Abstract
Purpose
Chronic bladder pain is a debilitating condition often accompanied by alterations in affective and autonomic function. Many of the symptoms associated with chronic bladder pain are mediated by the central nervous system. In this review, data from preclinical animal models and human neuroimaging studies were analyzed and a theoretical supraspinal bladder pain network was generated.
Materials and Methods
A comprehensive literature review was performed using PubMed and Google Scholar. Relevant reviews, original research articles, and their cited references were summarized then organized on a neuroanatomical basis.
Results
The following brain loci are the most predominant in the bladder pain literature: thalamus, parabrachial nucleus, cerebral cortex, amygdala, hypothalamus, periaqueductal gray, and rostral ventromedial medulla. This review highlights each of these regions, discussing the molecular and physiological changes that occur in each during the context of bladder pain.
Conclusions
A complex network of brain loci is involved in bladder pain modulation. Studying these brain regions and the changes they undergo during the transition from acute to chronic bladder pain will provide novel therapeutic strategies for those suffering from chronic bladder pain diseases such as interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Keywords: pain, urinary bladder, animal models, neuroimaging
Introduction
The brain is responsible for integrating both the sensory and affective components of bladder pain. Many of the hallmarks associated with chronic bladder pain are mediated by the central nervous system (CNS). For instance, bladder pain is diffuse and referred to somatic structures like the knees and lower back due to the convergence of multiple primary sensory afferents on second order neurons within the spinal cord 1. Bladder pain is also associated with strong autonomic and emotional responses, implying the involvement of higher order processing within the brain itself. Undoubtedly, the peripheral nervous system plays a large role in acute bladder pain processing; intravesical lidocaine regimens ameliorate bladder pain for up to 10 days following application 2. However during the transition from acute to chronic bladder pain, a network of nociceptive brain centers is recruited to sustain pain despite a lack of constant peripheral input. Furthermore, it is likely that in the absence of effective alleviation of symptoms, the brain is responsible for frequent comorbidities associated with chronic bladder pain including depression, anxiety, and cognitive changes 3. Although numerous studies have evaluated the role of the brain in processing and mediating aspects of somatic pain, the exploration of the CNS in interstitial cystitis/bladder pain syndrome (IC/BPS) and other visceral conditions is relatively limited. A number of important distinctions between somatic and visceral pain suggest that extrapolating data from somatic studies to visceral pain may not be appropriate. These distinctions include the relatively low innervation of the viscera by primary sensory afferents, lack of conscious perception of visceral nociceptor activation, broad arborization of visceral afferents within the spinal cord, independent nerve networks within visceral organs, and distinct spinal projection tracts, amongst others 1.
Chronic bladder pain is most commonly diagnosed as IC/BPS or chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). The specific cause of these diseases is unknown and patients are only diagnosed with the disease after all other pelvic conditions with known pathologies are excluded. Research efforts have focused on addressing bladder pain from within the organ itself, however new evidence suggests that the brain plays a critical role in bladder pain maintenance and should be further investigated as a therapeutic target. This review will summarize what is currently known about the brain and brain stem's involvement in bladder pain processing.
Materials and Methods
A comprehensive literature review was performed using PubMed and Google Scholar. Keywords included supraspinal, central nervous system, brain, brainstem, bladder pain, and bladder nociception. Secondary searches were completed using the keywords thalamus, parabrachial nucleus, cortex, hypothalamus, amygdala, periaqueductal gray, rostral ventral medulla, and bladder pain after these brain regions had been identified in the primary literature search. Articles written in a language other than English were not included in the analysis. Relevant reviews, original research articles, and their cited references were summarized then organized on a neuroanatomical basis.
