Abstract
Background
Niacin is an anti-dyslipidemic agent that may cause blood sugar elevation in patients with diabetes, but its effects on glucose and insulin values in non-diabetic statin-treated subjects with cardiovascular disease and at high risk for diabetes are less well known.
Methods
This was a pre-specified, intent-to-treat analysis of 3414 participants in the Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes trial randomized at 92 centers in the United States and Canada to ERN plus simvastatin/ezetimibe (ERN) or simvastatin/ezetimibe plus placebo (Placebo). Baseline and annual fasting glucose and insulin values were measured. Those experiencing an adverse event indicative of diabetes or starting medications for diabetes were considered to have confirmed diabetes. In addition, non-diabetic subjects with 2 annual follow up glucose measurements were categorized into normal, impaired fasting glucose or newly diagnosed diabetes (presumed or confirmed) states.
Results
Compared to placebo, ERN increased annual fasting glucose from baseline to 1 year in both those with normal (7.9±15.8 vs 4.3±10.3 mg/dl; p<0.001) and impaired fasting glucose (4.1±18.7 vs 1.4±14.9 p<0.02) and increased insulin levels. Both effects waned over the next 2 years. There were less consistent effects in those with baseline diabetes. There was an increased risk of progressing from normal to presumed or confirmed impaired fasting glucose (ERN 197/336 cases (58.6%) versus placebo 135/325 cases (41.5%) p<0.001) over time, but no difference in diabetes development in the two treatment groups except in those with normal fasting glucose at baseline.
Conclusions
The addition of ERN to simvastatin/ezetemibe had marginal effects on glycemia in those with diabetes at baseline, and there was a trend toward increased development of new onset diabetes. In addition ERN increased the risk of developing impaired fasting glucose which may have deleterious consequences over time and warrants further study.
Keywords: Extended release niacin, clinical trial, heart disease, glucose and insulin, diabetes, impaired fasting glucoses
Introduction
Niacin has been widely used to treat dyslipidemia. One of its well-known side-effects is the tendency to increase blood glucose levels (1) which is thought to be related to an increase in insulin resistance (2). This has been well-studied in subjects with pre-existing diabetes though the effect has been demonstrated to be modest in most reports (3) except in those with poorly controlled diabetes (4). Thus in those with known diabetes it is appropriate that physicians monitor glucose levels carefully when niacin is being used in the event that modification of glycemic control is required. Because it is generally recommended that patients with diabetes perform home glucose self-monitoring, potential worsening of glycemic control due to niacin therapy may thereby be corrected.
By contrast, the effect of niacin treatment in non-diabetic subjects, particularly those at high risk for diabetes has been less well known. Since such patients are likely not monitoring their glucose levels, niacin treatment in this group may lead to undetected diabetes A recent meta-analysis of controlled clinical trials of niacin treatment reporting the development of new onset diabetes concluded that niacin therapy was associated with a moderate increased risk of incident diabetes (5). However this study did not examine whether trial participants were normoglycemic or dysglycemic at baseline and not all of the trials were performed in high risk subjects with atherosclerotic cardiovascular diseases which would influence the incidence of new onset diabetes. Furthermore none of these trials evaluated the effect of niacin on the development of dysglycemia in previously euglycemic individuals. Since even mild elevations in glucose levels may be associated with increased morbidity (6), it would be of value to assess the impact of niacin therapy on glucose levels in the prediabetic range particularly among those with atherosclerotic cardiovascular disease because of their high risk for dysglycemia and diabetes (7). Furthermore since the majority of these individuals are receiving statins, which also increase the risk of diabetes development (8), addition of niacin to statin therapy may further aggravate the risk for diabetes in some subjects. Recent, well-conducted randomized trials have failed to demonstrate a reduction in long-term clinical events with extended-release niacin (ERN) when added to statin therapy (9,10) making the future place of ERN therapy in the management of dyslipidemia unclear (11). We felt it important to report the effects of ERN on glucose and insulin levels in a prespecified secondary analysis in the Atherothrombosis Intervention in Metabolic syndrome with low high density lipoprotein/high triglycerides: Impact on Global Health (AIM-HIGH) trial. This study compared the effects of ERN with simvastatin and ezetimibe (ERN) therapy versus placebo with simvastatin and ezetimibe (Placebo) therapy in subjects with atherosclerotic cardiovascular disease (9).
Methods
Study design
AIM-HIGH was a randomized, placebo-controlled clinical trial designed to test the hypothesis that in patients with atherosclerotic cardiovascular disease and atherogenic dyslipidemia, treatment with ERN (Niaspan™, AbbVie, Inc.) to raise baseline levels of HDL-C would decrease the rate of cardiovascular endpoints (coronary artery disease death, non-fatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome or symptom driven coronary or cerebral revascularization) (9). Entry criteria for AIM-HIGH have been described in detail (12). Briefly, patients were included if they were at least 45 years old and had established stable atherosclerotic cardiovascular disease with high density lipoprotein cholesterol (HDL-C) <40 mg/dl for men, <50 mg/dl for women, high triglycerides (100 to 400 mg/dl) and low density lipoprotein cholesterol (LDL-C) <180 mg/dl (conversion factor to SI units for cholesterol X 0.0259 and for triglyceride X 0.0113). Lipoprotein inclusion criteria were adjusted according to baseline treatment to account for estimated effects of ongoing treatment. Established atherosclerotic cardiovascular disease was defined as stable coronary heart disease with prior documented myocardial infarction or acute coronary syndrome or documented multivessel coronary artery disease; cerebrovascular or carotid disease with ischemic sequelae, carotid revascularization, or asymptomatic carotid stenosis >70%: or peripheral arterial disease with ankle-brachial index <0.85 or prior revascularization. Subjects with significant comorbidities (e.g., left ventricular ejection fraction <30%) or increased risk for medication adverse effects were excluded.
