Abstract
Background
An imbalance in the excitatory/inhibitory systems with abnormalities in the glutamatergic pathways has been implicated in the pathophysiology of autism. Furthermore, chronic redox imbalance was also recently linked to this disorder. The goal of this pilot study was to assess the feasibility of using oral N-acetylcysteine (NAC), a glutamatergic modulator and an antioxidant in the treatment of behavioral disturbance in children with autism.
Methods
This is a 12-week, double-blind, randomized, placebo-controlled study of NAC in children with autistic disorder. Subjects randomized to NAC were initiated at 900 mg daily for 4 weeks, then 900 mg twice-daily for 4 weeks and 900 mg three-times-daily for 4 weeks. The primary behavioral measure (Aberrant Behavior Checklist – Irritability subscale) and safety measures were performed at baseline, 4, 8, and 12 weeks. Secondary measures included the ABC-Stereotypy subscale, Repetitive Behavior Scale – Revised (RBS-R), and Social Responsiveness Scale (SRS).
Results
Thirty-three subjects (31 males, 2 females; aged 3.2–10.7 years) were randomized in the study. Follow-up data was available on fourteen subjects in the NAC group and fifteen in the placebo group. Oral NAC was well-tolerated with limited side effects. Compared to placebo, NAC resulted in significant improvements on ABC-Irritability subscale (F=6.80; p<.001; d=.96).
Conclusions
Data from this pilot investigation support the potential usefulness of NAC for treating irritability in children with autistic disorder. Large randomized controlled investigations are warranted.
ClinicalTrials.gov Identifier
Keywords: Autism, glutathione, glutamate, irritability, disruptive behaviors, anti-oxidant
Introduction
Autistic disorder is a pervasive developmental disorder characterized by impairment in communication and reciprocal social interaction, as well as stereotypic/repetitive behaviors. Causes of autism remain elusive yet clearly combine genetic, developmental, and environmental factors (1). Several neurobiologic models have recently been proposed including the existence of a glutamatergic dysfunction (2) and excessive oxidative stress (3). These models have sparked hope for the development of targeted therapeutic agents leading to disease-specific interventions.
Increased ratio of excitation:inhibition (E:I) in sensory, mnemonic, social and emotional systems have been proposed as a model underlying at least some forms of autistic disorder (2). While glutamatergic pathways modulate excitatory neurotransmission, GABAergic pathways modulate inhibitory neurotransmission predominantly. This hypothesis is supported by evidence of increased glutamatergic transmission from neuropathologic and neurobiologic studies. Postmortem investigations have reported increases in expression of the mRNA of several genes associated with glutamatergic pathways including excitatory amino acid transporter 1 and glutamate receptor AMPA type 1, two members of the glutamate system (4). Genetic studies have also reported a link between autism and specific glutamate receptor genes (5–8). Glutamic acid decarboxylase, an enzyme that catalyzes the decarboxylation of glutamate to GABA, has also been reported to be reduced in parietal and cerebellar cortices of individuals with autism (9). Finally, increases in glutamate levels in serum (10, 11) and cerebrospinal fluid (12, 13) have also been observed in children with autism.
Another emerging hypothesis in autism suggests that the condition is a result of redox imbalance (3) – i.e. disequilibrium between oxidants and antioxidants in the body – which leads to accumulation of reactive oxygen species (ROS). Ordinarily, ROS are removed by superoxide dismutase (SOD), catalase, and glutathione (GSH; tripeptide γ glutamyl-cysteinyl-glycine) related enzymes including GSH peroxidase and GSH reductase. Accumulation of ROS can cause chemical modifications and functional changes of DNA, RNA, protein, lipid, and carbohydrate moieties, thereby resulting in cellular dysfunction. The potential involvement of redox imbalance in the pathogenesis of autism has been suggested by neuropathologic (3), genetic (14), and clinical studies (15). Differences in allele frequency and/or significant gene interaction between individuals with autism and typically developing controls were found for relevant genes encoding GSH-S-transferases, a key family of enzymes that detoxify pro-oxidative compounds by coupling them to the body’s main antioxidant molecule, glutathione (14). Peripherally, decreased levels of antioxidant enzymes such as erythrocyte GSH peroxidase and SOD (16), decreased cellular and mitochondrial GSH (15) were found in several investigations. Decreased plasma S-adenosyl-L-homocysteine (SAH) as well as S-adenosyl-L-methionine (SAM), two intermediates in the synthesis of cysteine (14) which is a key precursor of GSH had also been reported. Finally, it is suggested that redox imbalance may cause, at least partly, the neuronal insult and dysfunction seen in autism (15).
