Summary
Background
While CMV viral load (CMV-VL) is commonly used to guide preemptive therapy in the post-transplant setting, there is little data correlating viremia with clinical endpoints. We therefore investigated the association of CMV-VL with mortality in the first year after hematopoietic cell transplantation (HCT).
Methods
This cohort study included patients who received an allogeneic HCT between 01 January 2007 and 28 February 2013, were CMV seropositive or had a seropositive donor, and underwent weekly plasma CMV monitoring by PCR through day 100 post-transplant. Cox proportional hazards models were used to estimate the association of CMV-VL at different thresholds with overall by 1 year post-transplant, adjusting for the use of preemptive therapy and other factors such as neutropenia, and graft-versus-host disease. Secondary endpoints were non-relapse mortality and CMV end organ disease by 1 year post-transplant.
Findings
Among 926 patients, the cumulative overall mortality was 30·0% (95% CI 26·9–33·0) by 1 year. CMV-VL of ≥250 IU/ml was associated with increased risk of early (day 0–60 post-transplant) death (adjusted HR 18·1, 95% CI 8·8–37·4). The risk was attenuated after day 60 (adjusted HR 1·8, 95% CI 1·4–2·4). Similar associations were observed for higher CMV-VL thresholds. CMV-VL was also associated with increased risk of non-relapse mortality and demonstrated a dose-response relationship. The adjusted HR (95% CI) for CMV-VL of any positive CMV-VL below 500, 501–1000, and >1000 IU/ml were 1·4 (0·9–2·1), 2·6 (1·3–4·9), and 5·0 (3·1–8·1), respectively.
Interpretation
CMV viremia is associated with increased risk of overall and non-relapse mortality in the first year after HCT, independent of the use of preemptive therapy and with evidence of a postitive dose-response relationship. These data establish the suitability of viral load as a surrogate clinical endpoint for clinical trials for CMV vaccines, biologics, and drugs.
Funding
Merck & Co., Inc., National Institute of Health (K23-AI097234, K24HL093294, HL088021, CA78902, CA18029, HL122173)
Introduction
Cytomegalovirus (CMV) is a highly prevalent herpesvirus that is an important cause of morbidity and mortality in immunocompromised patients such as those receiving hematopoietic cell transplantation (HCT). Current prevention strategies that utilize antiviral agents such as ganciclovir or foscarnet at the onset of viremia (preemptive therapy), have successfully limited the incidence of CMV end organ disease to 3–6% in the first three months after HCT.1–3 Yet these therapies have important toxicities, viral resistance does occur, and CMV pneumonia remains a deadly disease. Clinicians caring for immunocompromised patients are in need of better preventative agents; however without an accepted virologic endpoint, clinical trials powered to prevent CMV end organ disease are likely too costly and time consuming.1
While CMV DNA viral load testing by quantitative PCR is increasingly used to guide preemptive therapy, data linking specific viral load thresholds with clinical outcomes are lacking.4–6 Several studies have described the early kinetics of viral replication in bone marrow transplant recipients and the association of viral load with CMV disease, but few of these patients received preemptive therapy.7–9 Furthermore, the development of a standard method of quantitation (WHO standard IU/ml) has reduced the heterogeneity in the performance of different assays, making comparison of viral load values between different laboratories feasible.10 The purpose of this study was to estimate the association of CMV viral load, using the new international standard, with non-relapse mortality and overall mortality during the first year after HCT. Mortality was chosen for the endpoint not only because of the now relatively rare incidence of CMV disease, but also to account for the indirect effects of CMV infection and its treatment, such as neutropenia, and death from fungal infection and gram negative bacteremia.6,11
Methods
Study Design
This was retrospective non-interventional cohort study of previously collected CMV viremia and clinical outcome measures among HCT patients who were closely followed for 1 year post-transplant for CMV-associated disease and death. As part of the study, a detailed chart review was conducted to confirm disease status and extract additional information regarding end organ involvement and other important clinical details.
Participants and Routine Clinical Care Setting
Patients receiving their first allogeneic HCT at the Fred Hutchinson Cancer Research Center (Fred Hutch) between 01 January 2007 and 28 February 2012 who were CMV seropositive (R+) or had a seropositive donor (D+) were included in this study. Since 2007, allogeneic HCT recipients undergo weekly CMV testing by quantitative PCR measured in blood plasma at least until day 100 post-transplant (using BioRad master mix and Roche MP96 instrument for extraction).12 The lower limit of detection for this assay (95% reproducibility limit) is 20 IU/ml. The conversion factor to the WHO standard is 4 copies = 1 IU.
