Abstract
Since the discovery of the genetic basis of DOCK8 immunodeficiency syndrome (DIDS) in 2009, several hundred patients worldwide have been reported, validating and extending the initial clinical descriptions. Importantly, the beneficial role of hematopoietic stem cell transplantation for this disease has emerged, providing impetus for improved diagnosis. Additionally, several groups have further elucidated the biological functions of DOCK8 in the immune system that help explain disease pathogenesis. Here, we summarize these recent developments.
Keywords: DOCK8, combined immunodeficiency, hyperimmunoglobulinemia E syndrome, tissue Resident Memory T cells (TRM), cytothripsis, cutaneous virus infection, somatic reversion, genetics, eczema, food allergy, hematopoietic stem cell transplantation
Introduction
With the completion of the Human Genome Project and the adoption of newer genomic technologies such as comparative genomic hybridization and whole exome sequencing to complement linkage and homozygosity mapping, the pace of discovery of new genetic defects underlying human primary immunodeficiency diseases (PID) has rapidly accelerated to encompass ~230 disorders (1, 2). As part of this trend, in 2009 we and others discovered autosomal recessive loss-of-function mutations of the gene DOCK8 in cohorts of autosomal recessive Hyper-IgE Syndrome (HIES) patients (3, 4). This discovery established DOCK8 immunodeficiency syndrome (DIDS) as a clinical entity distinct from autosomal dominant HIES caused by dominant negative STAT3 mutations, or from other immunodeficiencies sometimes expressing elevated serum IgE such as Tyrosine Kinase (TYK2) deficiency (which was initially considered an autosomal recessive HIES). This distinction has been key to better understanding of the diverse and complex clinical phenotype of the disease, as reflected through its natural history with or without hematopoietic stem cell transplantation (HCST) or other therapy. This distinction has also led to the development of improved diagnostic tools, and has revealed several unusual mechanisms contributing to disease.
Since the original reports, over 170 cases of DIDS have been published worldwide, which is a likely underestimate. In this review, we will build upon previous published reviews (5, 6) to paint a more complex and nuanced picture of this disease based upon accumulating data, which we hope to be useful to the practicing clinical immunologist and the PID researcher. We will begin by summarizing recent updates about the genetics of this disease and its implications for diagnosis, clinical outcome and unusual features of disease, followed by recently elucidated disease mechanisms and future areas of research.
Genetics: Loss-of-function mutations in DOCK8
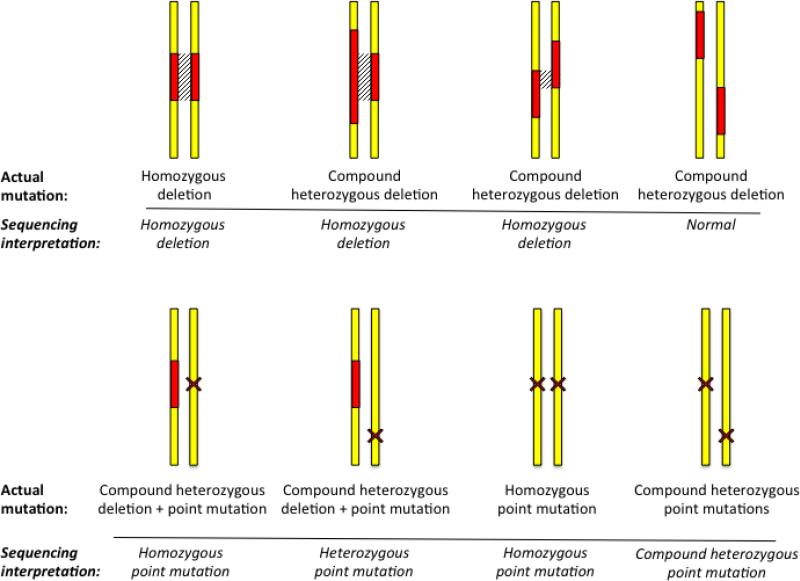
DIDS is caused by autosomal recessive loss-of-function mutations in the DOCK8 gene. These mutations universally lead to loss of DOCK8 protein expression, which is normally restricted to the immune system. Most DOCK8 mutations involve large deletions in the gene that are unique to each family. In over half of the families we study, a large deletion on one or both DOCK8 alleles is observed. For a PID, this is an unusual characteristic that reflects recombination occurring between repetitive DNA sequence elements over a large stretch of the DOCK8 locus near the telomere. A deletion on one allele can be accompanied by the same deletion on the other allele (homozygous deletion), a different deletion on the other allele (compound heterozygous deletions), or a different type of mutation on the other allele. Consequently, sequencing results can sometimes be difficult to interpret (Figure 1). For example, two non-overlapping large deletions can give rise to an apparently normal Sanger sequencing result. Upon closer inspection, occasionally the regions coinciding with the heterozygous large deletion(s) show loss of heterozygosity when the patient's single nucleotide polymorphisms (SNP) in these regions are compared to those of both parents. However, informative SNP and parental DNA samples are not always available for every patient. Next generation sequencing can also suggest a deletion but this interpretation requires comparing the actual sequence coverage for a patient to expected coverage over regions of the DOCK8 gene. Identifying large deletions is more definitively performed by using comparative genomic hybridization arrays or multiplex ligation-dependent probe amplification assays (3, 7), both of which are now commercially available for DOCK8.
Fig. 1. Possible combinations of germline mutations in DIDS.
