagic organisms as well. The association of Ba with fine particles (e.g., clay and fine barite) and the incorporation of Ba into biogenic shells allowed for its prolonged residence time in seawater.
Estimates of Spill-Related Sedimentation Rates of Hydrocarbons and Drilling Mud Components
As discussed in Results and Discussion, elevated levels of alkanes and barium in the first six cups of the trap (25 August 2010 to 29 December 2010) were spill-related, whereas contributions from crude oil or drilling mud was less significant in later cups (after January 2011). To assess total sedimentation due to the DwH spill and mediating measures, background (not spill related) sedimentation rates of alkanes, olefins, and barium were estimated based on their flux after January 2011 (cups 7–19). The sedimentation rates in excess of these background values were assumed to be derived from the spill. These estimates may be considered conservative, because low levels of DwH spill-related pollutants likely continued to sink in 2011.
About 2,980 (±200) μg⋅m−2 of the sum of C25 to C36 normal alkanes was derived from crude oil. These alkanes make up 3.5% of fresh Louisiana sweet crude oil, suggesting that about 85.0 (±7.2) mg⋅m−2 of crude oil sank between late August and December 2010. Two studies have estimated the total surface area impacted by DwH spill: (i) Valentine et al. (7) estimated 3,200 km2 based on the hopane concentrations and (ii) Chanton et al. (8) computed affected areas based on spatial distribution of 14C data in core tops. In this study, we use 8,400 km2, which is the total area with detectable fossil carbon signature calculated in Chanton et al. (8). The reason for not using Valentine’s estimation is that it only includes heavily polluted areas with direct fallout of crude oil, whereas oil settled to the deep traps had lingered in the water for several months and would have been well distributed to a much wider area than those identified in Valentine’s study. Using 8,400 km2 as the total surface area impacted, the total amount of oil settled in this period (25 August 2010 through 8 January 2011), denoted here as Toil-trap, was about 7.2 (±0.6) × 108 g. It is about ∼0.14 (±0.01)% of the total amount of crude oil released, assuming 3.2 million barrels (∼5.1 × 1011 g) released to the GoM during the DwH spill; Toil-trap is 0.8–8.1% of the 1.6 (±1.3) × 1010 g of petrocarbon estimated by Chanton et al. (8) (Tchanton) and 0.7–6% of the total crude oil as determined by Valentine et al. (7). Error propagation has been used to calculate the uncertainty (101). For example, the fraction uncertainty of Tchanton is about 0.81, and fractional uncertainty of Toil-trap is ∼0.09, so the propagated fractional uncertainty of the percentage of Toil-trap to Tchanton is 0.82, which is the square root of the sum of 0.812 and 0.092.
ΣPAH flux ranges from 0.14 μg⋅m−2⋅d−1 to 0.87 μg⋅m−2⋅d−1 (Table 1), which is comparable to several trap studies in Mediterranean (47, 48, 61, 62, 102), but it is about one order of magnitude lower than PAH flux reported in Adhikari et al. (93) (1.95–7.78 μg⋅m−2⋅d−1). Adhikari et al. deployed three shallow (150, 250, 350 m) sediment traps in 2012 and 2013 in two sites close to DwH well (103). The difference between the two studies is expected: (i) ΣPAH, in our work, comprises a much smaller number of compounds than was used to calculate ΣPAH in Adhikari et al.; Adhikari et al. summed 43 individual or groups of PAHs, but some groups of PAH, e.g., C2-P/A, can include about 10 individual isomer compounds; therefore, the sum probably includes more than 100 individual compounds. In contrast, our study only summed 34 PAH compounds (Table S2). In addition, our study did not include an analysis of fluorene and its C1,C2, and C3 alkylated compounds; those compounds are the most abundant compounds in Adhikari’s study. (ii) The traps in Adhikari’s study were deployed in April for only 2–3 d in shallow depths. Spring is the time with the highest discharge of suspended PM from the Mississippi River (Fig. S2). As demonstrated in many studies, including those mentioned above, PAH concentrations, especially of HMW ones, were largely affected by fine particles from terrestrial sources. Our study also showed a significant correlation between PAHs and PIM (P < 0.001), and the Fl/(Fl+Py) ratio did show a significant pyrogenic PAH source, starting from cup 2. Thus, in this study, we do not intend to use the PAH fluxes to indicate input of petroleum sources, because of the significant noncrude oil input.