Results
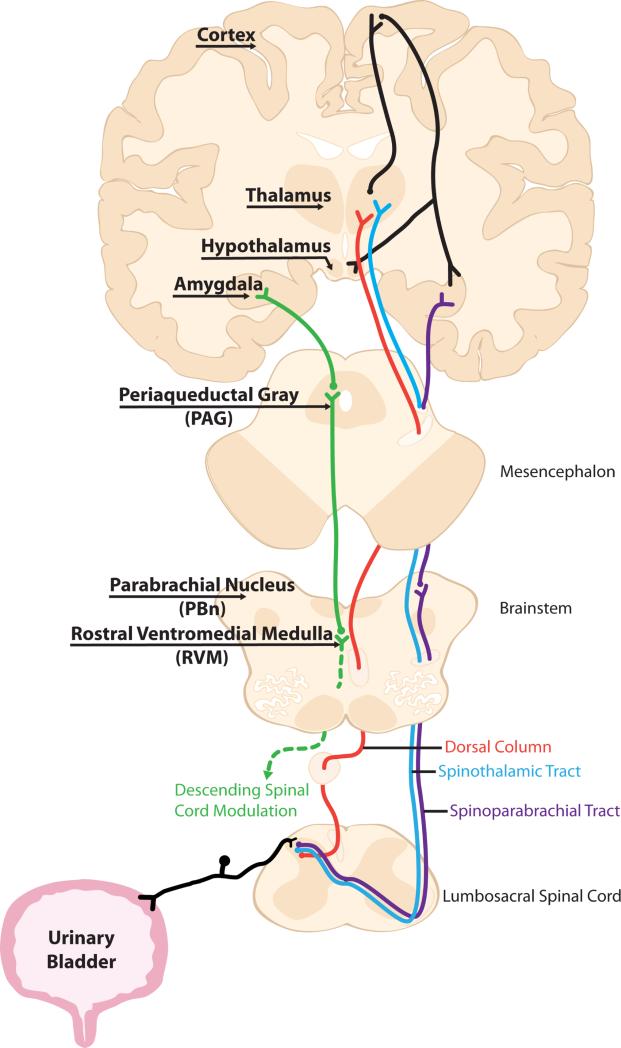
Bladder pain's complex neuroanatomical profile starts at the level of the primary afferent and compounds as it ascends to the brain. Sensory information from the urinary bladder is detected by both lightly myelinated Aδ and unmyelinated C fibers carried in the hypogastric, pelvic, and pudendal nerves 4. Second order neurons receiving visceral input are primarily located in the superficial dorsal horn (laminae I and II), and laminae V and X of the spinal cord 1. There are three main ascending tracts that visceral information is conveyed through: the spinothalamic tract (STT), spinoparabrachial tract (SPT), and dorsal column (DC) pathway (Fig 1). The remainder of this review will highlight the major supraspinal sites that have been linked to bladder pain in both preclinical animal models and chronic bladder pain patient imaging studies.
Fig 1. Ascending and descending anatomical routes capable of transmitting bladder pain.
Primary sensory afferents detect noxious signals in the bladder and relay information to second order neurons in the lumbosacral spinal cord. Sensory information is transmitted to subcortical relay centers like the thalamus and parabrachial nucleus via three main ascending tracts, the spinothalamic tract, spinoparabrachial tract, and postsynaptic dorsal column. From here, the sensory and affective components of bladder pain are processed in a number of supraspinal regions that are highly interconnected with one another. Descending bladder pain information is transmitted to the PAG before being processed by the RVM and finally returning to the spinal cord where it acts on primary sensory afferents, spinal interneurons, and motor neurons.
Thalamus
As the termination point of the STT, the thalamus is one of the first supraspinal sites involved in bladder pain processing. Recent imaging studies have revealed that women with chronic pelvic pain display decreases in left thalamus gray matter compared to healthy controls 5. Additionally, DTI studies revealed reduced fractional anisotropy (i.e. reduced white matter integrity) in the right anterior thalamic radiation to the prefrontal cortex that correlated with increased pain and urinary dysfunction, and decreased quality of life in IC/BPS patients 6.
In macaques, urinary bladder distension (UBD) primarily increases activity in ventroposterolateral nucleus (VPL) neurons, some of which directly project to the primary somatosensory cortex 7. Conversely a second smaller population of VPL cells is inhibited during UBD. Both populations however exhibit analogous changes in activity during noxious somatic stimulation of the lower body (i.e. tail, groin, hip regions); innocuous somatic stimuli does not alter cell excitability 7. In the cat and rat, UBD also excites and inhibits distinct populations of cells in the ventrobasal (VB) thalamic nucleus, a more broad anatomical area that encompasses the VPL 8, 9. However unlike in the monkey, these cells also respond to both innocuous and noxious somatic stimuli suggesting species-specific differences in thalamic mediation of peripheral inputs. Furthering this species specificity is the fact that UBD-evoked changes in neuronal activity were blocked by lesioning the dorsal midline of the rat spinal cord, suggesting that nociceptive information from the bladder in this species is primarily transmitted to the VB via the dorsal column pathway and not the traditional STT 9.
Cellular activation, as measured by transcriptional increases in the immediate early gene cFos, was noted in the paraventricular and mediodorsal nuclei of the mouse thalamus 1 and 2 hours after injection with cyclophosphamide (CYP), a cystitis-inducing compound 10. Increased cFos protein levels were also observed, but only in the paraventricular nucleus 2 hours post-injection. These CYP-induced changes in thalamic activation are partially mediated by peripheral c-fiber input; when capsaicin-sensitive c-fiber populations were depleted, there was less cFos mRNA in the thalamus following CYP injection 10.