Preemptive antiviral therapy was initiated once the viral load reached 125 IU/ml for most patients as previously described.6 Patients considered high-risk, such as those receiving ≥1mg/kg body weight of prednisone, or cord blood transplant recipients, were started at any positive viral load. After day 100 post-transplant, patients who are considered at risk for late CMV were recommended to continue weekly CMV PCR testing and to start preemptive therapy if the viral load was ≥250 IU/ml.
Patients were excluded from the study if more than 40% of the expected weekly CMV surveillance tests were missed, more than 2 consecutive weekly tests were missed, or if they received CMV prophylaxis as part of a clinical trial. The institutional review board at Fred Hutch approved this protocol for accessing and analyzing these data.
Data sources
Demographic, clinical, and laboratory data for this study were accessed from an ongoing research database. The records also include communications and reports from referring providers. Patients remain at the transplant center until approximately day 100 post-transplant after which they are often referred back to their primary oncologists. The Long Term Follow Up Clinic remains in contact with patients and their providers as needed. Chart review was performed to identify all cases of CMV end organ disease as typically defined,13 record all episodes of preemptive therapy occurring in the first year after HCT, and determine cause of death. Any death occurring after relapse was considered due to relapse.14 Death without relapse was further classified as due to graft-versus-host-disease (GVHD), organ failure, infection, or other; these classifications were not mutually exclusive.
Statistical Methods
Kaplan-Meier and cumulative incidence estimation methods were used to initially estimate the incidence of overall and non-relapse mortality, initiation of preemptive therapy, and of CMV reactivation and disease following transplant. The cumulative incidence of CMV disease, initiation of preemptive therapy, and CMV reactivation was estimated treating death as a competing risk event. For non-relapse mortality, relapse was a competing risk event. Cox proportional hazards were used to estimate the association of CMV plasma viral load following transplant with overall and non-relapse mortality by 1 year post-transplant and with CMV disease by day 100. The proportional hazards assumption was tested and variables that violated the assumption were stratified by time. Models for CMV disease and non-relapse mortality were also fit using the methods of Fine and Gray (Appendix p.3). All analyses were performed using SAS v.9.4 (SAS Institute Inc.,Cary, NC, USA).
Viral load was evaluated (1) as a categorical variable to assess for a dose response relationship (Non-viremic, >0–500, 501–1000, and >1000 IU/ml), and (2) as a series of binary variables based on viral load cut-points selected a priori (any positive, >150, >250, >500, >750, and >1000 IU/ml). The lowest threshold of 150 IU/ml was chosen as this is approximately the lower limit of quantification of a commonly used assay. The upper threshold of 1000 IU/ml was chosen since many centers use this threshold to initiate preemptive therapy. In both the categorical and cut-point metrics, viral load was treated as time-dependent but such that it could only increase. For the categorical variable, if the viral load increased to the next category threshold the patient would then be assigned to the next risk strata going forward, otherwise they would remain at the highest category attained. For the binary cut-points, at the time of the first detectable viral load level above a given cut-point, the variable was fixed as “above” throughout follow-up. This was done to allow for long-term effects of viral load on mortality. If a CMV PCR test was missing, the value from the prior week’s test was carried over for up to three weeks. Demographic and clinical factors evaluated as potential covariates in each of the models were age at HCT, donor age, race, donor race, sex, donor sex, HLA matching, underlying disease risk, hematopoietic cell transplantation specific comorbidity index (HCT-CI), conditioning regimen, cell source, transplant year, GVHD prophylaxis regimen, peak acute GVHD grade (time-dependent), NIH chronic GVHD (time-dependent), and neutropenia (time-dependent). Factors were included in the final models if they were significant themselves or if their inclusion in the model markedly modified the association between CMV viremia and disease (>10%). Karnofsky scores were not consistently collected for the patients however their addition would be expected to have little additional impact on mortality and non-relapse mortality after adjusting for HCT-CI. 15 Duration of underlying disease was also not evaluated as it was not thought to have an effect on the association between CMV viremia and the endpoints of interest and the underlying health of the patient was already taken into account with both the HCT-CI score and disease risk which incorporates disease stage.
Landmark analyses were also performed among patients who survived to day 100 post-transplant to estimate the association between maximal CMV viral load and the viral AUC before day 100 on non-relapse mortality and overall mortality by 1 year after transplant.
