Abstract
Immunomodulatory therapies, targeting the immune checkpoint receptor-ligand complex PD-1/PD-L1 have shown promising results in early phase clinical trials in solid malignancies, including carcinomas of the head and neck. In this context, PD-L1 protein expression has been proposed as a potentially valuable predictive marker. In the present study, expression of PD-L1 and PD-1 was evaluated by immunohistochemistry in 80 patients with predominantly HPV-negative oral squamous cell carcinomas and associated nodal metastasis. In addition, CD274/PD-L1 gene copy number status was assessed by fluorescence in situ hybridization analysis. PD-L1 expression was detected in 36/80 (45%) cases and concordance of PD-L1 expression in primary tumor and corresponding nodal metastasis was present in only 20/28 (72%) cases. PD-1 expression was found in tumor-infiltrating lymphocytes (TILs) but not in tumor cells. CD274/PD-L1 gene amplification was detected in 19% of cases, with high level PD-L1 amplification present in 12/80 (15%), and low level amplification in 3/80 (4%). Interestingly, CD274/PD-L1 gene amplification was associated with positive PD-L1 immunostaining in only 73% of cases. PD-L1 copy number status was concordant in primary tumor and associated metastases. Clinically, PD-L1 tumor immunopositivity was associated with a higher risk for nodal metastasis at diagnosis, overall tumor related death und recurrence. Based on our findings we propose to include PD-L1 copy number status in addition to protein status in screening programs for future clinical trials with immunotherapeutic strategies targeting the PD-1/PD-L1 axis.
Keywords: PD-L1, PD-1, oral squamous cell carcinoma, fluorescence in situ hybridization, gene amplification, Pathology Section
INTRODUCTION
Squamous cell carcinomas comprise over 95% of malignant tumors in the head and neck region, including the oral cavity. The disease is associated with a poor prognosis, with an overall 5-year survival rate of 60%. Two-thirds of the tumors present with locally advanced or metastatic disease (stages III and IV). Historically, oral cavity squamous cell carcinoma (OCSCC) was viewed as a tobacco- and alcohol-related cancer, but infection with human papillomaviruses (HPVs) has emerged as an important risk factor for OCSCCs (reviewed in [1]) Treatment recommendations according to the National Comprehensive Cancer Network include surgery for patients with early-stage tumors and surgery or definitive concurrent chemoradiotherapy for those with advanced-staged tumors [2].
Tumor immunotherapy targeting the immune regulatory molecules programmed death 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1) have shown antitumor effects in a subset of patients with solid tumors and have become an interesting prospect for the treatment of head and neck squamous cell carcinoma (HNSCC). HNSCC is an immunosuppressive disease, with tumor cells evading the host immune system through a number of mechanisms, including alteration of their own immunogenicity and production of immunosuppressive mediators (reviewed in [3]). The PD-1/PD-L1 pathway is important for tumor immune escape and promotion of the tumor microenvironment. PD-L1 is a 290aa type I transmembrane surface glycoprotein constitutively expressed on T-cells, B-cells, myeloid dendritic cells and tissue macrophages. PD-L1 is encoded by the CD274/PD-L1 gene, located on chromosome 9p24.1, in close proximity to PDCD1LG2 (programmed cell death ligand 2). PD-L1 can be upregulated in tumor cells and in a number of different tissue types in response to INF-γ and other inflammatory mediators [4]. Its receptor PD-1 is an immune checkpoint protein, expressed on the surface of several immune cells, particularly cytotoxic T-cells [4-6]. Tumor immune escape can occur through several potential mechanisms, mediated by high tumor expression of PD-L1 and/or tumor immune infiltration by PD-1-positive T-lymphocytes: (1) Binding of PD-L1 by PD-1 on T-cells can lead to functional anergy or apoptosis of T-cells. (2) In regulatory T-cells (Tregs), ligation of PD-1 by PD-L1 can potentially induce immunologic tolerance. (3) Reverse signaling from PD-L1 may prevent tumor cells from apoptosis and (4) PD-L1 interaction with the CD80 ligand can promote inhibition of immune response [7-13].