Parabrachial Nucleus (PBn)
In addition to its well-established role in autonomic function, the PBn is a major relay in sensory processing. cFos expression is increased in over half of the PBn projections at the level of the sacral parasympathetic nucleus following instillation of formalin, a noxious chemical, in the bladder 11. cFos expression is also increased in the lateral PBn nucleus following this treatment and in the single injection CYP-cystitis model 12, 13. Additionally, increases in tyrosine hydroxylase were observed in the PBn of cats diagnosed with idiopathic feline IC 14. Tyrosine hydroxylase is one of the rate-limiting enzymes involved in the production of catecholamines like dopamine and norepinephrine. Increases in norepinephrine are strongly associated with the stress response, thus potentially linking PBn activation to the affective component of IC/BPS 14.
The firing rate of PBn neurons is also affected by nociceptive cues arising from the bladder. Specifically, intravesicular acetic acid increases the firing rate of a subset of neurons in the lateral PBn 15. A second subset of previously silent neurons also begins to fire following this manipulation 15. The additional nociceptive input coming from this second set of cells may contribute to central sensitization by decreasing the activation threshold of PBn projection terminations.
Cerebral Cortex
Recent imaging studies generated from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) network data have provided insight into many of the changes that occur in the cortex of bladder pain patients. Specifically, bilateral increases in gray matter are observed in the primary somatosensory cortex (SI) of CP/CPPS patients and the right SI of IC/BPS patients 16, 17. In the second patient population, volumetric increases in the pelvic area of the homunculus correlated with increased pain, anxiety, and urinary dysfunction 17. The bilateral nature of the increases observed in CP/CPPS patients is likely due to the fact that the bladder sends sensory information to both sides of the spinal cord. It is unknown why IC/BPS patients exhibit lateralized increases in SI gray matter.
In addition to gray matter volumetric changes, resting cortical oscillation states also change in the context of bladder pain. Compared to healthy controls, IC/BPS patients exhibit increased oscillation frequency powers in SI, primary motor cortex, and ventral/medial supplemental motor areas (SMA); the opposite trend was observed in the posterior insula 18. Increased connectivity was observed between sensorimotor regions like the ventral/medial SMA and the cerebellum and red nucleus. Alterations in this sensorimotor network positively correlated with increased bladder pain during filling, and might underlie pelvic floor dysfunction that typically accompanies IC/BPS 18. Animal models have also been used to study cortical state during active instances of bladder pain. In anesthetized rats, both topical application of capsaicin to the bladder and UBD result in cortical desynchronization (i.e. cortical arousal) 19. The latency to return to a synchronized state varies between the two stimuli however, with chemically induced desynchronization far outlasting that produced by acute mechanical perturbations. This suggests that chronic bladder pain patients may be in a constant state of cortical desynchronization due to sensitization of primary afferents that may result from a barrage of inflammatory mediators continuously assaulting those fibers located in the bladder epithelium.
Like many other regions highlighted in this paper, the cerebral cortex shows increased cFos expression following a single injection of CYP 20. This cortical activation may be partially mediated by peripheral purinergic signaling as intraperitoneal administration of a P2X7 receptor antagonist prior to CYP significantly reduces both cortical and spinal cFos expression. Physiologically, increased activity in the cerebral cortex has been observed in certain cell populations during UBD. Conversely, a second set of cortical neurons are inhibited during UBD 21. Both populations maintain a receptive field in the hand as well as in the bladder.
Amygdala
The amygdala, and specifically the central nucleus (CeA), is member of the limbic system that is strongly indicated in visceral pain processing. Neuroimaging studies have shown increased gray matter in the left amygdala of patients suffering from chronic pelvic pain 16. As a preliminary indicator of bladder pain involvement in rodents, increased levels of cFos protein were observed in the CeA following a single CYP injection 22. In an opposing top-down approach, non-specific optogenetic activation of the right CeA resulted in increased pain-like responses during bladder distension, demonstrating this loci's ability to modulate sensory information arising from the viscera 23.
On the molecular level, bladder pain has been linked to a specific G-protein coupled receptor in the CeA. Administration of agonists and antagonists of metabotropic glutamate receptor 5 (mGluR5) into the CeA increase and decrease UBD-evoked pain-like responses respectively by altering CeA neuronal excitability 23. Furthermore, genetic disruption of mGluR5 in the right CeA decreases both bladder pain and dorsal horn spinal phosphorylation of extracellular signal-regulated kinase (ERK), a common marker of nociceptive-induced neuronal activation, following UBD 23. Asymmetrical involvement of the left and right CeA in somatic pain processing has been reported, but the specific contribution of the left CeA in bladder pain remains unknown 24.