The background concentration of barium was 423 (±94) ppm, in the same range as background levels of 250–550 ppm reported in Boothe and Presley (64), yielding excess sedimentation rates of barium of ∼64 mg⋅m−2 between late August and the end of December (cups 1–6). Olefins compounds matching the unique fingerprint of drilling mud were not observed after December, and the total calculated olefins input between late August and December 2010 (cups 1–6) was 88 (±4) µg⋅m−2.
Acknowledgments
The deployment of sediment trap could not be performed without the help of several people, including U. Bathmann and G. Rohard (Alfred Wegener Institute), T. Kumbier (K.U.M. Umwelt und Meerestechnik), Joe Montoya (Georgia Institute of Technology), and the crew of RV Oceanus. We also thank Drs. Yongming Han (Chinese Academy of Sciences), Alan Shiller (University of Southern Mississippi), Benjamin Harlow (Washington State University), James Ross (LDEO), Jared Singer (RPI), Samantha Bosman (Florida State University), and Alexander Cherkinsky (UGA Center for Applied Isotope Studies) for careful analysis of samples. Previous versions of the manuscript were improved by comments from Andrew Juhl (LDEO) and Charles Fisher (Pennsylvania State University). The sample collection was funded by two National Science Foundation Rapid projects (OCE-1045330 and 1059103), and the lab analyses was funded by a grant from the Gulf of Mexico Research Initiative to support the “Ecosystem Impacts of Oil and Gas Inputs to the Gulf (ECOGIG)” consortium. Funding was also provided by the National Institute of Environmental Health Sciences Center for Environmental Health in northern Manhattan (p30 ES009089) to establish the Trace Organic lab at LDEO. This is ECOGIG Contribution 441 and LDEO Contribution 8010.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (doi: 10.7266/N7MK69V2, doi: 10.7266/n77942nj, doi: 10.7266/N7BR8Q6H).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513156113/-/DCSupplemental.
References
- 1.Federal Interagency Solutions Group . Oil Budget Calculator: Deepwater Horizon-Technical Documentation. Fed Interagency Solutions Group; Washington, DC: 2010. [Google Scholar]
- 2.McNutt MK, et al. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109(50):20260–20267. doi: 10.1073/pnas.1112139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joye SB, MacDonald IR, Leifer I, Asper V. Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nat Geosci. 2011;4(3):160–164. [Google Scholar]
- 4.Malakoff D. January 15, 2015. After geoscientists joust, judge rules BP Gulf spill totaled 3.19 million barrels of oil. Science. Available at www.sciencemag.org/news/2015/01/after-geoscientists-joust-judge-rules-bp-gulf-spill-totaled-319-million-barrels-oil. Accessed May 18, 2016.
