Abstract
Over the past decade, stem cell gene therapy has achieved unprecedented curative outcomes for several genetic disorders. Despite the unequivocal success, clinical gene therapy still faces challenges. Genetically-engineered hematopoietic stem cells (HSCs) are particularly vulnerable to attenuation of their repopulating capacity once exposed to culture conditions, ultimately leading to low engraftment levels post-transplant. This becomes of particular importance when transduction rates are low or/and competitive transplant conditions are generated by a reduced intensity conditioning in the absence of a selective advantage of the transduced over the unmodified cells. These limitations could partially be overcome by introducing mega-doses of genetically-modified CD34+cells into conditioned patients or by transplanting HSCs with high engrafting and repopulating potential. In the present review, based on the lessons gained from the cord blood transplantation, we summarize the most promising approaches to date of increasing either the numbers of HSCs for transplantation or/and their engraftability, as a platform towards the optimization of engineered stem cell grafts.
Keywords: Gene therapy, cord blood transplantation, stem cells, ex vivo expansion, engraftment
Introduction
Hematopoietic stem cell gene therapy (HSC-GT) represents an autologous therapeutic intervention by which a normal copy of a deficient gene is introduced into patient's own HSCs to reestablish effective gene function. As such, HSC-GT bypasses the immunological risks of allogeneic HSC transplantation and the immune suppression needed to prevent or control these risks. Nowadays, HSC-GT offers a curative potential to diseases where hematopoietic cell transplantation is suboptimal (i.e metachromatic leukodystrophy)[1] or the need for a well-matched donor precludes a significant number of patients from undergoing this therapeutic procedure (i.e hemoglobinopathies) [2].
Over the last decade, the proof of principle that the genetic modification of autologous HSCs can provide durable cures in monogenic disorders has been demonstrated for several diseases including primary immunodeficiencies and lysosomal storage diseases [3–8]. Despite the unequivocal success, depending on the underlying disease and transgene function, outcome may be suboptimal (chronic granulomatous disease- CGD, hemoglobinopathies), thus requiring improvements in culture conditions, vector design, infused cell numbers and quality, conditioning etc.
Although a single HSC is, theoretically, capable and sufficient to eventually repopulate the hematopoietic system in mice, in humans, the delayed reconstitution from a single cell or limited numbers of HSCs is not compatible with life and high numbers of infused cells are required for rapid engraftment and hematologic reconstitution after HSC transplantation[9-10]. In HSC-GT in particular, where the ex vivo transduction process negatively affects the competiveness and homing of gene-modified cells[11], the need for high numbers of transduced HSCs with the capacity to robustly engraft long-term, is further magnified.
Umbilical cord blood transplantation (UCB) and HSC-GT face common challenges such as suboptimal HSC doses for infusion and impaired engraftment of transplanted cells. Towards overcoming several of the current shortcomings of UCB and HSC-GT, investigators try to develop methods to ex vivo expand the HSCs or enhance their engraftment capacity. Based mostly on lessons gained in the UCBT setting, this review will summarize current approaches and considerations towards this goal, and deliberate on how these may be optimized for effective GT applications.
Current limitations to the efficacy of HSC-GT
Although the last decade granted clinical GT with sound achievements, successful implementation of GT still faces major constraints including, in certain cases, limited efficacy due to suboptimal transduction efficiency or engraftment incompetence of the gene-modified cells[6,12,13]. Despite highly successful transduction of HSCs in GT of immune deficiencies[8] or lysosomal storage diseases[7], efficient gene transfer to HSCs with vectors bearing large and complex gene expression cassettes, such as globin vectors, still remains challenging. The incorporation of elements of the human locus control region (LCR) in globin vectors improved gene transfer into HSCs at the expense, however, of a severe compromise of vector titers due to the substantial length of the micro-LCR cassettes [14, 15]. The problem is further intensified when chromatin insulators are inserted in already large vector constructs, to protect the transgene expression from chromosomal position effects and/or shield the target genome from genotoxic events[15–17]. Overall, in hemoglobinopathies, both gene transfer efficiency and titers remain suboptimal and consequently, large vector production batches associated with high costs as well as full myeloblation are necessary to reach clinically relevant levels of engraftment[18].
Another obstacle that HSC-GT needs to circumvent is the significant loss of repopulating cells due to culture conditions applied to facilitate successful gene transfer, which in turn, hampers the long-term engraftment of gene-modified cells. Indeed, culture supplementation with cytokines induces changes in cell cycle, apoptosis, and adhesion molecules[19–23], ultimately leading to differentiation and loss of the primitive phenotype[24–27] of the transduced HSCs. In addition, transduction per se compromises the homing and engraftment potential of HSCs through their exposure to vector particles and supernatant[28].
In order to minimize the peri-transplant toxicity of HSC-GT in myeloablated recipients, especially those with comorbidities, several groups proposed testing a non-myeloablative conditioning; in this case, successful HSC-GT becomes highly challenging, especially when the disease background (i.e thalassemia, CGD) does not allow for a selective pressure at the gene-corrected stem or early progenitor cell level upon engraftment[29, 30]. Due to the increased competiveness from the endogenous unmodified HSCs in this case, very high doses of HSCs, transduced with robustly performing vectors, will be needed to establish clinically relevant long-term engraftment.
For all the reasons mentioned above, various approaches have been widely sought in order to increase the numbers of infused cells, enrich the cell grafts in stem cells, expand the HSCs ex vivo or enhance the engrafting capacity of infused cells.
Enrichment of human stem/progenitor cells in gene-engineered cell grafts
Various sources of HSCs are being used in HSC transplantation, including bone marrow (BM), mobilized peripheral blood (mPB) and UCB. Mobilization, the pharmacological egress of HSCs from BM niches to the peripheral blood, yields by leukapheresis several fold higher numbers of CD34+ cells than BM [31–33] providing also earlier hematopoietic recovery upon transplantation. Therefore, not only the majority of autologous and allogeneic HSC transplantations are performed with mPBHSCs[34] but also, GT approaches use in most cases, the granulocyte-colony stimulating factor (G-CSF)-mPB as HSC source for genetic modification.
Purified CD34+ cell populations, obtained from mPB, remain markedly heterogeneous and contain, apart from HSCs with long-term repopulating capacity, a pool of multilineage progenitors with short-term engraftment potential [35, 36]. In order to increase the chance that HSCs endowed with high repopulating capacity are targeted by a therapeutic vector while reduce the number of target cells that need to be modified ex vivo and thereby the large vector load and relevant cost, further purification of hematopoietic stem and progenitor cells beyond the standard CD34+ cell fraction, may be needed.