Chronic bladder pain is often accompanied by affective disturbances, some of which may be mediated by the neuroendocrine workings of the amygdala. IC/BPS patients demonstrate amygdala-mediated defense responses when presented with a visceral threat, indicating increased limbic activity in IC/BPS pathophysiology 25. Additionally, increases in corticotropin releasing hormone (CRH) mRNA, a neurotransmitter released under stress conditions, are observed in the CeA following single-injection CYP treatment 26. Acute application of corticosterone (CORT), a stress hormone, to the CeA resulted in increased UBD-evoked pain-like responses 23. When CORT was chronically applied to the CeA, UBD not only triggered increased pain-like responses, but also increased the excitability of spinal sensory neurons receiving input from the bladder, indicating a role for this supraspinal center in the development of emotionally-evoked bladder hyperalgesia 27. Alternatively, increased spinal excitability may also be due to global increases in CORT brought on by augmentation of the stress hypothalamic pituitary adrenal (HPA) axis, which can be modulated by the CeA.
Hypothalamus
Like the amygdala, the hypothalamus is involved in neuroendocrine responses to bladder pain due to its role as the head of the HPA axis. Instillation of 5% formalin in the bladder caused increased cFos expression in the paraventricular nucleus (PVN), a region of the hypothalamus that maintains neural control of the pituitary gland 13. Additionally, both the PVN and arcuate nucleus show increased galanin expression for 48 hours following a single CYP injection 26. Galanin is an inhibitory neuropeptide that has different functional effects depending upon its site of action; increased galanin in the arcuate nucleus has antinociceptive effects in somatic pain models 28. If a similar anti-nociceptive role for galanin exists in visceral pain, then the arcuate nucleus may be responsible for on-going pain inhibitory tone during injury that would allow an animal to still respond to additional stimuli beyond an initial insult. In addition, if this anti-nociceptive system was dysregulated, it is possible that mild stimulation of the bladder (e.g. normal filling in an IC/BPS patient) may induce a pain response. UBD has no effect on neuronal activity in the lateral area of the anterior hypothalamus 29. This could be due to the site of recording or the mechanical nature of the stimulus and the fact that it is being applied to a lightly anesthetized animal as opposed to instillation of a chemically active mediator in a conscious animal.
The PVN of both mice and rats also exhibits increased levels of CRH following a single CYP injection 26, 30. Following release from the PVN, CRH induces adrenocorticotropic hormone (ACTH) secretion from the pituitary gland, leading to increased production of corticosteroids in the adrenal cortex which negatively feedback onto the hypothalamus and pituitary. Appropriately then, CYP injections also resulted in increased ACTH serum levels 30. As with clinical patient populations, increased stress can lead to increased bladder pain in animal models. Chronic footshock, which increases CRH expression in the PVN, has been shown to augment pain-like responses during noxious bladder distension 31. Administration of oxytocin, another hormone synthesized by the hypothalamus, decreases bladder pain-like responses acting as both an anxiolytic and an analgesic agent 32.
Periaqueductal Gray (PAG)
Located in the midbrain, the PAG is a well-characterized member of the descending modulatory pain nexus. However to date, most studies involving the bladder and PAG have focused on the spinal cord/PAG/pontine micturition center (PMC) connections responsible for maintaining urinary continence. Under normal circumstances, this arc is responsible for directing urethral sphincter and bladder wall muscle activity so as to maintain bladder control; inhibition of the PAG results in attenuation of these processes 33. The majority of micturition reflex communication is low threshold mechanosensory information. In one of the only studies to investigate PAG processing of chemosensory (i.e. nociceptive) cues, increased cFos expression was observed in the PAG following intravesical acetic acid instillation 34.
Rostral Ventromedial Medulla (RVM)
Located in the brainstem, the RVM is a structure, like its anatomically connected partner the PAG, that is commonly associated with descending pain modulation. cFos expression in the nucleus raphe magnus (NRM) subdivision of the RVM following bladder distension suggests that the RVM is involved in bladder pain processing 13. More detailed experiments examined the firing rates of these cells in both the cat and the rat. Two distinct classes of cells were identified: one set was inhibited by bladder distension and the second was excited; a subset of neurons from each class however was excited by PAG stimulation, providing evidence for the involvement of the PAG-RVM circuit in bladder pain processing 35. The bidirectional firing properties observed following bladder distension align well with the three classical RVM cell types: on-cells which fire immediately prior to nociceptive cues, off-cells which are inhibited prior to nociceptive instances, and neutral cells that do not exhibit activity changes in during nociception. Electrical stimulation of the RVM induced both inhibition and, less frequently, facilitation of UBD-evoked pain-like responses, once again echoing the nociceptive dichotomy demonstrated by on- and off-cells 36. The relative activity of these two RVM projection neuron classes likely determines whether bladder pain is increased or decreased by directly modulating activity in primary sensory afferents, including those arising from the bladder base and body, second and third order neurons, and interneurons within the dorsal horn 37.