- 5.Camilli R, et al. Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science. 2010;330(6001):201–204. doi: 10.1126/science.1195223. [DOI] [PubMed] [Google Scholar]
- 6.Spier C, Stringfellow WT, Hazen TC, Conrad M. Distribution of hydrocarbons released during the 2010 MC252 oil spill in deep offshore waters. Environ Pollut. 2013;173:224–230. doi: 10.1016/j.envpol.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Valentine DL, et al. Fallout plume of submerged oil from Deepwater Horizon. Proc Natl Acad Sci USA. 2014;111(45):15906–15911. doi: 10.1073/pnas.1414873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanton J, et al. Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the Deepwater Horizon oil spill. Environ Sci Technol. 2015;49(2):847–854. doi: 10.1021/es5046524. [DOI] [PubMed] [Google Scholar]
- 9.Prouty NG, et al. Impact of Deepwater Horizon spill on food supply to deep-sea benthos communities. Estuarine Coastal Shelf Sci. 2016;169:248–264. [Google Scholar]
- 10.Daly KL, Passow U, Chanton J, Hollander D. February 2, 2016 doi: 10.1016/j.ancene.2016.01.006. Assessing the impacts of oil-associated marine snow formation and sedimentation during and after the Deepwater Horizon oil spill Anthropocene. [DOI] [Google Scholar]
- 11.Brooks GR, et al. Sedimentation pulse in the NE Gulf of Mexico following the 2010 DWH blowout. PLoS One. 2015;10(7):e0132341. doi: 10.1371/journal.pone.0132341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passow U, Ziervogel K, Asper V, Diercks A. Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ Res Lett. 2012;7(3):035301. [Google Scholar]
- 13.Alldredge AL, Silver MW. Characteristics, dynamics and significance of marine snow. Prog Oceanogr. 1988;20(1):41–82. [Google Scholar]
- 14.Asper VL. Measuring the flux and sinking speed of marine snow aggregates. Deep Sea Res Part A. 1987;34(1):1–17. [Google Scholar]
- 15.Smetacek V. Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance. Mar Biol. 1985;84(3):239–251. [Google Scholar]
- 16.Asper VL, Deuser W, Knauer G, Lohrenz S. Rapid coupling of sinking particle fluxes between surface and deep ocean waters. Nature. 1992;357:670–672. [Google Scholar]
- 17.Passow U. Formation of rapidly-sinking, oil-associated marine snow. Deep Sea Research Part II: Topical Studies in Oceanography. October 2014. [DOI]
- 18.Peterson CH, et al. A tale of two spills: Novel science and policy implications of an emerging new oil spill model. Bioscience. 2012;62(5):461–469. [Google Scholar]
- 19.Gillis J, Robertson C. July 27, 2010 On the surface, gulf oil spill is vanishing fast; New York Times, p A1. [Google Scholar]
- 20.Turner RE, Rabalais NN. Coastal eutrophication near the Mississippi River delta. Nature. 1994;368(6472):619–621. [Google Scholar]
- 21.Hu C, et al. Did The northeastern Gulf of Mexico become greener after the Deepwater Horizon oil spill? Geophys Res Lett. 2011;38(9):L09601. [Google Scholar]
- 22.Hamm CE. Interactive aggregation and sedimentation of diatoms and clay‐sized lithogenic material. Limnol Oceanogr. 2002;47(6):1790–1795. [Google Scholar]
- 23.Parsons ML, Turner RE, Overton EB. Sediment-preserved diatom assemblages can distinguish a petroleum activity signal separately from the nutrient signal of the Mississippi River in coastal Louisiana. Mar Pollut Bull. 2014;85(1):164–171. doi: 10.1016/j.marpolbul.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Kiorboe T, Lundsgaard C, Olesen M, Hansen JLS. Aggregation and sedimentation processes during a spring phytoplankton bloom—A field experiment to test coagulation theory. J Mar Res. 1994;52(2):297–323. [Google Scholar]
- 25.Muschenheim DK, Lee K. Removal of oil from the sea surface through particulate interactions: Review and prospectus. Spill Sci Technol Bull. 2002;8(1):9–18. [Google Scholar]