N-acetylcysteine (NAC) is an orally bioavailable prodrug of cysteine which is well known for its role as an antidote against acetaminophen overdose-induced liver damage by maintaining or restoring hepatic concentrations of cysteine, leading to GSH synthesis (17). Cysteine supplied by NAC treatment can also be oxidized to cystine, a substrate for the glutamate-cystine antiporter. This antiporter allows for the cellular uptake of cystine, which causes the reverse transport of glutamate into the extracellular space. The non-vesicular glutamate released into the extracellular space stimulates the type 2/3 metabotropic glutamate receptors (mGluR2/3), which in turn inhibits the vesicular release of glutamate, thereby resulting in decrease in glutamatergic neurotransmission (18) and the E:I ratio. Collectively, the glutamatergic and the anti-oxidant properties of NAC have stimulated interest in examining its effectiveness in several neuropsychiatric disorders (19–23) including autism. The goal of this double-blind, randomized, placebo-controlled trial in children with autism was to examine the usefulness of oral NAC in targeting irritability in this population.
Methods and Materials
Study design
The present investigation is a 12-week, double-blind randomized, placebo-controlled study of oral NAC in children with autism. This study was conducted in the Autism & Developmental Disabilities Clinic in the Division of Child & Adolescent Psychiatry, Lucile Packard Children’s Hospital at Stanford University. Recruitment started in March 2009 and ended in September 2010. Subjects with and without intellectual disability were included. After obtaining informed consent, subjects were screened and inclusion and exclusion criteria were assessed. No changes in eligibility criteria were applied throughout the study. This investigation was approved by the institutional review board at Stanford University School of Medicine. An investigational new drug application (# 100905) was filed with the Food and Drug Administration. This study was registered in National Institutes of Health’s on-line database ClinicalTrials.gov (identifier NCT00627705). The full trial protocol is available upon request.
Inclusion and Exclusion Criteria
Inclusion criteria included: (a) Outpatients between 3 and 12 years of age; (b) males and females who were physically healthy; (c) diagnosis of autism based on DSM-IV-TR criteria, Autism Diagnostic Interview – Revised (ADI-R) (24) and/or Autism Diagnostic Observation Schedule (ADOS) (25), and expert clinical evaluation; (d) Clinical Global Impressions – Severity (CGI-S) rating of 4 or greater (26) based on a clinical evaluation of irritability; (e) care provider who could reliably bring subject to clinic visits, could provide trustworthy ratings, and interacted with the subject on a regular basis; (f) stable concomitant medications and biomedical treatments for at least 2 weeks prior to enrollment; and (g) no planned changes in psychosocial interventions during the trial.
Exclusion criteria included: (a) DSM-IV diagnosis of schizophrenia, schizoaffective disorder, or psychotic disorder not otherwise specified; (b) prior adequate trial of NAC; (c) active medical problems: unstable seizures, significant physical illness; (d) pregnancy or sexually active females; and (e) subjects taking antioxidant agents and GSH prodrugs except when they had been off these compounds for at least 4 weeks.
Interventions
After the screening phase, baseline measures were obtained from subjects continuing to meet inclusion and exclusion criteria. Subjects were randomly assigned (1:1) to either placebo or active based on age (below and above 7.5 years) and gender. Randomization was done by the Stanford pharmacy (M.H.) using www.randomization.com which randomizes each subject by using the method of randomly permuted blocks. Each participant received a supply of the compound (NAC or placebo) labeled with a reference number. The study coordinator (R.A.L.), who was not involved in randomization and clinical ratings, received information about the group assignments and distributed the compound to the parents. Parents and investigators involved in the study were blinded to participants’ status. As NAC is a nutritional supplement, the quality control is predictably variable and therefore purity is not as stringent as prescription medications. In the present study, the stability of the compound was ascertained and the integrity of the active agent was protected by individual packaging of each NAC dose. Provided by BioAdvantex Pharma Inc. (Mississauga, Ontario, Canada), the active compound and matching placebo had identical appearance, odor and taste. The same formulation and preparation were used in a recent study for cystic fibrosis (27). Subjects randomized to the active drug were initiated at the dose of 900 mg every day for the first 4 weeks, then 900 mg twice daily for 4 weeks and 900 mg three times daily for 4 weeks. The selections of dose and length of trial were based on previously published studies for other psychiatric conditions (19–21) and the previous experience of our group in studies of children with cystic fibrosis (27). If subjects could not tolerate a specific dose, they would be maintained at the highest tolerated dose. Subjects were evaluated at baseline, week 4, week 8 and week 12. The Aberrant Behavior Checklist (ABC) (28), Clinical Global Impressions – Improvement (CGI-I), CGI-S, and Dosage Record and Treatment Emergent Symptom Scale (DOTES) (26) were obtained at each visit. Additionally, the Social Responsiveness Scale (SRS) (29, 30) and Repetitive Behavior Scale-Revised (RBS-R) (31) were obtained at baseline and at week 12.