Actual mutations with sequencing interpretations below. Yellow, DOCK8 allele. Red, deletion. X, point mutation (missense, nonsense, splicing mutation, small indel). Gray hatches, sequence that appears to be deleted.
Loss-of-function mutations in DOCK8 immunodeficient patients are also caused by point mutations and small indels that cause splicing alterations, as well as nonsense mutations or frameshift alterations that cause nonsense-mediated decay predominantly. In contrast, promoter mutations leading to loss of DOCK8 expression have not been identified. DOCK8 has two conserved protein domains including a DOCK Homology Region (DHR)2 domain that mediates guanine-nucleotide exchange factor activity. Theoretically, small in-frame indel or missense mutations within either of these conserved domains could disrupt the function of DOCK8 without affecting its expression. However, this situation has not yet been reported in a patient. In the one patient who has a homozygous missense mutation (NP_0011800465.1: p.Cys1447Arg), such a mutation was protein-destabilizing (8). Thus, it seems that loss-of-function mutations that simultaneously preserve DOCK8 expression are likely to be extremely rare.
Somatic reversions complicate DIDS
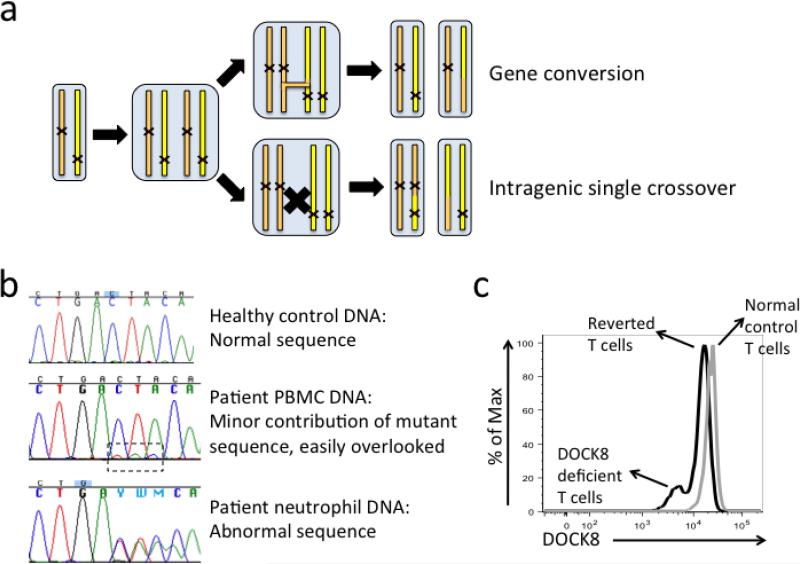
In a surprising twist complicating the genetics of this disease, we found that over half of DIDS patients in the NIH cohort have somatic reversions that partially restore DOCK8 expression (9). This has subsequently also been observed by other centers (10). Somatic reversions have been reported in several PID, especially the Wiskott-Aldrich syndrome where it occurs in up to 11% of patients (11). However, the much higher proportion of DIDS patients with somatic reversions reflects DOCK8's location in a recombination hotspot. This characteristic explains not only why so many large germline deletions arise but also why somatic reversion occurs so often in DIDS. Recombination can create a healthy DOCK8 allele by stitching together the good parts from both alleles (intragenic single crossover), or by copying the good part from one allele over the corresponding bad part of the other allele (gene conversion) (Figure 2a). Occasionally, a single nucleotide change at the original mutation site or at a compensatory second site abolishes the point mutation or small indel, creating a new sequence code that is in frame. The end result is a reverted cell expressing one healthy DOCK8 allele. However, none of these processes can happen when there are large homozygous deletions or overlapping large heterozygous deletions.
Fig. 2. Somatic reversions can obscure a molecular diagnosis of DIDS.
(a) During replication of DOCK8 alleles, either gene conversion or intragenic single crossover can occur to generate somatic reversions. (b) An example of DIDS patient with somatic reversion. Notice the small peaks in the dashed frame showed abnormal sequence that was barely detectable in Sanger sequencing of PBMC DNA as opposed to neutrophil DNA. (c) Intracellular flow cytometic detection of DOCK8 in the same patient. A distinctive double peak (black) indicates this was a DIDS patient with somatic reversion in T cells. The level of reversion was exceptionally high in this patient, which resulted in the apparently normal Sanger sequencing result in (b).
Somatic reversion in PID can be detected when the wild-type allele confers a survival or growth advantage to mutant cells, leading to expansion of those cells. Somatic reversion occurs to a variable degree in DIDS, primarily in T cells and NK cells. Nevertheless, because somatic reversion only occurs in a small proportion of blood cells overall, the genomic DNA obtained from such a patient represents a mixture of normal alleles and defective alleles. When mutational analysis is performed using this genomic DNA, if there is a high level of reversion the reverted DNA can make it seem that the patient has a heterozygous mutation only, similar to what is seen in a healthy carrier (Figure 2b). In this way, somatic reversion has the potential to further obscure and delay a genetic diagnosis. However, since somatic reversion in DIDS has not been detected in neutrophils, this potentially confounding factor can be removed from consideration by performing germline mutational analysis on genomic DNA from either purified neutrophils or fibroblasts.