Procedures
The study was conducted at 92 centers in the United States and Canada. After providing written informed consent, subjects entered a 4-to-8-week open-label phase during which they received simvastatin 40 mg daily, plus ERN at doses increasing weekly from 500 mg to 2000 mg per day. Subjects tolerating at least 1500 mg ERN daily were randomly assigned, in a 1:1 ratio, to ERN or matching placebo tablets. To mask treatment assignment to ERN, placebo tablets included 50 mg immediate-release niacin in each 500 or 1000 mg tablet. Therefore, subjects in the control group on study drug at apparent 1500–2000 mg doses received 100–150 mg niacin daily. To limit confounding by differences in LDL-C, both groups underwent an aggressive ongoing effort to suppress LDL-C to 40 to 80 mg/dL throughout the follow up period, thus helping to assure that differences between the groups were attributable to HDL-C effects. The trial was stopped at 36 months of follow-up before its planned conclusion on the recommendation of its Data and Safety Monitoring Board based on demonstrated inability to prove benefit of ERN on the primary outcome.
Laboratory Measurements
Laboratory samples were analyzed by the Northwest Lipid and Diabetes Research Laboratory (University of Washington) using standardized procedures. Fasting glucose (FG), insulin, HbA1c, lipid profiles and apo B were measured at baseline and FG was measured annually in all participants and insulin at the year 1 and year 3 follow-up visits. HOMA-IR (homeostatic model assessment for insulin resistance) was estimated in mass units using the following formula: (Glucose X Insulin)/405 and HOMA-β (%) was estimated as: (360 x Insulin)/(Glucose – 63) (13). Management of elevated glucose levels was the responsibility of the patient’s primary care physician or endocrinologist. Laboratory values were reported to the participant at each follow-up and extremely high levels triggered an alert whereby laboratory staff telephoned the clinical site regarding the laboratory result.
Categorization of glycemic status
Glycemic status was categorized at baseline and at follow-up;
Baseline glycemic status
Previously diagnosed diabetes was defined as a positive history of diabetes and/or taking one or more antihyperglycemic medications. Participants without previously diagnosed diabetes at baseline were categorized according to the American Diabetes Association (ADA) criteria (14) as having normal FG (NFG; FG <100 mg/dl (SI units conversion factor X 0.0555), impaired FG (IFG; FG 100–125 mg/dl) or newly diagnosed diabetes (new diabetes; FG >125 mg/dl).
Follow-up glycemic status among non-diabetic subjects
Over the course of the study subjects reported to have developed diabetes by report of an adverse event with Medical Dictionary for Regulatory Activities (MedDRA) preferred term ‘Diabetes mellitus’, ‘Diabetes mellitus non-insulin-dependent’, ‘Diabetes mellitus inadequate control’, ‘Diabetic ketoacidosis’, or ‘Insulin resistant diabetes’, or started for the first time on antihyperglycemic medication by their primary physicians were designated as having confirmed diabetes. For the remaining subjects glycemic status was based on annual glucose testing. Because of the variability in plasma glucose measurements, the American Diabetes Association (ADA) has recommended that a confirmatory test be performed to establish the diagnosis of diabetes, preferably within 6 weeks of the initial test (14). Since this was not possible in AIM HIGH, we used the annual follow-up FG measurements to confirm changes from baseline glycemic status to more rigorously characterize the effect of the two treatments on subsequent glycemic status. Duration of follow-up depended on when the participant was randomized and the availability of FG measurements at each annual visit. Year 1 follow-up data was available for 2,869, year 2 for 2,693 and year 3 for 1,774 individuals. Because of the drop-off in available follow-up data at year 3, we restricted the analysis to those participants who had both year 1 and year 2 FG values or who were diagnosed with diabetes by their physicians or started antidiabetic medications during this period. Of the total 1080 participants with NFG and the 1010 with IFG at baseline, 663 and 675 participants, respectively, were included in this analysis.
Using the two follow-up FG measurements we then categorized participants with either NFG or IFG at baseline into follow-up NFG, IFG or new diabetes categories during the follow-up period, based upon whether they had normal, impaired or diabetic FG levels at the 2 follow-up visits. Deterioration of glucose tolerance status from baseline at only one of the two follow-up visits was defined as “presumed” follow-up IFG or new diabetes, whereas deterioration of glycemic status at both visits was designated as “confirmed” follow-up IFG or new diabetes (Table 1). Improvement in those with IFG at baseline to normal was only designated follow-up NFG if both follow-up tests were in the normal range. For analyses of the effects of therapy on new onset diabetes, participants who self-reported new diabetes or new antidiabetes medication starts within the first 2 years of follow-up were added to participants with new onset diabetes confirmed by successive annual FG testing. Presumed and confirmed follow-up IFG and new diabetes were studied separately as well as combined together. Participants with indeterminate glycemia that could not be categorized (Table 1) were excluded from this analysis.
Table 1.
Definition of follow up glucose tolerance status in the subgroup of participants with normal or impaired baseline fasting glucose based on year 1 and year 2 fasting glucose categories
| Baseline FG | Year 1 FG | Year 2 FG | Follow-up Status |
|---|---|---|---|
| Normal | Normal | Normal | NFG |
| Impaired | Normal | Presumed IFG | |
| Normal | Impaired | Presumed IFG | |
| Impaired | Impaired | Confirmed IFG | |
| Diabetes | Normal | Indeterminate | |
| Diabetes | Impaired | Presumed new DM | |
| Normal | Diabetes | Presumed new DM | |
| Impaired | Diabetes | Presumed new DM | |
| Diabetes | Diabetes | Confirmed new DM* | |
| Impaired | Normal | Normal | NFG |
| Impaired | Normal | IFG | |
| Normal | Impaired | IFG | |
| Impaired | Impaired | IFG | |
| Diabetes | Normal | Indeterminate | |
| Diabetes | Impaired | Presumed new DM | |
| Normal | Diabetes | Presumed new DM | |
| Impaired | Diabetes | Presumed new DM | |
| Diabetes | Diabetes | Confirmed new DM* |
Also includes subjects who were diagnosed with new diabetes or started on antihyperglycemic medications by their personal physicians in either year 1 or 2
Statistical Methods
Randomization was stratified on history of diabetes and sex. Baseline characteristics were well balanced between the treatment arms. Thus the study population was compared among the three glycemic states at baseline using a chi-square test for categorical data or t-test for continuous data. The proportion of participants with NFG at baseline with NFG, IFG or new diabetes at follow-up was compared by treatment group using a chi-square test. A similar comparison was made for those with IFG at baseline. Separate multinomial logistic regression models were fitted to examine the effect of ERN versus Placebo therapy on progression from baseline NFG to follow-up IFG, from NFG to new diabetes and from IFG to new diabetes. Follow-up glycemic status was categorized into 3 groups: follow-up NFG (reference group); presumed-plus-confirmed follow-up IFG; presumed-plus-confirmed new diabetes. The odds ratios associated with the treatment groups and confidence intervals were calculated. The model assumption and behaviors of the data were examined to decide on the use of multinomial logistic models. Separate logit models were run and the diagnostics tools were used on each model in order to detect outliers or influential data points. Hosmer and Lemeshow’s goodness-of-fit test showed that the predicted frequency and observed frequency matched closely. Number needed to harm (NNH) was computed using computational methods for number needed to treat based on incidence using an online calculator (http://graphpad.com/quickcalcs/NNT1/). All other analyses were performed using SAS Version 9.3 (SAS Institute, Cary NC). A two-tailed p <.0.05 was required to reject the null hypothesis.