- 26.Koelmans AA, et al. Black carbon: The reverse of its dark side. Chemosphere. 2006;63(3):365–377. doi: 10.1016/j.chemosphere.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Mari X, et al. Effects of soot deposition on particle dynamics and microbial processes in marine surface waters. Global Biogeochem Cycles. 2014;28(7):662–678. [Google Scholar]
- 28.Perring AE, et al. Characteristics of black carbon aerosol from a surface oil burn during the Deepwater Horizon oil spill. Geophys Res Lett. 2011;38(17):L17809. [Google Scholar]
- 29.Allen AA, Jaeger D, Mabile NJ, Costanzo D. The use of controlled burning during the Gulf of Mexico Deepwater Horizon MC-252 oil spill response. Int Oil Spill Conf Proc. 2011;2011(1):abs194. [Google Scholar]
- 30.Mabile N. Proceedings of the 2012 Interspill Conference and Exhibition. Interspill Ltd; Poole, UK: 2012. The coming of age of controlled in-situ burning. [Google Scholar]
- 31.API . A Decision-Maker's Guide To In-Situ Burning. Am Petrol Inst; Washington, DC: 2005. Publ no 4740. [Google Scholar]
- 32.Buist I. Window-of-opportunity for in situ burning. Spill Sci Technol Bull. 2003;8(4):341–346. [Google Scholar]
- 33.Flores-Cervantes DX, Plata DL, MacFarlane JK, Reddy CM, Gschwend PM. Black carbon in marine particulate organic carbon: Inputs and cycling of highly recalcitrant organic carbon in the Gulf of Maine. Mar Chem. 2009;113(3–4):172–181. [Google Scholar]
- 34.Aeppli C, et al. Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ Sci Technol. 2012;46(16):8799–8807. doi: 10.1021/es3015138. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Fingas M, Page DS. Oil spill identification. J Chromatogr A. 1999;843(1):369–411. [Google Scholar]
- 36.Yunker MB, et al. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem. 2002;33(4):489–515. [Google Scholar]
- 37.Gustafsson Ö, Gschwend PM, Buesseler KO. Using 234 Th disequilibria to estimate the vertical removal rates of polycyclic aromatic hydrocarbons from the surface ocean. Mar Chem. 1997;57(1):11–23. [Google Scholar]
- 38.Wang ZD, et al. Quantitative characterization of PAHs in burn residue and soot samples and differentiation of pyrogenic PAHs from petrogenic PAHs—The 1994 Mobile Burn Study. Environ Sci Technol. 1999;33(18):3100–3109. [Google Scholar]
- 39.Yan B, et al. Molecular tracers of saturated and polycyclic aromatic hydrocarbon inputs into Central Park Lake, New York City. Environ Sci Technol. 2005;39(18):7012–7019. doi: 10.1021/es0506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy CM, et al. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109(50):20229–20234. doi: 10.1073/pnas.1101242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryerson TB, et al. Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution. Proc Natl Acad Sci USA. 2012;109(50):20246–20253. doi: 10.1073/pnas.1110564109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valentine DL, et al. Propane respiration jump-starts microbial response to a deep oil spill. Science. 2010;330(6001):208–211. doi: 10.1126/science.1196830. [DOI] [PubMed] [Google Scholar]
- 43.Khadikar PV, Mandloi D, Bajaj AV, Joshi S. QSAR study on solubility of alkanes in water and their partition coefficients in different solvent systems using PI index. Bioorg Med Chem Lett. 2003;13(3):419–422. doi: 10.1016/s0960-894x(02)00953-8. [DOI] [PubMed] [Google Scholar]
- 44.Puls W, Kühl H, Frohse A, König P. Measurements of the suspended matter settling velocity in the German Bight (North Sea) Dtsch Hydrogr Z. 1995;47(4):259–276. [Google Scholar]
- 45.Migon C, Sandroni V, Marty J-C, Gasser B, Miquel J-C. Transfer of atmospheric matter through the euphotic layer in the northwestern Mediterranean: Seasonal pattern and driving forces. Deep Sea Res Part II. 2002;49(11):2125–2141. [Google Scholar]
- 46.Payne JR, Clayton JR, Kirstein BE. Oil/suspended particulate material interactions and sedimentation. Spill Sci Technol Bull. 2003;8(2):201–221. [Google Scholar]