Outcome measures
Primary outcome measures include: (a) the ABC-Irritability subscale; and (b) DOTES, which provides information on the presence, frequency and severity of side effects (26). Secondary outcome measures included: (a) ABC-Stereotypy subscale; (b) RBS-R subscales; (c) SRS; and (d) CGI-I.
The ABC is a standardized scale comprising 58 items, for assessing problem behavior in subjects with intellectual and developmental disabilities (28). The items resolve into five subscales: irritability, lethargy/social withdrawal, stereotypic behavior, hyperactivity, and inappropriate speech. High scores indicate more severe behavioral symptoms.
The RBS-R (31) is a rating scale for measuring the presence and severity of a variety of forms of restricted, repetitive behavior that are characteristic of individuals with autism. It has 6 subscales: stereotpyies, self-injurious behaviors, compulsions, rituals, insistence on sameness, and restricted. High scores indicate more severe behavioral symptoms.
The SRS (29, 30) is a 65-item parent report questionnaire designed for use with children aged 4 through 18, and more recently, a special version of the SRS has been developed for preschoolers (32). The SRS provides age- and gender-referenced, as well as raw scores for the following domains: total score (which reflects severity of social deficits pertaining to autism), receptive, cognitive, expressive, and motivational aspects of social behavior, and autistic preoccupations. High scores indicate more severe behavioral symptoms.
The CGI-S and CGI-I were used to monitor baseline symptoms and clinical improvement, but were not used as outcome measures. The CGI-S scale is a 7-point scale that requires the clinician to rate the severity of the patient’s illness at the time of assessment, relative to the clinician’s past experience with patients who have the same diagnosis. Considering total clinical experience, a patient is assessed on severity of mental illness at the time of rating with 1=normal, not at all ill; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; or 7=extremely ill. The CGI-I scale is a 7-point scale that requires the clinician to assess how much the patient’s illness has improved or worsened relative to baseline. A subject is rated as 1=very much improved; 2=much improved; 3=minimally improved; 4=no change; 5=minimally worse; 6=much worse; or 7=very much worse.
Statistical analyses
To examine the primary hypothesis that active treatment with oral NAC would decrease irritability associated with autism, we computed mixed effects regression models with ABC-Irritability score as the primary dependent variable. Treatment Group (2 levels: NAC vs. placebo) and Time (4-levels: baseline, week 4, week 8, and week 12) and their interaction were covariates. The interaction of Treatment Group X Time directly tests the hypothesis by examining whether treatment groups showed a different pattern of change in symptoms across study time points. Additional mixed effect regression models were computed using scores in other ABC sub-scales, SRS Total and sub-scales, and RBS Total and sub-scales as dependent variables. These exploratory analyses examined specific treatment effects for lethargy, stereotypy/repetitive behavior, hyperactivity, inappropriate speech, and social communication behavior. All models were fit using an autoregressive covariance structure. All randomized subjects with a minimum of one baseline assessment were included in the analyses.
Results
Study population
Fifty-one potential subjects inquired about the study (see Figure in supplemental information for patient disposition throughout the study). Forty-three of them signed a consent form. Seven were excluded because they did not meet criteria for autistic disorder. Three decided not to participate in the study before baseline measures were obtained. Thirty-three subjects (31 males, 2 females; aged 3.2–10.7 years) were randomized in the study. Fifteen were randomized to receive the NAC and eighteen were randomized to the placebo group. Four were unwilling to take the compound because of its taste (1 active and 3 placebo) and analyses were conducted on all subjects with at least one follow-up assessment (NAC, N=14; placebo, N=15). There were no differences between the placebo group and active group on any of the demographic and clinical baseline measures (Table 1). Twenty-five subjects (NAC N=13, placebo N=12) completed the study. Mean age of subjects randomized in the NAC and placebo groups were 7.0 ± 2.1 and 7.2 ± 2.2 years, respectively. Fourteen subjects were on at least one psychotropic medication with three being on more than one. The most common prescribed classes of medications were second generation antipsychotics and selective serotonin re-uptake inhibitors. ADI-R or ADOS information was available on all subjects with the exception of 4 who had a history of having an autistic diagnosis based on ADOS but documentation was not provided.
Table 1.