Clarification as to whether somatic reversion is occurring can be provided by intracellular flow cytometric analysis of DOCK8 protein expression in single cells (9). The flow cytometric assay requires a small amount of blood and can be completed in several hours, making it also suitable for rapid clinical screening and diagnosis (9, 12). Either fresh or previously cryopreserved peripheral blood mononuclear cells (PBMC) can be used in this assay. Somatic reverted cells express low levels of DOCK8 protein comparable to that seen in cells from healthy carriers. When both DOCK8-deficient and reverted cells are present in the same sample, a double peak in the histogram can be observed (Figure 2c). However, due to the low signal to noise ratio in this assay, a true DOCK8-deficient control might need to be included when the assay is initially set up. As somatic reversion often occurs in a small proportion of T and NK cells, appropriate surface staining is essential to differentiate lymphocyte subsets and maximize detection efficiency. A well-tested combination of surface markers to delineate DOCK8 expression in these subsets contains CD3, CD19, CD56, CD45RA, and CCR7. Given its many advantages, this method should supplant immunoblotting except in the rare case where loss of DOCK8 function without loss of DOCK8 expression is suspected. The latter case is unlikely to occur given the large size of DOCK8 gene (47 exons), since nearly all mutations that introduce a premature truncation would result in nonsense-mediated decay with loss of DOCK8 expression, rather than a truncated protein that could only be detected up by immunoblotting.
Clinical progression of disease and its treatment
The initial clinical description of DIDS was that of a combined immunodeficiency characterized by recurrent infections, severe allergic disease, and predisposition to cancers, with disease disproportionately involving the skin (3, 4). Since then, an estimated 200 or more mutation-positive patients (published and unpublished) have been identified worldwide, with multiple reports confirming and adding to the original characterization of this disease (8, 13-17). Cumulatively, these data establish that patients can be afflicted with a wide spectrum of microbes beyond the Staphylococcus aureus and herpes simplex virus, human papillomavirus, and molluscum contagiosum skin infections that predominate, leading to rarer presentations such as sclerosing cholangitis; that candidal infections are common but mild; and that chronic Epstein-Barr virus besides poorly controlled human papillomavirus infections likely contribute to the increased lymphoma and skin cancer risk. Additionally, these data also reinforce the observations that autoimmunity including autoimmune hemolytic anemia, lupus, and cerebral vasculitis are serious but rare complications (18); that vasculopathy can involve other large vessels outside the central nervous system (19, 20); and that connective tissue, dental, and skeletal abnormalities are features primarily of autosomal dominant STAT3-mutant hyper-IgE syndrome rather than DIDS.
A long-awaited, retrospective survey from multiple international centers of 136 DIDS patients was recently published, detailing the natural history of this disease including its outcome (13). Half of the patients were dead by age 20 years, usually because of infection or cancer, and most patients had at least one life-threatening complication by age 25. Of note, the high morbidity and mortality occurred despite treating some patients with prophylactic antimicrobials, replacement immunoglobulin G, or IFN-α. The last was reported as helpful for some cases of severely incapacitating herpes simplex virus or warts (21-23). However, all of these modalities seem to have partial efficacy or have been limited by side effects when they have been used in a larger number of DIDS patients, making them more suitable as adjunctive therapy (13). In contrast, the only form of definitive therapy, borne out through the experience of multiple centers, has been hematopoietic stem cell transplantation (HSCT) (24-31). HSCT for DIDS has been successfully performed using reduced intensity myeloablative conditioning regimens with matched related or matched unrelated allogeneic donor cells (24-26, 29, 30). In one case, engraftment of matched related allogeneic donor cells, sufficient for development of severe GVHD, occurred even without any conditioning, emphasizing the intrinsic survival advantage of DOCK8-expressing cells (32). More recently, haploidentical donor cell engraftment was successful in a patient following TCRαβ/CD19 cell depletion (31). If confirmed in additional patients, this approach could potentially expand the number of patients who otherwise would not be considered HSCT candidates due to lack of a fully HLA-matched donor. Analysis of survival outcomes with HSCT have yet to be published but are in progress (13).
Because it cures infection susceptibility, which is the main cause of early death, HSCT is now considered standard of care for DIDS when an appropriate donor is available. HSCT also cures the eczematous dermatitis, as well as food allergies in some but not all patients (26-29, 33, 34). Whether HSCT also cures the autoimmune complications and reduces the risk of cancers are as yet undetermined. It is important to identify DIDS patients at a young age so that they can receive definitive treatment before non-reversible parenchymal organ damage from infections and other serious complications arise. While the disease is easily recognized when patients have developed widespread and difficult to treat viral skin infections in late childhood or early adolescence, as infants and toddlers, patients might only have atopic dermatitis, recurrent otitis media, and few pneumonias. Most frighteningly, patients can be seemingly “healthy” before suddenly developing cancers or cerebrovascular complications (19). Although several algorithms have been proposed to help clinicians distinguish DIDS from other conditions, they are of limited utility at an early stage of disease (14, 35). Laboratory screening for lymphopenia, decreased serum IgM levels, and selective decreases in CD4 T cells, naïve CD8 T cells, and memory B cells, may help to flag DIDS patients from severely atopic patients (36). An elevated serum IgE may also be helpful but its absence does not rule out DIDS. The development of a rapid flow cytometry-based assay to detect DOCK8-deficient cells is a major step in lowering barriers to definitive testing for this disease.
As discussed above, somatic reversion frequently occurs in DIDS, raising the question whether this self-correction alters the natural history of disease. We have found that patients with somatic reversion tend to be older before they either die or require HSCT for worsening disease (9). They have some measures of improved disease, but not enough to effect cure. This can be explained by spontaneous corrections in T cells occurring only in a limited repertoire of T cells, in contrast to HSCT which corrects the full T cell repertoire. Thus, although patients might have repaired T cells able to respond well to one virus, they might not have repaired T cells that can respond well to other viruses. Interestingly, patients from regions of the world having a high level of consanguinity (and hence increased likelihood of large homozygous deletions incapable of somatic reversion) seem to have more severe systemic disease manifestations and die younger than patients from outbred populations. Thus, knowing the exact mutations for any given patient facilitates predictions of likely disease severity and progression.