Contrary to stimulation, lesions of the RVM resulted in increased pain-like responses to UBD, but only in animals with inflamed bladders 36. Recruitment of pro-nociceptive RVM function during inflammatory bladder pain may arise from the enlistment of serotonergic signaling 38 or the development of central sensitization. This phenomenon has been observed in other visceral pain states; following intracolonic instillation of capsaicin, on-cells exhibited increased firing to noxious colorectal distension and novel firing to previously innocuous distension pressures 39. Whether or not this sensitization also exists following bladder inflammation remains to be seen.
Discussion
Bladder pain is a complex physiological process that involves the integration of sensory and affective signals across the brain (Table 1). Following primary afferent activation in the periphery, sensory information traverses either the STT or dorsal column to arrive in the thalamus, the SPT to arrive in the PBn, or the STT to arrive in the medullary lateral reticular nucleus 4, 40. All three of these regions exhibit electrophysiological and molecular changes during bladder pain. Of note, neurons in both the thalamic and medullary nuclei also exhibit changes during noxious stimulation of the colon and skin implicating these regions as potential generation sites for the anatomical phenomenon known as referred pain 7-9, 40. Resulting from the convergence of somatic and visceral sensory afferents onto the same cortical projection neurons, referred pain is the tangible representation of nociceptive events occurring internally; in the case of IC/BPS patients, bladder pain manifests as pain in the lower back knees.
Table 1.
Summary of molecular, structural, and physiological changes in the brain during bladder pain.
| Brain Region | Experimental Manipulation | Clinical Presentation | |||
|---|---|---|---|---|---|
| CYP Injection | Intravesical Inflammatory Agent | UBD | IC/BPS | CP/CPPS | |
| Thalamus | ↑ cFos expression10 | ↑/↓ activity7,8,9 | ↓ white matter integrity6 | ||
| PBn | ↑ cFos expression12 | ↑cFos expression13 ↑ activity15 |
↑ tyrosine hydroxylase14 (feline IC) | ||
| Cortex | ↑ cFos expression20 | ↑ desynchronization19 | ↑ desynchronization19 ↑/↓ activity21 |
↑ gray matter17 ↑/↓ oscillation frequency powers18 |
↑ gray matter16 |
| Amygdala | ↑ cFos expression22 ↑ CRH expression26 |
↑ gray matter16 | |||
| Hypothalamus | ↑ galanin expression26 ↑ CRH expression26,30 |
↑ cFos expression13 | no change29 | ||
| PAG | ↑ cFos expression34 | ||||
| RVM | ↑/↓ activity35 ↑ cFos expression13 |
||||
The thalamus is the main source of input to the cerebral cortex. During innocuous bladder distension, the insular, anterior cingulate (ACC), and prefrontal cortices all show increased activity 41. Neither innocuous nor noxious bladder distension has been performed in chronic bladder pain patients as of yet, however resting state fMRI and voxel based morphometry have identified alterations in regional connectivity, intrinsic cortical oscillations, and gray matter densities in IC/BPS and CP/CPPS patients. CP/CPPS patients exhibit activity differences in the anterior insula during spontaneous pain and increased gray matter density in the anterior insula and ACC 42, while no study has shown activity or gray matter density changes in these regions in female IC/BPS patients.
The PBn sends substantial projections to the hypothalamus and the CeA 43. Following CYP treatment, expression of CRH, a stress response trigger, is observed in both of these regions positioning them as supraspinal initiators of the affective disturbances commonly experienced by bladder pain patients 26. Centrally mediated pharmacological management of these symptoms can reduce bladder pain; daily administration of amitriptyline, a tricyclic antidepressant, decreased clinical IC symptoms in cats suffering from idiopathic feline IC despite no improvement in urothelium integrity 44. Similarly, oxytocin-induced reductions in pain-like responses during bladder distension are partially mediated by the hormone's anxiolytic effects 32. Unlike the hypothalamus, which also receives thalamic input via cortical connections, the PBn and amygdala are not activated during fMRI in the context of innocuous bladder distension 41. Following repeated episodes of bladder pain however, the spinoparabrachio-amygdaloid pathway may be recruited to sustain nociceptive input to subcortical regions in the absence of peripheral input, facilitating the transition from acute to chronic bladder pain.
Despite extensive inquiry in the context of normal micturition, very little is known about the PAG and its role in bladder pain. Aside from a report of increased cFos expression following acetic acid intravesical instillation, the PAG has only been referenced in studies that focus on its anatomical partner, the RVM. Via endogenous PAG opioid signaling, the RVM is capable of inhibiting nociceptive signaling in the dorsal horn. Intraperitoneal administration of the opioid receptor antagonist naloxone reduced RVM-stimulation-induced inhibition of pain-like responses to bladder distension reinforcing the established role of endogenous opioids signaling in bladder nociception 36. This signaling is disrupted in a rodent model of early life bladder inflammation, mimicking patient populations in which previous urinary insults increase the probability of developing IC/BPS later in life 45. As the final supraspinal location on bladder pain's descending route, the RVM is also a prime candidate for central sensitization. Evidence for this phenomenon is the fact that lesions of the RVM only increase pain-like responses to bladder distension in the context of pre-existing inflammation, suggesting that prior injury lowers activation thresholds 36.