Currently, there is no unique immunophenotypic profile to safely and clearly demarcate primitive HSCs from transiently engrafting multipotent progenitors (MMP). The similarity of the immunophenotypic profile between long-term engrafting HSCs and MMPs[37] complicates the identification of a functional measure of the stem and progenitor cell content. Short-term (colony-forming units-CFUs) as well as long-term culture-initiating cell (LTC-IC) culture-based assays, together with xenogeneic transplantation models are frequently used to assess the potency of human HSCs to sustain functional hematopoiesis and characterize “true” HSCs[38]. Based on such studies, populations of CD34+/CD90+/CD133+ cells[38], CD34+/CD38-/CD133+ and CD34+/CD38-/CD45RA-/CD90+ cells[37] or CD34+/CD38-/CD45RA-/CD90+/CD49f+ cells[39] are considered to be enriched in the most primitive human HSCs. Very recently, using a multi-step screening instead of multiparametric flow cytometry analysis, Chen JY et al have identified HoxB5, a gene whose expression is limited to the BM, as a marking gene of functional long-term HSCs in the mouse. By this approach, they demonstrated that only 7 to 35% of cells identified as HSCs with the previous solely immunophenotype-based approaches, were LT-HSCs[40].
A number of groups utilized various CD34+ enriched cell populations in order to exploit the higher repopulating capacity of specific, competitive, CD34+ cell subpopulations or to indirectly improve the suboptimal transduction efficiencies or even reduce the high vector production costs by targeting lower cell numbers.
The group of D. Kohn showed increased susceptibility to transduction with markedly less vector amounts by further purification of HSCs beyond the standard CD34+cell enriched fraction, through the isolation and transduction with lenti-viral vectors of CD34+/CD38- cells as compared with the unfractionated CD34+ cells[41]. These sorted and gene-modified CD34+/CD38- cells were approximately 100 fold more competitive for engraftment than their CD34+/CD38+ counterparts when xenotransplanted into NSG mice. These data imply that in a clinical setting, transduction of CD34+/CD38- cells would allow the use of limited amounts of viral vector to achieve high rates of transduced HSCs with good engraftment capacity thus generating treatment opportunities for more patients.
In an another approach to target HSCs with a more primitive phenotype, the group of M. Crez has recently shown that direct gene transfer into unstimulated CD34+ cells by a receptor-targeting CD133-LV lentiviral vector which uses the CD133 surface marker of primitive HSCs as entry receptor, allowed for sustained long-term engraftment of gene-corrected cells in immunodeficient mice even after secondary transplantation[42]; an effect that was attributed to preferential gene transfer into cells with higher engrafting and repopulating capacity.
Novel mobilization approaches combining Plerixafor with G-CSF have resulted in increased mobilization of the CD34+/CD38- or CD34+/CD133+/CD38- cell subsets, thus providing pharmacological enrichment in HSCs of mobilized cell grafts[43–45]. Extensive studies from our group using thalassemia as a model, have defined Plerixafor+G-CSF-mobilized cells as an optimal graft source for thalassemia GT but also for HSC-GT applications in general; they do not only contain very high numbers of CD34+ cells obtained through single collections [46], but importantly also, demonstrate superior competitive long-term engraftment in a thalassemic mouse model [47] or enhanced early human chimerism after transduction/transplantation under a non-myeloablative conditioning, in xenografts[48]. Despite in fact, that transduction rates with a lentiviral globin vector were lower for Plerixafor+G-CSF-cells compared to cells mobilized with either agent alone, there was increased β-globin expression per vector copy, implying that a given required level of expression might be achieved at a lower vector copy number, thus providing higher biosafety in GT applications. Since cells bearing a more primitive phenotype maybe less permissive to gene transfer, optimization of the culture process, yet with preservation of their primitive cell nature may be needed, as it was recently suggested[49].
Ex vivo expansion of transduced HSCs
The need for high numbers of transplantable HSCs becomes particularly challenging in UCB transplantation because of the size of suitable grafts in blood banks and the low number of recovered stem cells as well as in HSC-GT approaches where the culture conditions, suboptimal transduction rates or/and a non-myeloablative conditioning may lead to impaired engraftment.
Ex vivo expansion of HSCs would be a most satisfactory resolution in a gene therapy setting by providing high numbers of gene-corrected cells, should the expanded cells retain their competiveness and repopulating capacity in vivo. However, culturing the HSCs ex vivo, is neither easy nor always successful, as a plethora of intrinsic and extrinsic mechanisms are responsible for the fate and renewal of HSCs.
Initial attempts to expand HSCs using various combinations of cytokines generated significant expansions in myeloid progenitors but at best, modest increases in long-term repopulating cells[50, 51]. Early clinical trials of UCB transplantation, conducted with the expectation that the increased committed progenitors would at least enhance the delayed neutrophil count recovery, failed to achieve, in most cases, an improvement in neutrophil or platelet recovery[52, 53]. Therefore, current approaches to HSC expansion target molecular pathways governing HSC self-renewal or cell-fate decisions.
Cell-based expansion approaches
In an attempt to simulate the HSC niche and bone marrow microenvironment that allows self-renewal and proliferation of the more primitive hematopoietic cells as well as to recapitulate the molecular signals and interactions directing these properties, coculture of HSCs with feeder, non-hematopoietic, cells has been assayed by numerous groups in order to ex vivo expand HSCs. Hematopoietic stem and progenitor cell (HSPC) expansion was achieved by culturing hematopoietic cells on feeder cells derived from various sources including bone marrow[54–59], fetal liver[60–62], UCB[63] or central nervous system[64].
Amongst feeder cells, mesenchymal stromal cells (MSCs) have gained the most momentum as a means to promote HSC expansion. MSCs regulate vital functions of HSCs including migration, survival and development. SDF-1, one of the various factors secreted by MSCs is not only a pivotal HSC chemoattractant but also a master regulator in the maintenance of HSC quiescence and HSPC pool size and niche functionality[65].