Disease mechanisms
DOCK8 is an atypical guanine nucleotide exchange factor (GEF) that activates CDC42 in immune cells to influence their functioning. Research into DIDS has revealed multiple pathogenic mechanisms whereby loss of DOCK8 contributes to the complex disease phenotype seen in humans. Results from earlier studies, summarized in previous reviews (5, 6), showed that patients had T cell and NK cell lymphopenia and fewer memory CD8 T cells, and that decreased T cell production of the antiviral cytokines IFN-γ and TNF-α could contribute to virus susceptibility, decreased TH17 cells to fungal susceptibility, TH2 skewing to severe atopy, and defective antibody maturation responses to recurrent sinopulmonary infections. More recent research findings, which we describe below, have deepened our understanding of disease mechanisms and have led to interesting new concepts into how DOCK8 normally regulates the immune system, which we describe below.
1. DIDS is the first disease caused by tissue-resident memory T cells (TRM) deficiency
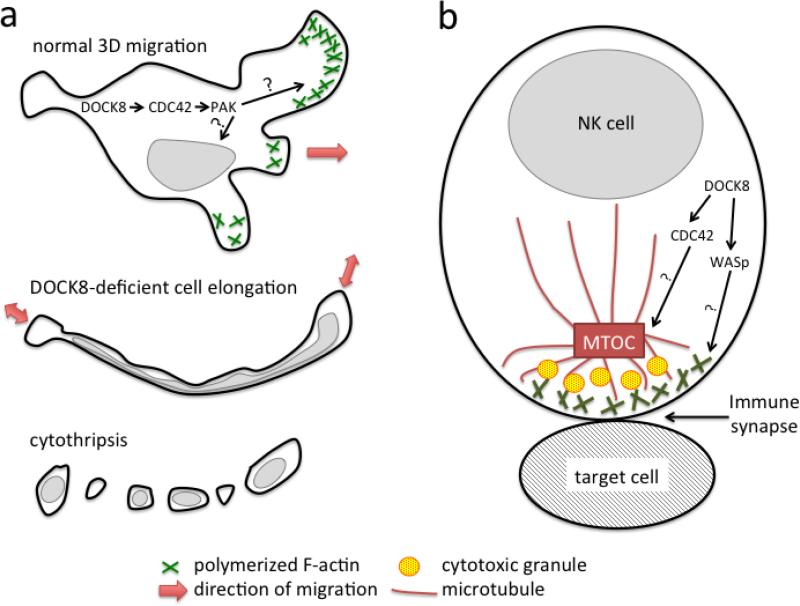
Although patients having other combined immunodeficiencies can develop atopic dermatitis and virus skin infections, the skin disease in DIDS is more extreme in the range of pathogens, severity and recurrence rate, and disproportionate involvement compared to other tissues (17). Similar to DIDS patients, Dock8-deficient mice also have worse disease with higher virus replication when infected in the skin with herpes simplex virus (37, 38). Thus, we consider this to be the signature of DIDS. Multiple studies have shown various ways by which loss of DOCK8 could worsen virus infections, such as decreased production of antiviral cytokines by T cells and dendritic cells (3, 23), decreased NK cell cytotoxicity (39, 40), impaired dendritic cell migration for priming of T cell responses in the lymph nodes (41), and decreased survival of memory CD8 T cells (42, 43). However, these mechanisms do not clearly explain why virus infections targeting the skin predominate over systemic virus infections. We have recently discovered that this distinctive feature can be explained by a selective survival defect of DOCK8-deficient T cells and NK cells as they migrate within the skin, while their short-term chemotaxis remains intact (37, 43). DOCK8 is crucial for maintaining cell shape integrity when lymphocytes move through the highly confined spaces that are characteristic of skin but not in other tissues (Figure 3a). DOCK8 normally controls lymphocyte shape integrity by activating CDC42 and p21-activated kinase (PAK1/2), to coordinate cytoskeletal structures both spatially and temporally during cell locomotion. Without DOCK8, lymphocytes in the skin are subjected to physical stresses that distort their morphology and tear the cells apart, in a process we call cytothripsis for “cell shattering.” Tissue-resident memory CD8 T cells (TRM), which do not recirculate through the lymphatics and blood but instead reside indefinitely within the skin, are particularly susceptible to cytothripsis. These cells constantly move within the epidermis, where their function is to respond rapidly when latent viruses re-emerge or when or reinfection occurs. Thus, the loss of these particular cells results in impaired local antiviral immunity within the skin.
Fig. 3. A critical role for DOCK8 during cytoskeletal rearrangement.
(a) A DOCK8-CDC42-PAK pathway regulates cytoskeletal proteins such as F-actin to keep the cell body and nuclei intact during 3D migration of lymphocytes. The coordination of migration, not the mobility per se, is impaired when DOCK8 is absent and leads to cytothripsis. (b) DOCK8 regulates CDC42 and WASp to rearrange cytoskeletal proteins such as F-actin and microtubules in the immunological synapse. This contributes to NK cell killing of target cells.