Results
Baseline Status
Of the 3,414 randomized participants, all but 2 could be classified by glycemic status at baseline. Approximately 34% (n=1,158) had previously diagnosed diabetes; a further 164 (4.8%) were found to have diabetes by baseline FG testing, making a total of 1322 subjects (38.7%) with baseline diabetes. Almost 30% (n=1,010) had IFG and 32% (n=1,080) had NFG. Those with diabetes at baseline were older and had higher HbA1c but lower LDL-C levels than those without diabetes, and participants with diabetes and IFG had higher FG and triglyceride and lower HDL-C values and a higher prevalence of metabolic syndrome than those with NFG (Table 2). Baseline insulin and HOMA-IR were significantly higher and HOMA-β lower in the IFG and diabetes groups versus the normal FG group. There were also significant differences between the diabetes and IFG groups for glucose, HbA1c, insulin, HOMA-IR, HOMA β, HDL-C and triglyceride values.
Table 2.
Baseline clinical and metabolic data in participants with normal fasting glucose (NFG), impaired fasting glucose (IFG) by fasting glucose status and presence of either previously diagnosed or newly diagnosed diabetes
| Normal Fasting Glucose | Impaired Fasting Glucose | P-value (1) | Diabetes | P-value(1) | P-value (2) | |
|---|---|---|---|---|---|---|
| n | 1080 | 1010 | 1322 | |||
| Sex | ||||||
| Male | 916 (85%) | 895 (89%) | 0.01 | 1097 (83%) | 0.23 | <.001 |
| Female | 164 (15%) | 115 (11%) | 225 (17%) | |||
| Age (yrs) | 62.9±9.2 | 63.2±8.7 | 0.54 | 64.7±8.3 | < 0.001 | <.001 |
| BMI (kg/m2) | 29.8 ± 5.0 | 31.0 ± 4.8 | < 0.001 | 32.6 ± 5.7 | < 0.001 | <.001 |
| Metabolic syndrome (%) | 65 | 80.5 | < 0.001 | 94.6 | < 0.001 | <.001 |
| Fasting glucose (mg/dL) | 92±7 94 (89, 97) |
109±7 107 (103, 113) |
< 0.001 | 127±26 128 (110, 145) |
< 0.001 | <.001 |
| HbA1c (%) | 5.5±0.4 5.5 (5.2, 5.7) |
5.7±0.4 5.7 (5.4, 5.9) |
< 0.001 | 6.6±0.9 6.5 (6.0, 7.1) |
< 0.001 | <.001 |
| Insulin (mU/L) | 15.1±14.8 11.9 (7.9, 18.3) |
18.8±28.3 14.4 (9.5, 22.2) |
< 0.001 | 25.2±28.9 17.5 (11.1, 28.4) |
< 0.001 | <.001 |
| HOMA-IR | 3.4±3.5 2.7 (1.8, 4.2) |
5.1±7.4 3.9 (2.5, 6.0) |
< 0.001 | 8.0±10.1 5.5 (3.3, 8.9) |
< 0.001 | <.001 |
| HOMA-β | 210.2 ± 455.4 147.4 (95.5, 230.8) |
151.2 ± 249.0 114.2 (75.9, 177.4) |
< 0.001 | 206.0 ± 728.4 99.6 (61.1, 188.0) |
< 0.001 | <.001 |
| LDL-C (mg/dL) | 77±23 74 (62, 88) |
76±23 74 (61, 86) |
0.40 | 71±23 69 (56, 83) |
< 0.001 | <.001 |
| HDL-C (mg/dL) | 35±6 35 (31, 39) |
35±6 35 (31, 39) |
0.04 | 34±6 34. (30, 38) |
< 0.001 | 0.11 |
| Triglyceride (mg/dL) | 173±62 157 (126, 203) |
182±65 163 (131, 218) |
0.002 | 192±71 175 (136, 227) |
< 0.001 | 0.002 |
| Non-HDL-C (mg/dL) | 111±27 108 (94, 124) |
112±27 108 (95, 124) |
0.46 | 109±27 106 (91, 123) |
0.06 | 0.009 |
| Apo B (mg/dL) | 84±20 82 (70, 95) |
84±21 81 (70, 93) |
0.50 | 82±20 80 (68, 93) |
0.02 | 0.08 |
P-values compare against Normal Fasting Glucose.
