Abstract
Purpose
vascularized fibular grafting has been used to treat osteonecrosis of the femoral head in younger patients. Although the results described in the literature are promising, the failure rate is still significant, especially in steroid users. This study was undertaken to learn more, on a histopathological level, about the mechanism of vascularized fibular graft failure.
Methods
fifteen femoral heads removed at conversion to total hip arthroplasty were analyzed. The case load comprised 10 men and 5 women. They ranged in age from 28 to 39 years and had a median age of 35 years. The interval between the vascularized fibular implant procedure and the conversion to total hip arthroplasty ranged from 22 months to 30 months; the median interval was 26 months. All the patients were steroid users. The heads were sectioned and axial and coronal sections were taken and stained using the WHO method (hematoxylin, phloxine, saffron and Alcian green). A quantitative and qualitative analysis of graft-host interaction at the head (zone 1), neck (zone 2) and epiphysis (zone 3) was performed.
Results
all the specimens showed recognizable collapse of the articular surface over the area of necrosis. Thirteen femoral heads showed the presence of an osteochondral flap attached only at the margins of the area of avascular necrosis, and 10 of these 13 femoral heads also showed loss of the articulating surface with an ulcer crater corresponding to the exposed area of avascular necrosis.
Conclusions
vascularized fibular graft failure seems to be related to a negative effect of creeping substitution: the revascularization becomes a negative force as it supports unbalanced bone resorption, which, as is well known, is enhanced by corticosteroids.
Clinical relevance
creeping substitution is an undermining force in the repair and revascularization of the necrotic area in the femoral head.
Keywords: vascularized fibular graft, osteonecrosis, creeping substitution, corticosteroid, femoral head
Introduction
Osteonecrosis (ON) of the femoral head is a disabling disease caused by ischemia of the bone and bone marrow of the femoral head. Numerous non-traumatic conditions are associated with ON: alcohol abuse, steroid therapy, autoimmune disease, hyperlipidemia, pancreatitis, hypercoagulopathies, hemoglobinopathies, Gaucher’s disease and autosomal dominant inheritance of the disease, with mapping of the chromosomal position of the gene to 12q13 (1–4).
Treatment for ON of the femoral head depends on the stage of the disease (5). Today, in the mature adult population, total hip replacement is a popular and successful method of treatment, although it is not advisable in younger patients (6–9). Over the years several surgical procedures and non-surgical salvage treatments have been developed for this patient group (10). Restricted weight bearing, core decompression (11), electrical stimulation (12), transtrochanteric rotation osteotomy (13), and non-vascularized (14) and vascularized (15) structural grafts have been used and have been reported to show encouraging results. A vascularized graft is believed not only to provide support for the articular surface but also to introduce mesenchymal stem cells into the affected area of the femoral head (16–18). The failure rate of vascularized grafts, as reported in the literature, varies, ranging from 4 to 30% (19, 20). Most of these failed grafts were found in chronic steroid users (21).
The purpose of the present study was to outline the histopathological features of femoral heads retrieved at revision surgery after failure of vascularized fibular grafts used to treat ON of the femoral head.
Methods
The Laboratory of Bone & Joint Pathology of the Department of Laboratory Medicine at St. Michael’s Hospital (Toronto, CA) processed 25 femoral heads removed from 23 patients at conversion of failed implantation of vascularized fibular grafts to total hip arthroplasty.
A review of the clinical files of the patients included in the study protocol was carried out to determine the primary disease process and the treatment that had preceded the ON of the femoral head. The following clinical information was obtained: patient age, activity level, general health, comorbidities and use of corticosteroids. Only specimens retrieved from patients who were chronic corticosteroid users were included in this study.
The pathological assessment was conducted in full knowledge of the patients’ clinical and imaging data and operative findings. The study was carried out on materials in the files of the Pathology Division. After initial selection, 15 out of 25 femoral heads were included in the study.
The case load comprised 10 men and 5 women. They ranged in age from 28 to 39 years and had a median age of 35 years. The interval between the vascularized fibular implant procedure and the conversion to total hip arthroplasty ranged from 22 months to 30 months; the median interval was 26 months.