Aberrant PD-L1 expression has been demonstrated in many solid cancers [14], including melanoma [15, 16], lung cancer [17, 18] and colon cancer [19, 20]. Moreover, selective 9p24.1 gene amplification has recently been identified as an important mechanism for increased PD-L1 protein expression in nodular sclerosing Hodgkins lymphoma and primary mediastinal large B-cell lymphoma [21]. Subsequently, 9p24.1 gene amplification has been detected in a subgroup of gastric carcinoma, colon carcinomas, triple-negative breast cancers and glioblastomas [22, 23]. In a meta-analysis of 25 studies, Wu et al showed that overexpression of PD-L1 is associated with worse 3-year overall survival (OS) in esophageal, hepatocellular and urothelial carcinoma as well as gastric cancer whereas this association was not found in carcinomas of the lung and melanoma [14].
In HNSCC, limited data suggest that PD-L1 is expressed in tumor cells in 50-70% of the cases and that PD-1-positive tumor infiltrating lymphocytes (TIL) may be more common in cancers that are HPV positive than HPV-negative [24-30]. PD-L1 expression is found in various HNSCC tumor cell lines and upregulation was demonstrated in response to the proinflammatory cytokines INF-γ, TNF-α and IL-1β. Interestingly, in a preclinical HNSCC model system, PD-1 mediated signals on T-cells can inhibit antitumor immune response [27, 31].
To further explore the PD-1/PD-L1 axis in HNSCC, we evaluated and compared frequencies of PD-1 and PD-L1 immunohistochemical expression in a clinically well characterized cohort of OCSCCs of 80 primary tumors and 28 associated nodal metastasis and determined its prognostic impact. In addition we analyzed the PD-L1 gene locus on 9p24.1 by fluorescence in situ hybridization (FISH) and identify PD-L1 amplification as an important mechanism of PD-L1 upregulation in OCSCC.
RESULTS
Expression of PD-L1 and PD-1
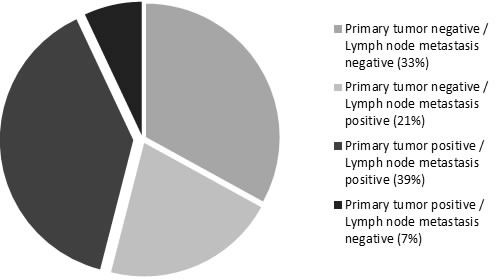
PD-L1 expression was evaluated in tumor cells of primary oral cavity squamous cell carcinoma and associated nodal metastasis by immunohistochemistry. PD-L1 expression in at least 5% of tumor cells was found in 36/80 (45%) of OCSCCs. Mean percentage of positive tumor cells (any strength of expression) was 60% (range: 15%-90%). Strong, membranous staining (3+) was found in 13/36 (36%) cases, intermediate staining (2+) in 13/36 (36%) cases, whereas 10/36 (28%) cases demonstrated weak staining (1+) (Figure 1a-1c). In 28 of the 80 analyzed cases, primary tumors and corresponding nodal metastases were available for assessment. Evaluation of the immunohistochemical PD-L1 staining results in these 28 cases was performed both on tissue cores as well as whole tissue slides to exclude confounding effects due to possible tissue heterogeneity. Among 13 primary tumors positive for PD-L1 expression, 11/13 (85%) of corresponding nodal metastases showed concomitant positive expression of PD-L1, whereas 2/13 (15%) cases displayed loss of staining in the metastasis. A discrepancy between staining results in tissue cores and whole slides was only seen in one case, which showed positive PD-L1 staining in the whole tissue section and negative staining in the tissue cores. In 15 primary tumors negative for PD-L1 expression, 9/15 (60%) of matching nodal metastases were PD-L1 negative, whereas 6/15 (40%) cases were PD-L1 positive. Altogether, in 20/28 (72%) cases, concordant PD-L1 expression was found in matched primary tumors and nodal metastases, and in 8/28 (28%) cases a discordance of PD-L1 expression was present in primary tumor specimen and corresponding nodal metastases (Figure 2).
Figure 1. Squamous cell carcinoma of the head and neck, showing membraneous PD-L1 expression in tumor cells with.