In order to develop more appropriate treatment options for chronic bladder pain patients, we need to learn about the supraspinal changes that occur during the development of chronic conditions like IC/BPS. To probe this question however, more appropriate animal models need to be developed. By definition, most animal models of bladder pain are more acute in nature than chronic. UBD is a transient stimulus, with ramifications lasting only minutes after stimulus completion. In the single CYP injection and inflammatory instillation (e.g. formalin, acetic acid, etc.) models, measures are taken shortly after treatment when there is still significant damage to the bladder urothelium. Repeated low dose administration of CYP is somewhat extended since little urothelium damage remains at the end of the injection paradigm. However, if this treatment were able to maintain pelvic hypersensitivity for weeks in the absence of additional CYP doses, it would be even more valid. The early life bladder inflammation model accurately reflects many of the symptoms that chronic bladder pain patients experience; following repeated inflammatory insult to the urothelium during the neonatal period, adult rodents experience increased urinary frequency, decreased micturition thresholds, and increased pain-like responses to bladder distension 46. Perhaps the most reliable of all animal models is naturally occurring feline IC. However, due to the sporadic development of this disease, limited data has been generated using this model.
Since CP/CPPS is a diagnosis limited to men and there is a 10:1 preponderance of female IC/BPS patients, supraspinal disparities between these diseases are most likely due to sex hormones, the estrous/menstrual cycle, or psychosocial factors that differentially affect the sexes 47. Ovariectomy in rats decreased pain-like responses to bladder distension while dynamic changes in estrogen levels increased responses 48. Despite colloquial speculation, there is limited research to support the effects of the menstrual cycle on bladder pain in healthy subjects. IC/BPS patients report the highest pain during the perimenstural period, the two days prior to and following the start of a new menstrual cycle; healthy controls do not exhibit cycle-dependent changes in bladder pain 49. Similarly, rats with inflamed bladders show increased pain-like responses to bladder distension during metestrus and proestus, the phases of the estrous cycle during which the rodent is not sexually receptive; saline-treated animals do not exhibit cycle-dependent fluctuations in pain 50. These data suggest a role for sex hormones in pain sensitivity, but only in chronic pain states and not in acute instances of bladder pain.
Conclusions
The neuroanatomical route of bladder pain is complex. Although the peripheral nervous system is responsible for the initial response to noxious stimuli, it is the CNS that is responsible for the affective and autonomic disturbances that accompany bladder pain. In chronic diseases like IC/BPS, the CNS undergoes molecular, physiological, and structural changes that result in pain despite a lack of noxious input from the periphery. Investigating these supraspinal changes will identify new therapeutic strategies to help those suffering from IC/BPS and other chronic pelvic diseases.
Abbreviations
- ACTH
adenocorticotropic hormone
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CNS
central nervous system
- CORT
corticosterone
- CP/CPPS
chronic prostatitis/chronic pelvic pain syndrome
- CRH
corticotropin releasing hormone
- CYP
cyclophosphamide
- DTI
diffusion tensor imaging
- fMRI
functional magnetic resonance imaging
- HPA
hypothalamus pituitary adrenal
- IC/BPS
interstitial cystitis/bladder pain syndrome
- MAPP
Multidisciplinary Approach to the Study of Chronic Pelvic Pain
- NIDDK
National Institute of Diabetes, Digestive, and Kidney Diseases
- PBn
parabrachial nucleus
- PMC
pontine micturition center
- PSDC
postsynaptic dorsal column
- PVN
paraventricular nucleus of the hypothalamus
- RVM
rostral ventromedial medulla
- SI
primary somatosensory cortex
- SPT
spinoparabrachial tract
- STT
spinothalamic tract
- UBD
urinary bladder distension
- VMR
visceromotor response
- VPL
ventroposterolateral nucleus of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910. doi: 10.1111/j.1464-410X.2008.08162.x. [DOI] [PubMed] [Google Scholar]