Baseline Comparison of Participants with Autism Assigned to Receive N-Acetylcysteine or Placebo
| Placebo | NAC | |
|---|---|---|
| # in group | 15 | 14 |
| Male/Female | 15/0 | 12/2 |
| Age (years) | 7.2 (2.2) [3.2–10.7] | 7.0 (2.1) [4.4–10.4] |
| ABC irritability score | 14.8 (9.6) [5–41] | 16.9 (7.9) [1–27] |
| CGI severity score | 5.3 (0.8) [4–6] | 5.1 (0.7) [4–6] |
| SRS total | 104.7 (28.1) [48–158] | 111.9 (28.3) [64–150] |
| RBS-R total | 38.2 (24.0) [16–115] | 33.1 (16.2) [8–66] |
ABC, Aberrant Behavior Checklist; CGI, Clinical Global Impression; SRS, Social Responsiveness Scale; RBS-R, Repetitive Behavior Scale-Revised. p value for gender was based on Fisher’s exact test; no statistical differences between the two group (NAC and placebo) on any of the demographic measures and baseline clinical characteristics.
Behavioral Outcomes
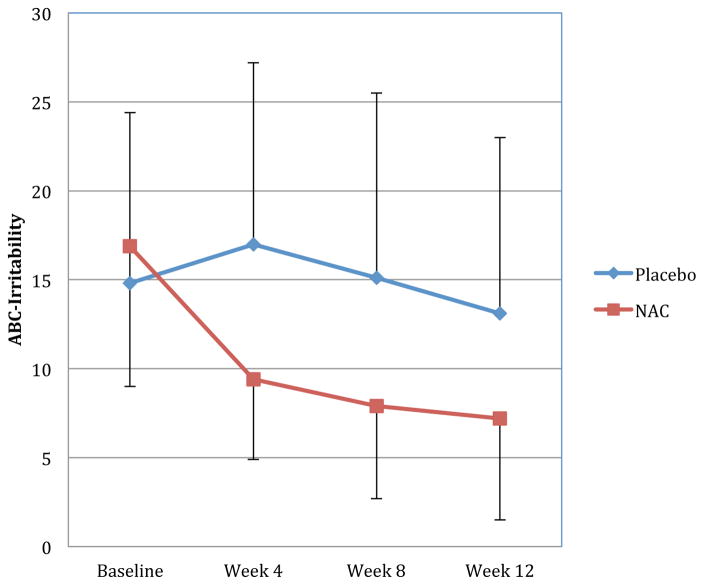
Figure 1 presents results for the primary outcome measure, ABC-Irritability, across the four study time points. When examining all participants, oral NAC treatment significantly improved irritability (F(3,66)=6.80; p<.001; d=.96). Oral NAC treatment resulted in a trend toward significance in improvement of stereotypic/repetitive behavior on the ABC (F(3,67)=2.21; p=.096; d=.72) and significant improvement on the RBS-Stereotypies (F(1,24)=7.07; p=.014; d=.90). Additionally, NAC treatment did not significantly influence SRS-Total raw scores (F(1,20)=2.36; p=.141; d=.44), but there were significant improvements in SRS-Social Cognition (F(1,20)=4.99; p=.037; d=.99) and SRS-Autism Mannerisms (F(1,20)=4.56; p=.045; d=.95) sub-scales. However, the improvements in SRS-Social Cognition appear to be due to greater baseline impairment in the NAC group followed by regression to the mean rather than a true treatment effect. There were no significant treatment effects for any other SRS subscales or for ABC hyperactivity, lethargy, and inappropriate speech subscales (all p>.100), although interestingly there was a large reduction (d=.72) in hyperactivity at Week 12 with changes in from baseline to end of the trial reaching statistical significance (Δ NAC = −10.4±9.7; Δ placebo = −3.2±3.3; F(1,22)=5.57, p=.028). Differences were less striking between the two groups at either Week 4 ((Δ NAC= −5.3 ± 8.2; Δ placebo = 0.5 ± 7.5; F(1,23)=3.49, p=.074) or Week 8 (Δ NAC=−7.7±9.7; Δ placebo = 0.3±10.9; F(1,22)=3.63, p=.070). Global improvement was assessed using CGI-I subscale. In the NAC group, five subjects were judged to be “much improved”, six were “minimally improved’, two were “no change,” and one was “much worse.” In the placebo group, two subjects were judged to be “much improved”, five were “minimally improved’, five were “no change,” one was “minimally worse,” and one was “much worse.”
Figure 1.
Significant improvements with NAC treatment for the primary outcome measures: Aberrant Behavior Checklist-Irritability subscale (ABC-Irritability) (F=6.80; p<0.001; d=0.96) with improvement being observed in week 4 and continuing through week 8 and Week 12. Error bars denote standard deviations. For clarity, positive error bars are shown for the placebo group, and negative error bars are shown for the NAC group.