P-values compare Diabetes against Impaired Fasting Glucose
Data shown are mean ± SD, and median (25th and 75th percentile) for lipid and glycemic measures. Conversion to SI units; glucose X 0.055, cholesterol X 0.0259, triglyceride X 0.0113
Effects of Treatment on Follow-Up Fasting Glucose or Insulin Measures by Baseline Glycemic Status
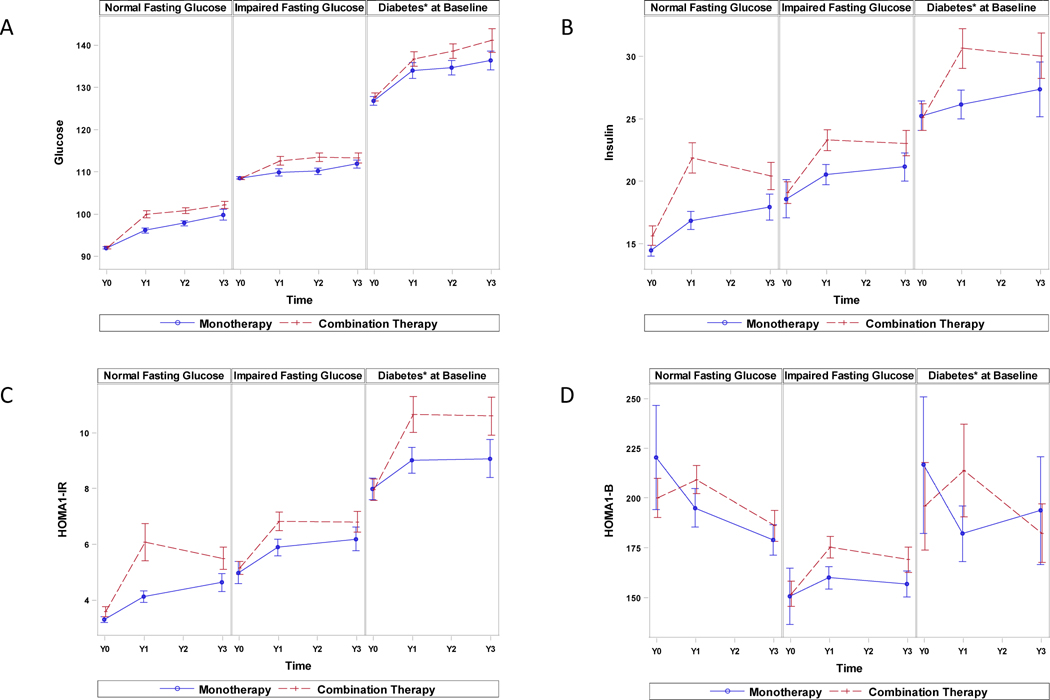
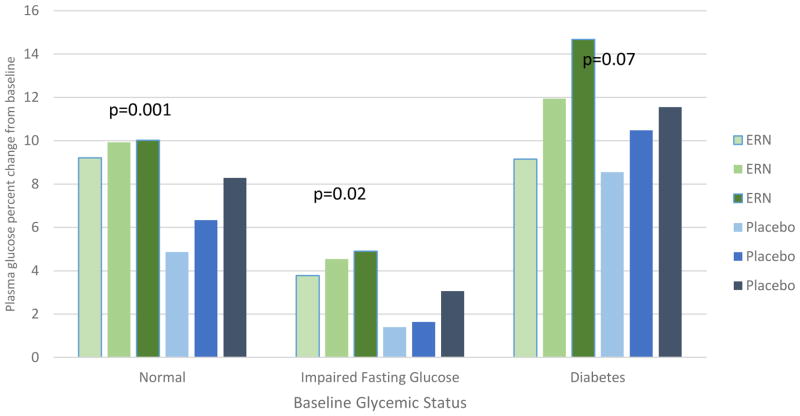
Figure 1A compares annual FG values by treatment modality stratified according to baseline glycemic status. There were no differences between baseline FG levels in the two treatment groups for each glycemic category. FG levels gradually increased during follow-up in both groups and were significantly higher at each yearly visit in those assigned to ERN versus Placebo therapy for both the NFG (p<0.001) and IFG (p=0.02) groups but only at year 2 in those with diabetes. Table 3 and Figure 2 illustrate the corresponding annual FG changes from the baseline level by baseline glycemic status, demonstrating the same pattern except there were no significant differences between any treatment groups for year 3 and no difference at any year in those with diabetes. The FG increment with ERN versus Placebo therapy was greatest in year 1 (NFG 9.5% vs 4.5%; IFG 4% vs 1.5%) with smaller increments in years 2 and 3 in the non-diabetic groups (Figure 2).
Figure 1.
Comparison of baseline and annual follow-up fasting glucose, insulin, HOMA-IR and HOMA-β values between ERN Therapy and Placebo Treatment groups by fasting glucose status or diabetes (for fasting glucose only). Mean and standard error are depicted.
Table 3.
Change from baseline in fasting glucose (annually), and insulin, HOMA-IR and HOMA-β (years 1 and 3) by baseline fasting glucose status for ERN versus Placebo treatment groups.