The specimens received (in formaldehyde) by the Pathology Laboratory consisted of the femoral heads, all with comparable amounts of the femoral neck.
Following external gross examination, the heads were sectioned to produce slabs in different planes: axial, coronal and sagittal. X-rays of the slabs were taken to confirm the correct location of the implanted fibular graft, the pattern of the host bone reaction to the graft, and the extent of the changes at the articular surface.
The heads were sectioned with a circular band saw.
The axial slabs were harvested across the femoral neck at the resection margin and at the base of the femoral head. This best demonstrated the placement of the fibular graft in the femoral neck and lower head. It also best showed the position and state of the vascular pedicle. In all the specimens the slabs showed that the graft was well placed, just under the area of ON, and the pedicle was patent.
Coronal slabs of the entire distal femoral head were then harvested from the remaining distal portion of the femoral head, and sagittal slabs of the remaining anterior and posterior halves of the head were taken. The sections were stained using the WHO method (hematoxylin, phloxine, saffron and Alcian green) (22). This complex but routine multicolor staining system allowed identification of: mucopolysaccharide in cartilage (Alcian green), the amount of fibrous tissue (saffron), the presence or loss of nuclei to identify viability or necrosis (hematoxylin), and areas of fibrinoid or coagulative necrosis (phloxine). Selected sections were embedded in Spurr’s medium and submitted undecalcified for sectioning using the Exakt precision saw (Exakt Technology Inc., Oklahoma City, OK, USA). The resulting 1–2 mm thick slices were mounted on plastic backing, ground to a thinness of less than ten microns, and treated by surface etching with acid and surface staining with toluidine blue (modification of the Schenk method) (23).
The fine detail radiographs were examined with the naked eye and with up to 25× magnification. The histological preparations were examined by transmitted and incident white light and polarized light microscopy. The undecalcified plastic embedded sections were examined using a combination of transmitted and incident light.
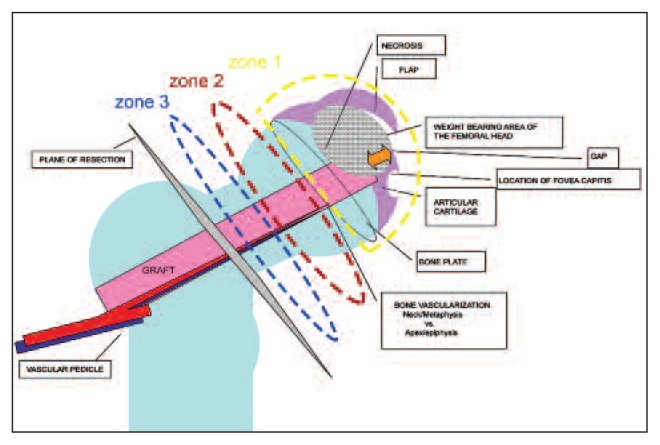
Both the decalcified and undecalcified sections were carefully examined, not only to determine overall integrity of the structure but also to identify the interfaces between the graft, pedicle and host bone in the three defined zones of the construct in the head and the neck (Fig. 1): zone 3, the femoral neck, zone 2, the lower part or “metaphysis” of the femoral head, and zone 1, the apical or “epiphyseal” part of the femoral head.
Fig. 1.
Diagrammatic view of the anatomical features of the implanted graft and its relationship with the host. The length of the graft was divided into three zones: zone 3, the femoral neck; zone 2, the lower femoral head or “metaphysis”; zone 1, the more apical or epiphyseal component of the femoral head. The intention was to track the vascularization of the pedicle and the changes in the three different areas of the specimen.
The intention was to examine the graft pedicle to study the health of the pedicle tissue and blood vessels and see what the tissue changes were present in the three different areas of the specimen. The selection of these three zones allowed us to distinguish the mainly epiphyseal area (the site of avascular necrosis, collapse and remodeling at the apex of the graft) and then compare it with changes in the host bone and graft in the metaphyseal and neck regions. The assessment was carried out using a semi-quantitative method with changes scored as mild (occasional presence of the features studied), moderate (presence of changes in up to half the microscopic field but not in all fields), or severe (changes in all fields) (scoring: mild = one, moderate = two and severe = three).