Safety Evaluation
Minimal adverse effects (AE’s; see Table 3) were observed with the exception of one subject in the active group who experienced worsening of baseline agitation and irritability requiring early termination, which was followed by symptom resolution. This participant exhibited the same behavioral worsening 6 weeks after being terminated from the study, which led to a medical evaluation that revealed severe constipation. Most AE’s were gastrointestinal, consistent with previous reports. However, no statistical significance between the NAC and placebo groups was detected from chi-square tests for individual side effects (p = .198) or all gastrointestinal adverse effects combined (p = .199).
Table 3.
Treatment Emergent Adverse Events
| Placebo (n=15) | NAC (n=14) | |
|---|---|---|
| Total N with GI adverse events | 7 (47%) | 11 (79%) |
| constipation | 2 (13%) | 3 (21%) |
| nausea/vomiting | 3 (20%) | 6 (43%) |
| diarrhea | 1 (7%) | 3 (21%) |
| increased appetite | 0 | 2 (14%) |
| decreased appetite | 3 (20%) | 2 (14%) |
| Other adverse events | ||
| Akathisia | 0 | 1 (7%) |
| excitement/agitation | 3 (20%) | 2 (14%) |
| increased motor activity | 3 (20%) | 2 (14%) |
| Tremor | 1 (7%) | 0 |
| syncope/dizziness | 1 (7%) | 0 |
| depressed affect | 0 | 1 (7%) |
| nasal congestion | 6 (40%) | 4 (29%) |
| increased salivation | 2 (13%) | 0 |
| sweating | 1 (7%) | 0 |
Discussion
In this pilot investigation, we completed a double-blind, randomized, controlled trial to examine the usefulness of NAC in the treatment of irritability in children with autism. A significant decrease in the ABC-Irritability subscale was observed in the active group when compared to placebo. NAC was overall well-tolerated and might be helpful in targeting irritability in children with autism. This finding is important because the currently FDA-approved agents have a propensity to cause serious side effects (weight gain, metabolic abnormalities and tardive dyskinesia) which have limited their use considerably. Managing irritability, which can be manifested by aggression, tantrums, self-injurious behaviors, and anger, with an effective and safer agent can improve overall functioning in individuals with autism and alleviate burdens on the individual and family. However, the level of irritability in the present study (baseline ABC-Irritability = 17) tended to be on average lower compared with the previous studies with aripiprazole (baseline ABC-Irritability = 28 (33)) and risperidone (baseline ABC-Irritability = 19 (34)).
In addition to the benefits in decrease of irritability observed with NAC, other improvements were also found in this pilot study, although it is of lower significance. Decreased repetitive/stereotyped behaviors were observed on several subscales but reached statistical differences only on RBS-R Stereotypies and SRS-Autism Mannerism subscales. These findings add to a growing list of studies reporting benefits from NAC in various neuropsychiatric disorders. In a 12-week, double-blind, placebo-controlled investigation, NAC was found to be effective in decreasing hair-pulling symptoms in adults with trichotillomania (21). NAC was also beneficial in decreasing the severity of illness in a serotonin reuptake inhibitor resistant patient with obsessive compulsive disorder (23). In a randomized, double-blind, multicenter, placebo-controlled study of individuals with bipolar disorder, augmentation of usual medications with NAC resulted in significant improvement in the Montgomery Asberg Depression Rating Scale (20). Finally, in a double-blind, randomized, controlled trial in individuals with chronic schizophrenia, participants treated with NAC improved more than placebo-treated subjects as assessed by change in the Positive and Negative Symptoms Scale scores (19). These investigations suggest that NAC is potentially useful in the treatment of several neuropsychiatric disorders that share common pathologic pathways and is not a disease-specific agent.
As discussed above, the action of NAC might be related to two mechanisms: glutamatergic modulation and anti-oxidation. NAC modulates the glutamatergic system via increase in extracellular cystine (oxidized form of cysteine), which causes an increase in the non-vesicular transport of glutamate to the extracellular space via the glutamate-cystine antiporter. This action stimulates the inhibitory mGluR2/3, thereby reducing the synaptic vesicular release of glutamate and the E:I ratio. The use of glutamateric modulator is not novel in autism and several studies examining the effectiveness of such agents have been conducted in autism. Amantadine, a noncompetitive NMDA antagonist (35), has been examined in a 4-week double-blind, placebo-controlled trial in children with autism and difference between the two groups were found in ABC-Hyperactivity and Inappropriate Speech subscales, as assessed by clinicians but not parents (36). D-cycloserine, a partial agonist of the NMDA receptor, resulted in significant improvement in social withdrawal in a single-blind, placebo-lead-in study in ten subjects with autism (37); in this case, D-cycloserine acted as an antagonist in the presence of glutamate. However, a recently completed randomized, double-blind, controlled trial in children with autism failed to show a separation between the D-cycloserine group and placebo (38). Finally, several case reports and uncontrolled trials have been published suggesting the benefits of memantine, an uncompetitive NMDA receptor antagonist, in the treatment of children (39, 40) and adults (41) with autism. However, no randomized controlled trial has been published to date.