| Normal Fasting Glucose | Impaired Fasting Glucose | Diabetes* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ERN | Placebo | p | ERN | Placebo | p | ERN | Placebo | p | |
| Year 1 | |||||||||
| n | 423 | 410 | 388 | 415 | 547 | 506 | |||
| Glucose (mg/dl) | 7.9±15.8 5.0 (0.0, 14.0) |
4.3±10.3 3.0 (−2.0, 9.0) |
< 0.001 | 4.1±18.7 1.5 (−5.0, 9.5) |
1.4±14.9 −1.0 (−7.0, 6.0) |
0.02 | 7.7±41.3 5.0 (−15.0, 22.0) |
7.0±40.9 2.0 (−14.0, 21.0) |
0.77 |
| Insulin (mU/L) | 5.3±26.7 4.5 (−0.7, 9.4) |
2.4±13.6 1.5 (−2.3, 5.8) |
< 0.001 | 4.4±15.2 3.9 (−1.8, 9.8) |
1.5±38.2 1.4 (−2.5, 6.0) |
<0.001 | 5.1±33.4 3.0 (−3.1, 10.0) |
0.7±28.6 0.7 (−4.3, 6.7) |
<.001 |
| HOMA-IR | 2.3±13.2 1.1 (−0.0, 2.7) |
0.8±3.8 0.4 (−0.5, 1.6) |
< 0.001 | 1.8±6.1 1.1 (−0.4, 3.0) |
0.8±10.5 0.4 (−0.8, 1.8) |
<0.001 | 2.4±14.2 1.1 (−1.2, 4.0) |
1.0±11.2 0.4 (−1.5, 2.8) |
0.007 |
| HOMA-β | 1.0±220 19.2 (−38.1, 71.0) |
−29.5±664 5.4 (−41.9, 52.9) |
0.08 | 24.1±107 21.7 (−20.8, 70.4) |
5.7±343 11.1 (−16.7, 57.6) |
0.10 | 26.4±768 10.0 (−28.7, 63.2) |
−1.4±477 0.9 (−36.4, 46.1) |
0.017 |
| Year 2 | |||||||||
| n | 420 | 410 | p | 395 | 420 | p | 510 | 489 | p |
| Glucose (mg/dl) | 8.5±13.6 7.0 (0.0, 15.0) |
5.7±12.3 4.0 (−1.0, 10.0) |
0.002 | 5.0±19.6 4.0 (−4.0, 12.0) |
1.7±14.8 0.0 (−6.0, 7.5) |
0.007 | 10.6±41.8 6.0 (−13.0, 28.0) |
8.9±40.5 4.0 (−14.0, 26.0) |
0.50 |
| Year 3 | |||||||||
| n | 274 | 264 | 265 | 292 | 325 | 315 | |||
| Glucose (mg/dl) | 9.2±14.1 7.0 (1.0, 14.0) |
7.4±21.8 5.0 (−2.0, 12.0) |
0.26 | 5.2±18.2 4.0 (−5.0, 13.0) |
3.3±15.8 0.0 (−7.0, 10.0) |
0.18 | 14.8±51.7 5.0 (−12.0, 34.0) |
10.8±41.0 7.0 (−12.0, 30.0) |
0.28 |
| Insulin (mU/L) | 5.3±25.5 4.5 (−0.2, 10.1) |
3.8±17.5 1.9 (−1.8, 7.9) |
0.003 | 5.2±18.9 4.3 (−1.1, 12.0) |
2.2±46.1 3.2 (−1.4, 8.0) |
0.05 | 4.8±37.4 2.9 (−3.9, 11.5) |
3.3±42.4 2.4 (−3.1, 9.3) |
0.47 |
| HOMA-IR | 2.0±7.7 1.4 (0.2, 3.0) |
1.4±5.2 0.6 (−0.3, 2.0) |
< 0.001 | 2.0±6.5 1.4 (−0.3, 3.7) |
1.1±12.6 1.1 (−0.3, 2.3) |
0.06 | 2.5±14.4 1.4 (−1.0, 4.4) |
1.5±1.0 1.2 (−1.1, 3.8) |
0.39 |
| HOMA-β | −2.5±263 25.3 (−38.7, 71.9) |
−0.3±157 11.6 (−37.3, 53.5) |
0.16 | 25.3±138 32.6 (−10.1, 69.4) |
1.8±409 21.9 (−15.0, 63.4) |
0.23 | 4.1±289 10.3 (−50.2, 62.8) |
19.5±467 4.2 (−37.0, 39.5) |
0.41 |
P values compare the differences in change in value between ERN and Placebo groups.
Conversion to SI units; glucose X 0.055, cholesterol X 0.0259, triglyceride X 0.0113
Figure 2.
Percent change in plasma glucose from baseline to year 1, year 2 and year 3 by glycemic status at baseline and randomization treatment assignment. P-values from longitudinal models comparing treatment groups within glycemic status groups.
Figure 1B and C and Table 3 show the insulin and HOMA-IR values and their changes from baseline as a function of baseline glycemic status and treatment assignment. There were no baseline differences between treatment groups. At year 1 baseline insulin and HOMA-IR values and their changes from baseline increased in both treatment groups. Insulin and HOMA-IR increased to a greater extent in the ERN arm for all three glycemic status groups versus the Placebo arm, but the changes were not significant in year 3 in those with IFG or diabetes. There were no HOMA-β differences between treatment arms (Table 3, Figure1D).
Effects of Treatment on Changes in Glycemic Status
The effects of treatment on glycemic status was further assessed in the subset of participants without diabetes at baseline (n=1338) who had self-reported diabetes or who had follow-up FG measurements at both year 1 and 2, allowing for confirmation of a change in glycemic status. Table 4 illustrates the effects of ERN versus Placebo on follow-up glycemic status using only confirmed or presumed-plus-confirmed glycemic status categories. In the 1338 participants without diabetes at baseline, the overall incidence of presumed-plus-confirmed new diabetes was slightly but not significantly (p=0.18) higher in the ERN group (n=115 [17.9%]) compared to the Placebo group (99 [14.7%] during the 3 year follow-up period. The incidences of confirmed new diabetes were respectively 9.8% and 7.4% in the two treatment groups, (p=0.12). Most of the new diabetes cases developed in those who had IFG at baseline. In the Placebo group 5.8% of those with NFG at baseline developed presumed-plus-confirmed new diabetes (3.4% for confirmed cases), far fewer when compared to 23.0% of those with baseline IFG (11.2% for confirmed cases). The corresponding percentages developing new diabetes in the ERN arm were slightly but not significantly higher; for NFG at baseline 7.1% developed presumed-plus-confirmed diabetes (3.6% for confirmed diabetes) –again far fewer when compared to 27.8% of those with IFG at baseline (16.2% for confirmed cases).
Table 4.