- 47.Theodosi C, et al. Downward fluxes of elemental carbon, metals and polycyclic aromatic hydrocarbons in settling particles from the deep Ionian Sea (NESTOR site), Eastern Mediterranean. Biogeosciences. 2013;10(7):4449–4464. [Google Scholar]
- 48.Dachs J, Bayona JM, Fowler SW, Miquel J-C, Albaigés J. Vertical fluxes of polycyclic aromatic hydrocarbons and organochlorine compounds in the western Alboran Sea (southwestern Mediterranean) Mar Chem. 1996;52(1):75–86. [Google Scholar]
- 49.Aeppli C, Reddy CM, Nelson RK, Kellermann MY, Valentine DL. Recurrent oil sheens at the Deepwater Horizon disaster site fingerprinted with synthetic hydrocarbon drilling fluids. Environ Sci Technol. 2013;47(15):8211–8219. doi: 10.1021/es4024139. [DOI] [PubMed] [Google Scholar]
- 50.Reddy CM, et al. Identification and quantification of alkene-based drilling fluids in crude oils by comprehensive two-dimensional gas chromatography with flame ionization detection. J Chromatogr A. 2007;1148(1):100–107. doi: 10.1016/j.chroma.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Neff JM. Composition, Environmental Fates, and Biological Effect of Water Based Drilling Muds and Cuttings Discharged to the Marine Environment: A Synthesis and Annotated Bibliography. Am Petrol Inst; Washington, DC: 2005. [Google Scholar]
- 52.Emam EA. Modified activated carbon and bentonite used to adsorb petroleum hydrocarbons emulsified in aqueous solution. Am J Environ Prot. 2013;2(6):161–169. [Google Scholar]
- 53.Joung D, Shiller AM. Trace element distributions in the water column near the Deepwater Horizon well blowout. Environ Sci Technol. 2013;47(5):2161–2168. doi: 10.1021/es303167p. [DOI] [PubMed] [Google Scholar]
- 54. Schlumberger (2007) M-I Wate – 4.1 Sg BarIte - Introducing a 4.1 SG grade of barite to significantly extend reserves of acceptable drilling grade barite: www.slb.com/%7E/media/Files/miswaco/product_sheets/m-i_wate_barite.ashx.
- 55.Eagle M, Paytan A, Arrigo KR, van Dijken G, Murray RW. A comparison between excess barium and barite as indicators of carbon export. Paleoceanography. 2003;18(3):1021. [Google Scholar]
- 56.Gonneea ME, Paytan A. Phase associations of barium in marine sediments. Mar Chem. 2006;100(1-2):124–135. [Google Scholar]
- 57.Lea DW, Spero HJ. Experimental-determination of barium uptake in shells of the planktonic-foraminifera Orbulina universa at 22°C. Geochim Cosmochim Acta. 1992;56(7):2673–2680. [Google Scholar]
- 58.Hönisch B, et al. Planktic foraminifers as recorders of seawater Ba/Ca. Mar Micropaleontol. 2011;79(1):52–57. [Google Scholar]
- 59.Lira VF, et al. Effects of barium and cadmium on the population development of the marine nematode Rhabditis (Pellioditis) marina. Mar Environ Res. 2011;72(4):151–159. doi: 10.1016/j.marenvres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Polonini HC, et al. Ecotoxicological studies of micro- and nanosized barium titanate on aquatic photosynthetic microorganisms. Aquat Toxicol. 2014;154:58–70. doi: 10.1016/j.aquatox.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Bouloubassi I, et al. PAH transport by sinking particles in the open Mediterranean Sea: A 1 year sediment trap study. Mar Pollut Bull. 2006;52(5):560–571. doi: 10.1016/j.marpolbul.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Dachs J, Bayona JM, Raoux C, Albaigés J. Spatial, vertical distribution and budget of polycyclic aromatic hydrocarbons in the western Mediterranean seawater. Environ Sci Technol. 1997;31(3):682–688. [Google Scholar]
- 63.Chanton JP, et al. Radiocarbon evidence that carbon from the Deepwater Horizon spill entered the planktonic food web of the Gulf of Mexico. Environ Res Lett. 2012;7(4):045303. [Google Scholar]
- 64.Boothe PN, Presley BJ. 1989. Trends In sediment trace element concentrations around six petroleum drilling platforms in the northwestern Gulf of Mexico, Drilling Wastes. Proceedings of 1988 International Conference on Drilling Wastes, ed Engelhardt FR, Ray JP, Gilliam HH (Elsevier, New York), pp 3−22.