The effectiveness of NAC has also been linked to its anti-oxidative properties. NAC increases cysteine levels, thereby increasing the size of the glutathione pool (42). The relevance of an antioxidant strategy in autism is supported by mounting evidence suggesting the existence of redox imbalance in autism (14, 15). In fact, the effectiveness of several antioxidants has been examined in autism including omega-3 fatty acids and vitamin C. In contrast to NAC, these antioxidants promote glutathione recycling by facilitating the conversion of oxidized glutathione into reduced glutathione; hence the size of the glutathione pool remains unchanged. Studies examining omega-3 fatty acids have reported mixed findings (43, 44). While Meiri et al. reported that 8 of 9 subjects who completed the study showed improvement of about 33% on the Autism Treatment Evaluation Checklist in an open-label study (44), a pilot randomized study with 27 children did not find a statistically significant benefit from omega-3 fatty acids (43). In a double-blind, placebo controlled study with 18 children with autism, administration of vitamin C resulted in significant improvements in stereotypical behaviors, compared with placebo (45). Interestingly, no replication of this study has been published to date and a follow-up of this investigation is warranted.
Overall, oral NAC was very well tolerated in the present investigation. This observation is consistent with previous studies of oral NAC in other psychiatric conditions (19–22). Gastrointestinal side effects were most commonly observed and included nausea, vomiting, and diarrhea. These adverse events have been reported in previous studies involving the use of NAC in medical disorders (46). Interestingly, these side effects have not been reported in NAC studies of adults with neuropsychiatric disorders. The reason for this discrepancy is unclear and could be related to the age of participants in the present study with higher rates of GI adverse events in children compared to adults.
This study suffers from several methodological limitations. The sample size was relatively small and the age range was narrow which limits the generalizability of the findings. Most subjects were taking psychotropic medications and were receiving behavioral interventions, but including children not participating in any treatment program is impractical and unethical. The attrition rate was relatively high (25%), which mostly related to 4 subjects not able to take the compound. A higher number of participants in the placebo group refused to take the compound because of its taste, which warrant the implementation of supplemental strategies, such as a taste panel, to refine the placebo matching in a larger trial. Additional limitations include the use of informant-based scale such as the ABC, SRS, and RBS-R and the absence of direct laboratory observations or performance-based instruments. Also, the entry criteria did not exclude subjects with low irritability score on the ABC-Irritability subscale and the unblinded status of the study coordinator who could have influenced parent’s ratings. Finally, not all children were diagnosed using the ADI-R and/or the ADOS. Despite these limitations, our results suggest that potential modulation of glutamatergic neurotransmission and GSH metabolism in autism via NAC supplementation represents a potentially useful new approach that warrants further investigation. Future studies are definitely needed to replicate our findings in a larger sample size of well-characterized children with autism using both informant- and performance–based instruments, while examining the effect of NAC on glutamatergic transmission and GSH metabolism.