Comparison of the effects of ERN therapy versus Placebo in subgroups with normal or impaired baseline fasting glucose at baseline on presumed and/or confirmed follow-up glycemic status
| NORMAL FASTING GLUCOSE AT BASELINE (n=663) | |||
|---|---|---|---|
| Category at follow-up | Placebo n=327;19.2%* |
ERN n=336;19.6%* |
P*** |
| NFG | 173 (52.9)** | 115 (34.2)** | < 0.001 |
| Confirmed IFG | 55 (16.8) | 92 (27.4) | 0.002 |
| Presumed plus Confirmed IFG | 135 (41.3) | 197 (58.6) | <0.001 |
| Confirmed DM | 11 (3.4) | 12 (3.6) | 0.859 |
| Presumed plus Confirmed DM | 19 (5.8) | 24 (7.1) | 0.469 |
| IMPAIRED FASTING GLUCOSE AT BASELINE (n=675) | |||
| Category at follow-up | Placebo (n=348;20.5%*) | ERN (n=327;19.0%*) | p |
| NFG | 35 (10.0) | 18 (5.5) | 0.016 |
| IFG | 233 (67.0) | 218 (66.7) | 0.365 |
| Confirmed DM | 39 (11.2) | 53 (16.2) | 0.156 |
| Presumed plus Confirmed DM | 80 (23.0) | 91 (27.8) | 0.437 |
Percent of all Placebo or ERN therapy participants respectively;
number in parentheses refers to percent of the treatment subgroup
Comparison of proportion in the two treatment subgroups by follow-up glycemic status category
NFG=normal fasting glucose; IFG=impaired fasting glucose; DM=newly diagnosed diabetes
Among those with NFG at baseline, there were fewer subjects that remained with NFG at follow-up in the ERN group (34.2% vs 52.9%; p <0.001) and there were more participants with presumed-plus-confirmed follow-up IFG (58.6% vs 41.3%; p<0.001). In those with IFG at baseline there were fewer participants who reverted to NFG on follow-up in the ERN versus the Placebo group (5.5% vs 10.1%; p=0.016). The multinomial logistic regression model confirmed the effect of ERN compared to Placebo on worsening of glycemic status. The odds ratio associated with ERN compared to Placebo for the outcome of converting to IFG versus remaining with NFG was 1.82 (95% confidence interval [CI] 1.29, 2.57, p=0.0007; NNH = 8 [95% confidence interval (CI) 4–9], Table 5). However, the odds ratio associated with ERN compared to Placebo for the outcome of converting to new diabetes versus remaining with IFG at follow-up was 1.03 (CI 0.76, 1.39) p=0.85, or versus the entire cohort without diabetes at baseline (NFG+IFG) the odds ratio was 1.22 (CI 0.91, 1.64) p=0.18.
Table 5.
Logistic regression model comparing effects of ERN therapy versus Placebo therapy in subgroups with normal (NFG) or impaired baseline fasting glucose (IFG) at baseline on changes in follow-up glycemic status
| COMPARISON | ODDS RATIO (5–95 CI) | P value |
|---|---|---|
| Baseline NFG as referent | ||
| Presumed plus Confirmed follow-up IFG vs NFG | 1.82 (1.29,2.57) | 0.0007 |
| Baseline IFG as referent | ||
| Presumed plus Confirmed follow-up diabetes vs Presumed plus Confirmed IFG | 1.03 (0.76,1.39) | 0.8481 |
| Baseline NFG+IFG as referent | ||
| Presumed plus Confirmed follow-up diabetes vs IFG or NFG | 1.22 (0.91,1.64) | 0.1818 |
Discussion
The principal findings of this analysis of the AIM HIGH population comparing the effects of ERN versus Placebo added to simvastatin/ezetimibe over a mean 3 year follow-up period on glucose and insulin levels, were that FG levels increased modestly but significantly more in the ERN than the Placebo group irrespective of whether participants had NFG, IFG or diabetes at baseline. This difference was accompanied by a significant worsening of insulin resistance as assessed by insulin and HOMA-IR values, although this effect did not persist beyond 1 year of follow-up in those with baseline IFG or diabetes. There was also an increased occurrence of confirmed IFG in those with baseline NFG as well as a reduced likelihood of reversion from IFG to NFG during follow-up in the ERN compared to Placebo. Though there were numerical increases in the development of presumed or confirmed new diabetes cases in the ERN group, these differences did not achieve statistical significance.
These findings need to be viewed from the perspective of the metabolic characteristics of the AIM HIGH study population, all of whom had atherosclerotic cardiovascular disease and were receiving moderate to high dose statin therapy. In addition to the high prevalence of previously diagnosed diabetes (33.9%) a further 4.8% were found to have newly diagnosed diabetes and 29.6% had IFG. Thus a total of 68.3% of AIM HIGH participants had some form of hyperglycemia using these definitions which is similar to what has been reported in other studies of populations with atherosclerotic cardiovascular disease (7). Furthermore, as was noted for the subgroup with diabetes, participants with IFG at baseline had a higher body mass index (BMI), higher fasting insulin and HOMA-IR values signifying a greater degree of insulin resistance, lower HDL-C levels and a greater frequency of metabolic syndrome than did participants with NFG. This unfavorable risk profile may contribute to increased risk for morbidity and mortality, as has been reported for subjects with atherosclerotic cardiovascular disease who have dysglycemia as compared to those with normoglycemia (6). In addition participants with baseline NFG levels were significantly overweight, had a higher prevalence of metabolic syndrome than would be expected for their age group (15) and were comparatively hyperinsulinemic, suggesting that they were a relatively insulin resistant group and at increased risk for the development of dysglycemia or diabetes. Thus the non-diabetic population of AIM HIGH constituted a high risk population appropriate for examining whether the addition of ERN to simvastatin/ezetimibe therapy might significantly increase the development of dysglycemia and diabetes over that associated with simvastatin/ezetimibe treatment alone.
The effect of ERN versus Placebo therapies was first assessed in the entire study population stratified by their baseline glycemic status. Although there was a gradual rise in FG levels in all three glycemic categories in both treatment groups over the three year period, accompanied by increases in fasting insulin and HOMA-IR but not HOMA-β consistent with worsening insulin resistance over time, the increases were significantly greater in the ERN as compared to the Placebo group especially at 1 year, following which the differences diminished. The same trend in glucose levels was noted in a post-hoc analysis of the Coronary Drug Project, a trial of niacin monotherapy in subjects with coronary heart disease, in whom baseline glycemia was also categorized into NFG, IFG and diabetes subgroups (16). This suggests an initial effect of ERN to increase FG levels and insulin resistance which decreased with time. A similar observation was noted for the FG response to ERN in a recently published 64 week study comparing ERN versus placebo combined with simvastatin/ezetimibe therapy in a cohort selected to have hyperlipidemia but mostly free of clinical atherosclerotic cardiovascular disease; it was suggested that this resulted from a desensitization to the metabolic effect of niacin (17). In that report most of the effect of ERN to increase FG in non-diabetic individuals compared to placebo occurred within the first 16 weeks and then declined to pretreatment values by 64 weeks. The reason for the difference in the timing of the declining effect of ERN therapy on FG patterns in the two studies may be related to differences in the respective study populations. It is also possible that there was an even greater effect of ERN on glycemia in AIM HIGH earlier than was noted at 1 year but this was not tested in this study. In addition increasing weight and age over time could counteract this declining effect. Fasting insulin levels and HOMA-IR were also increased in the ERN subgroup with diabetes at 1 year suggesting an effect of ERN in these individuals, although this was not accompanied by a significant change in FG levels possibly because corrective antihyperglycemic therapy was applied. There were too few cases receiving antihyperglycemic therapy in the non-diabetic groups to influence overall glycemic responses.