MCSs were reported to improve HSC expansion when co-cultured with various cytokine cocktails including SCF, TPO, FLT-3L, G-CSF, FGF-1[58, 66–72]. In order to further enhance MSCs performance, several groups have used either genetically modified MSCs expressing proteins shown to support ex vivo expansion of HSCs (angiopoietin-like-proteins, hTERT)[73, 74] or purified MSCs expressing surface markers (Stro-1+ or Stro-3+)[56, 75]. Goncalves et al [56], one of the few groups to use adult HSCs rather than CB cells, demonstrated that allogeneic, Stro-1 purified MSCs can most successfully expand CD34+/CD38- cells, compared to either autologous MCSs or to the most commonly used, Dexter's long-term BM stroma[59]. In a clinical setting, transplantation of 31 adults with 2 cord-blood units, 1 of which was expanded ex vivo on allogeneic (from a related donor or “off-the-shelf, Stro-3 selected) MSCs, proved to be both safe and efficient[75]. Overall, the patients had a shorter time to engraftment and higher cumulative incidences of neutrophil and platelet recovery by days 26 and 30, respectively, as compared to control patients receiving 2 unmanipulated UCB units. However, long-term engraftment was uniformly originated from the unmanipulated unit implying that the culture process depleted cells capable of long-term repopulation or that graft-versus-graft interactions were responsible for an immunological rejection of the expanded unit. Khoury et al., reported improved long-term performance of CD34+/CD133+ cells by co-culturing them with MSCs engineered to secrete angiopoietin-like-5. This approach resulted in 60-fold expansion in cells repopulating NSG mice for up to 24 weeks post transplant and after secondary transplant[57].
Endothelial cells (ECs) were also shown to express inhibitory and stimulatory angiocrine factors that regulate the quiescence and proliferation of HSPCs and significantly augment the expansion of HSPCs[64, 76–78]. However, long-term maintenance of ECs requires stimulation with essential EC-growth factors and serum, that may affect their angiogenic properties and the HSPC expansion[79]. The group of Rafii S. has devised an angiogenic model functioning as a vascular niche through the introduction of the adenoviral (Ad) E4ORF1 gene into primary ECs[79]. They have carried out extensive studies showing that hematopoietic progenitor and primitive stem cells incubated in direct contact with ECs and in the presence of minimum concentration of exogenous cytokines, can proliferate, expand and support long-term multi-lineage engraftment [80–82].
These studies justify a potential role for cell-based HSC expansion approaches but also highlight the need for further investigation in order to maintain the pool of undifferentiated HSCs and obtain clinically meaningful grafts.
Pathways controlling cell fate decisions
Hox-homeobox gene products were identified as essential factors in the processes of HSC commitment and differentiation as well as in the regulation of proliferation of the more primitive hematopoietic subpopulations [83, 84]. Retroviral introduction of human HoxB4 cDNA in murine BM cells led to the increase of the proliferative potential of primitive hematopoietic cells without detectable effects on their maturation in vivo[85]. Although, HoxB4-transduced cells proved to expand well in vitro, concerns on disturbance of normal hemopoiesis with impaired lymphomyeloid differentiation[86], have led to the pursuit of safer and virus-free methods, based on the ability of the HOX proteins to passively translocate through cell membranes. Treatment of mouse and human hematopoietic cells with the HoxB4 protein increased the numbers of the primitive cells by at least 2.5 fold [87, 88]. A recent study reported that, activation of OCT4 by Oct4-activating compound 1 (OAC1) with subsequent upregulation of HoxB4 in CB CD34+ cells, increased 3.5-fold the number of SCID-repopulating cells and both, the short and the long-term repopulation capacity in NSG mice[89]. Enforced expression of a modified fusion gene of Nucleoporin 98 with HOXA10 (NUP98-HOXA10HD)[90] in mPB by lentiviral vector transduction, resulted in significantly more engrafting cells and their in vivo enrichment upon xenotransplantation, over control groups[91].
SALL4 is a zinc-finger transcriptional factor shown to play an essential role in the maintenance of embryonic stem cell pluripotency[92] and in the regulation of the HSC/HSPC self-renewal[93]. In one of the few studies attempting to expand mobilized PB rather than UCB, SALL4-transduced human CD34(+)/CD38(-) and CD34(+)/CD38(+) cells were rapidly and efficiently expanded ex vivo by >10.000-fold in the presence of appropriate cytokines[94]. On the other hand, constitutive expression of SALL4 was shown to possess oncogenic potential[95, 96] whereas Sall4 overexpression had a detrimental effect on hematopoiesis resulting in engraftment failure[97]. Consequently, care must be taken to achieve appropriately balanced Sall4 expression in ex vivo stem cell expansion applications and further studies are needed to ultimately determine its role in normal versus malignant hemopoiesis.
Thrombopoietin signaling has been implicated in maintaining HSC number in vivo and in modulating survival and enabling expansion of stem cells in vitro[98, 99]. A derivative of the thrombopoietin receptor (mpl) incorporated into a fusion protein (F36Vmpl) has the potential to respond to a nontoxic chemical inducer of dimerization (CID) and selectively expand transduced hematopoietic cells of mostly erythroid and lymphoid lineage [100–103]. It has been reported that by targeting primitive human progenitors (CD34+CD38-Lin-CD7-), mpl dimerization induces, both in vitro and in vivo, a rapid expansion of not only differentiated but also of multipotent progenitors, in the absence of exogenous cytokines [104].
Another pathway responsible for cell-fate decisions by inhibiting particular differentiation programs or by permitting either self-renewal or alternate differentiation pathways[105] is the Notch pathway. Notch signaling in human HSCs is activated by binding of the ligands Delta, Jagged1, or Jagged2 [106]. Addition of a soluble form of human Jagged-1 to cultures of purified primitive human blood cells (CD34+/CD38-/Lin-) only modestly improved cytokine-induced proliferation of progenitors. However, transplantation of human Jagged-1 cultured cells into immunodeficient mice enhanced the survival and expansion of human stem cells capable of multipotent repopulating capacity[107]. Bernstein's group engineered an immobilized form of the Delta1 ligand by fusing the extracellular domain to the Fc portion of human IgG-1[108] to coat tissue-culture plates prior to expansion, ultimately leading to an activation of the endogenous Notch signaling in vitro and a several-log increase in murine[109] and human[110, 111] hematopoietic progenitors. These Notch-expanded human hematopoietic cells promoted the short- term repopulating capacity in xenografts[26] and the myeloid engraftment and neutrophil recovery in a phase I clinical trial of subjects with acute leukemias who were transplanted with two cord blood units, one unmanipulated and one ex vivo-expanded, and compared with a concurrent cohort of patients undergoing double UCB transplantation (median time of neutrophil engraftment 16 vs 26 days, respectively). In the majority of patients however, there was a lack of long-term engraftment of the expanded unit, either due to loss of HSC self-renewal capacity following ex vivo culture or to immune-mediated rejection of the expanded, T-cell depleted, unit[26].
Small molecules
High-throughput screening of large chemical libraries identified a number of small molecules as potential CD34+ agonists and revealed some very promising new tools for HSPC expansion.