The following histological features were assessed:
Bone activity in the host bone: osteoblastic and osteoclastic activity, changes in trabecular size, and the presence and structure of woven and lamellar bone. Bone formation and resorption were used as indicators of bone activity; they were evaluated in a semi-quantitative manner; scores had a maximum of 3. Resorption was defined as the extent of trabecular and cortical surface occupied by osteoclasts in Howship’s lacunae (erosive lacunae devoid of osteoclasts that showed abrupt interruption of bone lamellae were included in the bone resorption data).
Bone marrow activity in the host bone: hematopoiesis versus adipose and fibrous tissue, the presence of necrosis, evidence of inflammation, patency of blood vessels and recanalization of occluded vessels and neovascularization.
State of vascularization of the pedicle: viability of components including blood vessels, fat, and transplanted muscle fibers, thrombosis, recanalization of occluded vessels and neovascularization, and inflammation, focusing particularly on zone 3.
Graft activity: evidence of increase or loss of substance, acellularity, loss of tissue detail due to tissue degeneration, inflammation, and integration between the graft and the femoral bone in which it had been placed. Integration at zones 2 and 3 was calculated in the cross sections while in zone 1 this was attempted in the coronal sections of the head using both histology and fine detail radiographs.
Articular cartilage status: only in zone 1 (cellularity, appearance of matrix, fraying, erosion, ulceration, collapse of the joint surface, presence and appearance of osteoarticular flap).
Gap: distance from the tip of graft to the joint surface (assessment of resorption, tissue degeneration and/or necrosis, vascularization, inflammation, fibrosis, sub-articular cyst formation, reactive cartilage or bone, and viability of tissues).
Assessments were inclusive of external gross examination, fine detail imaging and histological examination at the three selected zones. Data for each zone were gathered and analyzed and the zones were then compared.
Results
The patients included in this study (n=15) were all on steroid therapy when the ON was diagnosed. The free vascularized fibular graft had been implanted by the same orthopedic and plastic surgery team in all of them, and they were all in stage two of the disease (Ficat-Arlet scale).
The availability of clinical and fine-detail slab X-rays of the specimens allowed us to combine the information of two imaging modalities in order to better delineate the necrotic zone. All the specimens showed recognizable collapse of the articular surface over the area of necrosis, 13 femoral heads showed the presence of an osteochondral flap attached only at the margins of the area of avascular necrosis, and 10 of these 13 femoral heads also showed loss of the articulating surface with an ulcer crater corresponding to the exposed area of avascular necrosis (Figs. 2–4).
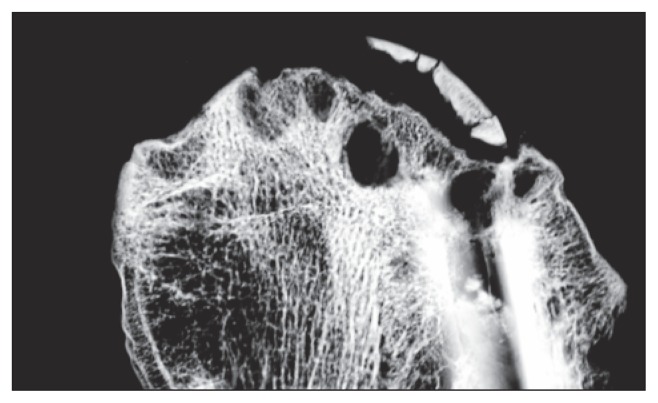
Fig. 2.