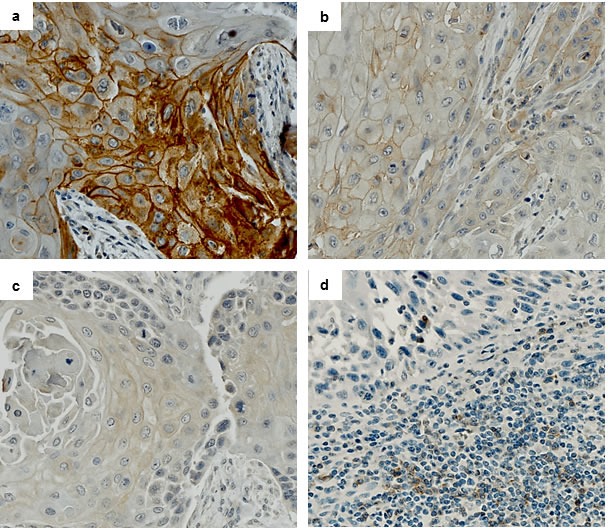
a. strong staining (3+), b. intermediate staining (2+) and c. weak staining (1+) intensity. Fibroblasts within tumor stroma are negative. d) PD-1 staining in squamous cell carcinoma with intermingled tumor-infiltrating lymphocytes (TILs). PD-1 staining is only seen in TILs, while carcinoma cells are negative. (a.-c. PD-L1 immunohistochemistry, d. PD-1 immunohistochemistry).
Figure 2. Concordance and discordance of PD-L1 expression in primary tumor and matched lymph node metastases.
We next evaluated PD-1 expression in both neoplastic as well as inflammatory cells by immunohistochemistry. 79 cases were available for IHC. PD-1 was not expressed in tumor cells whereas tumor-infiltrating lymphocytes (TILs) showed PD-1 staining. 41/79 (52%) cases contained PD-1 positive TILs. Mean percentage of PD-1 positive TILs was 6% (range 3%-20%). 25/36 (69%) PD-L1 positive carcinomas and 16/43 (37%) of PD-L1 negative carcinomas contained PD-1 positive TILs (Figure 1d). There was no significant correlation of PD-L1 and PD-1 expression.
Evaluation of CD274/PD-L1 copy number status by fluorescence in situ hybridization
Given the broad range of PD-L1 immunohistochemical expression in OCSCC, which could not be easily explained by autocrine or paracrine upregulation, we further explored the copy number status of the CD274/PD-L1 gene locus by FISH. PD-L1 FISH analysis was performed in all 80 primary tumors and in 16 corresponding lymph node metastases. 15/80 (19%) primary tumors fulfilled the criteria for amplification: in 12/80 cases (15%), high level amplification of the PD-L1 gene locus, as defined by PD-L1/CEP9 ratio ≥ 4.0 was seen (Figure 3a, 3b) and 3/80 (4%) cases showed low-level PDL-1 amplification as defined by PD-L1/CEP9 ratio ≥ 2.0 and ≤ 4.0 (Figure 3c). Gene copy number gain was restricted to tumor cells and was not present in the inflammatory cell component. A polysomy defined as average PD-L1 copy number > 3 signals/cell was seen in 16/80 (20%) cases (Figure 3d). 49/80 (61%) cases were disomic for the PD-L1 gene locus at 9.24.1 (Figure 3e). The PD-L1 copy number status was concordant in the primary tumor and corresponding lymph node metastases.
Figure 3. Fluorescence in situ-hybridization (FISH) for PD-L1.
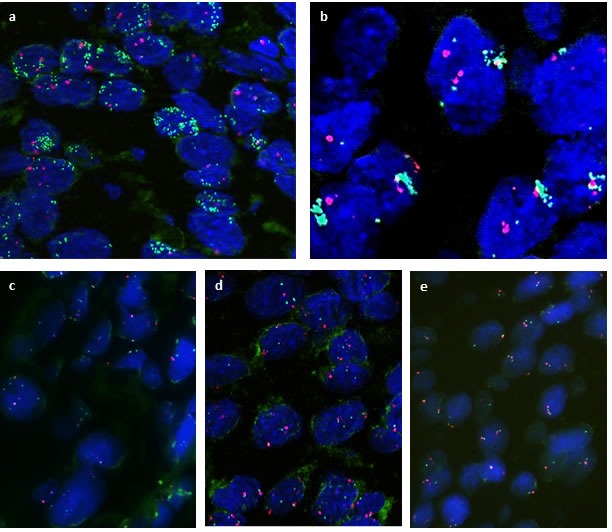
PD-L1 gene is labelled in green, centromer 9 in red. FISH analysis showing a., b. high level amplification, with PD-L1/CEP9 ratio ≥ 4, indicated by clusters of green fluorochrome labeling PD-L1 c. low level amplification, PD-L1/CEP9 ratio ≥ 2.0 - < 4; d. Polysomy of PD-L1 gene locus; e. PD-L1 disomy. (a.-d. SPEC CD274, PDCD1LG2/CEN9 Dual Color Probe).