Role of funding source
This was an investigator-initiated study funded by Merck and Co. Inc. Drs. Green and Boeckh receive significant support from NIH grants (K23-AI097234 and K24HL093294, respectively). Additional resources were provided by NIH grants (HL088021, CA78902; CA18029; HL122173). Authors from Fred Hutch led the study design, data collection, analysis, and interpretation. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Of the 1037 patients initially selected for inclusion in this cohort, 87 (8%) patients were excluded due to missing CMV testing and 24 (2%) were excluded due to participation in CMV prophylaxis trials. The demographic and clinical characteristics of the 926 patients in the analytic cohort are presented in Table 1.16,17 Follow up was terminated on 3 May 2014. Median time of follow up was 483 days (IQR 209-1110) post-transplant.
Table 1.
Cohort demographic and clinical characteristics
| Patient/Transplant characteristics (N=926) | n | % | |
|---|---|---|---|
| Age (years) | 0–18 | 129 | 14% |
| 18–40 | 195 | 21% | |
| 41+ | 602 | 65% | |
|
| |||
| Sex | Male | 506 | 55% |
| Female | 420 | 45% | |
|
| |||
| Race | White | 620 | 67% |
| Other | 248 | 27% | |
| Unknown | 58 | 6% | |
|
| |||
| Underlying disease | Acute Leukemia | 415 | 45% |
| Chronic Leukemia | 100 | 11% | |
| Lymphoma | 110 | 12% | |
| Other* | 301 | 33% | |
|
| |||
| Disease risk** | High | 343 | 37% |
| Intermediate | 76 | 8% | |
| Low | 507 | 55% | |
|
| |||
| Prior autologous HCT | 151 | 16% | |
|
| |||
| HCT-CI score | Low (0) | 154 | 17% |
| Intermediate (1–2) | 289 | 31% | |
| High (≥3) | 483 | 52% | |
|
| |||
| CMV serostatus | D+/R+ | 333 | 36% |
| D−/R+ | 459 | 50% | |
| D+/R− | 134 | 14% | |
|
| |||
| Conditioning regimen | Myeloablative | 559 | 60% |
| RIC | 367 | 40% | |
|
| |||
| Cell source | Bone Marrow | 194 | 21% |
| PBSC | 630 | 68% | |
| Cord Blood | 102 | 11% | |
|
| |||
| HLA matching | Matched, related | 288 | 31% |
| Matched, unrelated | 423 | 46% | |
| Mismatched | 62 | 7% | |
| Haploidentical | 50 | 5% | |
| Cord Blood | 102 | 11% | |
PBSC- Peripheral blood stem cells, RIC- Reduced intensity conditioning, HCT-CI score- hematopoietic cell transplantation specific comorbidity index16
Other diseases include (n, %): myelodysplastic syndrome (MDS) (106, 11%), multiple myeloma (MM) (55, 6%), myelofibrosis (37, 4%), aplastic anemia (26, 3%), and others each with frequency <20 patients (77, 8%).
Disease risk- High: Acute myeloid leukemia (AML) evolved from MDS, high-grade non-Hodgkin lymphoma (NHL) not in complete remission (CR), Hodgkin disease, secondary MDS, AML not in CR, Chronic myeloid leukemia (CML) in second chronic phase (CP) or accelerated phase/blast crisis, and Acute lymphoblastic leukemia not in first CR; Intermediate: chronic lymphocytic leukemia (CLL) not in CR, MM not in CR, AML in CR; low: CLL in CR, low-grade NHL, high-grade NHL in CR, MM in CR, CML in first CP, and ALL in first CR.17