Supplementary Material
Table 2.
Treatment Responses of Participants with Autism Assigned to Receive N-Acetylcysteine (NAC) or Placebo
| Mean (SD) [Range] | F | p | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | ||||||
| Placebo (n=15) | NAC (n=14) | Placebo (n=15) | NAC (n=14) | ||||
| ABC | |||||||
| ABC-Irritability | 14.8 (9.6) [5–41] | 16.9 (7.9) [1–27] | 13.1 (9.9) [4–41] | 7.2 (5.7) [0–18] | 6.80 | <.001 | .96 |
| ABC-Lethargy | 12.1 (7.8) [1–24] | 15.2 (9.5) [2–31] | 8.3 (7.7) [1–23] | 11.0 (9.4) [0–32] | 1.93 | .134 | −.30 |
| ABC-Stereotypy | 8.9 (6.5) [0–21] | 9.1 (5.5) [2–21] | 8.0 (7.0) [1–18] | 5.6 (5.7) [0–19] | 2.21 | .096 | .72 |
| ABC-Hyperactivity | 23.8 (9.3) [8–37] | 23.4 (9.0) [6–37] | 21.0 (11.5) [3–31] | 12.4 (11.4) [1–27] | 1.97 | .130 | .72 |
| ABC-Inappropriate Speech | 4.1 (3.7) [0–11] | 4.9 (3.2) [0–11] | 3.6 (3.6) [0–11] | 2.5 (2.6) [0–7] | 1.25 | .297 | .28 |
| RBS-R | |||||||
| RBS-Stereotypies | 8.1 (5.3) [1–17] | 6.7 (3.8) [2–14] | 6.9 (5.2) [0–18] | 4.6 (3.4) [0–11] | 7.07 | .014 | .90 |
| RBS-Self-injurious behavior | 3.4 (3.8) [0–14] | 3.9 (4.4) [0–14] | 3.0 (3.6) [0–13] | 2.2 (2.3) [0–8] | 2.47 | .129 | .63 |
| RBS-Compulsions | 5.8 (4.8) [2–22] | 4.7 (3.7) [0–12] | 5.2 (5.0) [1–21] | 2.5 (2.1) [0–6] | 2.48 | .128 | .70 |
| RBS-Rituals | 6.6 (4.5) [0–18] | 5.3 (3.7) [0–14] | 5.6 (4.9) [0–18] | 4.3 (3.4) [0–12] | .24 | .631 | .17 |
| RBS-Sameness | 9.2 (8.1) [2–33] | 7.8 (7.2) [0–23] | 7.9 (6.2) [1–23] | 5.3 (4.7) [0–14] | 1.26 | .273 | .46 |
| RBS-Restricted | 5.2 (3.7) [0–12] | 4.7 (3.4) [0–11] | 4.8 (3.6) [1–12] | 3.5 (2.3) [0–8] | 3.77 | .064 | .73 |
| SRS Total | 104.7 (28.1) [48–158] | 111.9 (28.3) [64–150] | 98.5 (37.8) [35–148] | 93.8 (26.7) [44–135] | 2.36 | .141 | .44 |
| SRS social awareness | 13.5 (3.7) [8–20] | 12.7 (3.4) [6–18] | 13.4 (4.7) [8–23] | 11.5 (3.3) [6–16] | 0.34 | .565 | .26 |
| SRS social cognition | 21.2 (5.8) [11–33] | 21.9 (6.3) [7–29] | 18.9 (5.6) [8–26] | 18.8 (7.0) [5–28] | 4.99 | .037 | .99 |
| SRS social communication | 39.3 (8.6) [25–52] | 39.6 (11.3) [22–56] | 34.5 (14.5) [10–52] | 33.3 (10.9) [15–50] | 0.01 | .998 | .04 |
| SRS social motivation | 16.9 (6.5) [6–27] | 16.6 (6.3) [6–24] | 14.5 (7.0) [5–25] | 13.0 (4.7) [6–20] | 0.29 | .597 | .24 |
| SRS autism mannerisms | 21.4 (7.3) [7–33] | 21.7 (5.6) [8–29] | 20.3 (6.9) [8–30] | 16.0 (6.1) [5–30] | 4.56 | .045 | .95 |
| CGI severity | 5.3 (0.8) [3–6] | 5.1 (0.7) [4–6] | 4.9 (0.9) [3–6] | 4.5 (0.8) [3–6] | 1.73 | .170 | .57 |
| CGI improvement | -- | -- | 3.2 (0.9) [2–5] | 2.9 (1.1) [2–6] | .81 | .449 | .30 |
Note. ABC, Aberrant Behavioral Checklist; SRS, Social Responsiveness Scale; RBS-R, Repetitive Behavior Scale-Revised. Means and standard deviations were derived from all observed data at the respective time points. F-values were derived from the interaction of Participant Group (NAC vs. Placebo) and Time (Week) in mixed effects regression models. Cohen’s d was computed based on the standardized mean difference in the change from Baseline to Week 12. For ABC, regression estimated degrees of freedom were 3,66 or 3,67. For SRS and RBS, degrees of freedom were 1,22 and 1,24, respectively.
Acknowledgments
This study was supported by a grant from the Escher Fund at the Silicon Valley Community Foundation to Dr. A.Y. Hardan. Dr. Frazier was supported by KL2 RR024990 (PI: P. Davis). We wish to thank J. Laval, A.G. Banerjee, and M. Panganiban for their help as well as Dr. M. Hamilton for her help in the randomization process. We would also like to gratefully acknowledge the effort and commitment of the participants and their families in this study.