Chromatin-modifying drugs such as the demethylating agents 5aza 2′deoxycytidine (5azaD) and trichostatin A (TSA), were among the first small molecules shown to promote the expansion of SCID-repopulating cells (SRCs) derived from BM[112], CB[113] or mPB[114]. More recently, Valproic Acid (VPA) and Nicotinamide (NAM), aSirtuin 1 deacetylace inhibitor, gained interest for their potential to enhance HSPC activity ex vivo. HSPCs exposed to VPA and cytokines were dramatically expanded and produced 36-fold greater numbers of SRCs capable of engrafting primary and secondary xenorecipients[115]. In addition to driving HSC proliferation and expansion of primitive HSCs, VPA was shown to upregulate CXCR4 expression on the surface of cord blood HSPCs and enhance their migration towards the BM[116]. Treatment of CB CD34+ cells with NAM not only resulted in an increase of CD34+/CD38- cell numbers, but also improved the homing capacity of the cells by 3-fold[117]. In a recently published trial, NAM-expanded CD133+ CB cells co-infused with the CD133-noncultured fraction of the same unit which contained immunocompetent T- and NK-cells, provided early hematopoietic recovery and long-term engraftment in the majority of patients[118].
Copper chelation-based expansion using tetraethylenepentamine (TEPA) was shown to result in 89-fold increase of CD34+cells capable of enhanced SRC ability[119]. A phase I/II clinical trial using two fractions of the same UCB unit, one nonexpanded and one (smaller) expanded in the presence of TEPA, demonstrated that by using this approach, a single expanded unit is able to achieve engraftment comparable to the unmanipulated double CB transplantation[25].
A library of 100.000 heterocycling chemical compounds was screened by Boitano et al., on mobilized peripheral blood (mPB) CD34+ cells, and identified StemRegenin 1 (SR1), a purine derivative and aryl hydrocarbon receptor antagonist that reversibly promotes the CD34+cell expansion by blocking HSC differentiation. Using mPB, SR-1 expanded CD34+cells by 2.6 fold after a 5-7 day-culture and by 73-fold after a 3-week culture in the presence of cytokines compared to cells cultured with cytokines only. Using CB, a 5-week culture with SR-1 plus cytokines led to 17.100-fold increase in the number of CD34+cells. Most importantly, Sr-1-expanded cells promoted both the early and sustained multilineage engraftment in NSG mice by increasing 17-fold the numbers of SRCs[120]
In a very recently published clinical trial evaluating the ability of SR-1 to expand CB CD34+cells ex vivo, a remarkable 330-fold expansion of CD34+cells was reached after 2-week culture. Seventeen patients received an unmanipulated UCB unit followed by the infusion of an SR-1 expanded UCB unit along with its recryopreserved CD34- cell fraction containing T-cells. They were all engrafted at a median of 15 days for neutrophils and 49 days for platelets, significantly faster than patients treated with unmanipulated dUCB. Long-term persistence of the SR-1-treated cells was observed in 11/17 patients whereas in the rest 6 patients who were engrafted with the unmanipulated graft, graft-versus-graft interactions were probably responsible for the loss of the SR-1 expanded UCB unit, rather than loss of its engraftment potential[121]. In a running clinical trial, the SR-1-expanded graft (HSC835, Novartis Pharmaceuticals) is now used in the context of single UCB transplantation (Clinicaltrial.gov NCT01930162).
Using similar high-throughput approaches, UM729 and its more potent analog UM171, that act independently of AhR pathway, were identified as small molecules with the ability to expand SRCs derived from human CD34+CD45RA–mobilized PB cells by 13-fold[122]. UM171 was thought to target phenotypically more primitive cells than SR1, as it supported greater expansion of SRCs (13 fold vs 2 fold) when used in combination with a controlled fed-batch culture approach[122, 123]. Indeed, the transcriptional profile of CD34+cells pulsed with SR1 or UM171 demonstrated only few commonly regulated genes[122]. UNC0638 is another small molecule identified as an inducer of the vitro expansion of human HSPCs. UNC0638 delayed the differentiation and promoted the maintenance of CD34+CD49f+ and CD34+CD38-CD45RA- cells while it synergized with SR1 leading to 5-fold expansion of SRCs[124].
Priming of stem cells to enhance homing/engraftment
Successful clinical outcome of HSC transplantation and gene therapy depends not only on high cell numbers but also on efficient homing and engraftment of cells to the BM. Methods for ex vivo treatment of HSCs to enhance the chemotactic migration of HSCs to the BM or improve cell adhesion to the BM stroma have been extensively studied.
To facilitate extravasation of HSCs and lodgment into BM post transplantation, a concerted action of adhesion molecules is taking place, in which endothelial P- and E-selectins play a key role. Selectin ligands on CD34+UCB cells are inadequately fucosylated as compared to BM or mPB[125, 126] leading to an impaired interaction with the BM microvascular network and at least partially, to a homing defect. Modulating selectin binding activity by enforced ex vivo fucosylation of UCB stem cells was shown to increase affinity to selectins and improve their engraftment into immunodeficient mice[126–128]. In a recent dUCBT clinical trial, 22 patients with hematologic malignancies received 2 CB units, one of which was shortly treated ex vivo with fucosyltransferase-VI and guanosine diphosphate fucose. Compared with 31 historical controls who underwent unmanipulated dUCBT, the median time to neutrophil and platelet engraftment in the trial was shortened by 9 and 10 days respectively[129].
The SDF-1–CXCR4 axis plays a principal role in the retention of HSPCs in BM niches and directs their migration from PB and lodging at specialized niches within BM[130, 131]. Towards improving the homing efficiency of HSCs, several agents that either directly or indirectly affect this axis have been investigated as a means to promote engraftment.
ProstaglandinE2 (PGE2) was initially identified as a regulator of HSC homeostasis capable of enhancing HSC formation in zebrafish[132]. Later it was shown that pulse exposure of murine and human hematopoietic cells to PGE2-treatment induced cell proliferation and cycling and decreased cell apoptosis as well as upregulated CXCR4 and survivin expression. These effects were translated to enhanced HSC engraftment in competitive transplantation settings, maintained also after secondary transplant[133, 134]. Preclinical studies with UCB xenotransplantation showed enhanced engraftment of PGE2-treated CB cells and in nonhuman primate autologous transplantation, dmPGE2-treated CD34(+) cells from mPB showed stable multilineage engraftment over 1 year post infusion[135]. However, when the repopulation kinetics of PGE2-treated hematopoietic grafts were followed through 5 serial transplantations, the effect of PGE2 on HSCs was transient as it did not alter long-term HSC competitiveness, lineage bias, or proliferative potential[136]. In a phase I clinical trial, double UCB transplantation including one unmanipulated and one PGE2-exposed unit, resulted in accelerated neutrophil recovery compared to historical control group (17.5 vs 21 days) coupled with preferential, long-term engraftment of the PGE2-treated unit in 10 of 12 treated recipients[137].