Besides being found in the skin, TRM with slightly different surface markers and TCR repertoires reflecting different pathogen or antigen specificities can also be found within other tissues, such as the respiratory and gastrointestinal tracts (44). These other mucosal surfaces are composed of substantially less collagen than the skin, resulting in a less extensive network of confined spaces as compared to skin. However, the possibility exists that TRM migrating for longer times within these non-skin sites could eventually undergo a low level of cytothripsis. This might contribute to the recurrent sinopulmonary and occasional gastrointestinal infections seen in DIDS, but still needs to be tested. Regardless, our study and those of others have recently highlighted the dichotomy between peripheral blood lymphocytes and tissue lymphocytes (37, 45, 46). In humans, “representative” blood samples are often used to evaluate immunity. For example, T cell lymphopenia measured in the blood is typically assumed to reflect decreased T cell counts throughout the body. However, peripheral blood contains a very small proportion of total T cells, with up to 98% of memory T cells actually located in non-lymphoid tissues, making accurate extrapolations about memory T cell numbers, phenotypes, and functions difficult. Thus, as illustrated in DIDS, a closer examination of regional immunity in other PID displaying disproportionate involvement of different organ systems might reveal additional gene defects targeting TRM in different tissues.
2. DOCK8 is critical for the development, survival, and functions of multiple immune cell types
Besides causing not only an exaggerated cell death of migrating lymphocytes in the skin, the absence of DOCK8 also compromises the survival of multiple lymphoid cell types elsewhere in the blood, secondary lymphoid organs, and other non-lymphoid tissues such as liver and gastrointestinal tract. Cell types affected include natural killer T cells (NKT) and memory CD8 T cells in both mice and humans, as well as type 3 innate lymphoid cells (ILC3) in mice (42, 43, 47, 48). The mechanism(s) by which impaired development and survival of these cell types occur remain unclear, but seems to reflect increased apoptosis, sometimes associated with decreased expression of the prosurvival factor Bcl-2, and is possibly related to impaired trafficking or localization to access survival signals in vivo (42, 43, 47). Proliferation is also sometimes decreased depending upon the experimental conditions, with impaired proliferation seen in chronically stimulated human T cells within peripheral blood mononuclear cell populations, as reflected by the expansion of replicatively senescent T effector memory CD45RA+ (TEMRA) cells, but normal proliferation of mouse T cells (42, 43, 47). In Dock8-deficient mice, NKT cells have reduced proliferative responses to the prototypic glycolipid antigen α-galactosylceramide with decreased production of IFN-γ and TNF-α, which could contribute to increased virus susceptibility and cancer predisposition (47). Furthermore, the decreased numbers of ILC3 in turn decreases production of IL-22, thereby partly impairing protective responses against enteric pathogens such as Citrobacter rodentium (48). Decreased numbers and suppressive functions of regulatory T cells (Treg) have also been reported in some patients, although not observed in mouse models (49, 50). The Treg defects could contribute to the increased autoreactive naïve B cells in patients reflecting compromised peripheral B cell tolerance and leading to increased risk of autoimmunity in some (49). DIDS patients have fewer memory B cells, including marginal zone-like cells that are also lacking in the mice (24, 48, 50, 51). The reason for their failed development is unknown, but in the natural infectious environment DIDS patients can have grossly normal-appearing lymphoid follicles with germinal centers (52).
Whether absence of DOCK8 affects the development and survival of myeloid cells has not been as carefully examined. Although numbers of peripheral blood neutrophils and monocytes in DIDS patients are normal, numbers of plasmacytoid dendritic cells are fewer, resulting in impaired antiviral IFN-α production in response to CpG DNA (21). Moreover, through their failure to activate Cdc42, Dock8-deficient mouse dendritic cells are compromised in their ability to migrate in 3-dimensional collagen matrices (41, 53). The dendritic cells become round and non-motile, differently from T cells and NK cells which develop an abnormally elongated morphology leading to cell death. In vivo, the abnormal dendritic cell morphology manifests not only as decreased trafficking into the dermis, but also decreased trafficking to the draining lymph nodes, which globally impairs priming of T cell responses. Additionally, increased numbers of eosinophils and basophils are observed in DIDS patients (54), presumed secondary to the increased TH2 skewing. However, further study of numbers and functions of these myeloid cell types in the tissues is needed to determine whether they could also be affected by a trafficking defect when DOCK8 is absent.
Finally, by regulating the rearrangement of cytoskeletal structures, DOCK8 can regulate the formation of the immunological synapse, which is necessary for normal cytolytic function in lymphocytes. Granule polarization and immunological synapse formation can be compromised in DOCK8-deficient NK cells, resulting in partially impaired target cell killing (39, 40). However, in contrast to NK cells, cytolytic function can be normal despite an abnormal immunological synapse in DOCK8-deficient CD8 T cells (3, 42, 55). One caveat of these studies is that impaired cytotoxicity may reflect the poor viability of these effector cells when not used immediately for functional studies after collection from DIDS patients, and that the defective cytotoxicity might be overcome depending upon how the effector lymphocytes were initially stimulated. An abnormal immunological synapse is also observed in Dock8-deficient B cells, which might contribute to the defective germinal center responses during affinity maturation of antibody responses observed in mice (51). However, in DIDS patients, functional antibody abnormalities do not consistently occur and germinal centers with marginal zones can be seen (3, 52), also suggesting that an abnormal immunological synapse may modulate but not completely interfere with downstream immune functions when DOCK8 is absent in lymphocytes.