CMV reactivation and disease
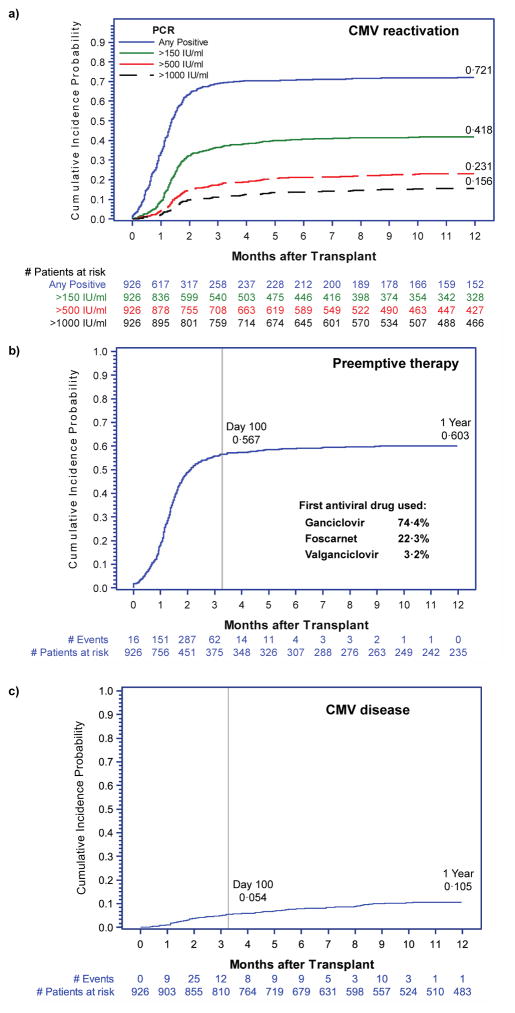
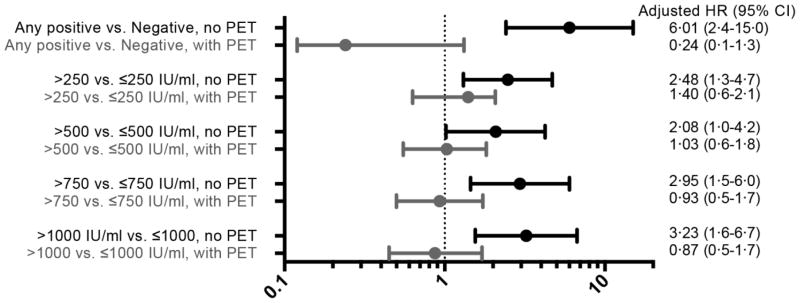
Sixty-eight percent (n= 643) of patients had CMV reactivation at any level of viremia by day 100 after HCT. In the same time period, 37% (n=346) of patients achieved a plasma viral load of >150 IU/ml, while a viral load of >1000 IU/ml was a relatively rare event occurring in only 12% (n=107) of patients (Figure 1a). Preemptive antiviral therapy was administered to 558 patients (60%) in total –512 patients received their first course before day 100 (Figure 1b). There were 95 patients with CMV disease identified in the first year after transplantation (cumulative incidence 10·5%)(Figure 1c). The majority of patients had GI tract disease (n=59, 62%), while CMV pneumonia and retinitis were less common occurring in 33 (35%) and 3 (3%) patients, respectively. Only 53% (n=50) of patients with CMV disease were diagnosed by day 100. As expected, CMV viremia was associated with an increased risk of CMV disease, but only when patients were not receiving preemptive therapy (Figure 2).
Figure 1. Cumulative incidence of: (a) CMV reactivation at different levels of viremia (any positive, >150 IU/ml, >500 IU/ml, and >1000 IU/ml); (b) Initiation of preemptive therapy*; and (c) CMV disease by 1 year after HCT for all subjects.
*Preemptive antiviral therapy was initiated with either induction-dose ganciclovir (5 mg/kg intravenously every 12 hours), foscarnet (90 mg/kg intravenously every 12 hours), or valganciclovir (900 mg orally every 12 hours). Induction therapy was continued for at least one week followed by at least two weeks of maintenance (once daily) therapy until cessation of CMV viremia.
Figure 2. Adjusted HR and 95% CI from multivariable Cox proportional hazards models evaluating CMV viral load as a time-dependent risk factor for CMV disease by 1 year after HCT, stratified by use of preemptive therapy (n= 926).
Models each adjusted for CMV serostatus, HLA matching, cell source, underlying disease, HCT-CI, and disease risk.
Overall and Non-relapse Mortality
The cumulative overall mortality and non-relapse mortality were 9·0% (95%CI 7·1–10·8) and 6·5% (95%CI 4·9–8·1) respectively by day 100, and 30·0% (95% CI 26·9–33·0) and 18·0% (95%CI 15·5–20·6) respectively by one year after HCT. Among the 95 patients with CMV disease, 35 (37%) died within the first year after HCT. However, death was directly attributable to CMV in only 3/263 (1%) patients who died in the first year after HCT; two patients died of CMV pneumonia and one had disseminated disease. Of the 263 deaths occurring by 1 year after HCT, 118 (45%) were due to relapse or disease progression (Table 2). Among the 145 non-relapse deaths, infection was either the primary or contributing cause in 109 (75%) cases and GVHD was implicated in 84 (58%) deaths.
Table 2.