Correlation between PD-L1 gene amplification and PD-L1 copy number gain with PD-L1 protein expression
Results for both PD-L1 immunohistochemistry and CD274/PD-L1 FISH were available for all 80 cases (Table 2 and Supplementary Table 1). Tumors with CD274/PD-L1 gene amplification displayed PD-L1 positivity by immunohistochemistry in only 11/15 (73%) cases. In contrast, 25/65 (38%) cases without CD274/PD-L1 amplification were PD-L1 immunopositive. Cases with CD274/PD-L1 amplification were PD-L1 immunopositive in a significantly higher frequency than cases without amplification (p = 0.01). 7/13 carcinomas with strong, membranous PD-L1 immunostaining (score 3+) showed high or low level CD274/PD-L1 amplification, 3/13 showed a polysomy and 3 cases displayed a normal copy number status. Interestingly, two of the highly amplified carcinomas and two of the tumors with low-level CD274/PD-L1 amplification were PD-L1 immunonegative (score 0). To confirm the findings and to exclude false negative staining due to tumor heterogeneity in these 4 cases, FISH analysis and immunohistochemistry were repeated on whole tissue sections, giving identical results. Altogether, 40/80 (50%) cases were negative for both PD-L1 immunohistochemistry and PD-L1 FISH.
Table 2. CD274/PD-L1 FISH and PD-L1 immunohistochemistry.
| CD274/PD-L1 FISH | Cases | PD-L1 IHC | |||
|---|---|---|---|---|---|
| n = 80 | Score 3+ |
Score 2+ |
Score 1+ |
Score 0 |
|
| High Level Amplification | 12 (15%) | 7/12 | 1/12 | 2/12 | 2/12 |
| Low Level Amplification | 3 (4%) | 1/3 | 0/3 | 0/3 | 2/3 |
| Polysomy | 16 (20%) | 3/16 | 3/16 | 3/16 | 7/16 |
| Disomy | 49 (61%) | 3/49 | 8/49 | 5/49 | 33/49 |
Correlation of PD-1, PD-L1 expression and CD274/PD-L1 amplification in tumor tissue with clinicopathologic features
All features of clinicopathological data in relation to PD-L1 immunohistochemical expression are summarized in Table 1. In 5 cases, HPV DNA was detected, indicating HPV associated carcinogenesis, with one of these cases showing CD274/PD-L1 high level amplification and PD-L1 immunopositivity. The remaining four cases did not express PD-L1. Patients with PD-L1 immunopositive tumors had a significantly higher risk for lymph node metastasis at diagnosis (p-value = 0.009), whereas PD-L1 immunohistochemical expression was not relevantly correlated with age, gender, grading, tumor size or HPV-positivity. In contrast, both CD274/PD-L1 copy number status and PD-1 immunohistochemical expression were found to be not relevantly correlated with the presence of nodal metastases and were also not relevantly correlated with age, gender, grading, tumor size, or HPV-positivity.
Table 1. Clinicopathological data in relation to PD-L1 immunohistochemical expression.
| Clinicopathologic Parameters | PD-L1 positive | PD-L1 negative |
|---|---|---|
| n (%) | 36 (45%) | 44 (55%) |
| Median age (years) Age range (years) |
57 38-86 |
60 44-84 |
| Gender Male Female |
23 (64%) 13 (36%) |
31 (70%) 13 (30%) |
| Differentiation G1 G2 G3 |
1 (3%) 29 (80%) 6 (17%) |
3 (7%) 27 (61%) 14 (32%) |
| Tumor Size T1 T2 T3 T4a/b |
4 (11%) 15 (42%) 5 (14%) 12 (33%) |
8 (18%) 17 (39%) 9 (20%) 10 (23%) |
| Lymph node metastasis Positive Negative |
26 (72%) 10 (28%) |
19 (43%) 25 (57%) |
| HPV (Type 16) Positive Negative |
1 (3%) 35 (97%) |
4 (9%) 40 (91%) |
Correlation of PD-L1 expression with clinical data
In the SCC patient cohort with PD-L1 immunopositive tumors, the risk of tumor related death was significantly increased (p = 0.01) (Figure 4a) which was also the case for the risk of recurrence (p = 0.05) (Figure 4b). In contrast, PD-1 expression, CD274/PD-L1 copy number, pT category, presence of lymph node metastases or tumor grading were not relevantly associated with these risks (data not shown).