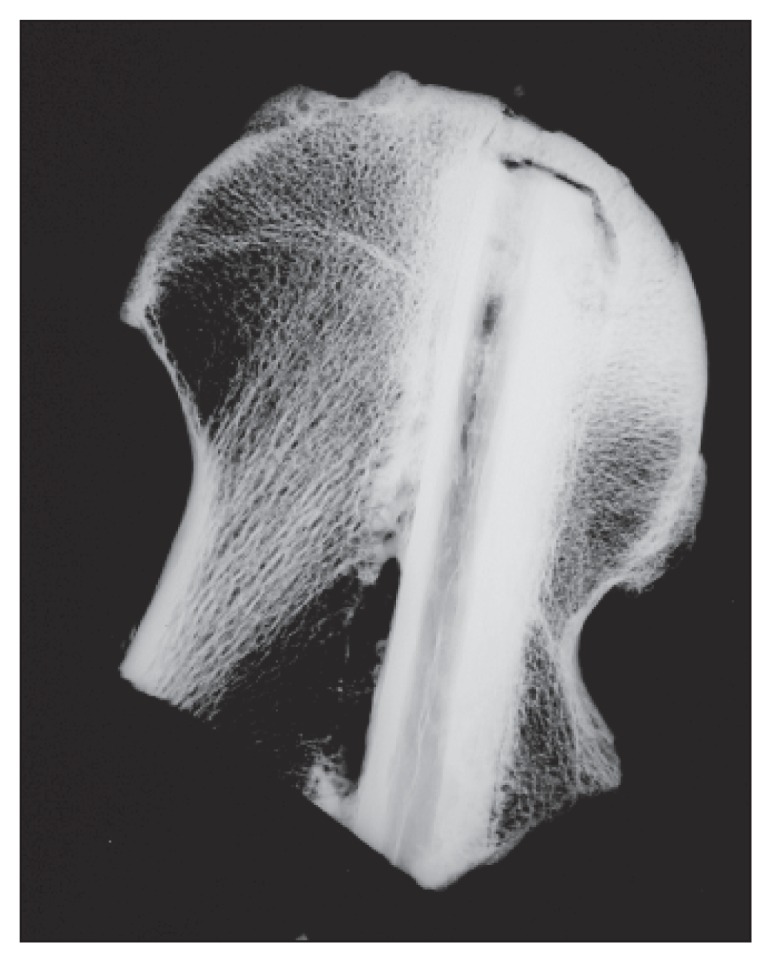
Low energy slab imaging showing the gap at the implant tip.
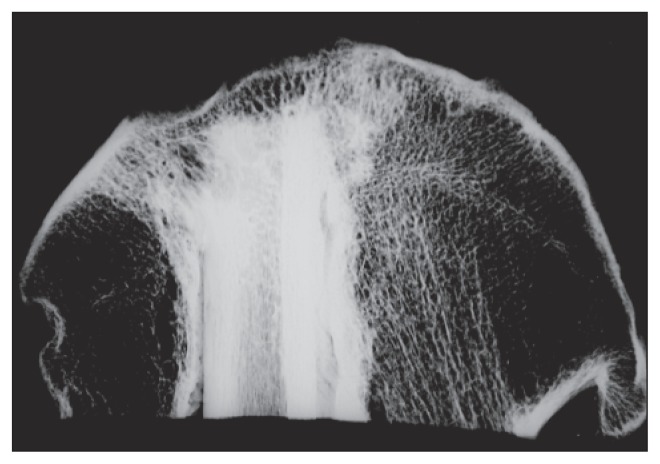
Fig. 3.
Low energy slab imaging showing a flap and cysts at the implant tip.
Fig. 4.
Low energy slab imaging showing sclerotic bone on growth on the implant.
The coronal slab X-rays showed that the necrotic area averaged 2.78±0.21 cm2. In all the specimens the fibular implant was centered in the weight-bearing area.