Causes of death in first year after HCT
| N = 263 | % | |
|---|---|---|
| Cause of Death (Mutually Exclusive) | ||
|
| ||
| Death due to relapse or disease progression | 118/263 | 44·9% |
|
| ||
| GVHD alone | 14/263 | 5·3% |
|
| ||
| Infection alone | 29/263 | 11·0% |
|
| ||
| Organ failure alone | 10/263 | 3·8% |
|
| ||
| No information available | 5/263 | 1·9% |
|
| ||
| Multiple contributing causes (NOT mutually exclusive groups) | 87/263 | 33·1% |
|
| ||
| GVHD | 70/87 | 80·5% |
| Infection | 80/87 | 92·0% |
| Organ failure | 36/87 | 41·4% |
| Other | 11/87 | 12·6% |
CMV Viral load and Overall Mortality
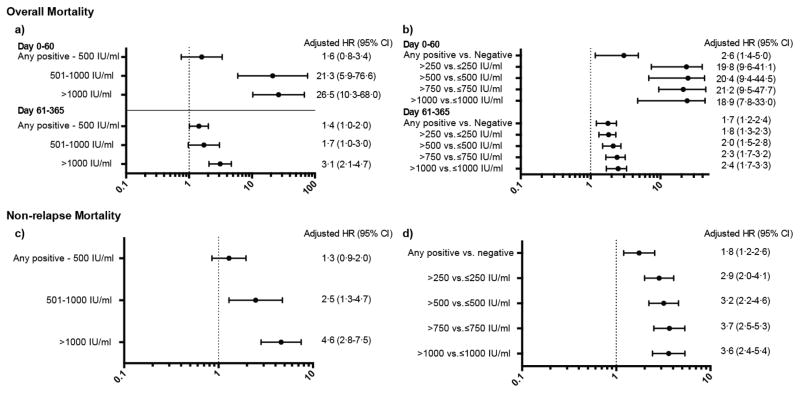
In Cox models adjusted for the use of preemptive therapy, neutropenia, and other important risk factors, patients with CMV viral loads >500 IU/ml experienced a significantly increased risk of death from any cause in the first year after HCT compared to patients who did not reactivate CMV (Figure 3a). The risk was highest in the first 60 days after transplant (adjusted HR 21·3 [95% CI 5·9–76·6] and 26·5 [95% CI 10·3–68·0] for viral loads of 500–1000 and >1000 IU/ml respectively) as compared to later periods (adjusted HR 1·7 [95%CI 1·0–3·0] and 3·1 [95% CI 2·1–4·7] for viral loads of 500–1000 and >1000 IU/ml respectively). The risk of death by day 60 among patients who had lower levels of viremia (any positive-500 IU/ml) was not significantly different from patients who did not reactivate (adjusted HR 1·6 [95% CI 0·8–3·4]), however after day 60, this comparison did reach statistical significance (adjusted HR 1·4 [95%CI 1·0–2·0]).
Figure 3. Adjusted HR and 95% CI from multivariable Cox proportional hazards models evaluating CMV viral load as a time-dependent risk factor for overall mortality with (a) viral load as a categorical variable and (b) viral load as a threshold; and non-relapse mortality with (c) viral load as a categorical variable and (d) viral load as a threshold by 1 year after HCT (n=926).
Comparator group for categorical models (a and c) is patients with no CMV reactivation.
Adjustment factors for overall mortality models: patient age,transplant year, underlying disease, disease risk, HCT-CI score, acute GVHD grade, neutropenia, and preemptive therapy. Adjustment factors for non-relapse mortality models: patient, HLA-matching, disease risk, HCT-CI score, acute GVHD, chronic GVHD, neutropenia, and preemptive therapy. Results of the categorical model for all factors are available in the appendix (page 2)
Similar results were observed in the multivariable models evaluating specific viral load thresholds as a risk factor for overall mortality (Figure 3b). In each model, having a viral load above the given threshold carried a significantly higher risk of death than having a viral load below the threshold. This was true even for the lowest threshold (any positive vs. negative) where the adjusted HR (95%CI) was 2·6 (1·4–5·0) until day 60 and 1·7 (1·2–2·4) after day 60. For the higher thresholds, as in the categorical models, the hazard ratios were higher before day 60 compared to after. These analyses were repeated, stratifying by CMV serostatus (D+/R+, D−/R+, D+/R−), and the point estimates were consistent between stratum. However, due to the low incidence of CMV viremia in D+/R− patients, the associations were not statistically significant in this group (Appendix p.2).