Another method of improving the homing capacity of HSPCs is by inhibition of Dipeptidylpeptidase 4 (DPP4 or CD26) on their surface. DPP4 is an enzyme that cleaves dipeptides from the N-terminus of proteins, including SDF-1. Cleavage of the N-terminus of SDF-1 deprives it from its chemotactic activity[138, 139]. Inhibition of DPP4, by small peptides such as Diprotin A (DipA) enhanced the responsiveness of murine and human HSPCs to the SDF-1 gradient, improving the overall cell homing and engraftment[140–141]. Combined pretreatment of murine BM cells with both DipA and PGE2 did not further enhance the engrafting capacity beyond that of priming with either agent alone. However, ex vivo pulse treatment of donor cells with PGE2 and subsequent cell transplantation into recipients treated with sitagliptin (another DPP4 inhibitor which has been originally approved for the treatment of type II diabetes), resulted in significantly higher levels of HSPC engraftment[142]. Systemic DPP-4 inhibition by sitaglipin was tested in a clinical trial as a means to enhance the engraftment of single-unit UCB transplants in adults with high-risk hematological malignancies. The area under DPP-4 activity-time curve (AUCA) significantly correlated with engraftment and the majority of patients with AUCA< 6,000 activity•h engrafted within 21 days[142].
Interestingly, in a targeted gene editing approach, Naldini's group used integrase-defective lentiviral vector-transduction and mRNA electroporation to deliver ZFNs and donor template in cord blood CD34+ cells. They were able to circumvent the limited permissiveness of the more primitive cells to gene transfer and gene targeting, by prolonging the stimulation period before gene targeting and making use of a combination of PGE2 and SR1 which significantly increased both the percentage of the transgene expressing cells in the more primitive subpopulations and the long-term engraftment up to 100%[49].
Current challenges and future prospects for improving engineered cell grafts
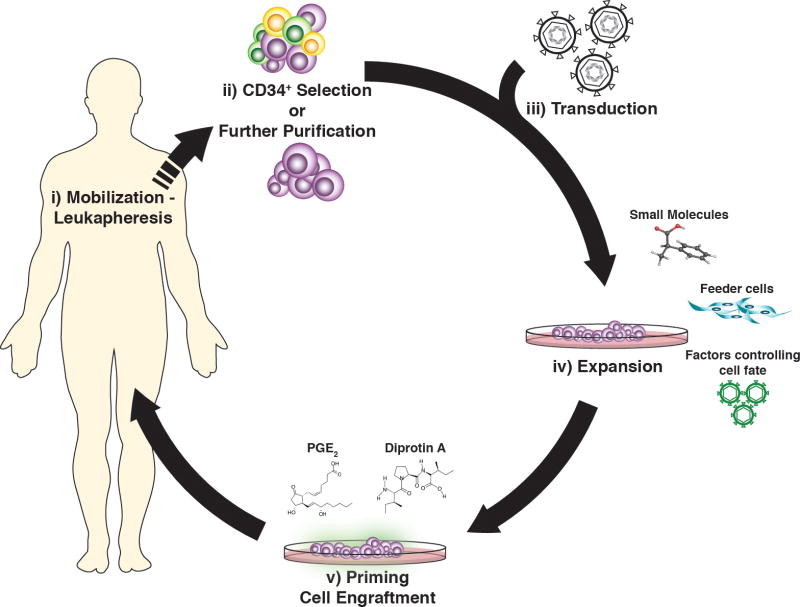
Gene therapy and CB transplantation face common, albeit aetiologically different, clinical challenges, including delayed hematologic reconstitution and impaired engraftment. Over the last decade, there has been an increased investigational interest and a fertile ground for optimization of transplanted grafts in the context of CB transplantation and gene therapy (Figure 1).
Figure 1. Approaches for optimization of the autologous gene-engineered cell grafts.
i) Mobilization with agents yielding leukaphereses products highly enriched in HSCPs ii) CD34+cell enrichment or further purification of the most “stem-cell” like HSPCs to reduce the number of cells for genetic modification and increase the chance of high in the hierarchy, HSCs to be targeted by the vector iii-iv) expansion of the transduced cells with preservation of their stemness phenotype by co-culturing the graft with feeder cells or pre-treat the gene-modified cells with factors that control intrinsing cell proliferation pathways or small molecules that allow cell division but prevent differentiation iv) priming of the transduced cells with effector molecules that improve engraftment upon transplantation, providing fast reconstitution and in vivo long-term persistence of the genetically-modified cells.
The first clear translational benefit of efforts to expand CB cells for transplantation was the shortening of the neutropenia period after double UCBT with one expanded and one unexpanded unit. Despite the obvious clinical relevance of this achievement, this benefit was derived from the expansion of committed myeloid progenitors rather than expansion of true stem cells, and ultimately, the long-term engraftment emanated from the unmanipulated unit, in the vast majority of cases[26, 75]. Recent evidence however, demonstrated that a SR-1- or NAM-expanded unit may support long-term hematopoiesis after dUCBT by maintaining the pool of undifferentiated HSCs[118, 121]. These data justify the future use of a single expanded CB unit, as a “stand-alone” graft, without co-infusion of a non-manipulated unit. While the unmanipulated unit used to serve as safety “back up” in case of engraftment failure of the expanded unit, the immunological graft-versus-graft interactions between the two HLA-mismatched units were often causing toxicities (higher GvHD, immunological rejection of the expanded unit, delayed platelet recovery). Provided that massive expansion of CB cells can be now reached, the field of UCBT will be likely revolutionized in the next years, should the transplantation of a single expanded CB unit is shown to improve the engraftment kinetics while reduce the hospital stays, risk of graft failure and treatment-related mortality. This novel UCB transplantation platform will considerably increase the number of available units as well as the possibility of finding a better HLA-matched graft.
The recent achievements in the UCBT field are of obvious relevance for the gene therapy field also. It is worth noting however, that the optimization of gene-modified cell grafts is far more challenging. The majority of the successful expansion methods described above have mostly been applied in cord blood HSPCs, yet data from mPB, the main graft source in GT applications, remain scarce. Given the different gene expression profiles, patterns of clonogenic efficiency and response to stimulatory cytokines of adult compared to fetal and CB HSCs[144, 145], further studies are required to demonstrate whether expansion strategies optimized in the CB transplantation context, may be effective in a gene therapy setting where the, more challenging to expand, mobilized CD34+ cells are commonly used[146]. In addition, the preservation of the undifferentiated status of HSCs during expansion of gene-modified HSCs is technically extremely challenging since both the gene transfer per se[28] and the long culture periods critically affect stemness[19–27]. Indeed, the efforts to expand genetically-modified HSCs from mPB with cytokine cocktails has not yet been successfully translated to large animal models or humans and in some cases, the expansion had a detrimental effect both on the short- and long-term marking[147, 148].