Bone activity in the host bone
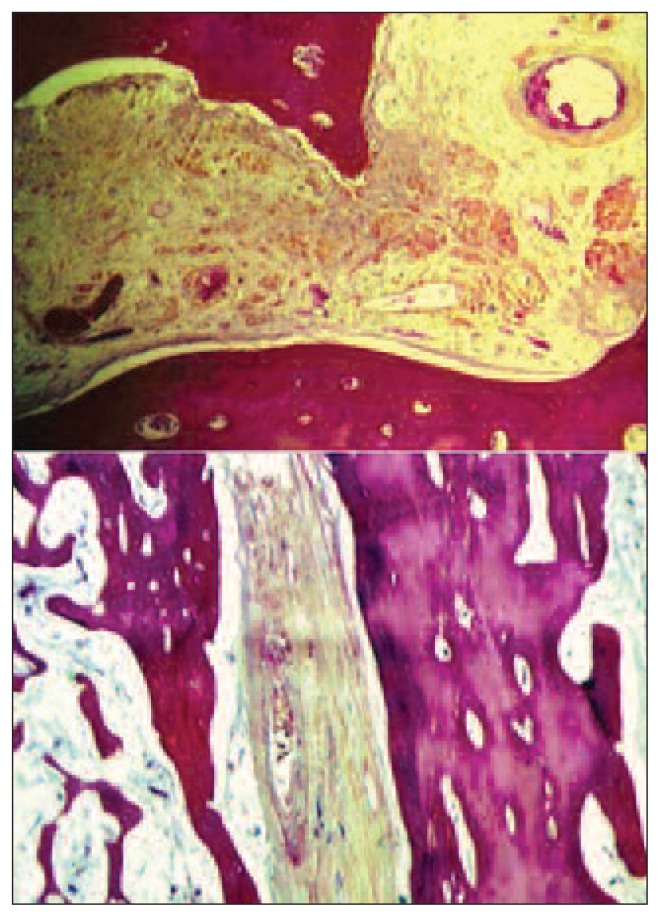
Zone 2 showed the highest osteoclastic and osteoblastic activity, followed by zone 3; the lowest scores were recorded in zone 1. Reactive host bone density was highest in zone 3 where actual bone shell was found to have formed to encase the pedicle and graft. The most striking osteoformative reaction to the graft was in the neck: this was the healthiest, best vascularized region of the femoral head where the graft was best incorporated by the femur; this is the most stable point furthest from the necrosis and degenerative state at the tip of the graft in the sub-articular zone of the femoral head. In zone 2 the host bone incorporated the graft in a well-organized manner; in this zone there was maturation to lamellar bone. The total amount of bone formed varied between the three zones. The average amount of viable bone expressed as a percentage of total bone present was highest in zone 3 (neck), lower in zone 2 and lowest in zone 1. The vessels in the Haversian canals of the host cortical bone were patent in all specimens (Fig. 5).
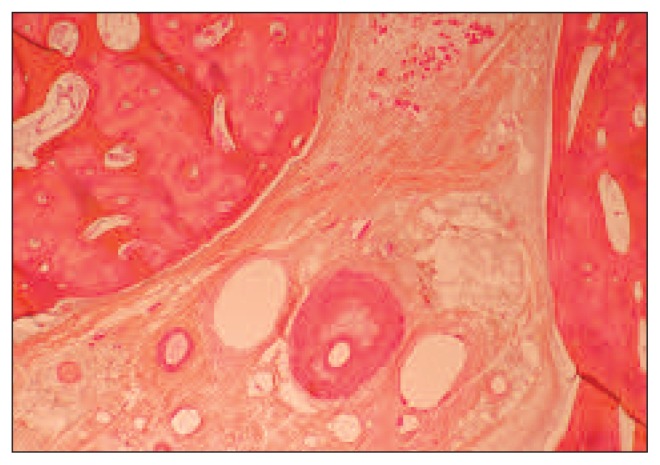
Fig. 5.
Pattern of recanalization of an artery in the pedicle.
Bone marrow activity in host bone
Healthy fatty tissue was better preserved in the neck, than in zone 2 or zone 1. Fibrovascular tissue and fibrosis were most prominent in zone 1 (at the tip of the graft) (Fig. 6). Hematopoietic activity gradually decreased from the neck to the epiphysis. Necrosis of the bone marrow was greatest in zone 2; resorption and remodeling had removed much of the graft bone and bone marrow in zone 1 and replaced it with reactive granulation tissue, degenerative cyst formation and detritus from collapse of the articular surface.
Fig. 6.
Fibrous tissue (top) and fibrovascular tissue (bottom) in the pedicle space.