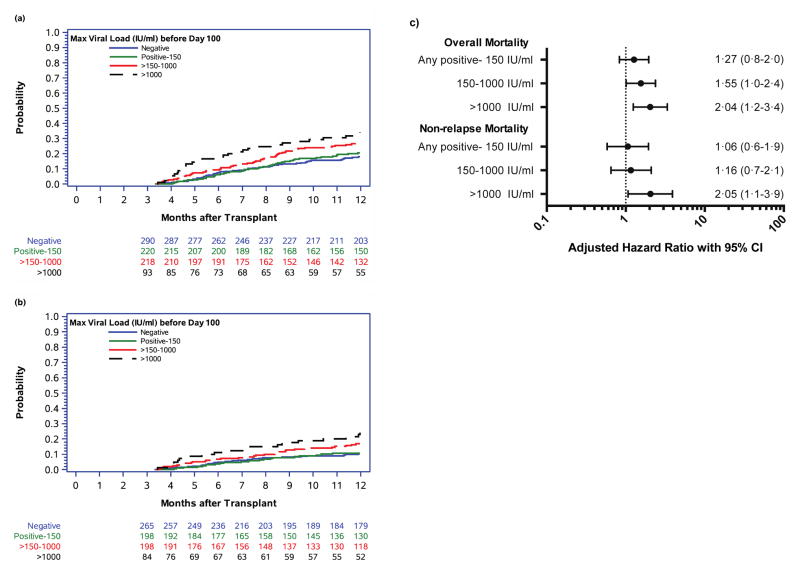
Among patients who survived to day 100 (N=832) the overall 1-year cumulative overall mortality was 23·1% (95% CI 20·1–26·1). These estimates varied according to the maximal viral load achieved before day 100 (Figure 4a). The cumulative incidence estimates (95% CI) were 20·5% (15·0–26·1), 27·1% (20·9–33·2), and 34·2% (24·3–44·2) respectively, among patients with a maximal viral load before day 100 of <150 IU/ml, 150–1000 IU/ml, and >1000 IU/ml. In multivariate analysis only maximal viral load >1000 IU/ml remained statistically significant (Figure 4c). We also examined the viral AUC before day 100 as a risk factor for death by 1 year. The median AUC was 1754·2 (interquartile range [IQR] 172·0–6358·9) for those patients that died by 1 year after HCT compared to 700·0 (IQR 95·0–3332·0) for those that survived (p<0·01). However, in multivariable Cox models, viral AUC was not significantly associated with overall mortality (Appendix p.2).
Figure 4. Cumulative incidence of (a) overall mortality and (b) non-relapse mortality by 1 year after HCT among day-100 survivors (n= 832) stratified by maximum CMV viral load before day 100 and (c) results from multivariable Cox proportional hazards models evaluating maximum CMV viral load before day 100 as a risk factor for overall and non-relapse mortality.
Covariates for overall mortality models include: age, donor relation, transplant year, underlying disease, disease risk, HCT-CI score, neutropenia before day 100, CMV viremia after day 100 (time-dependent).
Covariates for non-relapse mortality models include: age, donor relation, transplant year, disease risk, HCT-CI score, acute GVHD, chronic GVHD, neutropenia before day 100, CMV viremia after day 100 (time-dependent).
CMV Viral load and Non-Relapse Mortality
The adjusted HRs for viral loads of any positive-500, 500–1000, and >1000 IU/ml were 1·3 (95% CI 0·9–2·0), 2·5 (95% CI 1·3–4·7), and 4·6 (95% CI 2·8–7·5) respectively, providing evidence of a positive dose-response relationship (Figure 3c). Similar findings were observed when viral load was treated as a binary variable at specific cut-points (Figure 3d). However, in these models a potential dose-response relationship was less apparent due to the characteristics of a binary cut-point as opposed to a categorical variable.
Among patients who survived to day 100 without relapse the overall 1-year cumulative incidence of non-relapse mortality was 14·0% (95% CI 11·4–16·5). Similar to what was seen with overall mortality, the cumulative incidence estimates varied depending on maximal viral load before day 100 (Figure 4b). They were 10·7% (95% CI 6·3–15·2), 17·0% (11·5–22·4), and 24·2% (14·7–33·7) respectively, among those with a maximal viral load before day 100 of <150 IU/ml, 150–1000 IU/ml, and >1000 IU/ml. Again, in multivariable Cox models, only viral loads >1000 IU/ml remained significant (Figure 4c) and viral AUC was not significantly associated with non-relapse mortality by 1 year (Appendix p.2).