Footnotes
Financial Disclosures
Over the last 3 years, Dr. A.Y. Hardan has received research support from the following companies: Bristol-Myers Squibb Company, and Forest Pharmaceuticals. Dr. Frazier has received research support from, acted as a consultant to, or received travel support from Shire Development, Inc., and Bristol-Myers Squibb Company. Dr. L.A. Herzenberg and Dr. R. Tirouvanziam are listed as inventors on two patents licensed by Bioadvantex, Inc., the supplier of the NAC and placebo for this study, covering the use of NAC in cystic fibrosis. All other authors report no biomedical financial interests or potential conflicts of interest..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belmonte MK, Cook EH, Jr, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9:485–499. doi: 10.1080/10937400600882079. [DOI] [PubMed] [Google Scholar]
- 4.Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 5.Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, et al. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, et al. Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:48–50. doi: 10.1002/ajmg.b.30025. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S, Das S, Guhathakurta S, Sen B, Sinha S, Chatterjee A, et al. Glutamate receptor 6 gene (GluR6 or GRIK2) polymorphisms in the Indian population: a genetic association study on autism spectrum disorder. Cell Mol Neurobiol. 2007;27:1035–1047. doi: 10.1007/s10571-007-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serajee FJ, Zhong H, Nabi R, Huq AH. The metabotropic glutamate receptor 8 gene at 7q31: partial duplication and possible association with autism. J Med Genet. 2003;40:e42. doi: 10.1136/jmg.40.4.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 10.Tirouvanziam R, Obukhanych TV, Laval J, Aronov PA, Libove R, Banerjee AG, et al. Distinct Plasma Profile of Polar Neutral Amino Acids, Leucine, and Glutamate in Children with Autism Spectrum Disorders. Journal of autism and developmental disorders. 2011 doi: 10.1007/s10803-011-1314-x. [DOI] [PubMed] [Google Scholar]
- 11.Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Fuenmayor H, Borjas L, Arrieta A, Valera V, Socorro-Candanoza L. Plasma excitatory amino acids in autism. Invest Clin. 1996;37:113–128. [PubMed] [Google Scholar]
- 13.Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. Journal of autism and developmental disorders. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- 14.James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–2383. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yorbik O, Sayal A, Akay C, Akbiyik DI, Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids. 2002;67:341–343. doi: 10.1054/plef.2002.0439. [DOI] [PubMed] [Google Scholar]
- 17.Atkuri KR, Mantovani JJ, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 22.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006;184:254–256. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 26.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 27.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. Am J Ment Defic. 1985;89:492–502. [PubMed] [Google Scholar]
- 29.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 31.Bodfish JW, Symons FJ, Lewis MH. The Repetitive Behavior Scale: A test manual 1998 [Google Scholar]
- 32.Pine E, Luby J, Abbacchi A, Constantino JN. Quantitative assessment of autistic symptomatology in preschoolers. Autism. 2006;10:344–352. doi: 10.1177/1362361306064434. [DOI] [PubMed] [Google Scholar]
- 33.Marcus RN, Owen R, Kamen L, Manos G, McQuade RD, Carson WH, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 34.Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 35.Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl. 1994;43:91–104. [PubMed] [Google Scholar]
- 36.King BH, Wright DM, Handen BL, Sikich L, Zimmerman AW, McMahon W, et al. Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2001;40:658–665. doi: 10.1097/00004583-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. Am J Psychiatry. 2004;161:2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 38.Posey DJ, Seigler KA, Erickson CA, Azzouz F, Mullett J, Dienner J, et al. Double-Blind Placebo-Controlled Study of D-Cycloserine in Children with Autistic Disorder. American Academy of Child and Adolescent Psychiatry Annual Meeting; Chicago. 2008. [Google Scholar]
- 39.Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. Journal of child neurology. 2007;22:574–579. doi: 10.1177/0883073807302611. [DOI] [PubMed] [Google Scholar]
- 40.Erickson CA, Posey DJ, Stigler KA, Mullett J, Katschke AR, McDougle CJ. A retrospective study of memantine in children and adolescents with pervasive developmental disorders. Psychopharmacology. 2007;191:141–147. doi: 10.1007/s00213-006-0518-9. [DOI] [PubMed] [Google Scholar]
- 41.Erickson CA, Chambers JE. Memantine for disruptive behavior in autistic disorder. The Journal of clinical psychiatry. 2006;67:1000. doi: 10.4088/jcp.v67n0619h. [DOI] [PubMed] [Google Scholar]
- 42.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 43.Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A Pilot Randomized Controlled Trial of Omega-3 Fatty Acids for Autism Spectrum Disorder. J Autism Dev Disord. 2011;41:545–554. doi: 10.1007/s10803-010-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meiri G, Bichovsky Y, Belmaker RH. Omega 3 fatty acid treatment in autism. J Child Adolesc Psychopharmacol. 2009;19:449–451. doi: 10.1089/cap.2008.0123. [DOI] [PubMed] [Google Scholar]
- 45.Dolske MC, Spollen J, McKay S, Lancashire E, Tolbert L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:765–774. doi: 10.1016/0278-5846(93)90058-z. [DOI] [PubMed] [Google Scholar]
- 46.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clinical pharmacokinetics. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.