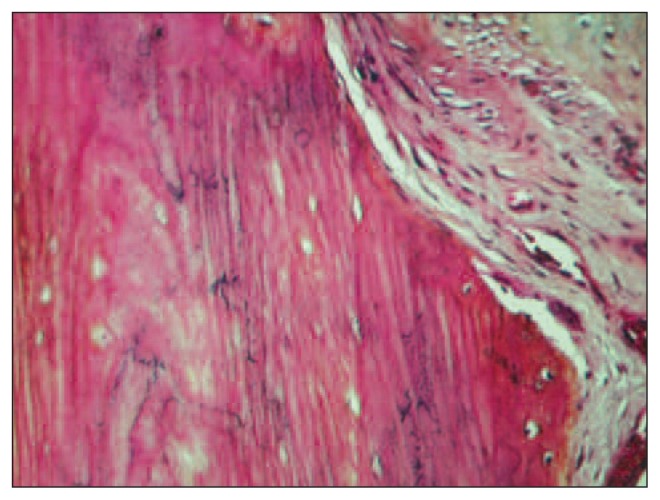
Graft activity
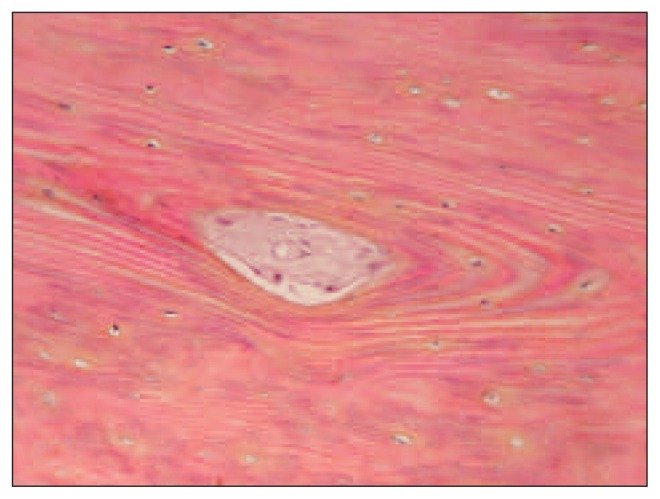
In zone 1, lysis of the tip was present in almost all the specimens (Fig. 7). Integration of the graft was found to be well developed in zones 2 and 3. Lysis along the periosteal surface of the implant was greater in zone 2 than in zone 3. The proportion of stainable nuclei, indicating a viable graft, was highest in zone 3 (Fig. 8). Cortical cellularity was best preserved in zone 2. The bone marrow content of the graft showed differences between zones 2 and 3. We found more hematopoietic activity and incorporated bone dust in zone 3, and more fibrovascular tissue in zone 2. Haversian canals in zones 2 and 3 had patent blood vessels without evidence of thrombosis or occlusion. Our study showed that there was active resorption and formation of bone in zones 2 and 3. This paralleled the accompanying pedicle and the preservation of vascularization. These results indicate a positive progression of tissue changes as indicated by the degree of incorporation and the preservation of the viability of the graft. This contrasted with the destructive changes at the tip of the graft in zone 1.
Fig. 7.
Lysis of the tip of the graft in zone 1.
Fig. 8.
Presence of nuclei, indicating graft viability.
Discussion
Published studies detailing the histological features of autogenous free vascularized fibular grafts in femoral head ON are limited.
Carter et al. (24) analyzed 13 retrieved femoral heads, which were divided into six transaxial segments (from the base to the articular surface). Their patients were similar to our group of patients in median age and range of the interval between fibular grafting and conversion to THA. Consistent with the present study, they found osteosclerotic bone encasing the graft. Unlike our findings, the cortices of all their grafts were reported to be totally necrotic with complete absence of revascularization, and of remodeling by osteoclastic resorption. The percentage of circumferential incorporation of the graft was the same as in our study (Carter et al. reported 54% osteo-incorporation in their zone “A” to zone “C” corresponding to our zone 3 in which it was 55%).