- 3.Nickel JC, Tripp DA, Pontari M, et al. Interstitial Cystitis/Painful Bladder Syndrome and Associated Medical Conditions With an Emphasis on Irritable Bowel Syndrome, Fibromyalgia and Chronic Fatigue Syndrome. The Journal of Urology. 2010;184:1358. doi: 10.1016/j.juro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.de Groat WC, Griffiths D, Yoshimura N. Neural Control of the Lower Urinary Tract. Comprehensive Physiology. 2015;5:327. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmer MA, Huang L, Martucci K, et al. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. J Urol. 2015;194:118. doi: 10.1016/j.juro.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler MJ, Hobbs SF, Fu Q-G, et al. Responses of neurons in ventroposterolateral nucleus of primate thalamus to urinary bladder distension. Brain Research. 1992;571:26. doi: 10.1016/0006-8993(92)90506-5. [DOI] [PubMed] [Google Scholar]
- 8.Brüggemann J, Vahle-Hinz C, Kniffki KD. Representation of the urinary bladder in the lateral thalamus of the cat. J Neurophysiol. 1993;70:482–491. doi: 10.1152/jn.1993.70.2.482. [DOI] [PubMed] [Google Scholar]
- 9.Robbins MT, Uzzell TW, Aly S, et al. Characterization of thalamic neuronal responses to urinary bladder distention, including the effect of acute spinal lesions in the rat. J Pain. 2006;7:218. doi: 10.1016/j.jpain.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Nishii H, Nomura M, Fujimoto N, et al. Thalamic neural activation in the cyclophosphamide-induced visceral pain model in mice. Neuroscience Research. 2008;60:219. doi: 10.1016/j.neures.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Ding YQ, Qin BZ, Li JS, et al. Induction of c-fos-like protein in the spinoparabrachial tract-neurons locating within the sacral parasympathetic nucleus in the rat. Brain Research. 1994;659:283. doi: 10.1016/0006-8993(94)90894-x. [DOI] [PubMed] [Google Scholar]
- 12.Bon K, Lantéri-Minet M, de Pommery J, et al. Cyclophosphamide cystitis as a model of visceral pain in rats. A survey of hindbrain structures involved in visceroception and nociception using the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1996;108:404. doi: 10.1007/BF00227263. [DOI] [PubMed] [Google Scholar]
- 13.Rodella L, Rezzani R, Gioia M, et al. Expression of Fos immunoreactivity in the rat supraspinal regions following noxious visceral stimulation. Brain Research Bulletin. 1998;47:357. doi: 10.1016/s0361-9230(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 14.Reche A, Jr, Buffington CAT. INCREASED TYROSINE HYDROXYLASE IMMUNOREACTIVITY IN THE LOCUS COERULEUS OF CATS WITH INTERSTITIAL CYSTITIS. The Journal of Urology. 1998;159:1045. doi: 10.1016/s0022-5347(01)63833-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Allen GV, Downie JW. Parabrachial nucleus influences the control of normal urinary bladder function and the response to bladder irritation in rats. Neuroscience. 2007;144:731. doi: 10.1016/j.neuroscience.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 16.Bagarinao E, Johnson KA, Martucci KT, et al. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014;155:2502. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kairys AE, Schmidt-Wilcke T, Puiu T, et al. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Patients with Interstitial Cystitis/Painful Bladder Syndrome. The Journal of Urology. 2015;193:131. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. The Journal of Urology. 2014;192:947. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conte B, Cutrufo C, Manzini S. Electrocorticographic desynchronization after application of visceral and somatic noxious stimuli in urethane-anesthetized rats: effect of intrathecal administration of tachykinin (NK 1 or NK 2) receptor antagonists. Journal of Pharmacology and Experimental Therapeutics. 1996;276:212. [PubMed] [Google Scholar]
- 20.Martins JP, Silva RBM, Coutinho-Silva R, et al. The role of P2X7 purinergic receptors in inflammatory and nociceptive changes accompanying cyclophosphamide-induced haemorrhagic cystitis in mice. British Journal of Pharmacology. 2012;165:183. doi: 10.1111/j.1476-5381.2011.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brüggemann J, Shi T, Apkarian AV. Viscero-somatic neurons in the primary somatosensory cortex (SI) of the squirrel monkey. Brain Research. 1997;756:297. doi: 10.1016/s0006-8993(97)00296-5. [DOI] [PubMed] [Google Scholar]
- 22.Bon K, Lantéri-Minet M, Michiels JF, et al. Cyclophosphamide cystitis as a model of visceral pain in rats: a c-fos and Krox-24 study at telencephalic levels, with a note on pituitary adenylate cyclase activating polypeptide (PACAP). Exp Brain Res. 1998;122:165. doi: 10.1007/s002210050504. [DOI] [PubMed] [Google Scholar]