Extensive studies to amplify gene-modified HSCs from mPB using the newly-identified expansion protocols are not yet available. Although platforms to enrich cell grafts in HSCs or to shortly prime HSCs with engraftment-enhancing effectors are likely to deliver a clinically relevant effect in the gene therapy context, it deserves to be further explored in large animal models and with mPB, the integration of combined techniques that preserve the stemness of the expanded genetically-modified cells with others that enhance their engraftability[49]. These novel evolving technologies, if succesfull, will enable a reduced intensity conditioning to be applied to patients undergoing gene therapy while significantly improve the outcome of gene and cell therapies, in general.
Highlights.
Challenges and interventions in optimizing cell grafts for gene therapy are discussed
High numbers and enhanced engraftment capacity of transduced HSCs are required for succesfull gene therapy
Highly enriched in HSCs grafts will reduce the target cell numbers and the vector load
Expansion of the transduced HSCs without differentiation will increase the effective dose of infused cells
Priming of the transduced HSCs with effector molecules will enhance their repopulating capacity
HSCs; hematopoietic stem cells
Acknowledgments
The authors gratefully acknowledge Dr Thalia Papayannopoulou for critically reading and commenting on the manuscript. This work was supported by NIH grant #P01 HL053750-19.
Footnotes
Disclosures of Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31:229–39. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 2.King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood. 2014;123:3089–94. doi: 10.1182/blood-2013-01-435776. quiz 3210. [DOI] [PubMed] [Google Scholar]
- 3.Cavazzana-Calvo M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 4.Cartier N, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 5.Cassani B, et al. Gene Therapy for Immunodeficiency Due to Adenosine Deaminase Deficiency. N Eng J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–22. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biffi A, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 8.Aiuti A, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science (80-) 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner JE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman E, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102–10. [PubMed] [Google Scholar]
- 12.Grez M, et al. Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther. 2011;19:28–35. doi: 10.1038/mt.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HJ, et al. Retroviral gene therapy for X-linked chronic granulomatous disease: results from phase I/II trial. Mol Ther. 2011;19:2092–101. doi: 10.1038/mt.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hargrove PW, et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol Ther. 2008;16:525–533. doi: 10.1038/sj.mt.6300394. [DOI] [PubMed] [Google Scholar]
- 15.Urbinati F, et al. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3′LTR. Mol Ther. 2009;17:1527–1536. doi: 10.1038/mt.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emery DW, et al. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: Flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100:2012–2019. doi: 10.1182/blood-2002-01-0219. [DOI] [PubMed] [Google Scholar]
- 17.Ramezani A, Hawley TS, Hawley RG. Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–24. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- 18.Walters MC, et al. Update of Results from the Northstar Study (HGB-204): A Phase 1/2 Study of Gene Therapy for Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex-Vivo with a Lentiviral Beta AT87Q-Globin Vector (LentiGlobin BB305. Blood. 2015;126:201. [Google Scholar]
- 19.Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Homing and engraftment defects in ex vivo expanded murine hematopoietic cells are associated with downregulation of beta1 integrin. Exp Hematol. 2001;29:1494–502. doi: 10.1016/s0301-472x(01)00751-2. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed F, et al. Impaired bone marrow homing of cytokine-activated CD34+ cells in the NOD/SCID model. Blood. 2004;103:2079–87. doi: 10.1182/blood-2003-06-1770. [DOI] [PubMed] [Google Scholar]
- 21.Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–52. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 22.Yong KL, Fahey A, Pizzey A, Linch DC. Influence of cell cycling and cell division on transendothelial migration of CD34+ cells. Br J Haematol. 2002;119:500–9. doi: 10.1046/j.1365-2141.2002.03837.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, et al. Homing defect of cultured human hematopoietic cells in the NOD/SCID mouse is mediated by Fas/CD95. Exp Hematol. 2003;31:824–832. doi: 10.1016/s0301-472x(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 24.Shpall EJ, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–76. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 25.de Lima M, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delaney C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang CC, Kaba M, Iizuka S, Huynh H, Harvey F. Hematopoiesis and Stem Cells : Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD / SCID transplantation Hematopoiesis and Stem. Blood. 2009;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall KM, Horvath TL, Abonour R, Cornetta K, Srour EF. Decreased homing of retrovirally transduced human bone marrow CD34+ cells in the NOD/SCID mouse model. Exp Hematol. 2006;34:433–42. doi: 10.1016/j.exphem.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Sadelain M, et al. Strategy for a multicenter phase I clinical trial to evaluate globin gene transfer in beta-thalassemia. Ann N Y Acad Sci. 2010;1202:52–8. doi: 10.1111/j.1749-6632.2010.05597.x. [DOI] [PubMed] [Google Scholar]
- 30.Yannaki E, Emery DW, Stamatoyannopoulos G. Gene therapy for β-thalassaemia: the continuing challenge. Expert Rev Mol Med. 2010;12:e31. doi: 10.1017/S1462399410001626. [DOI] [PubMed] [Google Scholar]
- 31.To LB, Haylock DN, Simmons PJ, Juttner Ca. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–58. [PubMed] [Google Scholar]
- 32.Champlin RE, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 33.Bensinger WI, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 34.Gratwohl A, et al. The EBMT activity survey 2006 on hematopoietic stem cell transplantation: focus on the use of cord blood products. Bone Marrow Transplant. 2008;41:687–705. doi: 10.1038/sj.bmt.1705956. [DOI] [PubMed] [Google Scholar]
- 35.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie JL, Gan OI, Doedens M, Wang JCY, Dick JE. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol. 2006;7:1225–1233. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- 37.Majeti R, Park CY, Weissman IL. Identification of a Hierarchy of Multipotent Hematopoietic Progenitors in Human Cord Blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 40.Chen JY, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baldwin K, et al. Enrichment of human hematopoietic stem/progenitor cells facilitates transduction for stem cell gene therapy. Stem Cells. 2015;33:1532–42. doi: 10.1002/stem.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brendel C, et al. CD133-targeted Gene Transfer Into Long-term Repopulating Hematopoietic Stem Cells. Mol Ther. 2015;23:63–70. doi: 10.1038/mt.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fruehauf S, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 44.Varmavuo V, et al. CD34+ cell subclasses and lymphocyte subsets in blood grafts collected after various mobilization methods in myeloma patients. Transfusion. 2013;53:1024–32. doi: 10.1111/j.1537-2995.2012.03848.x. [DOI] [PubMed] [Google Scholar]
- 45.Varmavuo V, et al. Blood graft composition after plerixafor injection in patients with NHL. Eur J Haematol. 2012;89:128–35. doi: 10.1111/j.1600-0609.2012.01794.x. [DOI] [PubMed] [Google Scholar]