- 65.Gordon ES, Goni MA. Controls on the distribution and accumulation of terrigenous organic matter in sediments from the Mississippi and Atchafalaya river margin. Mar Chem. 2004;92(1):331–352. [Google Scholar]
- 66.Gordon ES, Goñi MA. Sources and distribution of terrigenous organic matter delivered by the Atchafalaya River to sediments in the northern Gulf of Mexico. Geochim Cosmochim Acta. 2003;67(13):2359–2375. [Google Scholar]
- 67.Goñi MA, Ruttenberg KC, Eglinton TI. Sources and contribution of terrigenous organic carbon to surface sediments in the Gulf of Mexico. Nature. 1997;389(6648):275–278. [Google Scholar]
- 68.Montagna PA, et al. Deep-sea benthic footprint of the Deepwater Horizon blowout. PLoS One. 2013;8(8):e70540. doi: 10.1371/journal.pone.0070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murawski SA, Hogarth WT, Peebles EB, Barbeiri L. Prevalence of external skin lesions and polycyclic aromatic hydrocarbon concentrations in Gulf of Mexico fishes, post-Deepwater Horizon. Trans Am Fish Soc. 2014;143(4):1084–1097. [Google Scholar]
- 70.Fisher CR, et al. Footprint of Deepwater Horizon blowout impact to deep-water coral communities. Proc Natl Acad Sci USA. 2014;111(32):11744–11749. doi: 10.1073/pnas.1403492111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White HK, et al. Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. Proc Natl Acad Sci USA. 2012;109(50):20303–20308. doi: 10.1073/pnas.1118029109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi YH, Wang Y. Dynamics of carbon sequestration in a coastal wetland using radiocarbon measurements. Global Biogeochem Cycles. 2004;18(4):GB4016. [Google Scholar]
- 73.Vogel JS, Southon JR, Nelson DE, Brown TA. Performance of catalytically condensed carbon for use in accelerator mass-spectrometry. Nucl Instrum Meth Phys Res B. 1984;5(2):289–293. [Google Scholar]
- 74.Stuiver M, Polach HA. Reporting of 14C data. Radiocarbon. 1977;19(3):355–363. [Google Scholar]
- 75.McNichol AP, Aluwihare LI. The power of radiocarbon in biogeochemical studies of the marine carbon cycle: Insights from studies of dissolved and particulate organic carbon (DOC and POC) Chem Rev. 2007;107(2):443–466. doi: 10.1021/cr050374g. [DOI] [PubMed] [Google Scholar]
- 76.Trumbore S. Radiocarbon and soil carbon dynamics. Annu Rev Earth Planet Sci. 2009;37:47–66. [Google Scholar]
- 77.Brenna JT, Corso TN, Tobias HJ, Caimi RJ. 1997. High-precision continuous-flow isotope ratio mass spectrometry. Mass Spectrom Rev 16(5):227−258, and erratum (1997) 16(6):382. [DOI] [PubMed]
- 78.Fry B, Silva SR, Kendall C, Anderson RK. Oxygen isotope corrections for online δ34S analysis. Rapid Commun Mass Spectrom. 2002;16(9):854–858. doi: 10.1002/rcm.651. [DOI] [PubMed] [Google Scholar]
- 79.Coplen TB, Krouse HR. Sulphur isotope data consistency improved. Nature. 1998;392(6671):32. [Google Scholar]
- 80.Utermöhl H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt Int Verh Limnol. 1958;9:1–38. [Google Scholar]
- 81.Han YM, et al. The effect of acidification on the determination of elemental carbon, char-, and soot-elemental carbon in soils and sediments. Chemosphere. 2009;75(1):92–99. doi: 10.1016/j.chemosphere.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 82.Han Y, et al. Evaluation of the thermal/optical reflectance method for quantification of elemental carbon in sediments. Chemosphere. 2007;69(4):526–533. doi: 10.1016/j.chemosphere.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 83.Chow JC, et al. The dri thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. Air quality studies. Atmos Environ Part A. 1993;27(8):1185–1201. [Google Scholar]