In summary, multiple defects together could contribute to the infection susceptibility to a wide spectrum of pathogens and other immune manifestations in the patients. A major goal will be to understand how DOCK8 regulates these seemingly disparate processes at the molecular level; this should shed light into the role of the cytoskeleton in different immune cell types.
3. Biochemical functions of DOCK8
The phenotypic overlap between DIDS and the Wiskott-Aldrich syndrome, which both share atopic dermatitis, virus skin infections, and immunodeficiency, raises the possibility that DOCK8 functions in the same pathway as the Wiskott-Aldrich syndrome protein (WASp) in regulating cytoskeletal structures. DOCK8 activates CDC42, which when overexpressed results in filamentous (F-) actin where lamellipodia form, and WASp is a CDC42 effector that also induces F-actin polymerization (56). In T cells, B cells, and NK cells, absence of DOCK8 results in abnormalities in F-actin polymerization at the immunological synapse (39, 40, 42, 51) (Figure 3b). At least during NK cell cytolysis, DOCK8 also regulates microtubule polarization through its interaction via Hook-related protein 3 (HkRNP3) with microtubules and the dynein motor complex (57). During interstitial migration, absence of DOCK8, or of downstream CDC42 or PAK1/2, also leads to abnormalities in F-actin and microtubule structures concurrent with lymphocyte deformation (37). However, during such migration, DOCK8 functions through other effectors besides WASp, because absence of WASp is unable to recapitulate the loss of lymphocyte shape integrity. Different biological processes, i.e., immunological synapse formation, cell migration, and cell division, probably reflect different temporal and spatial requirements for rearranging cytoskeletal structures. Since different members of the small Rho family GTPases are known to exhibit cross-talk to regulate downstream cytoskeletal proteins, these are likely to be triggered differentially following initial activation of CDC42. Identifying other intermediate effectors besides WASp should help molecularly define the diverging signaling pathways regulated by DOCK8 that control cell shape processes in immune cells.
Additionally, the phenotypic overlap between hyper-IgE syndrome caused by dominant negative STAT3 mutations and DIDS raises the possibility that DOCK8 functions upstream in the same signaling pathway. As DOCK8 expression is restricted to the immune system, this might also account for why extraimmune manifestations of disease (except for those secondary to defective immunity) are lacking in DIDS. Supporting this hypothesis, DOCK8-deficient B cells fail to activate STAT3 when stimulated through Toll-like Receptor 9 (TLR9), resulting in impaired B cell proliferation and immunoglobulin production (58). In normal B cells, TLR9 stimulation stabilizes a pre-existing complex of DOCK8 with MyD88 and Pyk2, which promotes Pyk2 phosphorylation of DOCK8, Src kinase activation, and Syk activation, leading to STAT3 activation. Interruption of this signaling cascade might explain why absence of DOCK8 results in lack of marginal zone or marginal zone-like B cells, which express high levels of TLRs and have a greater dependency on TLR signaling for survival. Additionally, if DOCK8 is upstream of STAT3, absence of DOCK8 could impair STAT3 activation that is required for IL-17 production, thereby contributing to the decreased TH17 cells, although the mechanistic details linking DOCK8 to STAT3 for IL-17 production in T cells have not yet been examined.
Summary
With the identification of many more DOCK8-deficient patients and a better understanding of the natural history of DIDS, HSCT has become recognized as the only way to cure this highly morbid and lethal immunodeficiency. Early treatment has been aided by the development of a rapid flow cytometry based diagnostic method, which has also uncovered somatic reversions contributing to the variable clinical phenotype. Recent research delving into the mechanisms underlying this complex and fascinating disease has revealed multiple ways by which the immune system is compromised. Most notably, absence of DOCK8 impairs the ability of T cells and NK cells to maintain shape integrity when migrating within the skin, causing cell death and poor control of virus skin infections. Absence of DOCK8 also impairs the development, survival, and function of other lymphocyte subsets as well as dendritic cell migration and functions. Other work has linked DOCK8 to STAT3 activity, potentially explaining the overlapping clinical phenotype between DIDS and Job's syndrome. Future work focusing on how DOCK8 biochemically regulates these processes is critical for advancing our understanding this disease.
Multiple Choice Questions (correct answers are bolded)
- Less common clinical findings that occur in DIDS include all the following except
- Sclerosing cholangitis
- Lymphoma
- Vasculopathy
- Pneumatocele
- Eosinophilic esophagitis
- Laboratory abnormalities that are often seen in DIDS include all the following except
- Elevated serum IgE
- Decreased CD4 T cell numbers
- Increased serum IgM
- Eosinophilia
- DOCK8 protein in some lymphocytes
- Which of the following is a true statement about somatic reversion in DIDS?
-
a.Somatic reversion is observed in ~11% of patients.
-
b.Somatic reversion occurs preferentially in neutrophils.
-
b.Patients with somatic reversion tend to have less severe disease and die at an older age.
-
c.Hematopoietic stem cell transplantation can be avoided when somatic reversion is observed.
-
d.Mechanisms contributing to the high frequency of somatic reversions include original-site mutation, second-site mutation, gene conversion, intragenic crossover, and cytothripsis.
-
a.
- DIDS is effectively treated by:
- IVIG
- IFN-α
- HSCT
- Gene therapy
- All of the above.
- Mechanisms contributing to the infection susceptibility in DIDS include all the following except
- Death of tissue-resident memory T cells in the skin
- Decreased memory B cells including marginal zone B cells
- Decreased NK cell cytotoxicity
- Increased STAT1 activation
- Impaired dendritic cell production of IFN-α production
Acknowledgements
This work was supported by the Intramural Research Program, NIH, National Institute of Allergy and Infectious Diseases. Data in Figure 2 were generated after obtaining written informed consent from the patient on an NIAID IRB-approved research protocol.