- 46.Yannaki E, et al. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum Gene Ther. 2013;24:852–60. doi: 10.1089/hum.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Psatha N, et al. Superior Long-Term Repopulating Capacity of G-CSF+Plerixafor-Mobilized Blood: Implications for Stem Cell Gene Therapy by Studies in the Hbb(th-3) Mouse Model. Hum Gene Ther Methods. 2014;25:317–27. doi: 10.1089/hgtb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karponi G, et al. Plerixafor+G-CSF-mobilized CD34+ cells represent an optimal graft source for thalassemia gene therapy. Blood. 2015 doi: 10.1182/blood-2015-03-629618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genovese P, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–40. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhatia M, et al. Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture. J Exp Med. 1997;186:619–24. doi: 10.1084/jem.186.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda T, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105:1013–21. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shpall EJ, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8 doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 53.Jaroscak J, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–7. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 54.Robinson SN, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–66. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi M, et al. Serum-free coculture system for ex vivo expansion of human cord blood primitive progenitors and SCID mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol. 2001;29:174–82. doi: 10.1016/s0301-472x(00)00653-6. [DOI] [PubMed] [Google Scholar]
- 56.Gonçalves R, Lobato da Silva C, Cabral JMS, Zanjani ED, Almeida-Porada G. A Stro-1+ human universal stromal feeder layer to expand/maintain human bone marrow hematopoietic stem/progenitor cells in a serum-free culture system. Exp Hematol. 2006;34:1353–1359. doi: 10.1016/j.exphem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Khoury M, et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 2011;20:1371–1381. doi: 10.1089/scd.2010.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walenda T, et al. Synergistic effects of growth factors and mesenchymal stromal cells for expansion of hematopoietic stem and progenitor cells. Exp Hematol. 2011;39:617–28. doi: 10.1016/j.exphem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Dexter TM, Moore MAS, Sheridan APC. Maintenance of hemopoietic stem cells and production of differentiated progeny in allogeneic and semiallogeneic bone marrow chimeras in vitro. J Exp Med. 1977;147:1612–1616. doi: 10.1084/jem.145.6.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis ID, Verfaillie CM. Multi-lineage expansion potential of primitive hematopoietic progenitors: superiority of umbilical cord blood compared to mobilized peripheral blood. Exp Hematol. 2000;28:1087–95. doi: 10.1016/s0301-472x(00)00515-4. [DOI] [PubMed] [Google Scholar]
- 61.Chou S, Lodish HF. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc Natl Acad Sci U S A. 2010;107:7799–804. doi: 10.1073/pnas.1003586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–47. [PubMed] [Google Scholar]
- 63.Klein C, et al. Ex vivo expansion of hematopoietic stem- and progenitor cells from cord blood in coculture with mesenchymal stroma cells from amnion, chorion, Wharton's jelly, amniotic fluid, cord blood, and bone marrow. Tissue Eng Part A. 2013;19:2577–85. doi: 10.1089/ten.tea.2013.0073. [DOI] [PubMed] [Google Scholar]
- 64.Chute JP, Muramoto G, Fung J, Oxford C. Quantitative analysis demonstrates expansion of SCID-repopulating cells and increased engraftment capacity in human cord blood following ex vivo culture with human brain endothelial cells. Stem Cells. 2004;22:202–15. doi: 10.1634/stemcells.22-2-202. [DOI] [PubMed] [Google Scholar]
- 65.Tzeng YS, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–39. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 66.Andrade PZ, dos Santos F, Almeida-Porada G, da Silva CL, S Cabral JMS. Systematic delineation of optimal cytokine concentrations to expand hematopoietic stem/progenitor cells in co-culture with mesenchymal stem cells. Mol Biosyst. 2010;6:1207–15. doi: 10.1039/b922637k. [DOI] [PubMed] [Google Scholar]
- 67.Broxmeyer HE, et al. Experimental basis of cord blood transplantation. Bone Marrow Transplant. 2009;44:627–33. doi: 10.1038/bmt.2009.285. [DOI] [PubMed] [Google Scholar]
- 68.Levac K, Karanu F, Bhatia M. Identification of growth factor conditions that reduce ex vivo cord blood progenitor expansion but do not alter human repopulating cell function in vivo. Haematologica. 2005;90:166–72. [PubMed] [Google Scholar]
- 69.Luens KM, et al. Thrombopoietin, kit ligand, and flk2/flt3 ligand together induce increased numbers of primitive hematopoietic progenitors from human CD34+Thy-1+Lin- cells with preserved ability to engraft SCID-hu bone. Blood. 1998;91:1206–1215. [PubMed] [Google Scholar]
- 70.Murray LJ, et al. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp Hematol. 1999;27:1019–28. doi: 10.1016/s0301-472x(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 71.Nikougoftar Zarif M, et al. The High Yield Expansion and Megakaryocytic Differentiation of Human Umbilical Cord Blood CD133(+) Cells. Cell J. 2011;13:173–8. [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi M, et al. Serum-free coculture system for ex vivo expansion of human cord blood primitive progenitors and SCID mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol. 2001;29:174–182. doi: 10.1016/s0301-472x(00)00653-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhang CC, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawano Y, et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101:532–40. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- 75.de Lima M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chute JP. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 77.Rafii S, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–63. [PubMed] [Google Scholar]
- 78.Li N, et al. Human umbilical vein endothelial cells increase ex vivo expansion of human CD34(+) PBPC through IL-6 secretion. Cytotherapy. 2006;8:335–42. doi: 10.1080/14653240600845062. [DOI] [PubMed] [Google Scholar]
- 79.Seandel M, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–93. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butler JM, et al. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. bl. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perkins AC, Cory S. Conditional immortalization of mouse myelomonocytic, megakaryocytic and mast cell progenitors by the Hox-2.4 homeobox gene. EMBO J. 1993;12:3835–46. doi: 10.1002/j.1460-2075.1993.tb06062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sauvageau G, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci U S A. 1994;91:12223–7. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sauvageau G, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 86.Schiedlmeier B, et al. High-level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage on human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood. 2003;101:1759–68. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- 87.Krosl J, et al. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- 88.Amsellem S, et al. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–7. doi: 10.1038/nm953. [DOI] [PubMed] [Google Scholar]