Discussion
The results of this cohort study provide several important insights. First, the data indicate that higher viral loads carry an increased risk of both death and non-relapse death in the first year after transplant even when adjusting for the use of preemptive therapy and neutropenia that might occur as a result of preemptive therapy. Despite the relatively low viral load thresholds used to initiate preemptive therapy for our patients (125 IU/ml for most patients), viral loads of >500 or >1000 IU/ml, and their associated increased risk of death, were not completely preventable. Patients who developed viremia >500 IU/ml experienced a 20-fold increase in the risk of death by day 60 (Figure 2a and 2b). This risk was significantly diminished, however, after day 60. This is a critical advance since proof of a definitive association of specific viral load thresholds with important clinical endpoints such as mortality has previously been elusive. To our knowledge, this is the first report to examine this question in a large contemporary cohort using a standardized PCR measure. Other studies have identified CMV viremia as a risk factor for overall mortality and non-relapse mortality.17–19 However, these studies evaluated CMV viremia either as a predictor for mortality in non-transplant settings18 or analyzed it as a binary event.17,19
These data establish the suitability of using CMV viral load as a surrogate clinical endpoint for clinical trials and may provide evidence to preclude the collection of additional clinical endpoint data after fast track approval, as is presently required by some regulators. Our data provide strong epidemiologic evidence that drugs or biologic agents that can prevent high viral loads early after HCT may reasonably be expected to have an impact on mortality, even if they cannot completely prevent viremia or the initiation of preemptive antiviral therapy. In 1993, Kojima and colleagues predicted that using PCR to measure HIV-1 RNA copy number in the plasma of infected patients was “likely to be built into every clinical trial of anti-HIV-1 therapy in the near future.”20 It has been twenty years since the landmark papers correlating HIV-1 viral load with disease progression were published21–23 changing the regulatory environment to allow the use of a virologic endpoint, and thereby fostering the development of dozens of new antiretroviral agents.
The association of CMV viremia with increased risk of death may be a result of the immunomodulating effects of CMV infection. Indeed, CMV has been implicated in the pathogenesis of invasive bacterial and fungal infections as well as graft versus host disease,11,24 and our data show (Table 2) that the majority of non-relapse deaths were due to these complications, either alone or in combination. The association of high level CMV viremia with death was seen both for overall and non-relapse mortality, thus providing no evidence that a putative protective effect of CMV reactivation on relapse of the underlying malignancy would impact survival.17,25–27
Although the association of CMV viremia with CMV disease was not the focus of this study, our data also demonstrate that viral load is associated with an increased risk of disease only during times when patients were not receiving preemptive therapy. This finding confirms the excellent efficacy of preemptive therapy as shown in recent clinical trials.1–3,28
The study has several strengths, including large sample size, uniform management of patients, and thorough diagnostic approach for suspected cases of CMV disease. We also consider the use of the WHO standard (IU/ml) for quantifying CMV viral load a strength, although full commutability has not yet been achieved and measurements may still vary approximately two-fold between assays.10 A few limitations should be noted. First, since the mechanisms of CMV reactivation and its secondary effects are not yet known and could not inform more accurate assumptions, we assumed that the risks associated with viremia began as soon as the viremia occurred and continued even after treatment lowered the viral load. Another limitation to the study is that high-risk patients such as those who would receive steroids were included in the analyses as it could not be known at the time of transplant who would require treatment for GVHD. We attempted to control for this by adjusting the models for acute and chronic GVHD. Also, as a single center study, CMV management and transplant techniques might result in differences in the incidence of CMV viremia and the outcomes of interest. However our rates of CMV reactivation and preemptive therapy use are similar to other published cohorts.1,2,26,29
In conclusion, in this large cohort of allogeneic HCT patients who were monitored with a PCR-based preemptive therapy strategy, we have found that CMV viral load is associated with an increased risk of overall and non-relapse mortality in the first year after transplant even after controlling for the use of preemptive therapy. There is some evidence of a dose-response relationship with higher viral loads associated with higher risk of death and, for overall mortality, high viral loads have a greater impact early after transplant. Given these data, it seems reasonable that CMV viral load should be acceptable as a surrogate clinical endpoint for clinical trials going forward.
Supplementary Material
Acknowledgments
The authors would like to thank Sarah Aker, E. Lisa Chung, Elsa Garnace, Mathilda Loeffelholz, Elizabeth Nguyen, Jennifer Schaeffer, and Zach Stednick for additional data collection and management. We are also indebted to the patients and providers at Fred Hutch for their continued commitment to research participation.