Figure 4. Overall tumor-related survival and recurrence-free survival in patients with SCC of the oral cavity with respect to PD-L1 immunohistochemical status.
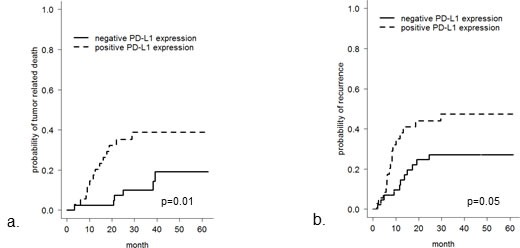
Overall tumor-related survival a. and recurrence-free survival b. is worse in SCC patients with tumoral PD-L1 immunopositivity (p = 0.01 and 0.05, respectively).
DISCUSSION
Malignant tumors can escape the host´s immune system by a process termed “immunoediting”, turning the immune microenvironment into an immunosuppressive state. An important pathway in the immune anti-tumor response is the PD-1/PD-L1 axis [10, 32, 33]. PD-L1 expression in tumor cells is thought to be predictive for tumor response to immunomodulatory therapies targeting the PD-1/PD-L1 pathway [34]. Data on PD-1 and PD-L1 expression in HNSCC concerning prevalence, prognostic impact and variation of expression in disease course is limited.
In the present study, we provide novel molecular genetic evidence that the CD274/PD-L1 gene is amplified in OCSCC and that gene amplification is accompanied by immunohistochemical PD-L1 protein overexpression in only a subset of amplified cases. We also demonstrate that the PD-L1 protein expression status in primary tumors and corresponding nodal metastasis is discordant in a significant number of cases investigated.
Using immunohistochemistry and a cut-off value for positivity defined as at least 5% tumor cells displaying membranous PD-L1 staining, we found PD-L1 expression in 36/80 (45%) of OCSCC, a range similar to published data. To date, only few studies have evaluated PD-L1 expression in OCSCC [27] [31] [24] [25] [28] with positivity rates ranging between 46%-87%. These variable positivity rates are likely due to several factors: (1) Use of different cut-off values for definition of positivity; for example, a study by Ukpo et al used a cut-off value of 5%, whereas Badoual et al and Strome et al defined positivity as any staining > 0% [27] [24] [28]; (2) cytoplasmic staining, in addition to membranous staining was counted as positive by some authors; (3) use of different antibodies clones for immunohistochemistry [27] [31] [24] [25] [28] and (4) inclusion of a high percentage of HPV-positive cases in one of the studies [28].
Up to date, there is limited data concerning changes of PD-L1 expression in tumors during metastatic progression and none of the studies up to date have evaluated SCCs of the head and neck. Results of our own study show that in only 72% of cases, the PD-L1-status was retained in nodal metastatic lesions. In two studies comparing matched tumor samples with corresponding metastatic lymph nodes involving patients with RCC and triple-negative breast cancer, respectively, the concordance rates were considerably higher [33, 35]. Tissue heterogeneity of PD-L1 protein expression or change of PD-L1 expression during metastatic disease progression might contribute to low concordance rates, but further functional follow up studies are needed to clarify this issue.
Tolerance of PD-L1 positive tumors by the immune system is mediated by interaction of PD-L1 on tumor cells with its receptor PD-1, preferentially expressed on Tregs and other immune cells. Upon activation of PD-1/PD-L1, the anti-tumor response of the immune system is attenuated as T-cell functions are suppressed by the mechanisms named above. In HNSCC, co-receptor signals on T-cells mediated by PD-1 can inhibit antitumor response in a pre-clinical model [8, 27, 33, 36, 37]. In our study cohort, all primary tumors were PD-1 negative, whereas PD-1 was expressed in TILs in 24/36 (67%) PD-L1 positive carcinomas and 16/43 (37%) of PD-L1 negative. Absolute numbers of positive TILs were low in comparison to previous studies [9], [26] and may reflect differences between the pathogenesis of OCSCC and other anatomical regions of the head and neck like the oropharynx, where HPV induced carcinogenesis is more prevalent.