- 89.Huang X, et al. Activation of OCT4 enhances ex vivo expansion of human cord blood hematopoietic stem and progenitor cells by regulating HOXB4 expression. Leukemia. 2015:1–10. doi: 10.1038/leu.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohta H, et al. Near-maximal expansions of hematopoietic stem cells in culture using NUP98-HOX fusions. Exp Hematol. 2007;35:817–30. doi: 10.1016/j.exphem.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abraham A, Kim YS, Zhao H, Humphries K, Persons DA. Increased Engraftment of Human Short Term Repopulating Hematopoietic Cells in NOD/SCID/IL2rγnull Mice by Lentiviral Expression of NUP98-HOXA10HD. PLoS One. 2016;11:e0147059. doi: 10.1371/journal.pone.0147059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–23. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 93.Yang J, et al. Enhanced self-renewal of hematopoietic stem/progenitor cells mediated by the stem cell gene Sall4. J Hematol Oncol. 2011;4:38. doi: 10.1186/1756-8722-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguila JR, et al. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood. 2011;118:576–85. doi: 10.1182/blood-2011-01-333641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma Y, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108:2726–35. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li A, et al. A SALL4/MLL/HOXA9 pathway in murine and human myeloid leukemogenesis. J Clin Invest. 2013;123:4195–207. doi: 10.1172/JCI62891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milanovich S, et al. Sall4 overexpression blocks murine hematopoiesis in a dose-dependent manner. Exp Hematol. 2015;43:53–64.e1. 8. doi: 10.1016/j.exphem.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piacibello W, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–53. [PubMed] [Google Scholar]
- 99.de Graaf CA, Metcalf D. Thrombopoietin and hematopoietic stem cells. Cell Cycle. 2011;10:1582–9. doi: 10.4161/cc.10.10.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagasawa Y, et al. Anatomical compartments modify the response of human hematopoietic cells to a mitogenic signal. Stem Cells. 2006;24:908–917. doi: 10.1634/stemcells.2005-0484. [DOI] [PubMed] [Google Scholar]
- 101.Neff T, et al. Pharmacologically regulated in vivo selection in a large animal. In Vivo (Brooklyn) 2002;100:2026–2031. doi: 10.1182/blood-2002-03-0792. [DOI] [PubMed] [Google Scholar]
- 102.Richard RE, et al. Expansion of genetically modified primary human hemopoietic cells using chemical inducers of dimerization. Blood. 2000;95:430–436. [PubMed] [Google Scholar]
- 103.Okazuka K, et al. Long-term regulation of genetically modified primary hematopoietic cells in dogs. Mol Ther. 2011;19:1287–1294. doi: 10.1038/mt.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdel-Azim H, et al. Expansion of multipotent and lymphoid-committed human progenitors through intracellular dimerization of Mpl. Blood. 2008;111:4064–74. doi: 10.1182/blood-2007-08-107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 106.Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29:1041–1052. doi: 10.1016/s0301-472x(01)00676-2. [DOI] [PubMed] [Google Scholar]
- 107.Karanu FN, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varnum-Finney B, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113 Pt 23:4313–8. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 109.Varnum-Finney B. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2002;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 110.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34+CD38– cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–9. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Milhem M. Modification of hematopoietic stem cell fate by 5aza 2′deoxycytidine and trichostatin A. Blood. 2004;103:4102–4110. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 113.Mahmud N, et al. Differential effects of epigenetic modifiers on the expansion and maintenance of human cord blood stem/progenitor cells. Biol Blood Marrow Transplant. 2014;20:480–9. doi: 10.1016/j.bbmt.2013.12.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saraf S, et al. Ex vivo expansion of human mobilized peripheral blood stem cells using epigenetic modifiers. Transfusion. 2015;55:864–874. doi: 10.1111/trf.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaurasia P, Gajzer DC, Schaniel C, Souza SD, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gul H, et al. Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells Dev. 18:831–8. doi: 10.1089/scd.2008.0235. [DOI] [PubMed] [Google Scholar]
- 117.Peled T, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342–355.e1. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 118.Horwitz ME, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124:3121–8. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peled T, et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32:547–55. doi: 10.1016/j.exphem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 120.Boitano aE, et al. Aryl Hydrocarbon Receptor Antagonists Promote the Expansion of Human Hematopoietic Stem Cells. Science (80-) 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wagner JE, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fares I, et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science (80-) 2014;345:1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Csaszar E, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218–29. doi: 10.1016/j.stem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 124.Chen X, et al. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev. 2012;26:2499–2511. doi: 10.1101/gad.200329.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110:559–69. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–6. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 127.Robinson SN, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rγ(null) mice. Exp Hematol. 2012;40:445–56. doi: 10.1016/j.exphem.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robinson SN, et al. Fucosylation with fucosyltransferase VI or fucosyltransferase VII improves cord blood engraftment. Cytotherapy. 2014;16:84–9. doi: 10.1016/j.jcyt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Popat U, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood. 2015;125:2885–92. doi: 10.1182/blood-2015-01-607366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang Y, Bonig H, Ulyanova T, Chang K, Papayannopoulou T. On the adaptation of endosteal stem cell niche function in response to stress. Blood. 2009;114:3773–82. doi: 10.1182/blood-2009-05-219840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 132.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pelus LM, Hoggatt J, Singh P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011;44 Suppl 1:22–9. doi: 10.1111/j.1365-2184.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goessling W, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–58. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoggatt J, Mohammad K, Singh P, Pelus L. Prostaglandin E2 enhances long-term repopulation but does not permanently alter stem cell competiveness. Blood. 2013;122:2997–3000. doi: 10.1182/blood-2013-07-515288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cutler C, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–81. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 139.Christopherson KW, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16:355–60. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 140.Peranteau WH, et al. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–4274. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–54. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 142.Broxmeyer HE, Pelus LM. Inhibition of DPP4/CD26 and dmPGE2 treatment enhances engraftment of mouse bone marrow hematopoietic stem cells. Blood Cells Mol Dis. 2014;53:34–8. doi: 10.1016/j.bcmd.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farag SS, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013;22:1007–15. doi: 10.1089/scd.2012.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp Hematol. 1999;27:1418–27. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 145.Mayani H. Biological differences between neonatal and adult human hematopoietic stem/progenitor cells. Stem Cells Dev. 2010;19:285–98. doi: 10.1089/scd.2009.0327. [DOI] [PubMed] [Google Scholar]
- 146.Tanavde VM, et al. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30:816–23. doi: 10.1016/s0301-472x(02)00818-4. [DOI] [PubMed] [Google Scholar]
- 147.Holyoake TL, et al. CD34 positive PBPC expanded ex vivo may not provide durable engraftment following myeloablative chemoradiotherapy regimens. Bone Marrow Transplant. 1997;19:1095–101. doi: 10.1038/sj.bmt.1700799. [DOI] [PubMed] [Google Scholar]
- 148.Tisdale JF, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–41. [PubMed] [Google Scholar]