To more rigorously evaluate the impact of ERN versus Placebo on the categorical change in glycemic status in the non-diabetic subgroup, we identified approximately 65% of the cohort without baseline diabetes who were subsequently diagnosed with diabetes or started for the first time on antidiabetic medications or who had repeated FG measurements at years 1 and 2 allowing for confirmation of a change in glycemic status as is recommended by the ADA (14). Using a definition of diabetes that included both presumed-plus-confirmed cases, 14.7% of the Placebo group developed diabetes during the first 2 years of follow-up versus 17.9% in the ERN group whereas using a definition including only confirmed cases the frequencies were 7.4% versus 9.8%% respectively. These represent incremental effects for ERN over Placebo therapy of 22% for the presumed-plus-confirmed definition and 32% for confirmed cases only although neither achieved statistical significance. In an earlier safety report from AIM HIGH, ERN was associated with a significantly higher incidence of adverse events with the MedDRA term “diabetes mellitus” than Placebo, but the criteria used in adverse event reporting were different to the more rigorous criteria we used in this study (18). These directional findings are similar to those reported by the larger Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), which tested the effect of adding ERN in combination with laropiprant – an anti-flushing agent - to effective statin-based LDL-C-lowering treatment in 25,673 high risk patients with prior vascular disease. New-onset diabetes was identified in 5.7% of the niacin-laropiprant group and 4.3% of the comparator group, comprising a significant 30% excess of diabetes for the niacin-laropiprant arm (10). HPS2-THRIVE had by far the greatest statistical weight among the 11 clinical trials included in the recently published meta-analysis reporting that niacin therapy was associated with a moderately increased relative risk (1.34 [95% confidence interval 1.21–1.49]) for new-onset diabetes (5). It is possible that with greater numbers in our study the differences would have achieved significance.
Although most of the participants developing diabetes in AIM HIGH had IFG at baseline, a few individuals with baseline NFG developed diabetes in both therapy arms, indicating that the occasional patient with atherosclerotic cardiovascular disease and NFG receiving statin treatment may progress within a year or two to diabetes and that the addition of ERN increased this tendency. Similar findings were noted in the Coronary Drug Project report (16). In addition to diabetes development, the risk of progressing from NFG at baseline to the IFG category was one third greater in those receiving ERN versus Placebo such that only a third of participants with baseline NFG in the ERN group remained with NFG at follow-up compared with one half of the Placebo group. Thus the addition of ongoing ERN therapy increases the finite possibility that normoglycemic individuals with atherosclerotic cardiovascular disease will become persistently dysglycemic, which could increase future risk for diabetes (18) and atherosclerotic cardiovascular disease events (6).
The strengths of the study are its multicenter, prospective, randomized design of ERN versus Placebo added to long-term statin therapy, three year duration and the availability of annual glucose and insulin measurements in the majority of the participants. There are several limitations. First we did not measure 2-hour post challenge glucose values so we could not fully categorize glycemic status. This may have led to an under-diagnosis of dysglycemia and diabetes (19,20). Second, it is recommended that confirmation of newly diagnosed diabetes be performed within 6 weeks, but this was not feasible by design in AIM HIGH. Nevertheless confirmation at 1 year strengthens the likelihood that a change in glycemic status is durable. Lastly, measurement of glycosylated hemoglobin levels would have been helpful particularly for the group with diabetes in assessing the effect of therapy on glycemia, but these measurements were not systematically measured in AIM HIGH. Future trials should assess glycosylated hemoglobin in normoglycemic as well as dysglycemic and diabetic subjects, because a retrospective study has suggested that normoglycemic patients taking niacin and/or statins may experience relatively greater increases of FG than of HbA1c (21).
In summary recent trials showing a lack of clear clinical benefit of using ERN with intensive LDL-C reduction therapy raises uncertainties regarding the future role of ERN in the management of dyslipidemia. Using a strategy that combined self-reported new onset diabetes together with repeated annual FG testing, this study found that new onset diabetes was relatively common in subjects with atherosclerotic cardiovascular disease receiving moderate-to-intensive LDL-C- lowering treatment and that there was trend toward a greater incidence of new onset diabetes in those receiving ERN, consistent with recent reports. In addition, almost 60% of patients with normal FG levels developed IFG with added ERN due to worsening insulin resistance - approximately one third more than occurred in the Placebo group, which in longer-term followup could have deleterious effects on the future risk of diabetes and recurrence of cardiovascular events. Thus the data support a recommendation that glucose measurements should be carefully and regularly evaluated in dyslipidemic patients with atherosclerotic cardiovascular disease especially in those treated with ERN, so that worsening glycemia may be managed with lifestyle or medication change.
Highlights.
Addition of extended release niacin to simvastatin/ezetimibe therapy (ERN) in subjects with cardiovascular disease increases fasting glucose and insulin levels compared to simvastatin/ezetimibe alone
There was a trend toward an increase in new onset diabetes in the ERN group
There was a significantly greater increase in the development of impaired fasting glucose among those with baseline normal fasting glucose in the ERN group
Acknowledgments
AIM-HIGH was supported by the National Heart, Lung, and Blood Institute (U01 HL081616 and U01 HL081649) and by an unrestricted grant from AbbVie, Inc. AbbVie donated the extended release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data.