In our OCSCC patient cohort, PD-L1 positive carcinomas had a significantly higher risk for nodal metastasis at the time of diagnosis (p-value 0.01). Moreover, the risk of disease-and overall tumor-related death was significantly higher in PD-L1 positive OCSCC. In comparison, results of previous studies have been contradicting in this regard, probably reflecting differences in methodologies used to assess PD-L1 status and differences in patient characteristics, including percentage of HPV-related SCC tumor subgroups [25] [28]. It is well known that human papillomavirus (HPV)-associated HNSCCs tend to have improved clinical outcomes when compared to tobacco-related head and neck cancers. In contrast to oropharyngeal cancer, where HPV has been recognized as an important causative factor for tumor development, its influence on OCSCC pathogenesis is less clear and prevalence rates are much lower.
Expression of PD-L1 in tumors cells is mediated by different mechanisms. It can be induced by autocrine or paracrine factors within the tumor microenvironment, especially INF-γ or HIF1α. In this case, PD-L1 expression is dynamic, with variable, time-dependent expression. On the other hand, expression can be driven by gene amplification events involving the 9p24.1 locus. The 9p24.1 chromosomal locus contains the CD274/PD-L1, PD-L2 and JAK2 genes [21, 23]. Selective 9p24.1 amplification has been recently recognized as an important mechanism for increased PD-L1 expression in nodular sclerosing Hodgkins lymphoma and primary mediastinal large B-cell lymphoma [21] and has also been demonstrated in gastric carcinoma, colon carcinomas, triple-negative breast cancers and glioblastomas [22, 23]. Other signaling pathways, which have been linked to oncogene-induced PD-L1 expression are, amongst others, loss of PTEN and dysregulation of the JAK/STAT pathway, the latter probably providing an additional “enhancer loop” for PD-L1 overexpression when JAK2 is amplified [16, 36, 38-40].
Using FISH analysis, we found amplification of 9p24.1, including the CD274/PD-L1 gene locus, in 19% of OCSCC cases. The amplification driven PD-L1 expression in a subgroup of OCSCC may identify a new subgroup of HNSCC with a disease-specific genetic alteration. Further studies are needed to evaluate the impact of PD-L1 amplification on pathogenesis and disease progression and on prognosis of this newly identified subgroup of HNSCC.
Blocking the PD-1/PD-L1 axis is a promising treatment option in multiple tumor types. Up to date, several clinical studies have been conducted, including especially patients with melanoma and non-small cell lung cancer (NSCLC), but also HNSCC (reviewed in [3]). These studies have demonstrated better response rates in patients with high PD-L1 expression. In addition, immune checkpoint blockage has shown remarkable response rates in lymphomas, especially Hodgkins lymphoma, with PD-L1 amplification [41]. Response to anti-PD-1 treatment was also found, in a significantly lower percentage, in PD-L1 negative cases [8, 33, 42-45]. Considering the fact that the interaction of PD-L1 on tumor cells with PD-1 on TILs leads to escape of cancer cells from the immune system, it is not surprising, that upregulation of PD-1 by recombinant cytokines, stimulatory antibodies or transfer of activated adoptive T-cells increases the effect of PD-1/PD-L1 pathway blockage [27, 46, 47]. The identification of CD274/PD-L1 gene copy number gain as a potential mechanism for PD-L1 overexpression in the present study may provide a rationale for treatment of HNSCC patients, especially in a subgroup of OCSCC with PD-L1 amplification. Future studies have to investigate whether PD-L1 copy number status, which is stable in the primary tumor and corresponding lymph node metastasis, might be a better predictive marker for tumor response to blockage of PD-1/PD-L1 pathway [34] than PD-L1 immunohistochemical status. In any case, the correlation between CD274/PD-L1 gene amplification and the PD-L1 immunohistochemical status is surprisingly poor, with 4 amplified cases being PD-L1 immunonegative. The reason for this discrepancy is currently not clear, but may involve technical problems with the antibody clone used or posttranscriptional or posttranslational modifications.