Footnotes
Authorship Contributions
Q.Z. and H.C.S. prepared the figures and wrote the manuscript. H.J. performed experiments in Figure 2.
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Zhang Y, Su HC, Lenardo MJ. Genomics is rapidly advancing precision medicine for immunological disorders. Nat Immunol. 2015;16(10):1001–4. doi: 10.1038/ni.3275. [DOI] [PubMed] [Google Scholar]
- 2.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361(21):2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124(6):1289–302. e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Ann N Y Acad Sci. 2011;1246:26–33. doi: 10.1111/j.1749-6632.2011.06295.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Davis JC, Dove CG, Su HC. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis Markers. 2010;29(3-4):131–9. doi: 10.3233/DMA-2010-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toth B, Pistar Z, Csorba G, Balogh I, Kovacs T, Erdos M, et al. Novel dedicator of cytokinesis 8 mutations identified by multiplex ligation-dependent probe amplification. Eur J Haematol. 2013;91(4):369–75. doi: 10.1111/ejh.12173. [DOI] [PubMed] [Google Scholar]
- 8.Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, et al. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J Clin Immunol. 2012;32(4):698–708. doi: 10.1007/s10875-012-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing H, Zhang Q, Zhang Y, Hill BJ, Dove CG, Gelfand EW, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133(6):1667–75. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kienzler AK, van Schouwenburg PA, Taylor J, Marwah I, Sharma RU, Noakes C, et al. Hypomorphic function and somatic reversion of DOCK8 cause combined immunodeficiency without hyper-IgE. Clin Immunol. 2015;163:17–21. doi: 10.1016/j.clim.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BR, Candotti F. Revertant somatic mosaicism in the Wiskott-Aldrich syndrome. Immunol Res. 2009;44(1-3):127–31. doi: 10.1007/s12026-008-8091-4. [DOI] [PubMed] [Google Scholar]
- 12.Pai SY, de Boer H, Massaad MJ, Chatila TA, Keles S, Jabara HH, et al. Flow cytometry diagnosis of dedicator of cytokinesis 8 (DOCK8) deficiency. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. 2015;35(2):189–98. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt KR, Gertz ME, Keles S, Schaffer AA, Sigmund EC, Glocker C, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015;136(2):402–12. doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Herz W, Ragupathy R, Massaad MJ, Al-Attiyah R, Nanda A, Engelhardt KR, et al. Clinical, immunologic and genetic profiles of DOCK8-deficient patients in Kuwait. Clin Immunol. 2012;143(3):266–72. doi: 10.1016/j.clim.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsum Z, Hawwari A, Alsmadi O, Al-Hissi S, Borrero E, Abu-Staiteh A, et al. Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty five patients. J Clin Immunol. 2013;33(1):55–67. doi: 10.1007/s10875-012-9769-x. [DOI] [PubMed] [Google Scholar]
- 17.Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, et al. Cutaneous Manifestations of DOCK8 Deficiency Syndrome. Arch Dermatol. 2011;148(1):79–84. doi: 10.1001/archdermatol.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jouhadi Z, Khadir K, Ailal F, Bouayad K, Nadifi S, Engelhardt KR, et al. Ten-year follow-up of a DOCK8-deficient child with features of systemic lupus erythematosus. Pediatrics. 2014;134(5):e1458–63. doi: 10.1542/peds.2013-1383. [DOI] [PubMed] [Google Scholar]
- 19.Sabry A, Hauk PJ, Jing H, Su HC, Stence NV, Mirsky DM, et al. Vaccine strain varicellazoster virus-induced central nervous system vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol. 2014;133(4):1225–7. doi: 10.1016/j.jaci.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Mutairi M, Al-Mousa H, AlSaud B, Hawwari A, AlJoufan M, AlWesaibi A, et al. Grave aortic aneurysmal dilatation in DOCK8 deficiency. Modern Rheumatology. 2013 doi: 10.3109/14397595.2013.874735. [DOI] [PubMed] [Google Scholar]
- 21.Keles S, Jabara HH, Reisli I, McDonald DR, Barlan I, Hanna-Wakim R, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: Rescue of severe herpetic infections with IFN-alpha 2b therapy. J Allergy Clin Immunol. 2014;133(6):1753–5. e3. doi: 10.1016/j.jaci.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papan C, Hagl B, Heinz V, Albert MH, Ehrt O, Sawalle-Belohradsky J, et al. Beneficial IFN-alpha treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133(5):1456–8. doi: 10.1016/j.jaci.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Al-Zahrani D, Raddadi A, Massaad M, Keles S, Jabara HH, Chatila TA, et al. Successful interferon-alpha 2b therapy for unremitting warts in a patient with DOCK8 deficiency. Clin Immunol. 2014;153(1):104–8. doi: 10.1016/j.clim.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuellar-Rodriguez J, Freeman AF, Grossman J, Su H, Parta M, Murdock H, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant. 2015;21(6):1037–45. doi: 10.1016/j.bbmt.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatz SA, Benninghoff U, Schutz C, Schulz A, Honig M, Pannicke U, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(4):552–6. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 26.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222(6):351–5. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 27.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Metin A, Tavil B, Azik F, Azkur D, Ok-Bozkaya I, Kocabas C, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. LID - 10.1111/j.1399-3046.2011.01641.x [doi]. Pediatr Transplant. 2012 doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 29.Boztug H, Karitnig-Weiss C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol. 2012;29(7):585–94. doi: 10.3109/08880018.2012.714844. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Schuster FR, Fuchs I, Laws HJ, Borkhardt A, Meisel R. Treosulfan-based conditioning in DOCK8 deficiency: complete lympho-hematopoietic reconstitution with minimal toxicity. Clin Immunol. 2012;145(3):259–61. doi: 10.1016/j.clim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Schuster FR, Adams O, Babor F, Borkhardt A, Comoli P, et al. Haploidentical stem cell transplantation in DOCK8 deficiency - Successful control of pre-existing severe viremia with a TCRass/CD19-depleted graft and antiviral treatment. Clin Immunol. 2014;152(1-2):111–4. doi: 10.1016/j.clim.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Al-Mousa H, Hawwari A, Alsum Z. In DOCK8 deficiency donor cell engraftment post-genoidentical hematopoietic stem cell transplantation is possible without conditioning. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2012.12.663. [DOI] [PubMed] [Google Scholar]
- 33.Happel CS, Stone KD, Freeman AF, Shah NN, Wang A, Lyons JJ, et al. Food allergies can persist after myeloablative hematopoietic stem cell transplantation in dedicator of cytokinesis 8-deficient patients. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azik F, Azkur D, Avci Z, Vezir E, Isik P, Tunc B, et al. Resolution of food-induced anaphylaxis in DOCK8-deficient patients following bone marrow transplantation. Turk J Pediatr. 2015;57(1):112–5. [PubMed] [Google Scholar]
- 35.Hagl B, Heinz V, Schlesinger A, Spielberger BD, Sawalle-Belohradsky J, Senn-Rauh M, et al. Key findings to expedite the diagnosis of hyper-IgE syndromes in infants and young children. Pediatr Allergy Immunol. 2016;27(2):177–84. doi: 10.1111/pai.12512. [DOI] [PubMed] [Google Scholar]
- 36.Janssen E, Tsitsikov E, Al-Herz W, Lefranc G, Megarbane A, Dasouki M, et al. Flow cytometry biomarkers distinguish DOCK8 deficiency from severe atopic dermatitis. Clin Immunol. 2014;150(2):220–4. doi: 10.1016/j.clim.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Dove CG, Hor JL, Murdock HM, Strauss-Albee DM, Garcia JA, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211(13):2549–66. doi: 10.1084/jem.20141307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flesch IE, Randall KL, Hollett NA, Di Law H, Miosge LA, Sontani Y, et al. Delayed control of herpes simplex virus infection and impaired CD4(+) T-cell migration to the skin in mouse models of DOCK8 deficiency. Immunol Cell Biol. 2015;93(6):517–21. doi: 10.1038/icb.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131(3):840–8. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ham H, Guerrier S, Kim J, Schoon RA, Anderson EL, Hamann MJ, et al. Dedicator of cytokinesis 8 interacts with talin and wiskott-Aldrich syndrome protein to regulate NK cell cytotoxicity. J Immunol. 2013;190(7):3661–9. doi: 10.4049/jimmunol.1202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119(19):4451–61. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208(11):2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8(+) T-cell memory. Eur J Immunol. 2011;41(12):3423–35. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161(4):737–49. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park CO, Kupper TS. The emerging role of resident T cells in protective immunity and inflammatory diseases. Nat Med. 2015;21(7):688–97. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814–28. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, et al. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122(12):2052–61. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AK, Eken A, Fry M, Bettelli E, Oukka M. DOCK8 regulates protective immunity by controlling the function and survival of RORgammat+ ILCs. Nat Commun. 2014;5:4603. doi: 10.1038/ncomms5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134(6):1365–74. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caracciolo S, Moratto D, Giacomelli M, Negri S, Lougaris V, Porta F, et al. Expansion of CCR4+ activated T cells is associated with memory B cell reduction in DOCK8-deficient patients. Clin Immunol. 2014;152(1-2):164–70. doi: 10.1016/j.clim.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Randall Kl, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10(12):1283–91. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.aan de Kerk DJ, van Leeuwen EM, Jansen MH, van den Berg JM, Alders M, Vermont CL, et al. Aberrant humoral immune reactivity in DOCK8 deficiency with follicular hyperplasia and nodal plasmacytosis. Clin Immunol. 2013;149(1):25–31. doi: 10.1016/j.clim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Krishnaswamy KJ, Singh A, Gowthaman U, et al. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc Natl Acad Sci USA. 2015;112(10):3056–61. doi: 10.1073/pnas.1501554112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–46. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Garcia R, Lermo-Rojo S, Martinez-Lostao L, Mancebo E, Mora-Diaz S, Paz-Artal E, et al. A case of partial dedicator of cytokinesis 8 deficiency with altered effector phenotype and impaired CD8(+) and natural killer cell cytotoxicity. J Allergy Clin Immunol. 2014;134(1):218–21. doi: 10.1016/j.jaci.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Ruusala A, Aspenstrom P. Isolation and characterisation of DOCK8, a member of the DOCK180-related regulators of cell morphology. FEBS Lett. 2004;572(1-3):159–66. doi: 10.1016/j.febslet.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 57.Ham H, Huynh W, Schoon RA, Vale RD, Billadeau DD. HkRP3 is a microtubule-binding protein regulating lytic granule clustering and NK cell killing. J Immunol. 2015;194(8):3984–96. doi: 10.4049/jimmunol.1402897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13(6):612–20. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]