According to González Della Valle et al. (25), who studied six retrieved femoral heads at an average of 16 months after the same grafting procedure, 50% of their grafts were not viable and none of the retrieved heads showed a patent anastomosis. Differently from the results of our study, these Authors stated that their six osteonecrotic femoral heads failed as a result of incorrect placement of the graft, which did not provide mechanical support for the subchondral plate.
Our observations were based on a larger number of patients (15) than were included in the studies of Carter et al. (24) and González Della Valle et al. (25). González Della Valle et al. (25) and Beris et al. (26) believed that precise placement of the bone graft supporting the subchondral plate is the most important factor; our results indicate that remodeling and removal of dead tissue in zone 1 also removes graft substance and therefore support for the articular cartilage.
The first phase after grafting is osteolysis of the cortical bone; this occurs within a period of several weeks and, in this interval of time, makes the graft weaker and reduces the support for the articular surface.
The integration of a vascularized cortical bone graft through ingrowth of capillaries, perivascular tissue and osteoprogenitor cells from the recipient bed into the graft is known as osteoconduction, and is followed by bone induction tissue forming osteogenic elements.
These processes of new tissue invading along channels made by invasive blood vessels in the transplanted bone constitute a phenomenon known as creeping substitution. The creeping substitution of cortical bone progresses transversally and parallel to the long axes of the graft segment. This might explain the better integration of the graft in zone 1 and 2 rather than in zone 3 found in our study.
Steroids enhance adipogenesis and inhibit osteogenesis and angiogenesis by marrow stem cells.
Several hypotheses regarding the cause of ON in patients using corticosteroids in general are based on the notion of small vessel occlusion secondary to a rise in intraosseous pressure from excessive marrow fat accumulation or a shift in the differentiation of marrow stem cells into adipocytes, leading to a reduction in the pool of stem cells available for osteoblast production, in turn resulting in insufficient repair and remodeling. The mechanism of failure of the vascularized graft in steroid users might be identical to the mechanism of primary ON (27–29).
In conclusion, from the present morphological study it can only be concluded that vascularized fibular grafts provide support for the joint surface in the presence of avascular necrosis of the femoral head. The graft shows incorporation with the host bone. In the pedicle there is preservation of vascular patency and tissue viability. This indicates that the concept of a graft maintaining joint support is valid. However, the restoration and/or maintenance of vascularization in zone 1 supports the reparative resorptive activity of “creeping substitution”, and this phenomenon, failing to adequately counterbalance the osteoformative components, eventually undermines the joint surface. The healing process fails: the revascularization becomes a negative force as it supports unbalanced bone resorption, which, as is well known, is enhanced by corticosteroids.
These findings suggest that the probability of success would be increased by careful selection of patients who do not have any evidence of collapse in the avascular necrotic zone. Unfortunately this may not be easy to establish in the pre-operative clinical investigations of these patients. At this time, one can only speculate that the recent introduction of non-resorbable materials in place of tissue grafts can be expected to avoid the negative effect of creeping substitution as an undermining force in the repair and revascularization of the necrotic area of the head. Additional osteoinductive materials and methods could be added to non-resorbable implants (e.g. tantalum) to enhance and extend support for the articular surface (30). This should gain valuable time for patients who are too young for total hip replacement arthroplasty and in whom vascularized fibular grafting may not be advisable.