Footnotes
Data access and author approval: All authors had access to the data and a role in writing the manuscript. All authors have reviewed and approved of this article prior to submission
Trial Registration: Niacin Plus Statin to Prevent Vascular Events (AIM-HIGH); https://clinicaltrials.gov/ct2/show/NCT00120289 (Clinicaltrials.gov Identifier NCT00120289
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health or the United States Department of Health and Human Services.
Conflicts of Interest: Dr. Goldberg reports receiving consultancies from Sanofi. Dr. Bittner reports grant funding for clinical trials from Janssen Pharma, Pfizer and Bayer Healthcare, participation in trials funded by Astra Zeneca, SANOFI/Regeneron and Amgen, consultancies for Eli Lilly, Amarin, Novartis and Amgen. Dr. Dunbar and Dr. Fleg report no conflicts of interests. Dr. Grunberger reports grant funding from Merck, NovoNordisk, Eli Lilly, Lexicon, Medtronic and Astra Zeneca and speakers bureau participation for NovoNordisk, Sanofi, Janssen, Eli Lilly, Berhinger Ingleheim, and GlaxoSmithKline. Dr. Guyton reports grant funding from Regeneron, Sanofi, Amgen, Amarin, Genzyme, Synageva and consultancies with Regeneron, Sanofi, and Amgen. Dr. Leiter reports grant funding, consultancies and honoraria from Aegerion, Amgen, Astra Zeneca, Eli Lilly, Merck, Pfizer, Regeneron, Valeant and Sanofi. Ms. McBride reports grant funding from AbbVie, Inc. (AIM-HIGH funding only). Dr. Robinson reports grant funding from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, GlaxoSmithKline, Merck, Pfizer, Sanofi/Regeneron and Takeda as well as consultancies from Akcea/Isis, Amgen, Eli Lilly, Esperion, Merck, Pfizer and Sanofi/Regeneron. Dr. Simmons reports no conflicts of interest. Dr. Wysham reports consultancies with Astra Zeneca, Behringer Ingleheim, Eli Lillly, Janssen, NovoNordisk and SANOFI. Ms. Xu reports not conflicts of interest. Dr. Boden reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ronald B. Goldberg, University of Miami Miller School of Medicine, Miami, FL.
Vera A. Bittner, University of Alabama School of Medicine, Birmingham AL.
Richard L. Dunbar, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Jerome L. Fleg, National Heart, Lung and Blood Institute, Bethesda, MD.
George Grunberger, Grunberger Diabetes Institute, Bloomfield Hills, MI.
John R. Guyton, Duke University Medical Center.
Lawrence A. Leiter, St. Michaels Hospital, Toronto, ON, Canada.
Ruth McBride, Axio Research, LLC, Seattle, WA.
Jennifer G. Robinson, University of Iowa Carver College of Medicine, Iowa City, IA.
Debra L. Simmons, University of Utah, Utah Diabetes and Endocrinology Center, Salt Lake City, UT.
Carol Wysham, Rockwood Diabetes and Endocrinology, Spokane, WA.
Ping Xu, PPD Development, Axio Research, LLC, Seattle, WA.
William E. Boden, Samuel S. Stratton VA Medical Center, Albany, NY.
References
- 1.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr Opin Lipidol. 2007;18(4):415–420. doi: 10.1097/MOL.0b013e3282364add. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JJ, Lawson JA, Campbell LV, et al. Effects of nicotinic acid on insulin sensitivity and blood pressure in healthy subjects. J Hum Hypertens. 2000;14(9):567–572. doi: 10.1038/sj.jhh.1001099. [DOI] [PubMed] [Google Scholar]
- 3.Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284(10):1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 4.Garg A, Grundy SM. Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. JAMA. 1990;264(6):723–726. [PubMed] [Google Scholar]
- 5.Goldie C, Taylor AJ, Nguyen P, et al. Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart. 2016;102(3):198–203. doi: 10.1136/heartjnl-2015-308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisman EZ, Motro M, Tenenbaum A, et al. Impaired fasting glucose concentrations in nondiabetic patients with ischemic heart disease: a marker for a worse prognosis. Am Heart J. 2001;141(3):485–490. doi: 10.1067/mhj.2001.113219. [DOI] [PubMed] [Google Scholar]
- 7.Anselmino M, Wallander M, Norhammar A, et al. Implications of abnormal glucose metabolism in patients with coronary artery disease. Diab Vasc Dis Res. 2008;5(4):285–290. doi: 10.3132/dvdr.2008.041. [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 9.The AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 10.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 11.Creider JC, Hegele RA, Joy TR. Niacin: another look at an underutilized lipid-lowering medication. Nat Rev Endocrinol. 2012;8(9):517–528. doi: 10.1038/nrendo.2012.22. [DOI] [PubMed] [Google Scholar]
- 12.AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011;161(3):471–477. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;38(Suppl 1):S8–16. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 16.Sazonov V, Maccubbin D, Sisk CM, et al. Effects of niacin on the incidence of new onset diabetes and cardiovascular events in patients with normoglycaemia and impaired fasting glucose. Int J Clin Pract. 2013;67(4):297–302. doi: 10.1111/ijcp.12089. [DOI] [PubMed] [Google Scholar]
- 17.Guyton JR, Fazio S, Adewale AJ, et al. Effect of extended-release niacin on new-onset diabetes among hyperlipidemic patients treated with ezetimibe/simvastatin in a randomized controlled trial. Diabetes Care. 2012;35(4):857–860. doi: 10.2337/dc11-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson TJ, Boden WE, Desvigne-Nickens P, et al. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371(3):288–90. doi: 10.1056/NEJMc1311039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartnik M, Rydén L, Malmberg K, et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007;93(1):72–77. doi: 10.1136/hrt.2005.086975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajanna V, Campbell KB, Leimberger J, et al. Elevation of fasting morning glucose relative to hemoglobin A1c in normoglycemic patients treated with niacin and with statins. J Clin Lipidol. 2012;6:168–173. doi: 10.1016/j.jacl.2011.12.008. [DOI] [PubMed] [Google Scholar]