- 23.Crock LW, Kolber BJ, Morgan CD, et al. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci. 2012;32:14217. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrasquillo Y, Gereau RW., 4th Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twiss C, Kilpatrick L, Craske M, et al. Increased Startle Responses in Interstitial Cystitis: Evidence for Central Hyperresponsiveness to Visceral Related Threat. The Journal of Urology. 2009;181:2127. doi: 10.1016/j.juro.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishii H, Nomura M, Aono H, et al. Up-regulation of galanin and corticotropin-releasing hormone mRNAs in the key hypothalamic and amygdaloid nuclei in a mouse model of visceral pain. Regul Pept. 2007;141:105. doi: 10.1016/j.regpep.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal Neuronal Responses to Urinary Bladder Stimulation in Rats With Corticosterone or Aldosterone Onto the Amygdala. J Neurophysiol. 2003;90:2180. doi: 10.1152/jn.00298.2003. [DOI] [PubMed] [Google Scholar]
- 28.Sun YG, Gu XL, Lundeberg T, et al. An antinociceptive role of galanin in the arcuate nucleus of hypothalamus in intact rats and rats with inflammation. Pain. 2003;106:143. doi: 10.1016/s0304-3959(03)00316-6. [DOI] [PubMed] [Google Scholar]
- 29.Snowball RK, Semenenko FM, Lumb BM. Visceral inputs to neurons in the anterior hypothalamus including those that project to the periaqueductal gray: a functional anatomical and electrophysiological study. Neuroscience. 2000;99:351. doi: 10.1016/s0306-4522(00)00203-7. [DOI] [PubMed] [Google Scholar]
- 30.Mineta K, Nomura M, Terado M, et al. Upregulation of corticotropin-releasing hormone gene expression in the paraventricular nucleus of cyclophosphamide-induced cystitis in male rats. Brain Research. 2004;1018:193. doi: 10.1016/j.brainres.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 31.Robbins MT, Ness TJ. Footshock-Induced Urinary Bladder Hypersensitivity: Role of Spinal Corticotropin-Releasing Factor Receptors. The Journal of Pain. 2008;9:991. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black LV, Ness TJ, Robbins MT. Effects of Oxytocin and Prolactin on Stress-Induced Bladder Hypersensitivity in Female Rats. The Journal of Pain. 2009;10:1065. doi: 10.1016/j.jpain.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura S, Allen GV, Downie JW. Volume-evoked micturition reflex is mediated by the ventrolateral periaqueductal gray in anesthetized rats. Am J Physiol. 1998;275:R2049–R2055. doi: 10.1152/ajpregu.1998.275.6.R2049. [DOI] [PubMed] [Google Scholar]
- 34.Mitsui T, Kakizaki H, Matsuura S, et al. Chemical bladder irritation provokes c-fos expression in the midbrain periaqueductal gray matter of the rat. Brain Research. 2003;967:81. doi: 10.1016/s0006-8993(02)04226-9. [DOI] [PubMed] [Google Scholar]
- 35.Snowball RK, Dampney RA, Lumb BM. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp Physiol. 1997;82:485. doi: 10.1113/expphysiol.1997.sp004041. [DOI] [PubMed] [Google Scholar]
- 36.Randich A, Mebane H, DeBerry JJ, et al. Rostral ventral medulla modulation of the visceromotor reflex evoked by urinary bladder distension in female rats. J Pain. 2008;9:920. doi: 10.1016/j.jpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 38.Randich A, Shaffer AD, Ball CL, et al. Serotonergic and noradrenergic facilitation of the visceromotor reflex evoked by urinary bladder distension in rats with inflamed bladders. Neuroscience Letters. 2008;442:253. doi: 10.1016/j.neulet.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanoja R, Tortorici V, Fernandez C, et al. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. European Journal of Pain. 2010;14:120, e1. doi: 10.1016/j.ejpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins MT, Uzzell TW, Aly S, et al. Visceral nociceptive input to the area of the medullary lateral reticular nucleus ascends in the lateral spinal cord. Neurosci Lett. 2005;381:329. doi: 10.1016/j.neulet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths D, Derbyshire S, Stenger A, et al. Brain control of normal and overactive bladder. J Urol. 2005;174:1862. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 42.Farmer MA, Chanda ML, Parks EL, et al. Brain Functional and Anatomical Changes in Chronic Prostatitis/Chronic Pelvic Pain Syndrome. The Journal of Urology. 2011;186:117. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- 44.Chew DJ, Buffington CA, Kendall MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc. 1998;213:1282. [PubMed] [Google Scholar]
- 45.DeBerry J, Ness TJ, Robbins MT, et al. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randich A, Uzzell T, DeBerry JJ, et al. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 47.Clemens JQ, Meenan RT, Rosetti MC, et al. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98. doi: 10.1097/01.ju.0000146114.53828.82. [DOI] [PubMed] [Google Scholar]
- 48.Robbins MT, Mebane H, Ball CL, et al. Effect of estrogen on bladder nociception in rats. J Urol. 2010;183:1201. doi: 10.1016/j.juro.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- 50.Ball CL, Ness TJ, Randich A. Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distention. J Urol. 2010;184:1529. doi: 10.1016/j.juro.2010.05.090. [DOI] [PubMed] [Google Scholar]