References
- 1.Kaushik AP, Das A, Cui Q. Osteonecrosis of the femoral head: An update in year 2012. World J Orthop. 2012;3:49–57. doi: 10.5312/wjo.v3.i5.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 3.Seamon J, Keller T, Saleh J, et al. The pathogenesis of non-traumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YF, Chen WM, Lin YF, et al. Type II collagen variants and inherited osteonecrosis of femoral head. N Engl J Med. 2005;352:2294–2301. doi: 10.1056/NEJMoa042480. [DOI] [PubMed] [Google Scholar]
- 5.Mont MA, Marulanda GA, Jones LC. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88( Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 6.Stavrakis AI, SooHoo NF, Lieberman JR. A comparison of the incidence of complications following total hip arthroplasty in patients with or without osteonecrosis. J Arthroplasty. 2015;30:114–117. doi: 10.1016/j.arth.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 8.Fevang BT, Lie SA, Havelin LI, et al. Improved results of primary total hip replacement. Acta Orthop. 2010;81:649–659. doi: 10.3109/17453674.2010.537807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polkowski GG, Callaghan JJ, Mont MA, et al. Total hip arthroplasty in the very young patient. J Am Acad Orthop Surg. 2012;20:487–497. doi: 10.5435/JAAOS-20-08-487. [DOI] [PubMed] [Google Scholar]
- 10.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 11.Fairbank AC, Bhatia D, Jinnah RH, et al. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42–49. [PubMed] [Google Scholar]
- 12.Steinberg ME, Brighton CT, Corces A, et al. Osteonecrosis of the femoral head. Results of core decompression and grafting with and without electrical stimulation. Clin Orthop Relat Res. 1989;(249):199–208. [PubMed] [Google Scholar]
- 13.Scher MA, Jakim I. Intertrochanteric osteotomy and autogenous bone-grafting for avascular necrosis of the femoral head. J Bone Joint Surg Am. 1993;75:1119–1133. doi: 10.2106/00004623-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357–1364. [PubMed] [Google Scholar]
- 15.Brunelli G, Brunelli G. Free microvascular fibular transfer for idiopathic femoral head necrosis: long-term follow-up. J Reconstr Microsurg. 1991;7:285–295. doi: 10.1055/s-2007-1006786. [DOI] [PubMed] [Google Scholar]
- 16.Soucacos PN, Beris AE, Malizos K, et al. Treatment of avascular necrosis of the femoral head with vascularized fibular transplant. Clin Orthop Relat Res. 2001;(386):120–130. doi: 10.1097/00003086-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Scully SP, Aaron RK, Urbaniak JR. Survival analysis of hips treated with core decompression or vascularized fibular grafting because of avascular necrosis. J Bone Joint Surg Am. 1998;80:1270–1275. doi: 10.2106/00004623-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Korompilias AV, Beris AE, Lykissas MG, et al. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2011;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 19.Urbaniak JR, Coogan PG, Gunneson EB, et al. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long-term follow-up study of one hundred and three hips. J Bone Joint Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zeng B, Xu Z, et al. Treatment of osteonecrosis of femoral head with free vascularized fibula grafting. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18:367–369. [PubMed] [Google Scholar]
- 21.Yoo MC, Chung DW, Hahn CS. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1992;(277):128–138. [PubMed] [Google Scholar]
- 22.Kreyberg L, Liebow AA, Uehlinger EA. Histological typing of lung tumors. WHO; Geneva: 1967. International histological classification of tumors. [Google Scholar]
- 23.Shenk R. On the histological processing of undecalcified bone. Acta Anat (Basel) 1965;60:3–19. [PubMed] [Google Scholar]
- 24.Carter JR, Furey CG, Shaffer JW. Histopathologic analysis of failed vascularized fibular grafts in femoral head osteonecrosis. Microsurgery. 1998;18:110–118. doi: 10.1002/(sici)1098-2752(1998)18:2<110::aid-micr9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.González Della Valle A, Bates J, Di Carlo E, et al. Failure of free vascularized fibular graft for osteonecrosis of the femoral head: a histopathologic study of 6 cases. J Arthroplasty. 2005;20:331–336. doi: 10.1016/j.arth.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Beris AE, Soucacos PN. Optimizing free fibula grafting in femoral head osteonecrosis: the Ioannina aiming device. Clin Orthop Relat Res. 2001;(386):64–70. doi: 10.1097/00003086-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(174):28–42. [PubMed] [Google Scholar]
- 28.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid- induced osteonecrosis. Clin Orthop Relat Res. 2000;(370):295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Asano T, Takahashi KA, Fujioka M, et al. Genetic analysis of steroid-induced osteonecrosis of the femoral head. J Orthop Sci. 2003;8:329–333. doi: 10.1007/s10776-003-0646-7. [DOI] [PubMed] [Google Scholar]
- 30.Veillette CJ, Mehdian H, Schemitsch EH, et al. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88( Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]