In summary, we show that PD-L1 is expressed in 45% of OCSCC, when a 5% cut-off of positive cells is applied. PD-L1 positive cases have a significantly higher risk for nodal metastasis at diagnosis. PD-L1 expression is associated with higher risk for overall tumor-related death and recurrence. Although PD-L1 expression status in corresponding nodal metastasis is equivalent to expression in primary tumors in the majority of cases, a considerable number of cases (32%) were discordant. CD274/PD-L1 amplification is a novel finding in OCSCC and is a frequent event, often (but not always) associated with high PD-L1 expression levels and stable during metastatic progression. Based on these data, we propose to include CD274/PD-L1 amplification analyses into studies recruiting patients for immune checkpoint protocols.
MATERIALS AND METHODS
Patient cohort
The retrospective cohort consisted of 80 patients, who underwent surgical resection of squamous cell carcinoma of the oral cavity between 2008 and 2010 at the Klinikum rechts der Isar of the Technical University of Munich, Germany. H&E stained sections were reviewed by three pathologists (MS, ED, KS). Grading and staging was undertaken according to the current World Health Organization Classification of Tumors of Head and Neck (2004) and the AJCC tumor, node, metastasis (TNM) classification (7th edition). Complete clinicopathologic data including age, gender, localization of primary tumor, local, nodal and distant recurrences and overall and disease-free survival were available for all patients (Supplementary Table 1). All patients underwent primary resection of tumors with curative intent following a standardized surgical procedure [48]. Patients did not have distant metastasis at diagnosis. Patients with lymph node metastasis at diagnosis received adjuvant radiotherapy. Mean follow-up was 31 months (range 2 - 63 months). Approval for the study was obtained from the Ethics Review Committee of the Technical University of Munich.
Tissue microarray construction
Formalin-fixed paraffin-embedded tumor samples were assembled into a tissue microarray (TMA) using a Tissue Microarrayer (Beecher Instruments, Sun Praierie, USA) with a core size of 0,6 mm. A minimum of 2 and (where feasible) up to 4 tumor cores from tumor invasion front and tumor center were taken from the primary tumors in areas previously marked by two pathologists (MS, ED). Corresponding regional nodal metastasis from 28 patients were also included in the TMA, selecting 1 or (where feasible) 2 different areas from the metastatic deposits, depending on size of metastasis.
Immunohistochemistry
IHC was performed on 2 μm sections from each TMA using a PD-L1 primary antibody (Cell Signaling, clone E1L3N) at a dilution of 1:100 and a PD-1 primary antibody (Cell Marque, clone 11RQ-22) at a dilution of 1:50. Stainings were run on an automated immunostainer with an iVIEW DAB detection kit (Ventana Medical Systems, Roche, Mannheim, Germany). Immunohistochemical expression of PD-L1 in tumor cells was assessed by counting the percentage of positive tumor cells and quantifying staining intensity in a 4-tiered grading system including “no staining” (0), “weak staining” (1+), “intermediate staining” (2+), “strong staining” (3+). PD-L1 positivity was arbitrarily defined as at least 5% tumor cells displaying membranous PD-L1 staining of any intensity. The cut-off value of 5% was chosen, because many clinical trials involving immunomodulatory therapeutics have chosen this value. Immunohistochemical expression of PD-1 in TILs was assessed by counting the percentage of PD-1 positive TILs. PD-1 positivity was counted as at least 1% positive TILs.
PD-L1 fluorescence in situ hybridization
Dual-color FISH analysis was performed on 2μm sections from formalin-fixed paraffin-embedded tissue. Sections were deparaffinized, dehydrated in 100% ethanol and air-dried. For in situ hybridization, the SPEC CD274, PDCD1LG2/CEN9 Dual Color Probe containing a mixture of fluorochrome direct labelled SPEC CD274, PDCD1LG2 probe specific for the CDC274/PD-L1 and PDCD1LG2/PD-L2 genes located at 9p24.1 and an orange fluorochrome labeled CEN 9 probe specific for the classical satellite III region of chromosome 9 (D9Z3) at 9q12 (Zytovision, Bremerhaven, Germany) was used according to the manufacturer´s instructions. At least 20 nuclei per sample were counted. PD-L1 amplification was defined as PD-L1/CEP9 ratio ≥ 2.0, with high level amplification defined as ≥ 4.0 and low level amplification defined as ≥ 2.0 and ≤ 4.0. Polysomy 9 was defined as average PD-L1 copy number > 3 signals/cell.