Footnotes
Contributors
MLG, WL, TCM, YC, KRJ, MAM and MB were responsible for the design of the study and interpretation of the data. MLG, WL, and HX performed the data analyses and created the figures. Data collection was performed by MLG, BMS, MLS, SG, SÖ, JY, FS and LEK. All authors contributed to the writing and revision of the paper and approved the final version.
Conflicts of Interest
Dr. Green reports grants from Merck & Co., Inc. during the conduct of the study; grants and personal fees from Astellas outside the submitted work. Dr. Leisenring and Ms. Xie received grants from Merck & Co., Inc. for the conduct of the study. Drs. Mast, Cui, and Marks are current employees of, and own stock in Merck and Co. Inc. Dr. Sorror reports personal fees from Jazz Pharmaceuticals, outside the submitted work. Dr. Boeckh reports grants and personal fees from Merck & Co., Inc., during the conduct of the study; grants and personal fees from Astellas, Shire, Roche/Genentech, Gilead and Chimerix; personal fees from Clinigen and Microbiotix, outside the submitted work. Drs. Sandmaier, Goyal, Özkök, Sahoo, Kimball, Jerome, and Ms. Yi have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. The Lancet Infectious Diseases. 2011;11:284–92. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 2.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to Prevent Cytomegalovirus Disease in Hematopoietic-Cell Transplantation. New England Journal of Medicine. 2013;369:1227–36. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 3.Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for Cytomegalovirus Prophylaxis in Hematopoietic-Cell Transplantation. New England Journal of Medicine. 2014;370:1781–9. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. [PubMed] [Google Scholar]
- 5.Gerna G, Lilleri D, Caldera D, Furione M, Zenone Bragotti L, Alessandrino EP. Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41:873–9. doi: 10.1038/sj.bmt.1705986. [DOI] [PubMed] [Google Scholar]
- 6.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1687–99. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. The Lancet. 2000;355:2032–6. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 8.Gor D, Sabin C, Prentice HG, et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- 9.Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med. 1999;190:177–82. doi: 10.1084/jem.190.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden RT, Preiksaitis J, Tong Y, et al. Commutability of the 1st WHO international Standard for Human Cytomegalovirus. Journal of Clinical Microbiology. 2015 doi: 10.1128/JCM.01495-15. published online Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J INFECT DIS. 2002;185:273–82. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 12.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. Journal of Clinical Microbiology. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical Infectious Diseases. 2002;34:1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo JD. [accessed March 21, 2015];Causes of Death. 2012 published online Feb 1. http://www.cibmtr.org/meetings/materials/crpdmc/pages/Feb12Cause.aspx.
- 15.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 16.Sorror ML. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–24. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deayton JR, Sabin CA, Prof, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004;363:2116–21. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 19.Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48:803–8. doi: 10.1038/bmt.2012.221. [DOI] [PubMed] [Google Scholar]
- 20.Kojima E, Shirasaka T, Anderson B, et al. Monitoring the activity of antiviral therapy for HIV infection using a polymerase chain reaction method coupled with reverse transcription. AIDS. 1993;7(Suppl 2):S101–5. doi: 10.1097/00002030-199311002-00019. [DOI] [PubMed] [Google Scholar]
- 21.Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 22.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Holland CA, Ellenberg JH, Wilson CM, et al. Relationship of CD4+ T cell counts and HIV type 1 viral loads in untreated, infected adolescents. Adolescent Medicine HIV/AIDS Research Network. AIDS Research and Human Retroviruses. 2000;16:959–63. doi: 10.1089/08892220050058371. [DOI] [PubMed] [Google Scholar]
- 24.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a Bidirectional Relationship between Cytomegalovirus Replication and acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 2010;16:1309–14. doi: 10.1016/j.bbmt.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–12. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka K, Nishida T, Asano-Mori Y, et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.07.019. published online July 23. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan M, Teira P, Battiwalla M, et al. Early CMV Reactivation Still Remains a Cause of Increased Transplant Related Mortality in the Current Era: A CIBMTR Analysis. Blood. 2014;124:47–7. doi: 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boeckh M, Nichols WG, Chemaly RF, et al. Valganciclovir for the Prevention of Complications of Late Cytomegalovirus Infection After Allogeneic Hematopoietic Cell Transplantation. Annals of internal medicine. 2015;162:1. doi: 10.7326/M13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito S, Pophali P, COW, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1313–6. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.