- 84.Louchouarn P, et al. Elemental and molecular evidence of soot- and char-derived black carbon inputs to New York City’s atmosphere during the 20th century. Environ Sci Technol. 2007;41(1):82–87. doi: 10.1021/es061304+. [DOI] [PubMed] [Google Scholar]
- 85.Gustafsson Ö, Haghseta F, Chan C, MacFarlane J, Gschwend PM. Quantification of the dilute sedimentary soot phase: Implications for PAH speciation and bioavailability. Environ Sci Technol. 1996;31(1):203–209. [Google Scholar]
- 86.Han Y, et al. Thermal/optical methods for elemental carbon quantification in soils and urban dusts: Equivalence of different analysis protocols. PLoS One. 2013;8(12):e83462. doi: 10.1371/journal.pone.0083462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han Y, et al. Evaluation of the thermal/optical reflectance method for discrimination between char- and soot-EC. Chemosphere. 2007;69(4):569–574. doi: 10.1016/j.chemosphere.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 88.Buist I, Trudel K, Morrison J, Aurand D. Laboratory studies of the properties of in-situ burn residues. Int Oil Spill Conf Proc. 1997;1997(1):149–156. [Google Scholar]
- 89.Masiello CA. New directions in black carbon organic geochemistry. Mar Chem. 2004;92(1):201–213. [Google Scholar]
- 90.Bray EE, Evans ED. Distribution of N-paraffins as a clue to recognition of source beds. Geochim Cosmochim Acta. 1961;22(1):2–15. [Google Scholar]
- 91.Wakeham SG, Schaffner C, Giger W. Polycyclic aromatic hydrocarbons in recent lake sediment I. Compounds having anthropogenic origins. Geochim Cosmochim Acta. 1979;44(3):403–413. [Google Scholar]
- 92.Sporstol S, et al. Source identification of aromatic hydrocarbons in sediments using GC/MS. Environ Sci Technol. 1983;17(5):282–286. [Google Scholar]
- 93.Yan B. 2004. PAH sources and depositional history in sediments from the lower hudson river basin. Ph.D. dissertation (Rensselaer Polytech Inst, Troy, NY)
- 94.Lima AL, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37(1):53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- 95.Aeppli C, et al. Recalcitrance and degradation of petroleum biomarkers upon abiotic and biotic natural weathering of Deepwater Horizon oil. Environ Sci Technol. 2014;48(12):6726–6734. doi: 10.1021/es500825q. [DOI] [PubMed] [Google Scholar]
- 96.Bence AE, Kvenvolden KA, Kennicutt MC. Organic geochemistry applied to environmental assessments of Prince William Sound, Alaska, after the Exxon Valdez oil spill—A review. Org Geochem. 1996;24(1):7–42. [Google Scholar]
- 97.Fine PM, Cass GR, Simoneit BRT. Chemical characterization of fine particle emissions from fireplace combustion of woods grown in the northeastern United States. Environ Sci Technol. 2001;35(13):2665–2675. doi: 10.1021/es001466k. [DOI] [PubMed] [Google Scholar]
- 98.Overton EB, Miles MS, Meyer BM, Gao H, Turner RE. Oil source fingerprinting in heavily weathered residues and coastal marsh samples. Int Oil Spill Conf Proc. 2014;2014(1):2074–2082. [Google Scholar]
- 99.Spear JW, Poore RZ. Seasonal Flux and Assemblage Composition of Planktic Foraminifera from the Northern Gulf of Mexico, 2008−2009. U. S. Geol Surv; Boulder, CO: 2011. [Google Scholar]
- 100.Sternberg E, Tang D, Ho T-Y, Jeandel C, Morel FM. Barium uptake and adsorption in diatoms. Geochim Cosmochim Acta. 2005;69(11):2745–2752. [Google Scholar]
- 101.Hughes I, Hase T. Measurements and Their Uncertainties: A Practical Guide to Modern Error Analysis. Oxford Univ Press; New York: 2010. [Google Scholar]
- 102.Lipiatou E, et al. Mass budget and dynamics of polycyclic aromatic hydrocarbons in the Mediterranean Sea. Deep Sea Res Part II. 1997;44(3):881–905. [Google Scholar]
- 103.Adhikari PL, Maiti K, Overton EB. Vertical fluxes of polycyclic aromatic hydrocarbons in the northern Gulf of Mexico. Mar Chem. 2015;168:60–68. [Google Scholar]