HPV analysis
DNA was isolated from formalin-fixed, paraffin-embedded tissue of primary tumor samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) in combination with the QiaCube (Qiagen, Hilden, Germany) according to the supplier's instructions. HPV analysis and determination of HPV subtypes was performed with the HPV LCD 3.5 Array - Kit (Chipron GmbH, Berlin, Germany) according to the manufacturer's recommendations.
Statistics
Descriptive and exploratory statistical analyses were performed using SPSS 21 (SPSS Inc, Chicago, IL, USA) and R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). The distribution of qualitative data was compared between groups using χ2-test or Fisher's exact test, depending on the cell counts of corresponding contingency tables. Likewise, quantitative data was compared between groups using the non-parametric Mann-Whitney U test. Cumulative incidence functions were estimated for censored outcomes to account for competing risks such as death. All statistical tests were performed on exploratory two-sided 5% significance levels.
SUPPLEMENTARY MATERIAL TABLE
Acknowledgments
The authors thank Melitta Bekesch, Klara Fizi, Petra Meyer and Kerstin Schrager for expert technical assistance and Axel Ullrich for support.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Editorial note
This paper has been accepted based in part on peerreview conducted by another journal and the authors’ response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. Journal of Cinical Oncology. 2015;33:3269–3276. doi: 10.1200/JCO.2015.61.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister DG. Head and Neck Cancers, Version 2. 2013 Featured Updates to the NCCN Guidelines (vol 11, pg 917, 2013) J Natl Compr Canc Ne. 2013;11:1458–1458. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 3.Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. Journal of clinical oncology. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O'Toole SA, McCaughan BC, Yearley JH, Horvath LG, Kao S, Boyer M, Scolyer RA. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malm IJ, Bruno TC, Fu J, Zeng Q, Taube JM, Westra W, Pardoll D, Drake CG, Kim YJ. Expression profile and in vitro blockade of programmed death-1 in human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck. 2015;37:1088–1095. doi: 10.1002/hed.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ni L, Ma CJ, Zhang Y, Nandakumar S, Zhang CL, Wu XY, Borthwick T, Hamati A, Chen XY, Kumaraguru U, Moorman JP, Yao ZQ. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunol Cell Biol. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 18.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. doi: 10.1371/journal.pone.0076012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y, Zhou H, Li R, Wang K, Wang W, Hua D, Zhang X. MiR-20b, -21, and -130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol. 2014;75:348–353. doi: 10.1016/j.humimm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O‘Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA. Integrative analysis reveals selective 9p24. 1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett MT, Anderson KS, Lenkiewicz E, Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE, Reddy SK, Ramanathan RK, Northfelt DW, Pockaj BA. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6:26483–26493. doi: 10.18632/oncotarget.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research N Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, Gey A, Rotem-Yehudar R, Pere H, Tran T, Guerin CL, Chauvat A, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 25.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47:1148–1153. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, Chen L, Drake CG, Topalian SL, Pardoll DM, Pai SI. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 28.Ukpo OC, Thorstad WL, Lewis JS., Jr B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113–121. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, Chang Y. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–1403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Liu Z, Cui Y, Wang G, Cao P. [The clinical significance of the expression of costimulatory molecule PD-L1 in nasopharyngeal carcinoma] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22:408–410. [PubMed] [Google Scholar]
- 31.Tsushima F, Tanaka K, Otsuki N, Youngnak P, Iwai H, Omura K, Azuma M. Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol. 2006;42:268–274. doi: 10.1016/j.oraloncology.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, Stanton ML, Hodi FS, McDermott DF, Atkins MB, Freeman GJ, Hirsch MS, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25:2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 39.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 41.Kline J, Bishop MR. Update on checkpoint blockade therapy for lymphoma. J Immunother Cancer. 2015;3:33. doi: 10.1186/s40425-015-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England Journal of Medicine. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 46.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 47.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, Romero P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 48.Kolk A, Jubitz N, Mengele K, Mantwill K, Bissinger O, Schmitt M, Kremer M, Holm PS. Expression of Y-box-binding protein YB-1 allows stratification into long- and short-term survivors of head and neck cancer patients. British Journal of Cancer. 2011;105:1864–1873. doi: 10.1038/bjc.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


